Abstract
Objective
To quantify health care resource utilization (HCRU) and costs associated with insomnia treated with commonly prescribed insomnia medications among patients with depression.
Methods
A retrospective cohort study was conducted using IBM MarketScan Commercial and Medicare Supplemental Databases to identify adults with: (1) ≥1 ICD-9/ICD-10 code for depression; (2) ≥1 commonly prescribed medication for insomnia (zolpidem immediate release [IR], zolpidem extended release [ER], trazodone, or benzodiazepines); and (3) ≥12 months of eligibility before and after initiating insomnia medication. A 1:1 age- and sex-matched control cohort with depression but without sleep-related disorders was identified. Adjusted HCRU and costs were compared using generalized linear models.
Results
A total of 21,027 patients (mean age = 48.3 years, 69.5% female) with depression and treated insomnia (D + TI; 1.9% zolpidem ER, 32.0% zolpidem IR, 50.0% trazodone, 16.1% benzodiazepines) were matched to controls. Although mean number of inpatient visits were similar (0.1 for both), relative to controls, D + TI had a higher mean number of ED (0.2 vs 0.1, p < .001) and outpatient visits (2.2 vs 1.3, p < .001). Adjusted total costs per patient per month were higher among D + TI patients ($2450 vs $1095, p < .001). Inpatient and ED costs were higher among patients prescribed zolpidem IR, trazodone, or benzodiazepines, but not zolpidem ER.
Conclusions
Relative to controls with depression but without sleep disorders, overall, health care costs for adults with D + TI were 2.2-fold higher; costs and HCRU varied by insomnia medication. Further study of the impact of newer insomnia treatments on patient outcomes in depression and comorbid insomnia is warranted.
Introduction
Depression incurs a major public health burden in the US and worldwide, and is associated with increased morbidity, mortality, and economic costsCitation1. Sleep disturbance is a key symptom of depression, and insomnia, defined as difficulty initiating and/or maintaining sleep with associated daytime impairment, is highly prevalent among individuals with depressionCitation2. Approximately 4 in 5 patients with depression have at least 1 insomnia symptomCitation3, and an estimated 3 in 4 patients with depression have difficulty initiating or maintaining sleepCitation4,Citation5. There is likely a bi-directional relationship between insomnia and depressionCitation6; relative to individuals without insomnia, those with insomnia are considerably more likely to suffer from clinically significant depressionCitation7,Citation8.
Between 2005 and 2010, the incremental economic burden of major depressive disorders (MDDs) in the US increased by 21.5% (from $173.2 billion to $210.5 billion), driven by a rise in direct medical costsCitation9. Total annual costs of insomnia in the US are estimated at $77–116B, largely due to indirect cost burden ($2–6B direct, $75–100B indirect)Citation10, and costs associated with insomnia may comprise a substantial proportion of depression-related indirect costs (i.e. lost productivity, absenteeism, presenteeism)Citation11. Among individuals with depression, sleep disturbances are associated with additional treatment-seeking for symptom management, thereby increasing the burden on health care systemsCitation2.
Treatment of insomnia in patients with depression might positively impact outcomes, particularly given the strong link between sleep disorders and MDD associated suicidal tendenciesCitation12,Citation13. The American Academy of Sleep Medicine (AASM) supports a multi-component approach to insomnia treatment including evaluation and treatment of comorbid conditions that may contribute to insomnia, use of cognitive behavioral therapies for insomnia (CBT-I), evaluation of prior treatment response, safety and availability of treatments, and patient preference and cost considerationsCitation10. Not all patients may have access to or benefit from CBT-I, and as such, pharmacotherapy plays a role in insomnia managementCitation10.
Pharmacotherapy options indicated for sleep onset and maintenance insomnia include non-benzodiazepine sedative hypnotics, or “Z-drugs,” such as eszopiclone, zaleplon and zolpidem, and various benzodiazepine drugsCitation10. While some benzodiazepines have an FDA approved indication for insomnia (e.g. temazepam), other benzodiazepines (e.g. alprazolam, lorazepam) do not but are still prescribed for insomniaCitation10. Trazodone is one of the most frequently prescribed medications for insomnia managementCitation14,Citation15, although it is not indicated for insomnia nor recommended by AASM to treat insomnia due to a lack of supporting efficacy dataCitation10. The most common adverse effects associated with trazodone include drowsiness and dizzinessCitation16, and among older patients, the use of low-dose trazodone may have a comparable risk of falls as compared to benzodiazepinesCitation17. Benzodiazepines have been associated with lingering next-day effects, cognitive and memory impairment, and increased risk of auto accidents, falls, and dependenceCitation18,Citation19. Mental health impacts associated with the coronavirus disease 2019 (COVID-19) pandemic have been linked to recent increases in benzodiazepine prescribing in the USCitation20, and more adults have reported experiencing depressive symptomsCitation21. Z-drugs have been associated with next day cognitive, memory, psychomotor, and balance impairments and a risk of dependenceCitation18,Citation19. Outcomes associated with insomnia management with commonly prescribed medications (including trazodone and benzodiazepines) among patients with depression have not been widely characterized, and adverse effects of these medications may be associated with poorer clinical outcomes as well as higher health care resource utilization (HCRU) and health care costs.
