Abstract
Objective
Evidence is needed on the impact of anticoagulation therapy on kidney function in patients with atrial fibrillation (AF). The objective of this analysis, which is part of the CALLIPER study, was to investigate the risk of worsening kidney function with rivaroxaban 15 mg once daily compared with warfarin in patients with AF and moderate-to-severe chronic kidney disease (CKD) in routine clinical practice in the United States.
Methods
CALLIPER was an observational, retrospective, new-user cohort study. Adult patients with AF in the US IBM Watson MarketScan databases who newly initiated anticoagulation with rivaroxaban 15 mg once daily or warfarin between January 2013 and December 2017 were included. Comparative analysis was performed using Cox proportional hazards regression after adjustment for potential confounding by the stabilized inverse probability of treatment weighting approach and propensity score matching. One of the main study outcomes was worsening kidney function (composite of progression to CKD stage 5, kidney failure, or need for dialysis), besides traditional AF-related outcomes.
Results
The cohort included 7368 patients: 5903 (80.1%) initiating warfarin and 1465 (19.9%) initiating rivaroxaban 15 mg once daily. Rivaroxaban 15 mg was associated with a significant 47% reduction in the risk of worsening kidney function versus warfarin (hazard ratio 0.53; 95% confidence interval 0.35–0.78). Similar results were observed in the subgroup of patients with type 2 diabetes.
Conclusions
Rivaroxaban 15 mg may be associated with a lower risk of worsening kidney function as compared with warfarin in the atrial fibrillation population with moderate-to-severe CKD.
Trial registration number
NCT03359876
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia worldwideCitation1,Citation2; the presence of AF increases the risk of stroke approximately fivefoldCitation1,Citation3. The global incidence and prevalence of AF have been increasing in the past two decades and are expected to continue to riseCitation4.
Many patients with AF have comorbid chronic kidney disease (CKD) of various etiologiesCitation1,Citation5. AF is associated with a higher risk of progression to end-stage kidney disease (ESKD) in patients with CKDCitation6,Citation7. In turn, CKD is associated with an increased risk of AF, suggesting a possible bi-directional relationship between the two diseasesCitation8. Shared risk factors may play a role in this relationshipCitation8. For example, the risk of both AF and CKD increases with ageCitation9,Citation10, and although diabetes is the main cause of CKDCitation11, patients with diabetes also have a higher risk of developing AF, especially those who have a history of kidney complicationsCitation12.
CKD can complicate the management of patients with AF because they are at increased risk of both stroke and bleedingCitation13,Citation14. The risk of stroke increases with decreasing kidney functionCitation14,Citation15. Once kidney function has been lost, it usually cannot be recoveredCitation16,Citation17; therefore, the preservation of kidney function in patients with AF is critical.
The standard treatment for preventing stroke in patients with AF is anticoagulation therapyCitation1,Citation18. However, the choice of anticoagulant can affect the rate of kidney function decline. The 2019 American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) guidelines on the management of AF and the 2020 European Society of Cardiology (ESC) guidelines on the diagnosis and management of AF generally recommend non-vitamin K antagonist oral anticoagulants (NOACs) over vitamin K antagonists (VKAs) for stroke prevention in AFCitation1,Citation18. Despite these recommendations and evidence that the use of VKAs is associated with increased vascular calcification and worsening kidney functionCitation19–25, VKAs are still widely used for stroke prevention in clinical practiceCitation26. In contrast, evidence from recent studies in patients with or without kidney disease receiving NOACs or VKAs for stroke prevention suggests that some NOACs, including rivaroxaban, may be associated with a lower risk of adverse kidney outcomes compared with VKAsCitation27–29. There is a need to investigate the impact of oral anticoagulants (OACs) on kidney function in patients with AF and comorbid CKD because these patients are at greater risk of progression to ESKDCitation6,Citation7.
The aim of the CALLIPER (Evaluation of Clinical outcomes among non-valvular Atrial fibriLLatIon PatiEnts with Renal dysfunction treated with warfarin or reduced dose rivaroxaban) study was to assess the effectiveness and safety of rivaroxaban 15 mg once daily compared with warfarin in patients with AF and moderate-to-severe CKD in routine clinical practice in the United States. Rivaroxaban 15 mg once daily is the label-recommended dose in this patient populationCitation30,Citation31. This analysis, which forms a part of the overall CALLIPER study, assessed the risk of worsening kidney function in patients with AF and moderate-to-severe CKD receiving rivaroxaban 15 mg once daily or warfarin for stroke prevention.
