Abstract
Objective
Chemotherapy-related adverse events (AEs) can negatively impact the care of patients. The prevention and management of AEs often require additional medications. This study evaluated the percentages of patients with metastatic pancreatic ductal adenocarcinoma (mPDAC) undergoing second-line therapy with 5-fluorouracil (5-FU)-based regimens that experienced AEs during treatment and received medication to manage those AEs.
Methods
We conducted a retrospective observational analysis utilizing the Flatiron Health database of adult patients with mPDAC who started second-line therapy between January 2016 and August 2020. The occurrence of diarrhea, fatigue, nausea and vomiting, neuropathy, and hematologic AEs including G3/G4 anemia, neutropenia, and thrombocytopenia was assessed. The use of concomitant medications including atropine and granulocyte colony stimulating factor (G-CSF) was assessed.
Results
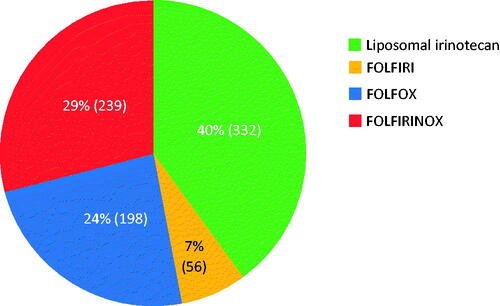
Of the 825 eligible patients, 29.0% (n = 239) received FOLFIRINOX, 24.0% (n = 198) received FOLFOX, 6.8% (n = 56) received FOLFIRI, and 40.2% (n = 332) received liposomal irinotecan-based regimens. FOLFIRI and FOLFIRINOX regimens were associated with the highest rates of anemia (16.1% and 15.5%), neutropenia (19.6% and 22.6%), and thrombocytopenia (14.3% and 9.6%). The liposomal irinotecan and FOLFOX regimens were associated with lower rates of anemia (11.8% and 12.1%), neutropenia (12.4% and 14.7%), and thrombocytopenia (2.4% and 8.1%). G-CSF use was observed among 63.6%, 34.9%, 33.9%, and 44.9% of patients treated with FOLFIRINOX, FOLFOX, FOLFIRI, and liposomal irinotecan-based regimens, respectively. Diarrhea was observed among 12.5%, 4.5%, 12.5%, and 10.2% of patients who were treated with FOLFIRINOX, FOLFOX, FOLFIRI, and liposomal irinotecan-based regimens, respectively. Nausea and vomiting occurred in 14.9%, 12.6%, 10.5%, and 13.1% of patients treated with FOLFIRINOX, FOLFOX, FOLFIRI, and liposomal irinotecan-based regimens, respectively. Atropine use was higher in patients treated with FOLFIRINOX and FOLFIRI (90.8% and 94.6%, respectively) than in patients treated with liposomal irinotecan-based regimens (75.6%).
Conclusions
In patients with mPDAC who received second-line therapy, those who received liposomal irinotecan-based regimens had the lowest rates of anemia, neutropenia, and thrombocytopenia compared to FOLFIRI, FOLFIRINOX, and FOLFOX, while requiring a similar or lower level of medication to treat and manage those adverse events. Patients treated with FOLFIRI received the highest dose of pegfilgrastim to manage neutropenia. The results of this real-world analysis are consistent with prior evaluations of patients with mPDAC and highlight the importance of managing adverse events and associated cost implications.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a lethal malignancy, accounting for an estimated 3.2% of new cancer diagnoses in the United States, and was the third leading cause of cancer-related deaths in the country in 2020Citation1. Non-modifiable risk factors for pancreatic cancer include older age (i.e., 70–80 years), African American race, and a family history or genetic susceptibility to the diseaseCitation2,Citation3. Modifiable risk factors include cigarette smoking, alcohol use, obesity, and diabetesCitation2,Citation3. A proposed mechanism for pathogenesis is a stepwise progression through genetically and histologically well-defined, non-invasive precursor lesionsCitation2,Citation3.