The objective of the current study was to quantify the impact of insomnia treated with commonly used, commonly prescribed insomnia medications (benzodiazepines, trazodone, and z-drugs) on health care cost and utilization among a national sample of patients with depression. We hypothesized that relative to matched individuals with depression but without sleep disorders, individuals with depression and treated insomnia (D + TI) would demonstrate considerably greater health care resource utilization and costs.
Methods
Data source
This retrospective cohort study used the IBM MarketScanFootnotei Commercial and Medicare Supplemental Databases. It included claims from January 1, 2011 to September 30, 2018 (study period) containing longitudinal data on health care services for >41 million persons in US with health-care coverage. The Commercial database encompasses a range of health plan types, including preferred provider organizations (PPOs), exclusive provider organizations (EPOs), point of service (POS) plans, indemnity plans, health maintenance organizations (HMOs), and consumer-directed health plans (CDHPs). The database contains administrative data that includes physician office visits; hospitalizations; retail, mail order and specialty pharmacy; and mental health care services. The Medicare Supplemental Database captures costs associated with employer-sponsored supplemental coverage to Medicare. MarketScan databases meet HIPAA requirements for fully de-identified data sets, and as the study was observational in nature and utilized de-identified patient data from employer-provided health insurance plans, it was exempt from Institutional Review Board (IRB) review.
Study population
Patients with D + TI were required to have a diagnosis of depression and at least 1 prescription for an insomnia medication of interest during the study period; the earliest fill date for an insomnia medication from January 1, 2012 to September 30, 2017 (identification period) was identified as the “index date.” Eligible patients were required to be aged 18 years or older at the study index date, with at least 12 months of continuous health plan enrollment with full medical and drug coverage before the index date (i.e. “baseline” period). Patients were also required to have at least 12 months of continuous enrollment post-index date (i.e. “follow-up” period). Patients were required to have ≥1 ICD-9 or ICD-10 corresponding to depression during the baseline period or on the index date. Depression was defined as patients with MDD, bipolar I depression, dysthymic disorder/persistent depressive disorder, adjustment disorder, other cerebral degeneration, chronic depressive personality disorder, post-schizophrenic depression, recurrent depressive disorder and/or mixed anxiety and depressive disorder. Patients with missing information related to age and/or sex were excluded.
D + TI patients were required to have at least 1 prescription fill for a medication of interest with an FDA-approved indication for insomnia treatment or trazodone ≤100 mg or at least 1 off-label insomnia treatment claim coupled with at least 1 physician-assigned ICD-9/ICD-10 insomnia diagnosis code within 12 months prior to first insomnia medication claim. Medications of interest included zolpidem immediate-release (IR), zolpidem extended-release (ER), trazodone, and benzodiazepines (as a class). Some benzodiazepines have an FDA-approved indication for insomnia (estazolam, flurazepam, temazepam, triazolam, quazepam) while others do not (clonazepam, lorazepam, alprazolam); patients prescribed benzodiazepines without an insomnia indication were required to also have an insomnia diagnosis code. Trazodone ≤100 mg daily was the threshold identified for use in insomnia management (≥150 mg/day is the recommended dosage for treatment of depression). Patients were excluded for the following: 1. the presence of a prescription drug claim for a fill of any insomnia medication of interest within the 12-month period before the index date; 2. claims for a single insomnia treatment with ≤5 days’ supply; and 3. an index prescription for benzodiazepines and an anxiety diagnosis during the 12-month baseline period. Patients on polypharmacy with at least two of the study medications of interest used concurrently during the study period were not excluded from the sample, as they represented <4% of the total population (data not shown). Patients with claims for two or more insomnia medications of interest on the index date were assigned to a treatment group based on the following hierarchy: zolpidem ER, zolpidem IR, trazodone, benzodiazepines.