Methods
CALLIPER was an observational, retrospective, new-user cohort study. Patients with AF and moderate-to-severe (stage 3 or 4) CKD who newly initiated anticoagulation therapy with warfarin or rivaroxaban 15 mg once daily were identified in the US IBM Watson MarketScan Commercial Claims and Medicare Supplemental databases. Individual patient-level data were retrieved for the time period between 1 January 2012 and 31 December 2017. This was the most recent data cut available at the initiation of the study conduct.
To be included in the study, patients were required to be ≥18 years of age and newly initiated on warfarin or rivaroxaban 15 mg (index event, index drug) between 1 January 2013 and 31 December 2017, and had to have at least 365 days of continuous medical and prescription benefits prior to the index event (baseline period). Patients with AF were identified by at least two diagnosis codes for AF on outpatient or inpatient claims on two different days, and those who had CKD were identified by one inpatient or outpatient diagnosis code for CKD stage 3 or 4 during the baseline period.
Patients were excluded from the study if they had a diagnosis of valvular AF, pregnancy, transient cause of AF, ESKD, or were on dialysis in the baseline period; had a diagnosis of venous thromboembolism, pulmonary embolism or deep vein thrombosis, or received hip or knee replacement 60 days prior to or on the index date. Patients were also excluded if they had a pharmacy claim for an OAC dispensation in the baseline period, or received both warfarin and rivaroxaban 15 mg once daily on the index date.
The initiation of warfarin or rivaroxaban was defined as the index event. The date of the first fill of these OACs was defined as the index date. Patients were followed up from the index date until the occurrence of an outcome, insurance disenrollment, index anticoagulant discontinuation/switch (with a grace period of 30 days and an outcome risk window of 5 days), or the end of data availability. One of the main study outcomes was a composite of progression to CKD stage 5, kidney failure or need for dialysis, besides traditional AF related outcomes such as the composite of ischemic stroke and intracranial hemorrhage, ischemic stroke alone, and major bleeding. Clinical characteristics and outcomes were defined based on diagnosis, procedure, and drug codes used in MarketScan databases. The kidney failure component of the study outcome was defined by the diagnosis code of ESKD, or diagnosis and procedure codes indicating kidney transplant status. Laboratory data were not captured in these databases. The composite outcome of ischemic stroke and intracranial hemorrhage, and ischemic stroke alone were defined by an appropriate inpatient diagnosis code in the primary position. Major bleeding rates were assessed using the validated Cunningham algorithm for the detection of bleeding-related hospitalizationsCitation32.
A comparative analysis was performed using Cox proportional hazards regression to evaluate the hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) in patients receiving rivaroxaban 15 mg once daily or warfarin. Potential confounding effects were addressed with propensity score (PS) methods; primary analysis was performed with stabilized inverse probability of treatment weighting (IPTW) and sensitivity analyses were performed using PS-based 1:1 matching. PS was defined as the patient’s probability to receive a treatment under investigation (rivaroxaban 15 mg) given a set of known patient baseline characteristics. While blinded to the outcomes, PSs were calculated by utilizing multiple logistic regressions on a relevant set of patient characteristics including patient demographics, comorbidities, concomitant medications, individual components of the CHA2DS2-VASc (Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke or transient ischemic attack, Vascular disease, Age 65–74, Sex category [female]) and modified HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio – not assessed, Elderly, Drugs/alcohol abuse, medication usage predisposing to bleeding) scores (), measured during the baseline period. The components of the HAS-BLED score that are normally assessed based on laboratory data, including abnormal kidney and liver function, were estimated based on diagnostic codes (Supplementary Table S1).
Table 1. Baseline characteristics before applying IPTW and the absolute standardized difference before and after IPTW.
The effectiveness of the IPTW method in balancing out the patient characteristics among the treatment groups was assessed using the absolute standardized differences (). A difference <0.1 was considered well-balanced for each variable. The distributions of PSs and stabilized weights were inspected for initial and IPTW-synthetic samples. A standardized difference of 0.1 or less was considered a negligible difference between groups.
PS matching was performed using 1:1 nearest neighbor matching with a maximum matching caliper. Differences in the confounder distributions between exposure groups were displayed to inspect successful confounder balance in measured characteristics. (Supplementary Table S2). A post-matching C-statistic was computed as a summary metric for confounder balance. C-statistics close to 0.5 represent a good overall balance.
All analyses were performed with the Aetion Evidence Generation Platform™ using the Effectiveness Evaluation Application, version r3.6.20180806_2223 0 g58f60b3. Statistical computations were conducted using R version 3.4.2 (2018-01-25). IPTW analysis was performed in R version 3.3.2 (2016-10-31) using the survey package.