Currently, surgical resection is the only curative treatment for PDAC, having a 5-year survival of 10–15% and median survival of 11–18 months. In patients with metastatzic PDAC, first line treatment with gemcitabine plus nab-paclitaxel or 5-FU/leucovorin plus oxaliplatin and irinotecan (FOLFIRINOX) is the standard of careCitation3,Citation4. Based on the results of the NAPOLI-1 trialCitation5, the US Food and Drug Administration (FDA) approved nanoliposomal irinotecan plus 5-FU and leucovorin as second-line therapy after previous gemcitabine therapy, and clinical practice guidelines have been updated accordinglyCitation6.
In clinical trials, eligibility criteria are designed to ensure the overall safety of enrolled patients and to narrowly define the patient population to obtain robust efficacy and tolerability data. Oftentimes, however, strict eligibility criteria do not reflect the heterogeneity of the general patient population that will receive the drug after approvalCitation7, and the generalizability of study findings may not be representative of the patient population in the clinical practice setting. Therefore, studies of real-world data from large databases can confirm clinical trial results and extend the assessment of safety in a broader patient cohort more representative of the general patient population of interest. FDA has used administrative claims data as part of its post-marketing surveillance of approval productsCitation8. With recent introduction of the Sentinel Initiative, FDA can proactively assess the safety of regulated drugs using the largest multisite distributed database in the worldCitation9.
In a retrospective, matched cohort study of patient data from a large, US-based claims database, Wong et al.Citation10 evaluated the incremental healthcare costs associated with treatment-related adverse events (TRAEs) in patients with prevalent types of cancer. Their findings showed TRAEs are associated with a significant economic burden. With the recent development and widespread adoption of clinical pathways, the cancer care process is becoming more standardized, and health systems are better able to manage cost, maintain quality of care, and determine the value of treatments for cancer patients, their healthcare providers, and payersCitation11,Citation12. In general, different clinical pathway programs (e.g., NCCN Value Pathways, AIM Cancer Treatment pathways) follow similar criteria to determine the optimal treatment regimen for cancer patients, and the regimens are ranked by efficacy, safety (especially those leading to hospitalization or affecting quality-of-life), strength of guideline recommendations, and cost factors first and then taking into account other factors such as comparative effectiveness, patient assistance programs, and administrationCitation11,Citation12.
In a recent systematic review and meta-analysisCitation13 evaluating the efficacy and safety of second-line 5-FU and oxaliplatin-based therapy, the most commonly reported Grade 3–4 treatment-related adverse events (TRAEs) associated with 5-FU/leucovorin plus oxaliplatin (FOLFOX) were neutropenia (21.5%) and fatigue (11.7%). Other Grade 3–4 TRAEs occurring in >10% of patients in any included study were neurotoxicity (5.3%), thrombocytopenia (4.9%), anemia (4.5%), diarrhea (4.2%), and vomiting (4.1%). While it is not clear why the NAPOLI trialCitation5 was not included in the systematic review and meta-analysis, indirect comparisons of efficacy and safety outcomes were discussed, prompting questions relating to the direct comparisons made herein.
The goal of our exploratory study was to evaluate the incidence of adverse events and use of supportive medications among patients with mPDAC treated with 5-FU based therapies in the second line metastatic setting in a hypothesis-generating fashion.
Methods
Study design and data source
This was a retrospective observational cohort study of patients with mPDAC who received second-line therapy for the disease. We used the Flatiron Health database, a longitudinal, demographically and geographically diverse database derived from electronic health records (EHR). This database contains information from > 280 cancer clinics (∼800 sites of care), representing > 2.2 million active US-based cancer patients available for analysis. Patient-level EHRs include structured (e.g., laboratory values, medication prescriptions) and unstructured data (e.g., physicians’ notes, biomarker reports), which were curated using technology-enabled abstraction. The majority of patients in the database originate from community-based oncology settings. Data provided to third parties are deidentified and provisions are in place to prevent re-identification to protect patient confidentiality.