To evaluate health care costs and HCRU associated with insomnia treated with older-generation medications, D + TI patients were matched to controls with a depression diagnosis, but no sleep disorders or procedures or insomnia treatment. Thus, D + TI patients were matched 1:1 by age and sex to patients with a depression diagnosis who were not treated for insomnia and did not have a diagnosis of sleep disorder. For the matched control group, the index date was defined as the index date of the matched D + TI patient; matched controls were required to have continuous health plan enrollment ≥12 months before, after, and including the index date. Control patients were excluded for any of the following reasons: 1. evidence of insomnia medication (insomnia study medications of interest); 2. insomnia diagnosis (ICD9 or ICD10 for insomnia); or 3. sleep-related disorders (ICD9 or ICD10 for hypersomnia, sleep-related breathing disorders, circadian rhythm sleep disorders, parasomnia, sleep-related movement disorders, and drug-induced sleep disorders, or procedure codes for sleep study procedures, sleep service codes, home sleep apnea testing, and durable medical equipment sleep medicine codes).
Study measures
HCRU and cost
Outcome measures included HCRU and health care cost, which included the following points of service: inpatient, emergency department (ED), outpatient visits, and prescription medication use. Inpatient visits were based on inpatient admission records, while ED visits were identified as outpatient visits with emergency room as a place of service. In addition, variables pertaining to provider type, point of service, and facility bill type were used to identify specific visit types. Prescription medications dispensed were assessed using administrative pharmacy claims. All-cause health care costs were calculated as the sum of all medical expenditure costs associated with each HCRU category during the study period; total costs included inpatient, emergency department (ED), outpatient visits, skilled nursing facility (SNF), hospice, home health agency (HHA) visits, and prescription medication use. Total prescription drug costs and those for insomnia and non-insomnia medications were analyzed separately. For each visit type, costs were evaluated among patients with ≥1 HCRU claim for a specific category of HCRU (e.g. total mean inpatient costs were calculated among the subset of patients with ≥1 inpatient visit). All costs were estimated per patient per month (PPPM), and were inflated to 2018 costs using the medical care component of the consumer price index (CPI)Citation22.
Other measures
Demographic characteristics included patient age on the study index date, sex, geographic region, and health plan type. Patient comorbid conditions were assessed using the Charlson Comorbidity Index (CCI); a higher score indicates greater overall comorbidity burdenCitation23,Citation24.
Statistical analysis
Descriptive analyses were performed for all study variables, including mean and standard deviation for continuous variables, and frequency counts and percentages for categorical variables. To compare HCRU between D + TI and matched control patients, generalized linear models with Poisson distribution and log link were used to estimate adjusted means and estimated mean ratios with 95% confidence intervals for HCRU visits per month and length of stay outcomes. For cost, generalized linear models with gamma distribution and log link were used to estimate mean cost per month for treated and control patients along with mean ratio and 95% confidence intervals. For all the adjusted models, P values and 95% confidence intervals between the cohort of treated insomnia patients and the matched control cohort were reported without adjusting for multiplicity. Covariates for models included age, sex, geographic region, baseline CCI score, health plan type [comprehensive, preferred provider organization (PPO), health maintenance organization (HMO), point-of-service (POS), and other].
Sensitivity analyses
Sensitivity analyses were planned to evaluate the robustness of our findings. We utilized alternate definitions of TI; while our main analysis did not exclude patients with TI who had other sleep disorders (i.e. comorbid insomnia), sensitivity analyses sought to confirm that costs were attributable to TI rather than other sleep-related diagnoses. Sensitivity analyses thus excluded D + TI patients who had any other, non-insomnia sleep-related diagnoses or procedures identified via claims data. Study outcomes, including number of inpatient visits, length of inpatient stay, number of ED visits, and total costs, were evaluated in sensitivity analyses for D + TI compared to matched controls.
Results
Study population attrition and characteristics
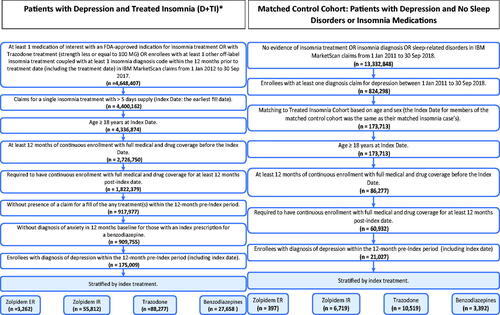
More than 4.6 M adults were identified as treated with a commonly prescribed insomnia medication from January 2012 through September 2017 (). After applying other exclusionary criteria, within the cohort of diagnosed depression patients, 175,008 insomnia-treated patients were identified. The matched cohort of adults with diagnosed depression (≥1 claim) and no sleep disorders was identified from 13.3 million individuals with no evidence of insomnia or sleep-related treatment, procedure, or diagnosis. The initial 1:1 matching process included 173,713 D + TI patients; after application of continuous eligibility criteria for controls, the final matched cohort was comprised of 21,027 adults in each patient group (D + TI or controls). Among patients treated with a commonly prescribed insomnia medication, 1.9% received zolpidem ER, 32.0% zolpidem IR, 50.0% trazodone, and 16.1% benzodiazepines.