Our institutional review board determined that CALLIPER is exempt from institutional review board oversight because it does not constitute research involving human subjects according to 45 CFR 46.102(f). The data included in the MarketScan database are de-identified and are in compliance with the Health Insurance Portability and Accountability Act (HIPAA) of 1996 to preserve participant anonymity and confidentiality, and as such this study followed the principles of the declaration of Helsinki without the requirement for review from a formal ethics review committee. CALLIPER was registered on ClinicalTrials.gov with the identifier NCT03359876.
Results
Baseline characteristics
A total of 7372 patients were included in the study cohort. Of these, 4 patients did not begin follow-up (). The final analysis cohort included 5903 (80.1%) patients who initiated warfarin and 1465 (19.9%) patients who initiated rivaroxaban 15 mg once daily. The median follow-up period was 115 days (interquartile range [IQR] 51–271) with warfarin and 119 days (IQR 33–304) with rivaroxaban. Reasons for censoring in the main patient cohort and for each exposure group are given in .
Figure 1. Patient disposition. Abbreviations. AF, atrial fibrillation; CKD, chronic kidney disease; DVT, deep vein thrombosis; ESKD, end-stage kidney disease; NOAC, non-vitamin K antagonist oral anticoagulant; PE, pulmonary embolism; VKA, vitamin K antagonist; VTE, venous thromboembolism
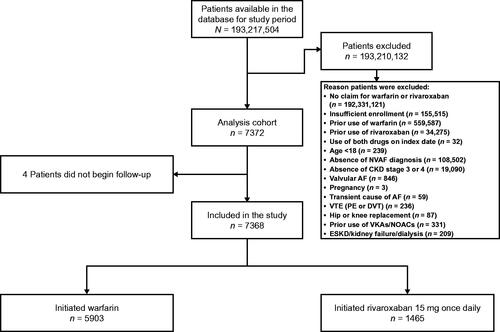
Table 2. Reasons for censoring in the main patient cohort and for each exposure group for the worsening kidney function outcome.
Baseline characteristics are summarized in . Most patients were male (n = 4440, 60.3%) and the median age was 79 (IQR 71–84) years. The median CHA2DS2-VASc score was 4.43 (IQR 3.40–5.62) and the median modified HAS-BLED score (not including the international normalized ratio, which was not captured in the database) was 3.00 (IQR 2.40–3.65). In the entire study cohort, more than half of the patients (53.0%) had diabetes. The percentage of patients with diabetes was similar in the warfarin (53.5%) and rivaroxaban (50.9%) groups. Other common comorbidities at baseline included hypertension (93.1%), heart failure (54.6%), anemia (40.7%), angina (36.2%), and cancer (26.5%). Most patients received concomitant treatment with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (64.0%) or beta-blockers (78.0%).
Clinical outcomes
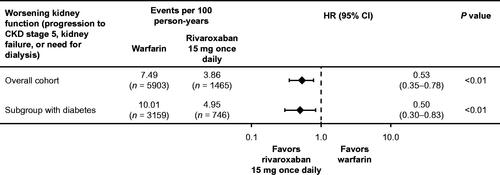
Rivaroxaban was associated with a significant 47% reduction in the risk of worsening kidney function (defined as a composite of progression to CKD stage 5, kidney failure, or need for dialysis) compared with warfarin (HR = 0.53; 95% CI 0.35–0.78) in patients with AF and CKD stage 3 or 4. The incidence rate of progression to CKD stage 5, kidney failure, or need for dialysis was 7.49 per 100 person-years with warfarin and 3.86 per 100 person-years with rivaroxaban. The results in the subgroup of patients with AF, CKD stage 3 or 4, and type 2 diabetes were similar to the overall study results (). Rivaroxaban was associated with a 50% reduction in the risk of worsening kidney function compared with warfarin (HR = 0.50; 95% CI 0.30–0.83) in this subgroup. The incidence rate of progression to CKD stage 5, kidney failure or need for dialysis was 10.01 per 100 patient-years with warfarin and 4.95 per 100 person-years with rivaroxaban in the subgroup with diabetes.
Figure 2. Worsening kidney function (progression to CKD stage 5, kidney failure, or need for dialysis) in the overall cohort and diabetes subgroup using stabilized IPTW methodology to adjust for differences in baseline characteristics. Abbreviations. CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; IPTW, inverse probability of treatment weighting
The results of a sensitivity analysis of worsening kidney function using 1:1 PS matching to adjust for differences in baseline characteristics were similar to the results of the analysis using the stabilized IPTW methodology (Supplementary Figure S2).