Patient population
Patients were eligible for inclusion if they were diagnosed with mPDAC on or after January 1, 2014 and had a record of activity (clinic visit and/or treatment administration) within 90 days of their metastatic diagnosis date. Eligible patients received first-line systemic therapy in the metastatic setting and were aged ≥ 18 years when they started a second-line therapy commonly used following gemcitabine-based first line therapy (i.e., liposomal irinotecan-based regimens, FOLFIRINOX, FOLFOX, or 5-FU/leucovorin plus irinotecan (FOLFIRI)) between January 1, 2016 and August 31, 2020. Finally, eligible patients had ≥ 1 record of a clinic visit and/or treatment administration after the start of second-line therapy.
Baseline characteristics
The following demographic and clinical characteristics were assessed: sex; race; geographic region; smoking history; cancer stage at initial diagnosis; site of primary tumor; practice type; age at and year of the start of second-line therapy; previous Whipple procedure; and Eastern Cooperative Oncology Group Performance Status (ECOG PS) score, serum albumin, and serum cancer antigen (CA) 19-9 (all ≤ 30 days before or ≤ 7 days after start of second-line therapy).
Study evaluations
The duration of therapy during which patients were at risk for adverse events was defined as the time between the first administration and the last activity date or start of subsequent treatment, whichever occurred first. A treatment cycle was defined as the number of days with an administration for a systemic agent during a line of therapy. A dose reduction was defined as a 20% decrease from the starting dose of irinotecan/liposomal irinotecan for patients treated with FOLFIRINOX, FOLFIRI, and liposomal irinotecan-based regimens and a 20% reduction from the starting dose of oxaliplatin for patients treated with FOLFOX. A dose delay was defined as a gap in treatment > 16 days between chemotherapy administrations.
AEs occurring between the database-defined start and end date for a given treatment were evaluated. Grade 3–4 AEs of interest included diarrhea, fatigue, nausea/vomiting, mucositis, and neuropathy. Hematologic AEs of interest included anemia, neutropenia, and thrombocytopenia. Elevations in liver enzymes were also evaluated. These AEs of interest were selected based on tolerability results from clinical trials evaluating the safety and efficacy of 5-FU-based regimensCitation3–5,Citation13. Laboratory-based AEs were graded based on the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03Citation14. The definition of Grade 3 anemia was modified to hemoglobin level 7 to < 8 g/dL. Grade 4 anemia was not evaluable using CTCAE criteria, as it is defined as “Life-threatening consequences; urgent intervention indicated”, which could not be assessed using the database. Thus, the definition of Grade 4 anemia was modified as hemoglobin level < 7 g/dL. Non-laboratory-related AEs were identified from structured EHR data using the relevant International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes.
Medication use to manage Grade 3–4 AEs during treatment was evaluated from the structured medication administration and order data, including: antiemetics, atropine (total administrations), antidepressants, antianemics, gabapentin, granulocyte colony stimulating factor [G-CSF], pregabalin, and systemic corticosteroids.
Statistical analysis
Results for continuous variables are summarized as means with standard deviations (SDs) or medians and interquartile ranges (IQRs), depending on the nature of their distributions. Results for categorical variables are summarized using absolute (frequencies) and relative (percentages) terms. All analyses were descriptive in nature and were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics
In total, 825 patients were included as having mPDAC and treated with second-line therapy in the metastatic setting. Overall, 52% of patients were male, 68% were white, and 41% were from the South region. The vast majority of patients received treatment from community-based practices. More than 50% of patients were current or previous smokers, most patients (n = 456) had an ECOG PS of 0 or 1, and nearly two-thirds of patients (n = 517) were initially diagnosed with Stage IV cancer. For 50% (n = 412) of patients, the primary tumor site was the head of the pancreas; and the vast majority of patients (n = 682) had not undergone Whipple surgery. Most patients had serum albumin levels < 40 g/L (n = 581) and CA 19-9 ≥ 40 U/mL (n = 559) ().
Table 1. Baseline demographic and clinical characteristics.
Most patients received treatment regimens containing liposomal irinotecan (40%), followed by FOLFIRINOX (29%), FOLFOX (24%), and FOLFIRI (7%) (). Overall, the median age at the start of second-line therapy was 69 years (IQR = 63–75). Patients who received FOLFIRINOX were notably younger than those who received the other second-line regimens. Most patients (n = 305) who received liposomal irinotecan-based regimens were treated in the community setting. Compared with the other second-line regimens, a notably smaller percentage of patients who received FOLFIRINOX had ECOG PS scores ≥ 2 ().