Figure 1. Sample attrition and cohort identification among patients with depression: depression and treated insomnia (D + TI) cohort matched to controls without sleep disorders. *The final sample size in the D + TI cohort was 21,027, following matching to the control cohort.
In the final matched cohort with depression (n = 21,027 in each group), 69.5% were female and the mean age at index was 48.3 years (SD 16.4) (). Most patients in both groups had a CCI score of 0 (61.9% in D + TI patients and 71.4% in controls). Mean CCI score was 0.9 among D + TI patients and 0.6 among controls. Finally, the proportion of patients with commercial claims and Medicare supplemental were similar across both D + TI patients and controls (87.2% and 12.8%, respectively).
Table 1. Demographic and baseline clinical characteristics among patients with depression: depression and treated insomnia (D + TI) cohort matched to controls without sleep disorders.
HCRU and costs
Relative to controls, a higher proportion of D + TI patients ≥1 hospitalization (17.9% vs 6.5%) (), and length of inpatient stay was also greater for D + TI patients (8.7 vs 5.9 days). A higher proportion of D + TI patients had ≥1 ED visits compared to controls (30.5% vs 16.7%). Among patients with depression, although mean number of inpatient visits were similar (0.1 for both), D + TI patients had higher mean number of ED visits (0.2 vs 0.1, p < .001), outpatient visits (2.2 vs 1.3, p < .001), and prescriptions (1.9 vs 1.4, p < .001) compared to controls (data not shown).
Table 2. Frequency of hospitalizations, ED visits, and outpatient visits among patients with depression: depression and treated insomnia (D + TI) cohort matched to controls without sleep disorders.
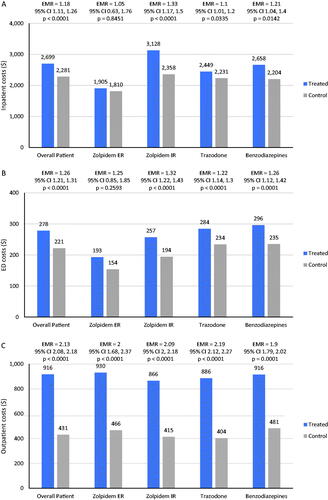
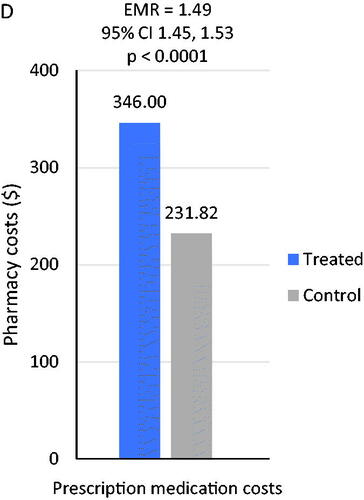
Relative to the matched control cohort, estimated mean PPPM costs were significantly higher among individuals with D + TI ($2450 vs $1095; estimated mean ratio, 2.24; 95% CI, 2.18–2.30; p < .001) (data not shown). Similarly, inpatient costs, ED costs, outpatient costs, and pharmacy costs were all significantly higher among individuals with D + TI (all ps < 0.001). Differences in total mean estimated health-care costs were largely attributable to differences in inpatient costs between D + TI patients and matched controls. Among D + TI patients, inpatient mean costs differed by insomnia medication. Inpatient costs were significantly higher for all patients treated with zolpidem IR ($3128 vs. $2358; estimated mean ratio= 1.33, 95% CI= 1.17–1.15, p < .001), trazodone ($2449 vs. $2231; estimated mean ratio= 1.1, 95% CI= 1.01–1.20; p = .034), or benzodiazepines ($2658 vs. $2204; estimated mean ratio= 1.21, 95% CI= 1.04–1.40; p = .014) compared with controls. However, no between-groups differences were observed for inpatient PPPM costs between controls and patients receiving zolpidem ER (). Mean ED costs also differed by insomnia medication; mean ED costs were significantly higher for patients treated with zolpidem IR ($257 vs. $194; estimated mean ratio= 1.32, 95% CI= 1.22–1.43; p < .001), trazodone ($284 vs. $234; estimated mean ratio= 1.22, 95% CI= 1.14–1.30; p < .001), or benzodiazepines ($296 vs. $235; estimated mean ratio= 1.26, 95% CI= 1.12–1.42, p < .001) compared with controls (), while zolpidem ER mean costs were not significantly different from controls. Outpatient costs PPPM were significantly higher for patients receiving each index treatment compared with matched controls (all p < .001) and estimated mean ratios were similar across each stratified treatment group (). Estimated total mean prescription medication costs were significantly higher for D + TI patients compared with controls ($346 vs $232, p < .001) (). Unadjusted cost results are available in Table A1.