Rivaroxaban was also associated with a reduction in ischemic stroke and intracranial hemorrhage (HR 0.61; 95% CI 0.30–1.24), and ischemic stroke alone (HR 0.77; 95% CI 0.33–1.82) in patients with AF and CKD stage 3 or 4 (Supplementary Table S3). Overall, no significant difference in Cunningham bleeding-related hospitalization was observed between rivaroxaban and warfarin users (HR 1.14; 95% CI 0.83–1.58) in this patient cohort. The incidence rate of the composite outcome of ischemic stroke and intracranial hemorrhage was 1.86 per 100 person-years with warfarin and 1.09 per 100 person-years with rivaroxaban. The incidence rate of ischemic stroke alone was 1.06 per 100 person-years with warfarin and 0.77 per 100 person-years with rivaroxaban, whereas the incidence rate of the Cunningham bleeding-related hospitalization was 5.04 per 100 person-years with warfarin and 5.87 per 100 person-years with rivaroxaban. Assessment of the traditional clinical outcomes in the subgroup of patients with AF, CKD stage 3 or 4, and type 2 diabetes was not performed due to the low number (≤5 in the rivaroxaban group) of the effectiveness events (there were 19 ischemic strokes in the warfarin subgroup and 3 ischemic strokes in the rivaroxaban subgroup).
The results of a sensitivity analysis of the composite outcome of ischemic stroke and intracranial hemorrhage, ischemic stroke alone and Cunningham bleeding-related hospitalization using 1:1 PS matching to adjust for differences in baseline characteristics were similar to the results of the analysis using the stabilized IPTW methodology (Supplementary Table S4).
Discussion
The CALLIPER study showed that rivaroxaban 15 mg once daily was associated with a significantly lower risk of worsening kidney function compared with warfarin in patients with AF and CKD stage 3 or 4 with or without diabetes in routine clinical practice.
Kidney function declines in patients treated with warfarin or NOACs, which is directionally consistent with the age-related decline observed in healthy adultsCitation33,Citation34. However, it has been hypothesized that NOACs may be associated with better preservation of kidney function than warfarin, as the inhibition of factor Xa or thrombin with NOACs may reduce vascular inflammation and consecutively have a protective effect on the kidneysCitation27. This might be one explanation for the CALLIPER study results. Another possible explanation is related to vascular calcification which leads to a decrease in kidney functionCitation35,Citation36. Vitamin K-dependent matrix Gla protein is an inhibitor of vascular calcification. Its function may be impaired with VKA anticoagulants such as warfarin, leading to the increased risk of vascular calcificationCitation19,Citation37. Furthermore, the use of warfarin is associated with anticoagulation-related nephropathy (ARN), which leads to the accelerated progression of CKDCitation21,Citation38. It should be noted that ARN may also occur in patients receiving NOACs, although further studies are needed to establish the prevalence of ARN in this settingCitation38.
The results of the CALLIPER study are consistent with the evidence from other real-world studies, which generally showed a lower risk of adverse kidney outcomes with rivaroxaban compared with VKAs. An Optum administrative claims database study on patients with AF in the United States reported that, compared with VKAs, rivaroxaban was associated with a significant 27% lower risk of ≥30% decline in estimated glomerular filtration rate (eGFR), 54% lower risk of doubling of serum creatinine, and 37% numerically lower risk of kidney failure (defined as eGFR <15 mL/min/1.73 m2, kidney transplant, or undergoing long-term dialysis)Citation27. A study on patients with AF identified in the US Truven MarketScan database demonstrated an 18% lower risk in the composite of progression to CKD stage 5 and the need for hemodialysis with rivaroxaban versus warfarinCitation28. An analysis of the ORBIT AF II (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II) did not show a significant difference in mean creatinine clearance CrCl decline with NOACs versus warfarinCitation33. However, worsening kidney function (defined as >20% decline in CrCl from baseline) was more common in patients receiving warfarin versus NOACs (28.0% vs 22.1% of patients, respectively)Citation33. Similar trends were observed for other kidney outcomes. An absolute serum creatinine increase of >0.3 mg/dL was observed in 18.0% of patients receiving warfarin and 12.9% of patients receiving NOACs, whereas a > 30% decrease in CrCl from baseline was observed in 14.3% of patients receiving warfarin and 9.8% of patients receiving NOACsCitation33. Rivaroxaban was associated with a 73% lower risk of the composite of progression to CKD stage 5 or need for dialysis compared with a VKA (phenprocoumon) in the RELOADED (Real-world Comparative Effectiveness of Stroke Prevention in Patients with Atrial Fibrillation Treated with Factor Xa Non-vitamin-K Oral Anticoagulants [NOACs] vs. Phenprocoumon) study, which included patients with AF and kidney disease identified from German claims dataCitation29. A US IBM MarketScan database analysis of patients with AF and diabetes showed an 18% (95% CI 4–30%) lower risk of progression to CKD stage 5 or hemodialysis with rivaroxaban compared with warfarinCitation39.