Study outcomes
Across all second-line therapy regimens, the median duration of therapy was 12.7 weeks (IQR = 6.4–26.6). Patients who received FOLFIRINOX had the longest treatment duration; patients who received FOLFOX had the shortest treatment duration (). Overall, 21% of patients had a dose reduction during the study. The largest and smallest percentages of patients that had dose reductions were in the FOLFOX (27%) and FOLFIRI (9%) treatment groups, respectively. Overall, 51% of patients experienced ≥ 1 dose delay during treatment. The largest and smallest percentages of patients that had dose delays were in the FOLFIRINOX (55%) and liposomal irinotecan-based regimens (48%) treatment groups, respectively ().
Table 2. Treatment characteristics.
Among the AEs of interest, the proportion of patients who experienced at least one AE was 45% for those treated with liposomal irinotecan-based regimens, 59% for patients treated with FOLFIRINOX, 44% among those treated FOLFOX, and 63% for those treated with FOLFIRI. For the Grade 3–4 AEs of interest, nausea and vomiting was experienced by the largest percentages of patients in all treatment groups, ranging from 12.5% for FOLFIRI to 15.9% for FOLFIRINOX. Rates for the other AEs of interest, including diarrhea, fatigue, neuropathy, and mucositis are shown by treatment group in . In terms of Grade 3–4 hematologic AEs, FOLFIRI and FOLFIRINOX regimens were associated with the highest rates of anemia (16.1% and 15.5%), neutropenia (19.6% and 22.6%), and thrombocytopenia (14.3% and 9.6%). The liposomal irinotecan and FOLFOX regimens were associated with lower rates of anemia (11.8% and 12.1%), neutropenia (12.4% and 14.7%), and thrombocytopenia (2.4% and 8.1%) (). The observed trends for rates of neutropenia were similar when stratified by prophylactic G-CSF use (). The percentages of patients experiencing Grade 3–4 elevations in liver enzymes are shown by treatment group in .
Figure 2. (a) Percentage of patients with Grade 3–4 AEs of interest during treatment. (b) Percentage of patients with Grade 3–4 hematologic AEs. (c) Percentage of patients with Grade 3–4 elevations in liver enzymes.
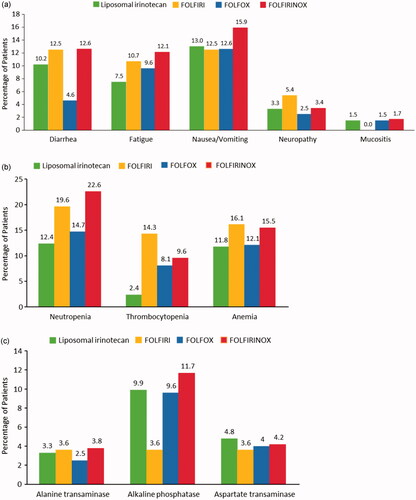
Table 3. Neutropenia rates by prophylactic G-CSF use.
In terms of concomitant medication use, antidepressants, antianemics, and gabapentin were used by the largest percentages of patients in all treatment groups; smaller percentages of patients used pregabalin or corticosteroids (). The vast majority of patients in the FOLFIRI and FOLFIRINOX groups received atropine (n = 53, 94.6% and n = 217, 90.8%). By contrast, 75.6% of patients in the liposomal irinotecan-based regimens group and 1% of patients in the FOLFOX group received atropine. This is consistent with the percentages of patients in each treatment group that experienced diarrhea. A majority of observed atropine use was initiated within 5 days of treatment (93.5%). However, with the exception of the FOLFOX group, between 2.9% and 14.3% of patients experienced diarrhea after atropine administration. Nearly 100% of patients included in the study received an antiemetic, as rates of nausea/vomiting were the highest in all treatment groups for all AEs evaluated.