Figure 2. Comparison of mean health care costs among patients with depression: depression and treated insomnia (D + TI) cohort matched to controls without sleep disorders. (A) Estimated mean inpatient costs stratified by treatment group. (B) Estimated mean ED costs stratified by treatment group. (C) Estimated mean outpatient costs stratified by treatment group. (D) Estimated mean pharmacy costs for overall patients.
Planned sensitivity analyses confirmed the robustness of the main study’s findings. Subsequent to excluding D + TI patients with sleep diagnoses and procedures other than insomnia (and their matched controls) from the study analyses, estimated mean ratios comparing treated insomnia patients to controls for primary study outcomes, including number of inpatient visits (estimated mean ratio= 1.08, 95% CI = 0.86–1.36), length of inpatient stay (estimated mean ratio = 1.47, 95% CI = 1.43–1.51), ED visits (estimated mean ratio = 1.23, 95% CI = 1.09–1.39), and total costs (estimated mean ratio = 2.16, 95% CI = 2.10–2.22), were consistent with findings from the main analyses. All sensitivity analysis results reflected similar estimates as the main analyses and all significant findings were confirmed.
Discussion
Results of this national analysis demonstrate that among patients with depression, insomnia treated with commonly prescribed medications is associated with a dramatic increase in all-cause health-care costs. Specifically, even after controlling for multiple covariates and relative to non-sleep disordered controls, D + TI patients demonstrated all-cause costs that were more than twice as high ($2450 vs $1094). These cost differences were largely driven by higher inpatient, ED, and outpatient costs, and longer inpatient stays among D + TI patients, rather than medication costs associated with insomnia treatment itself. The proportion of patients with inpatient visits was higher for D + TI patients as compared to matched controls (17.9% vs 6.5%), and inpatient length of stay was also greater among D + TI patients. Sensitivity analyses confirmed the robustness of our study’s findings.
Prior studies that similarly utilized real-world data found that insomnia is associated with increased health care costs and utilizationCitation25–27. Among Medicare beneficiaries, compared to non-sleep disordered controls, individuals with insomnia had higher HCRU and costs; total health care costs were higher by $63,607 (95% CI $60,532–$66,685), driven by inpatient costs which were $60,900 higher (95% CI $56,609–$65,191), although ED and prescription costs were also elevated among patients with insomniaCitation28. In another study of Medicare beneficiaries with insomnia, important baseline differences between treated and untreated patients accounted for cost and HCRU differences; treated patients had higher baseline rates of ED visits and prescription fills, and although health care costs increased subsequent to insomnia diagnosis. After adjusting for pre-insomnia differences, 1-year post-diagnosis costs were similar between treated and untreated patients ($113,799 vs 97,219)Citation29. In another large real-world study, more frequent ED and physician visits were associated with insomnia compared to non-sleep disordered persons, as ED visits increased by 50%, and physician visits by 120%Citation25. Fewer studies of cost and/or HCRU have been conducted among patients with depression and insomnia, although one study found untreated insomnia to be associated with higher health care costs among patients with depression. Asche et al., in a retrospective claims-based analysis, found that total direct costs for patients with MDD and untreated insomnia were significantly higher than MDD with no insomnia ($4858 vs $4007)Citation30. Additional research is needed to understand drivers of these cost differences among patients with D + TI. One possibility is that that this observed cost difference may be related to adverse events associated with use of commonly prescribed insomnia medications. Prior research has suggested an increased risk of falls and higher HCRU and costs among D + TI patients as compared to matched controls without sleep disordersCitation31, as well as an increased risk for falls and fractures among Medicare beneficiaries treated with z-drugs.Citation32
Consistent with other reportsCitation14,Citation15, trazodone and zolpidem IR were the most commonly prescribed insomnia medications in this real-world study. About 1 in 2 D + TI patients filled a prescription for trazodone ≤100 mg daily, while 32% filled a zolpidem IR prescription. Based on the dosage prescribed, which is lower than the starting dosage indicated for treatment of depression, trazodone use was likely used off-label for insomnia in our study sample. Other reports have indicated that trazodone is now more frequently used for insomnia than depression in clinical practiceCitation33, and its use is increasingCitation34. The high percentage of patients taking trazodone among our sample of D + TI patients may also be due to trazodone having an FDA-approved indication for depression and a perception that it could only help with the depressive symptoms. However, low-dose trazodone does not have a clinical effect on depressive symptomsCitation33. Many practitioners perceive trazodone to be a relatively safe medicationCitation35, although AASM Clinical Guidelines do not recommend trazodone due to limited efficacy and safety dataCitation10. Findings from the current, real-world study regarding trazodone use for insomnia add an important population health perspective to the literature.