Additional studies assessing the role of OACs in kidney function are being conducted. For example, XARENO (Factor XA inhibition in RENal patients with non-valvular atrial fibrillation – Observational registry) is an ongoing prospective, non-interventional, observational registry study assessing the progression of CKD and the clinical outcomes of OAC strategies in patients with AF and CKD in routine clinical practiceCitation40.
Different endpoints have been used to evaluate the decline in kidney function in patients with AF receiving anticoagulation for stroke preventionCitation27,Citation33,Citation41,Citation42. However, it is important to note that not all outcomes used to evaluate kidney function may have the same clinical relevanceCitation43,Citation44. For example, the development of ESKD is one of the most important clinical outcomes for patients with CKD, which is associated with increased morbidity, mortality and poor quality of lifeCitation43,Citation44. The use of ESKD as an endpoint in clinical studies is hampered by the varied definitions of ESKD among healthcare systems and the long follow-up times needed to obtain enough events for the results to reach significanceCitation43–45. The doubling of serum creatinine, or an approximately 57% decrease in eGFR, has been historically considered an acceptable surrogate endpoint, and, by expert consensus, even lower percentages of eGFR decline (e.g. 40% or 30% decline in eGFR) may be suitable as surrogate endpointsCitation43–46. It should be noted that the lower the percentage of eGFR decline, the weaker the association of such decline with the progression to ESKDCitation43,Citation44. However, all serum creatinine- and eGFR-based endpoints have limitationsCitation43,Citation44,Citation47. For example, lower decreases in eGFR may not directly translate to an effect on kidney functionCitation43,Citation44. Additionally, regulators, payers, clinicians, and patients may not accept these surrogates as robust and trustworthy primary endpointsCitation48,Citation49. Furthermore, the use of different methods to estimate eGFR values means that the results of different studies are not comparableCitation50.
The 2020 ESC guidelines on the diagnosis and management of AF highlight the fact that AF is rarely an isolated condition; patients with AF usually have comorbidities, such as CKD or diabetes, that complicate their treatmentCitation1. Therefore, it is important to consider kidney function in patients with AF receiving anticoagulation for stroke prevention, not only to identify label-recommended doses and manage the efficacy and safety of anticoagulation treatment but also to ensure that the treatment does not compromise kidney functionCitation27,Citation31,Citation33,Citation51–56. The current evidence supports the guideline recommendations for kidney function monitoring in all patients with AF, especially in those with AF and kidney impairmentCitation18,Citation33,Citation41,Citation42,Citation52,Citation53,Citation57,Citation58. The 2018 European Heart Rhythm Association (EHRA) guidelines on the use of NOACs in patients with AF and the 2019 AHA/ACC/HRS guidelines on the management of AF recommend more frequent kidney function assessment in patients with AF and CKD; when the patient’s eGFR is <60 mL/min/1.73 m2, the eGFR measurement frequency should be every (eGFR/10) monthCitation18,Citation58. Monitoring of kidney function enables physicians to tailor anticoagulation therapy to individual patients, ensuring optimal protection from stroke.
Study strengths and limitations
Healthcare databases (claims, electronic medical records) are increasingly used data assets to determine the comparative effectiveness and safety of medical products as used in routine clinical care. Large in size, such studies have their strengths and limitations.
CALLIPER was a retrospective new-user cohort database study designed to provide robust evidence on the association between clinical outcomes and OAC treatment among patients with AF in the ‘real world’ (i.e. in routine clinical practice). Unlike the highly selected patient populations in randomized controlled trials, the population in the CALLIPER study was heterogeneous and likely included patients who may have been excluded or underrepresented in such trials, for example, patients of advanced age or those with comorbidities such as CKD.
The source population of the CALLIPER study included all the insured individuals in the IBM Watson MarketScan databases. Therefore, the results are most generalizable to a US population. The MarketScan databases largely cover employees and their dependents, so patients with conditions that prevent them from being employed might be underrepresented.