Figure 3. (a) Percentage of patients that received concomitant medications. (b) Median dose of pegfilgrastim by second-line therapy group. (c) Percentage of type of G-CSF use by treatment type.
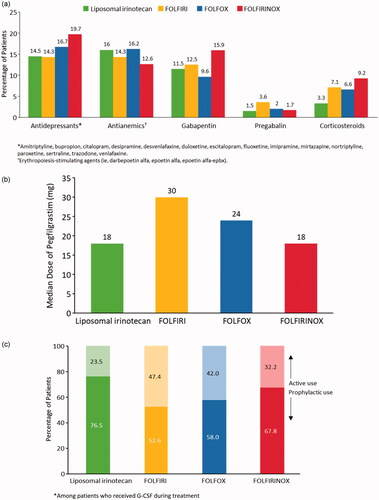
Nearly two-thirds of patients in the FOLFIRINOX group (63.6%) received G-CSF compared with 44.8% in the liposomal irinotecan-based regimens group, 34.9% in the FOLFOX group, and 33.9% in the FOLFIRI group. Across all four treatment groups, the most common G-CSF was pegfilgrastim (71.7%), and patients in the FOLFIRI group received the highest median dose (). Across all treatment groups, most patients used G-CSF prophylactically (within the first five days of initiating therapy). Prophylactic use of G-CSF was highest among patients in the liposomal irinotecan-based regimens group. The largest percentage of patients that received G-CSF more than 5 days after the initiation of therapy was in the FOLFIRI group (). Overall, despite the relatively high rates of G-CSF use, 24.4% of patients still experienced neutropenia.
Discussion
In this retrospective observational cohort study of patients with mPDAC who received second-line therapy, we used the EHR-based Flatiron Health database to evaluate the safety and tolerability of 5-FU based regimens as second-line therapy among patients with mPDAC. Our findings suggest there are important differences in Grade 3–4 AE rates and concomitant medication use among the four selected regimens.
Most of the mPDAC patients in our study received liposomal irinotecan-based regimens (40%) as second-line therapy, followed by FOLFIRINOX (29%), FOLFOX (24%), and FOLFIRI (7%). Our results are largely consistent with a recent systematic review of patients with pancreatic cancer who received 5-FU + oxaliplatin-based regimens where weighted mean percentages for Grade 3–4 AEs were: vomiting, 4.1%; diarrhea, 4.2%; and fatigue, 11.7%Citation13. For Grade 3–4 AEs ,hematologic AEs, larger percentages of patients in the FOLFIRI and FOLFIRINOX groups experienced anemia (16.1% and 15.5%), neutropenia (19.6% and 22.6%), and thrombocytopenia (14.3% and 9.6%) compared with patients in the liposomal irinotecan-based regimens and FOLFOX groups (11.8% and 12.1%; 12.4% and 14.7%; 2.4% and 8.1%, respectively). In a recent systematic review and meta-analysis, weighted mean percentages for Grade 3–4 hematologic AEs were: anemia, 4.5%; neutropenia, 21.5%; and thrombocytopenia, 4.9%Citation13. Generally, the prevalence of the toxicities noted in our study were lower than in respective clinical trials for the regimens of interest.
As Grade 3–4 hematologic AEs are common during the treatment of mPDAC, and because management costs can be significant, we reported G-CSF use to provide context to those costs. The percentages of patients that received G-CSF were consistent with the rates of Grade 3–4 neutropenia, and occurrence of neutropenia was lowest in the liposomal irinotecan-based regimens group. Prophylactic G-CSF use was generally high among patients in our study, consistent with guideline recommended care for regimens with higher risks of neutropeniaCitation15.
In this study, rates of diarrhea were similar across the cohorts. However, atropine use was highest (≥ 91%) among patients who received FOLFIRINOX or FOLFIRI. Seventy-six percent of patients in the liposomal irinotecan-based regimens group received atropine; 10.2% experienced diarrhea. Rates of diarrhea (4.6%) and atropine use (1%) were lowest in patients who received FOLFOX. From an economic perspective, lower AE rates lead to reduced use and cost of medications to treat these effects. These reduced costs help offset the cost of chemotherapy regimens, like liposomal irinotecan-based regimens.