Treatment of insomnia has important clinical and humanistic implications, yet insomnia is undertreatedCitation36. Pharmacologic treatment of insomnia can improve sleep onset latency and increase sleep durationCitation37, and improved sleep can in turn facilitate improved mental health outcomes and quality of life. A meta-analysis of 23 studies concluded that treating insomnia comorbid with depression resulted in moderate to large improvement in depressive symptomsCitation6. Further, failure to alleviate sleep problems after other depression symptoms have improved significantly increases the risk of depressive relapse and recurrenceCitation2. In terms of targeted treatment, additional research is warranted to provide insight into specific patient subgroups who respond positively to insomnia treatment, and to understand the underlying mechanisms linking insomnia treatment to reductions in depressive symptoms and improved moodCitation6. At the same time, results of this study suggest that there is a need for expanded treatment options for insomnia, including medications with fewer adverse effects than the very commonly prescribed medications included in this study. This need is more urgent given the current trend of increased insomnia prevalence and sleep medication prescribing that has occurred since the beginning of the COVID-19 pandemicCitation38,Citation39.
Strengths of the current study include a novel question of timely importance, the use of real-world data from a large, national US health care claims database, and a large study sample with geographic representation across the US. We also evaluated commonly used insomnia medications, including trazodone, that do not have an indication for use in insomnia, by use of an algorithm that utilized dosage dispensed and diagnostic codes to define use for insomnia. Relatively few studies have utilized real-world data to characterize outcomes associated with the use of trazodone for insomnia. Our findings should, however, be interpreted in the context of some important limitations. This study utilized administrative claims data for patient encounters; claims data incorporates diagnosis information using ICD-9/ICD-10 codes for billing purposes and is unable to provide insight into clinical variables of interest such as sleep continuity or depressive symptoms. While our analysis adjusted for CCI to adjust for comorbidity burden, we were unable to control for depression severity or duration. Severity of depression could have a significant impact on the amount of HCRU and on the severity of insomnia symptoms that patients experience. Our study did not consider treatment for depression, which may impact sleep, and additional research is warranted to understand the impact of treatment for both insomnia and depression on HCRU and costs, as well as other depression and insomnia symptoms and outcomes. Although matching methods were used to reduce confounding of observed covariates, some imbalance remained and with that the potential for unmeasured variables to influence the results. Since MarketScan databases include individuals with employer-sponsored health insurance, our findings may not be generalizable to populations with government-sponsored insurance or without health insurance coverage. Given the underreporting and underdiagnosis of insomnia and lack of validated algorithms to identify patients with insomnia in administrative claims data, we relied on prescription drug claims for on-label and off-label insomnia treatments to identify the treated insomnia population. However, we were cautious to exclude patients prescribed benzodiazepines to treat conditions other than sleep disorders, and patients receiving benzodiazepines who also had a diagnosis of anxiety were excluded. It is acknowledged that the observed differences in HCRU and costs among D + TI patients as compared to matched controls without sleep disorders could be a result of insomnia treatment in general. However, directly comparing treated vs. untreated insomnia was beyond the scope of this study, as we were unable to definitively identify an untreated insomnia population without over-the-counter or non-pharmacological treatment use. A nationally representative survey into prescription and non-prescription sleep product use among older adults in the US reported that 34.4% of participants using a sleep product used over-the-counter or herbal/natural aids, which would not have been reported in claims dataCitation40.