Kidney function and related outcomes are clinically assessed by using specific laboratory test results. The majority of administrative claims databases, including MarketScan, have poorly reported laboratory dataCitation59. These data, including international normalized ratios and estimated glomerular filtration rates, were not available in the IBM Watson MarketScan databases used for the CALLIPER study. A previous study showed that patients treated with NOACs in real-world clinical practice had lower rates of kidney outcomes than patients treated with warfarin with INR >3 as well as INR <2 or INR 2–3Citation27, suggesting that this limitation is not likely to have a significant impact on the results of CALLIPER. The unavailability of serum creatinine or eGFRs meant that worsening renal function could not be assessed based on surrogate endpoints such as a doubling in serum creatinine or an increase in estimated glomerular function. Instead of laboratory data, carefully chosen diagnosis and procedure codes were used to identify patients with moderate-to-severe CKD and to analyze the worsening kidney function endpoint. Although all efforts were made to capture patients with characteristics close to those eligible for the rivaroxaban 15 mg once daily dosing regimen, there was no possibility to assess their true kidney status. Our approach to identifying CKD based on the diagnosis codes might have missed patients who might have qualified for inclusion in the study based on the lab measures only. The confirmation of the clinical event in the composite endpoint by the lab measures would be beneficial for its assessment. Similarly, the other components of the HAS-BLED score that are normally assessed based on laboratory data, including abnormal kidney and liver function, were estimated based on diagnostic codes for liver and kidney diseases. These limitations must be considered when interpreting the results of the study.
In database analyses where randomization is not possible, residual confounding caused by unmeasured factors, missing data, miscoding or tactical coding issues, and over-the-counter drugs use (among other factors) remain present. Misclassification bias, for example, may impact the study’s internal validity. We attempted to mitigate the probability of misclassification bias by using a validated coding schema to define study variables whenever possible. It is also possible that unmeasured factors may have contributed to worsening renal function in the study. To minimize residual confounding in the CALLIPER study, several advanced confounding adjustment strategies were applied. The PS-based methods helped to generate cohorts of patients that were comparable in their demographic and clinical characteristics. However, only variables available in IBM Watson MarketScan databases were used for adjustment. For example, time from AF diagnosis could not be used for PS matching. The person–time in the MarketScan databases used in the study was relatively short, which meant that there was no way to determine when the first diagnosis of AF was recorded. Although there is no reason to expect the time from AF diagnosis to differ between the rivaroxaban and warfarin groups, residual confounding is possible.
Conclusions
Kidney function decline is common among patients with AF treated with OACsCitation33,Citation41,Citation42,Citation57. The choice of OAC may have an impact on the rate of kidney function declineCitation22,Citation23,Citation27–29. The CALLIPER study, which used one of the largest US administrative claims databases, demonstrated that the reduced dose of rivaroxaban was associated with a significant 47% and 50% risk reduction of progression to CKD stage 5, kidney failure or need for dialysis versus warfarin in AF patients with CKD stage 3 and 4 and in those who additionally have type 2 diabetes, respectively. These results suggest that rivaroxaban may be associated with a lower risk of adverse kidney outcomes compared with warfarin and may be considered over warfarin in patients with AF and stage 3 or 4 CKD with or without type 2 diabetes. Generated evidence adds to previous findings on non-traditional (stroke- and bleeding-related) outcomes in AF patients prescribed rivaroxaban or warfarin for stroke prevention.
Transparency
Declaration of funding
This study was funded by Bayer AG.
Declaration of financial/other relationships
TV, FK, BV and BS are employees of Bayer AG. CIC was contracted and funded by Bayer AG. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors have contributed to the design, analysis, interpretation of data, drafting and reviewing of the manuscript and have read and approved the final draft for submission.
Supplemental Material
Download MS Word (79.6 KB)Acknowledgements
The authors would like to acknowledge Milda Jakutavičiūtė from Chameleon Communications International, who provided editorial assistance with the preparation of the manuscript, with funding from Bayer AG. This analysis was previously presented at ESC 2019 Congress.
Data availability statement
This study used data from US IBM Watson MarketScan Commercial Claims and Medicare Supplemental databases.
References
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498.
- Turakhia MP, Conference Participants, Blankestijn PJ, Carrero JJ, et al. Chronic kidney disease and arrhythmias: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Eur Heart J. 2018;39(24):2314–2325.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22(8):983–988.
- Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. 2021;16(2):217–221.
- Zoni-Berisso M, Lercari F, Carazza T, et al. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213–220.
- Bansal N, Fan D, Hsu CY, et al. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. 2013;127(5):569–574.
- Bansal N, Xie D, Tao K, et al. Atrial fibrillation and risk of ESRD in adults with CKD. Clin J Am Soc Nephrol. 2016;11(7):1189–1196.
- Watanabe H, Watanabe T, Sasaki S, et al. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J. 2009;158(4):629–636.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528.
- Liu P, Quinn RR, Lam NN, et al. Progression and regression of chronic kidney disease by age among adults in a population-based cohort in Alberta, Canada. JAMA Netw Open. 2021;4(6):e2112828.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045.