There is a paucity of published studies on FOLFIRI-based regimens with which to compare, albeit indirectly, our results. Clinical trials supporting FOLFIRI’s continued use in mPDAC are nearly a decade old, have small sample sizes, and lack appropriate comparators in some studiesCitation16–18. Across the three studies, the AEs were inconsistently reported. Neuzillet et al.Citation17 reported 23.8% of patients experienced Grade 3–4 toxicities, mainly hematological (17.5%). Yoo et al.Citation16 and Zaniboni et al.Citation18 reported the following rates for Grade 3–4 events: neutropenia, 24% and 20%; thrombocytopenia, 3% and 2%; and anemia, 3% and not reported, respectively. Taken together, the small sample sizes and lack of a comparator make it difficult to draw conclusions regarding the tolerability of FOLFIRI for patients with advanced mPDAC.
Our analysis is quite distinct from other available work. The recent 5-FU + oxaliplatin comparative meta-analysis used different methodologies than our study and, therefore, a direct comparison of the results is not possible to this robust real-world datasetCitation13. This also holds true for comparisons between NAPOLI-1, the oxaliplatin meta-analysis and this dataset. Utilizing a different approach, Koeller et al.Citation19 used data from published real-world studies on patients with mPDAC who received second-line therapy with liposomal irinotecan + 5-FU + leucovorin and compared the findings with results from the NAPOLI-1 trialCitation5. The authors reported patients from the real-world studies were older, received more lines of therapy, and had poorer ECOG PS compared with the patients enrolled in NAPOLI-1. However, liposomal irinotecan + 5-FU + leucovorin showed similar efficacy and safety profiles to those reported in the NAPOLI-1 study.
Numerous real-world data studies have demonstrated the significant economic burden associated with treating patients with advanced or metastatic pancreatic cancer, including first-, second-, and third-line therapiesCitation20–25. Other real-world data studies have evaluated the costs associated with AEsCitation26–28. Importantly, budget impact model studies, which weigh all of the factors contributing to the treatment of patients with pancreatic cancer, have shown the higher costs of some drugs are offset by lower administration costs and reduced AE ratesCitation29. Although these important studies are very recent, their findings are likely to be strongly considered by those working on improving clinical pathway programs. The value of these real-world studies becomes even more evident when one considers a controlled clinical trial comparing these various treatment regimens is unlikely to be conducted.
Limitations
Our results should be interpreted in the context of some study limitations. First, data from the Flatiron Health database are collected prospectively for routine clinical care. We evaluated these data retrospectively and descriptively for research purposes, as such no power or sample size calculations were conducted for this analysis. Patient data included in the analysis were subject to non-random allocation bias because the reason(s) to forgo treatment is/are not available from the database. Additionally, reasons why the patients went on to receive their second line regimen are not directly available with these data. Within specific regimens, we did not assess the starting dose of each agent to categorize treatments into smaller categories (i.e., modified FOLFIRINOX), nor did we assess dosing schedules. Reasons why some laboratory testing was not done for some patients were unavailable. Similarly, hospitalization data are not available in the database; thus, AEs that required hospitalization may be under-reported. Symptomatic toxicities (e.g., fatigue, nausea, vomiting, diarrhea) are not well captured in the structured EHR data. We were not able to determine if symptoms observed during second therapy may have been caused by the late effects of first line therapy. Due to the source of our data we could not directly evaluate the costs associated with the care received by patients included in our study. Due to reporting limitations, we could not identify febrile neutropenia. Data on transfusions were unavailable to fully characterize the severity of anemia. Corticosteroids can be used to treat thrombocytopenia, but due to their broad indications their use identified during treatment may not be directly attributable to this adverse event. These data are collected from primarily the community setting and may not be generalizable to other settings of care. To protect patient confidentiality, the upper limit for patient age was 85 years, so the true age of some of the elderly patients was not available. Further analyses are necessary to account for risk factors of treatment tolerability. Finally, while such analyses are not presented here, our study should be considered in the context of real-world effectiveness.