Conclusions
In this real-world study and relative to matched patients with depression but without sleep disorders, patients with D + TI had greater HCRU. Overall health care costs for D + TI patients were more than twice that of controls, and cost differences between cohorts were largely attributable to higher mean inpatient costs, not insomnia medication costs. Observed differences in HCRU and costs by insomnia medication of interest may be associated with the clinical and safety profile of each medication or medication class, and the results suggest an unmet need for safer therapies among patients with comorbid insomnia and depression. Indeed, given the recent rise in prevalence of depression and insomnia and increased prescribing of sleep medications associated with COVID-19, effective and safe insomnia medication options, particularly among those with depression, are clearly needed, as long-term mental health consequences, including insomnia, associated with the pandemic will remain of great importance even after the pandemic ends. In summary, these findings highlight the importance of insomnia and insomnia treatments among individuals with depression as well as the need for additional research to better understand how insomnia treatment, including medications with novel mechanisms of action, might impact this patient population.
Transparency
Declaration of funding
This work was supported by Eisai Inc., Woodcliff Lake, NJ. Employees of the study sponsor were involved in the study design, as well as collection, analysis, interpretation of the data, and in critically revising the manuscript for important intellectual content. Funding for manuscript preparation was provided to Genesis Research (DTA, DG are employees of Genesis).
Declaration of financial/other relationships
EMW received compensation for consulting on this study. EMW has received research funding from the AASM Foundation, US DoD, Merck, and ResMed. EMW has served as a scientific consultant to DayZz, Eisai, Merck, and Purdue, and is an equity shareholder in WellTap. TRJ, FF and MM are employees of Eisai, Inc. DTA and DG are employees of Genesis Research, which received compensation for conducting this study. A reviewer on this manuscript disclosed that they have received manuscript or speaker’s fees from Astellas, Dainippon Sumitomo Pharma, Eisai, Eli Lilly, Elsevier Japan, Janssen Pharmaceuticals, Kyowa Yakuhin, Lundbeck, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD, Nihon Medi-Physics, Novartis, Otsuka Pharmaceutical, Shionogi, Shire, Takeda Pharmaceutical, Tsumura, Wiley Japan, and Yoshitomi Yakuhin, and research grants from Dainippon Sumitomo Pharma, Eisai, Mochida Pharmaceutical, Meiji Seika Pharma and Shionogi. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
Study conception and design: EMW, DTA, TRJ, FF, DG, MM; Analysis: DTA, DG; Interpretation of the data: EMW, DTA, TRJ, FF, DG, MM; Drafting of the paper and revising it critically for intellectual content: EMW, DTA, TRJ, FF, MM; Final approval of the version to be published: EMW, DTA, TRJ, FF, DG, MM. All authors agree to be accountable for all aspects of this work.
Wickwire_et_al._CMRO_Supplementary_Table_1FEB2022.docx
Download MS Word (22.9 KB)Acknowledgements
Portions of this manuscript were presented as a poster at the Academy of Managed Care Pharmacy (AMCP) Annual Meeting, April 12–16, 2021 (Virtual). The authors thank Jenifer Wogen (Genesis Research) for her writing support and assistance in preparation of this article. The authors can confirm that JW has given permission to be acknowledged for her part in this article.
Notes
i IBM MarketScan, IBM North America, New York, USA.
References
- Friedrich MJ. Depression is the leading cause of disability around the world. Jama. 2017;317(15):1517.
- Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008;10(3):329–336.
- Stewart R, Besset A, Bebbington P, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29(11):1391–1397.
- Yates WR, Mitchell J, John Rush A, et al. Clinical features of depression in outpatients with and without co-occurring general medical conditions in STAR*D: confirmatory analysis. Prim Care Companion J Clin Psychiatry. 2007;9(1):7–15.
- Hamilton M. Frequency of symptoms in melancholia (depressive illness). Br J Psychiatry. 1989;154:201–206.
- Gebara MA, Siripong N, DiNapoli EA, et al. Effect of insomnia treatments on depression: a systematic review and meta-analysis. Depress Anxiety. 2018;35(8):717–731.
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? Jama. 1989;262(11):1479–1484.
- Li L, Wu C, Gan Y, et al. Insomnia and the risk of depression: a meta-analysis of prospective cohort studies. BMC Psychiatry. 2016;16(1):375.
- Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–162.
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(2):307–349.
- Overland S, Glozier N, Sivertsen B, et al. A comparison of insomnia and depression as predictors of disability pension: the HUNT study. Sleep. 2008;31(6):875–880.
- Agargun MY, Cartwright R. REM sleep, dream variables and suicidality in depressed patients. Psychiatry Res. 2003;119(1–2):33–39.
