Abstract
Objective
The objective of this retrospective cohort study was to describe the adherence and discontinuation patterns of somatropin over 3 years among children with pGHD insured by Medicaid across the United States.
Methods
Eligible children were aged ≥3 and <16 years with Medicaid coverage, diagnosed with pGHD, and had ≥2 new prescriptions for somatropin between 1 July 2014 and 31 December 2018. Four non-exclusive patient cohorts were constructed (≥3, 12, 24, and 36 months of continuous enrollment after initial prescription). Suboptimal adherence was defined as medication possession ratio <0.80, and discontinuation as a gap of >60 days between somatropin fills. Logistic and proportional hazards regression methods were used to estimate odds of suboptimal adherence and time to discontinuation, respectively.
Results
In the 12-month cohort (n = 3623), mean age was 10.5 ± 3.2 years, 70.8% were male, 44.4% White, 29.1% Hispanic, 7.1% Black, and 1.7% Asian. At months 12, 24, and 36, the proportion with suboptimal adherence was 40.9, 50.4, 54.4%, respectively, and 49.2% of patients with ≥3 months of follow-up discontinued therapy. At 12 months, lower age and race/ethnicity (Black vs. White referent) had greater odds of suboptimal adherence. Discontinuation was associated with Black (vs. White referent) race and geographic region.
Conclusions
Sociodemographic characteristics may be risk factors for suboptimal adherence and/or discontinuation of prescribed somatropin therapy. Improving GH regimen adherence among this at-risk population, and specifically among subgroups at highest risk, is warranted to improve clinical outcomes.
Introduction
Pediatric growth hormone deficiency (pGHD) is treated with daily subcutaneous injections of recombinant human growth hormone (r-hGH), known as somatropin. The goal of somatropin therapy is to improve growth in childhood, to achieve final adult height to within 2 standard deviations of the population mean, and to prevent or improve metabolic impairments seen with GH deficiency. Worldwide, the prevalence of pGHD in different regions has been estimated at approximately 1 in 3500–10,000 children, and the Utah Growth Study estimated the prevalence of pGHD in the US as at least 1 in 3480Citation1–4. Some estimates of pGHD prevalence may be influenced by differential treatment access, particularly among subgroups that have barriers to care, such as those with lower socioeconomic status or racial/ethnic minorities. Thus, there is limited data related to pGHD prevalence among racial/ethnic subgroups, and those with barriers to healthcare may have historically resulted in an underestimated prevalence of pGHD. Data from the National Health and Nutrition Examination Survey (NHANES data) has suggested different growth trajectories for non-Hispanic White and non-Hispanic Black children; Black children’s growth outpaced that of White children during childhood, but growth of White children exceeded the growth of Black children during adolescenceCitation5. White children have been overrepresented among pGHD registries relative to the population, and Black children have been underrepresentedCitation6,Citation7. Other research has suggested that racial/ethnic biases may play a role in the underdiagnosis of GHD and less frequent referral to treatment among Black as compared to White childrenCitation7.
Adherence to somatropin has been demonstrated to be suboptimal in real-world studiesCitation8–10. The requirement for daily injections is burdensome and may contribute to suboptimal adherence. An estimated 2 in 3 children/adolescents with pGHD may miss more than one injection per weekCitation8. Suboptimal adherence to somatropin can be associated with poorer growthCitation8–10. Factors influencing adherence may include the following: (1) therapy related barriers (e.g. lack of disease awareness and benefits of treatment, improper use of device, injection pain and schedule burden), (2) payer hurdles (e.g. frequent health plan-required authorizations), (3) emotional or social barriers (e.g. peer pressure, forgetting doses), (4) the physician/patient relationship, and (5) socioeconomic barriers (e.g. chaotic households)Citation11. Strategies to improve adherence to prescribed somatropin therapy include injection devices which can track adherence, effective partnership between patient and physician, and education regarding the benefits of treatment. New long-acting growth hormones (LAGHs) are in development; a less frequent injection schedule may be less burdensome to patients and caregivers, facilitate better adherence, and long-term, may facilitate improved growth and improved quality of lifeCitation12. Recent data indicates that both children and adults, treated with daily r-hGH injections, indicated a preference for a less frequent injection regimenCitation13.