Conclusion
Liposomal irinotecan-based regimens had the lowest rates of anemia, neutropenia, and thrombocytopenia compared to FOLFIRI, FOLFIRINOX, and FOLFOX, while requiring a similar or lower level of medication to treat and manage those adverse events. Patients treated with FOLFIRI received the highest dose of pegfilgrastim. Rates of diarrhea were similar across treatment cohorts despite the larger percentages of patients that used atropine with FOLFIRINOX and FOLFIRI. The results of this study showed regimens commonly used as second-line therapies for mPDAC have distinct tolerability and concomitant medication profiles, have meaningful cost implications that must be further evaluated to improve quality-of-life for patients with mPDAC, and provide cost-effective care. The results of this real-world analysis are consistent with prior evaluations of patients with mPDAC and highlight the importance of managing adverse events and associated cost implications.
Transparency
Declaration of funding
This study was sponsored by Ipsen. The sponsor was involved in the design of the study, analysis, and interpretation as well as review of the manuscript.
Declaration of financial/other relationships
GK receives research funding from Ipsen and has an immediate family member who is an employee of Ipsen; PC is an employee of and has stock in Ipsen; AS and SW are employees of Genesis Research which receives research funding from Ipsen; Z.W has a consulting/advisory role with Ipsen, Array BioPharma, Five Prime Therapeutics, Novartis, Lilly, Merck, Merck KGaA, Bristol-Myers Squibb, Bayer, AstraZeneca/MedImmune, and Macrogenics, has been compensated for travel by Lilly, Merck, and Bayer, and his institution receives funding from Novartis, Plexxikon, Pfizer, Merck, and Five Prime Therapeutics. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
George Kim: conceptualization, methodology, visualization, review and editing, final approval. Paul Cockrum: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, visualization, final approval. Andy Surinach: conceptualization, formal analysis, investigation, methodology, project administration, resources, visualization, writing – review and editing, final approval. Shu Wang: analysis, resources, visualization, review and editing, final approval. Zev Wainberg: conceptualization, methodology, visualization, review and editing, final approval.
Acknowledgements
This study was funded by Ipsen. Linda A. Goldstein, PhD, CMPP from The Write Source MSC, LLC provided editorial and writing assistance funded by Ipsen in accordance with GPP3.
Data availability statement
The data that support the findings of this study have been originated by Flatiron Health, Inc. These de-identified data may be made available upon request, and are subject to a license agreement with Flatiron Health; interested researchers should contact <[email protected]> to determine licensing terms.
References
- Howlader N, Noone AM, Krapcho M, et al. (eds). SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020.
- Bond-Smith G, Banga N, Hammond TM, et al. Pancreatic adenocarcinoma. BMJ. 2012;344:e2476.
- McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24(43):4846–4861.
- Lambert A, Schwarz L, Borbath I, et al. An update on treatment options for pancreatic adenocarcinoma. Ther Adv Med Oncol. 2019;11:1758835919875568.
- Wang-Gillam A, Li CP, Bodoky G, NAPOLI-1 Study Group, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545–557.
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(4):439–457. Published 2021 Apr 1. doi:https://doi.org/10.6004/jnccn.2021.0017
- Beaver JA, Ison G, Pazdur R. Reevaluating eligibility criteria – balancing patient protection and participation in oncology trials. N Engl J Med. 2017; 376(16):1504–1505.
- U.S. Food and Drug Administration, FDA’s sentinel initiative – background; 2020 [cited 2020 Mar 23]. Available from: https://www.fda.gov/safety/fdas-sentinel-initiative/
- U.S. Food and Drug Administration, Sentinel initiative; 2008 [cited 2020 Mar 23]. Available from: https://www.sentinelinitiative.org/.
- Wong W, Yim YM, Kim A, et al. Assessment of costs associated with adverse events in patients with cancer. PLOS One. 2018; 13(4):e0196007.
- Daly B, Zon RT, Page RD, et al. Oncology clinical pathways: charting the landscape of pathway providers. J Oncol Pract. 2018; 14(3):e194–e200.
- Schroeder A. Clinical pathways: a current snapshot, and the journey ahead. J Clin Pathways. 2017;3(2):33–40.