- Seyed Ahmadi S, Svensson AM, Pivodic A, et al. Risk of atrial fibrillation in persons with type 2 diabetes and the excess risk in relation to glycaemic control and renal function: a Swedish Cohort Study. Cardiovasc Diabetol. 2020;19(1):9.
- Olesen JB, Lip GYH, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625–635.
- Roldán V, Marín F, Fernández H, et al. Renal impairment in a "real-life" cohort of anticoagulated patients with atrial fibrillation (implications for thromboembolism and bleeding). Am J Cardiol. 2013;111(8):1159–1164.
- Masson P, Kelly PJ, Craig JC, et al. Risk of stroke in patients with ESRD. CJASN. 2015;10(9):1585–1592.
- Weis L, Metzger M, Haymann JP, et al. Renal function can improve at any stage of chronic kidney disease. PLOS One. 2013;8(12):e81835.
- Ku E, Hsu RK, Johansen KL, et al. Recovery of kidney function after dialysis initiation in children and adults in the US: a retrospective study of United States renal data system data. PLOS Med. 2021;18(2):e1003546.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140(2):e125–e151.
- Willems BAG, Vermeer C, Reutelingsperger CPM, et al. The realm of vitamin K dependent proteins: shifting from coagulation toward calcification. Mol Nutr Food Res. 2014;58(8):1620–1635.
- Peeters FECM, Dudink EAMP, Kimenai DM, et al. Vitamin K antagonists, non-vitamin K antagonist oral anticoagulants, and vascular calcification in patients with atrial fibrillation. TH Open. 2018;2(4):e391–e398.
- Brodsky SV, Nadasdy T, Rovin BH, et al. Warfarin-related nephropathy occurs in patients with and without chronic kidney disease and is associated with an increased mortality rate. Kidney Int. 2011;80(2):181–189.
- Brodsky S, Eikelboom J, Hebert LA. Anticoagulant-related nephropathy. J Am Soc Nephrol. 2018;29(12):2787–2793.
- de Aquino Moura KB, Behrens PMP, Pirolli R, et al. Anticoagulant-related nephropathy: systematic review and meta-analysis. Clin Kidney J. 2019;12(3):400–407.
- Rizk DV, Warnock DG. Warfarin-related nephropathy: another newly recognized complication of an old drug. Kidney Int. 2011;80(2):131–133.
- Levy DS, Grewal R, Le TH. Vitamin K deficiency: an emerging player in the pathogenesis of vascular calcification and an iatrogenic consequence of therapies in advanced renal disease. Am J Physiol Renal Physiol. 2020;319(4):F618–F623.
- Washam JB, Holmes DN, Thomas LE, et al. Pharmacotherapy for atrial fibrillation in patients with chronic kidney disease: insights from ORBIT-AF. J Am Heart Assoc. 2018;7(18):e008928.
- Yao X, Tangri N, Gersh B, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. Am J Cardiol. 2017;70(21):2621–2632.
- Coleman CI, Kreutz R, Sood N, et al. Rivaroxaban's impact on renal decline in patients with nonvalvular atrial fibrillation: a US MarketScan claims database analysis. Clin Appl Thromb Hemost. 2019;25:107602961986853.
- Bonnemeier H, Kreutz R, Kloss S, et al. Comparative safety and effectiveness of non-vitamin-K oral anticoagulants vs phenprocoumon in patients with non-valvular atrial fibrillation and renal disease – results from the RELOADED study. Eur Stroke J. 2019;4(1_suppl):1–860.
- Janssen Pharmaceuticals Inc. Xarelto (rivaroxaban) Prescribing Information. Titusville, NJ: USA; January 2021. [cited 8 June 2021] Available from: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf.
- Bayer AG. Xarelto (rivaroxaban) Summary of Product Characteristics. Berlin: Germany; 2 February 2021. [cited 10 January 2022] Available from: https://www.ema.europa.eu/documents/product-information/xarelto-epar-product-information_en.pdf.
- Cunningham A, Stein CM, Chung CP, et al. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560–566.
- Inohara T, Holmes DN, Pieper K, et al. Decline in renal function and oral anticoagulation dose reduction among patients with atrial fibrillation. Heart. 2020;106(5):358–364.
- Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. 2016;23(1):19–28.
- Posch F, Ay C, Stoger H, et al. Exposure to vitamin K antagonists and kidney function decline in patients with atrial fibrillation and chronic kidney disease. Res Pract Thromb Haemost. 2019;3(2):207–216.
- Siltari A, Vapaatalo H. Vascular calcification, vitamin K and warfarin therapy – possible or plausible connection? Basic Clin Pharmacol Toxicol. 2018;122(1):19–24.