- Singareddy RK, Balon R. Sleep and suicide in psychiatric patients. Ann Clin Psychiatry. 2001;13(2):93–101.
- Jaffer KY, Chang T, Vanle B, et al. Trazodone for insomnia: a systematic review. Innov Clin Neurosci. 2017;14(7–8):24–34.
- Roy AN, Smith M. Prevalence and cost of insomnia in a state medicaid fee-for-service population based on diagnostic codes and prescription utilization. Sleep Med. 2010;11(5):462–469.
- Bayer AJ, Pathy MS, Ankier SI. Pharmacokinetic and pharmacodynamic characteristics of trazodone in the elderly. Br J Clin Pharmacol. 1983;16(4):371–376.
- Bronskill SE, Campitelli MA, Iaboni A, et al. Low-dose trazodone, benzodiazepines, and fall-related injuries in nursing homes: a matched-cohort study. J Am Geriatr Soc. 2018;66(10):1963–1971.
- Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70(2):197–245.
- Mokhar A, Tillenburg N, Dirmaier J, et al. Potentially inappropriate use of benzodiazepines and z-drugs in the older population-analysis of associations between long-term use and patient-related factors. PeerJ. 2018;6:e4614.
- Jones CM, Guy GP Jr, Board A. Comparing actual and forecasted numbers of unique patients dispensed select medications for opioid use disorder, opioid overdose reversal, and mental health, during the COVID-19 pandemic, United States, January 2019 to May 2020. Drug Alcohol Depend. 2021;219:108486.
- Czeisler ME, Lane RI, Petrosky E, et al. Mental health, substance use, and suicidal ideation during the COVID-19 Pandemic – United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049–1057.
- Consumer Price Index. Measuring Price Change in the CPI: Medical Care. 2020. (Accessed October 29, 2020). https://www.bls.gov/cpi/factsheets/medical-care.htm.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Quan H, Li B, Couris CM, et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- DiBonaventura M, Richard L, Kumar M, et al. The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLOS One. 2015;10(10):e0137117.
- Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30(3):263–273.
- Pollack M, Seal B, Joish VN, et al. Insomnia-related comorbidities and economic costs among a commercially insured population in the United States. Curr Med Res Opin. 2009;25(8):1901–1911.
- Wickwire EM, Tom SE, Scharf SM, et al. Untreated insomnia increases all-cause health care utilization and costs among medicare beneficiaries. Sleep. 2019;42(4):zsz007.
- Wickwire EM, Vadlamani A, Tom SE, et al. Economic aspects of insomnia medication treatment among medicare beneficiaries. Sleep. 2020;43(1):zsz192.
- Asche CV, Joish VN, Camacho F, et al. The direct costs of untreated comorbid insomnia in a managed care population with major depressive disorder. Curr Med Res Opin. 2010;26(8):1843–1853.
- Amari D, Juday T, Frech F, et al. Higher health care resource use and costs associated with falls among elderly medicare beneficiaries on commonly used insomnia medications in the United States. J Manag Care Pharm. 2020;26:S46.
- Tom SE, Wickwire EM, Park Y, et al. Nonbenzodiazepine sedative hypnotics and risk of fall-related injury. Sleep. 2016;39(5):1009–1014.
- Fagiolini A, Comandini A, Catena Dell'Osso M, et al. Rediscovering trazodone for the treatment of major depressive disorder. CNS Drugs. 2012;26(12):1033–1049.
- Wong J, Murray Horwitz M, Bertisch SM, et al. Trends in dispensing of zolpidem and low-dose trazodone among commercially insured adults in the United States, 2011-2018. Jama. 2020;324(21):2211–2213.
- Schroeck JL, Ford J, Conway EL, et al. Review of safety and efficacy of sleep medicines in older adults. Clin Ther. 2016;38(11):2340–2372.
- Leger D, Poursain B. An international survey of insomnia: under-recognition and under-treatment of a polysymptomatic condition. Curr Med Res Opin. 2005;21(11):1785–1792.
- Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133.
- Mandelkorn U, Genzer S, Choshen-Hillel S, et al. Escalation of sleep disturbances amid the COVID-19 pandemic: a cross-sectional international study. J Clin Sleep Med. 2021;17(1):45–53.
- Hamel L, Kearney A, Kirzinger A, et al. Health tracking Poll- July. Kaiser Family Foundation. 2020;2020.
- Maust DT, Solway E, Clark SJ, et al. Prescription and nonprescription sleep product use among older adults in the United States. Am J Geriatr Psychiatry. 2019;27(1):32–41.