- Wainberg ZA, Feeney K, Lee MA, et al. Meta-analysis examining overall survival in patients with pancreatic cancer treated with second-line 5-fluorouracil and oxaliplatin-based therapy after failing first-line gemcitabine-containing therapy: effect of performance status and comparison with other regimens. BMC Cancer. 2020; 20(1):633.
- U.S. Department of Health and Human Services, Common terminology criteria for adverse events (CTCAE) version 4.0 (v4.03): National Institutes of Health; 2010.
- Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015;33(28):3199–3212. doi:https://doi.org/10.1200/JCO.2015.62.3488
- Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer. 2009;101(10):1658–1663.
- Neuzillet C, Hentic O, Rousseau B, et al. FOLFIRI regimen in metastatic pancreatic adenocarcinoma resistant to gemcitabine and platinum-salts. WJG. 2012;18(33):4533–4541.
- Zaniboni A, Aitini E, Barni S, et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II study. Cancer Chemother Pharmacol. 2012;69(6):1641–1645.
- Koeller J, Surinach A, Arikian SR, et al. Comparing real-world evidence among patients with metastatic pancreatic ductal adenocarcinoma treated with liposomal irinotecan. Ther Adv Med Oncol. 2020;12:1758835920944052.
- Chang S, Long SR, Kutikova L, et al. Burden of pancreatic cancer and disease progression: economic analysis in the US. Oncology. 2006;70(1):71–80.
- DaCosta Byfield S, Nash Smyth E, Mytelka D, et al. Healthcare costs, treatment patterns, and resource utilization among pancreatic cancer patients in a managed care population. J Med Econ. 2013;16(12):1379–1386.
- Hirsch J, Pelizzari P, Cockrum P. Treatment patterns, survival rate, and total costs by line of therapy for FDA-approved/NCCN category 1 treatments for Medicare patients with metastatic pancreatic cancer(Poster C5). AMCP Nexus; 2019 October 29 to November 1; National Harbor, MD.
- Muldoon LD, Hirsch J, Dieguez G, et al. Treatment patterns, survival rate, and parts a and B costs by line of therapy for FDA-approved/NCCN category 1 treatments for patients with metastatic pancreatic cancer (abstract e18357). J Clin Oncol. 2019;37(15_suppl):e18357–e18357.
- Muldoon LD, Hirsch J, Dieguez G, et al. Comparing service utilization and costs for Medicare FFS patients with metastatic pancreatic cancer by chemotherapy regimen and line of therapy (Poster PCN302). ISPOR; 2019 May 18–22; New Orleans, LA.
- O'Neill CB, Atoria CL, O'Reilly EM, et al. Costs and trends in pancreatic cancer treatment. Cancer. 2012; 118(20):5132–5139.
- Dieguez G, Surinach A, Mercer D, et al. Real-world rates of hematologic laboratory abnormalities and associated cost among metastatic pancreatic cancer therapeutic regimens (J1). American Society for Clinical Oncology – Gastrointestinal Cancers Symposium; 2020 January 23–25; San Francisco, CA.
- Herrera-Restrepo O, Ferrufino CP, Bilir SP, et al. Budget impact in the USA of liposomal irinotecan as a post-gemcitabine treatment option for patients with metastatic pancreatic adenocarcinoma (mPC) (poster PCN80). ISPOR; 2019 May 18–22; New Orleans, LA.
- Hirsch J, Dieguez G, Cockrum P. The cost of adverse events for FDA-approved/NCCN category 1 treatments for medicare fee-for-service patients with metastatic pancreatic cancer (poster 4-138). ASHP Midyear Clinical Meeting; 2019 December 8–12; Las Vegas, NV.
- Prescott J, Pelletier V, Schilling A, et al. The state of the management of metastatic pancreatic cancer – a focus on recent real-world clinical & economic evidence with liposomal irinotecan. Clinical Brief; 2020 [cited April 7]. Available from: https://ajmc.s3.amazonaws.com/_media/_pdf/ajmc_a911_metastaticpancreaticcancer_clinicalbrief_web.pdf.