- van Gorp RH, Schurgers LJ. New insights into the pros and cons of the clinical use of vitamin K antagonists (VKAs) versus direct oral anticoagulants (DOACs). Nutrients. 2015;7(11):9538–9557.
- Wheeler D, Giugliano R, Rangaswami J. Anticoagulation-related nephropathy. J Thromb Haemost. 2016;14(3):461–467.
- Hernandez AV, Bradley G, Khan M, et al. Rivaroxaban versus warfarin and renal outcomes in non-valvular atrial fibrillation patients with diabetes. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):301–307.
- GWT-TUD GmbH, ClinStat GmbH [Internet]. Factor XA – Inhibition in renal patients with non-valvular atrial fibrillation – Observational registry (XARENO). [cited 8 June 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT02663076.
- Böhm M, Ezekowitz MD, Connolly SJ, et al. Changes in renal function in patients with atrial fibrillation: an analysis from the RE-LY trial. J Am Coll Cardiol. 2015;65(23):2481–2493.
- Fordyce CB, Hellkamp AS, Lokhnygina Y, et al. On-treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin: Insights from ROCKET AF. Circulation. 2016;134(1):37–47.
- Badve SV, Palmer SC, Hawley CM, et al. Glomerular filtration rate decline as a surrogate end point in kidney disease progression trials. Nephrol Dial Transplant. 2016;31(9):1425–1436.
- Hartung EA. Biomarkers and surrogate endpoints in kidney disease. Pediatr Nephrol. 2016;31(3):381–391.
- Kanda E, Kashihara N, Matsushita K, et al. Guidelines for clinical evaluation of chronic kidney disease : AMED research on regulatory science of pharmaceuticals and medical devices. Clin Exp Nephrol. 2018;22(6):1446–1475.
- Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the national kidney foundation in collaboration with the US food and drug administration and European Medicines Agency. Am J Kidney Dis. 2020;75(1):84–104.
- Samra M, Abcar AC. False estimates of elevated creatinine. Perm J. 2012;16(2):51–52.
- Mol PGM, Maciulaitis R, Vetter T. GFR decline as an end point for clinical trials in CKD: a view from Europe. Am J Kidney Dis. 2014;64(6):838–840.
- Ciani O, Grigore B, Blommestein H, et al. Validity of surrogate endpoints and their impact on coverage recommendations: a retrospective analysis across international health technology assessment agencies. Med Decis Making. 2021;41(4):439–452.
- Andrade JG, Hawkins NM, Fordyce CB, et al. Variability in non-vitamin K antagonist oral anticoagulants dose adjustment in atrial fibrillation patients with renal dysfunction: the influence of renal function estimation formulae. Can J Cardiol. 2018;34(8):1010–1018.
- Camm AJ, GARFIELD-AF Investigators, Cools F, Virdone S, et al. Mortality in patients with atrial fibrillation receiving nonrecommended doses of direct oral anticoagulants. J Am Coll Cardiol. 2020;76(12):1425–1436.
- Steinberg BA, Shrader P, Thomas L, et al. Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II registry. J Am Coll Cardiol. 2016;68(24):2597–2604.
- Santos J, António N, Rocha M, et al. Impact of direct oral anticoagulant off-label doses on clinical outcomes of atrial fibrillation patients: a systematic review. Br J Clin Pharmacol. 2020;86(3):533–547.
- Bristol Myers Squibb, Pfizer EEIG. Eliquis (apixaban) Summary of Product Characteristics. Uxbridge: UK; 25 May 2021. [cited 8 June 2021] Available from: https://www.ema.europa.eu/documents/product-information/eliquis-epar-product-information_en.pdf.
- Daiichi Sankyo Europe GmbH. Lixiana (edoxaban) Summary of Product Characteristics. Munich: Germany; 23 April 2021. [cited 8 June 2021] Available from: https://www.ema.europa.eu/documents/product-information/lixiana-epar-product-information_en.pdf.
- Boehringer Ingelheim International GmbH. Pradaxa (dabigatran etexilate) Summary of Product Characteristics. Ingelheim am Rhein: Germany; 17 March 2021. [cited 8 June 2021] Available from: https://www.ema.europa.eu/documents/product-information/pradaxa-epar-product-information_en.pdf.
- Hijazi Z, Hohnloser SH, Andersson U, et al. Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: Insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. 2016;1(4):451–460.
- Steffel J, Verhamme P, Potpara TS, et al. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–1393.
- Schneeweiss S, Rassen JA, Glynn RJ, et al. Supplementing claims data with outpatient laboratory test results to improve confounding adjustment in effectiveness studies of lipid-lowering treatments. BMC Med Res Methodol. 2012;12:180.