Evaluations of real-world GH adherence in pGHD are needed, particularly among populations of lower socioeconomic status or racial/ethnic subgroups in the US, where barriers to care continue to persist. Data from a large dataset, such as that contained within a US Medicaid Claims Database, offers an opportunity to understand current rates of suboptimal adherence with current therapy (i.e. daily injections of somatropin) among Medicaid recipients. The objectives of this study were to describe medication adherence and discontinuation among children diagnosed with pGHD, who were treated with daily somatropin injections over 3 years, from a US Medicaid population. Secondary objectives were to explore barriers to somatropin adherence, by characterizing the demographic and clinical profiles of a US Medicaid population with pGHD who have good somatropin adherence compared to those with suboptimal adherence.
Methods
Data source
This retrospective cohort study employed the Centers for Medicare and Medicaid Services (CMS) Medicaid database accessed by Clarify Health through the Virtual Research Data Center. The CMS Medicaid database contains 100% of the state-level Medicaid claims submitted to CMS. This database includes information on patient demographic and enrollment data, as well as inpatient and outpatient services and prescription drug claims, for more than 60 million people covered by Medicaid fee-for-service (FFS) or Managed Medicaid insurance. Approximately 24% of the US population is covered by Medicaid, and of this, Medicaid FFS and Managed Medicaid represent roughly 17% and 83%, respectively (approximately 4% and 20% of the US population, respectively). Individual states submit Medicaid claims to the Transformed Medical Statistical Information System (T-MSIS). CMS aggregates the T-MSIS data into T-MSIS Analytic Files (TAF), which are structured specifically for research and analytical purposes. Since 2016, the CMS Medicaid database represents over 90% of the Medicaid FFS and Managed Medicaid market in the US. However, data in the T-MSIS vary regionally due to differences in the Medicaid program at the state level. Data coverage is lower in 2014 and 2015 as states transitioned to the T-MSIS file structure at different times; during this time, only 18 states submitted T-MSIS data in 2014 and 25 sites in 2015, respectively. Since 2016, all 50 states have been participating in TAF. CMS provides assessments of TAF claims at the individual state level to ensure that the data are usable for research and analysis. In the CMS Medicaid database, diagnoses, procedures and prescription drugs are coded using the International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification (ICD-9/10-CM), the Current Procedural Terminology, Fourth Edition (CPT-4) and National Drug Code (NDC), respectively.
Study population
Patients were between 3 and 16 years of age who had at least 2 prescription claims for somatropin between 1 July 2014 and 31 December 2018. This was the most recent data available from the CMS Medicaid database at the time of analysis. The first somatropin claim during this study identification window denotes each individual’s “index date.” Eligible patients were further required to have at least 1 diagnosis code for GHD [ICD-9 CM (253.3, 253.4) or ICD-10 CM (E23.0, E23.1)] in the period 6 months prior to or on the index date (“baseline period”). Patients were further required to have continuous medical and pharmacy eligibility during the baseline period and during the variable study follow-up period. Patients were excluded for 1 or more of the following reasons: (1) prescription claims for somatropin during the baseline period (prior to initiation of new somatropin therapy); (2) diagnostic claim of psychosocial dwarfism (ICD-9-CM 259.4 or ICD-10-CM E34.3); (3) other identified causes of short stature, such as celiac disease ((ICD-9-CM 579.0 or ICD-10-CM K90.0), uncontrolled primary hypothyroidism (ICD-9-CM 244.9 or ICD-10-CM E03.9), or rickets (ICD-9-CM 268.0 or ICD-10-CM E55.0).
Four non-exclusive cohorts were constructed based on available follow-up time after somatropin initiation. Patients were selected based on follow-up time of up to 48 months, but insufficient sample size was obtained for the 48-month follow-up patient group (n < 11). Thus, cohorts were followed for up to 36 months post-index date and defined by follow-up ≥3 months, ≥12 months, ≥24 months, and ≥36 months. Discontinuation of somatropin therapy was evaluated in the 3-month follow-up cohort, which included patients with at least 3 months and up to 36 months of follow-up, while adherence was evaluated in the 12, 24 and 36-month follow-up cohorts.
Study measures
Primary outcome measures for this study were adherence to, and discontinuation of, somatropin therapy. Adherence was measured using the medication possession ratio (MPR), which was calculated for cohorts with 12, 24, and 36 months of follow up based on continuous eligibility during each follow-up period. MPR was calculated as total days’ supply of somatropin during the specific (12, 24, 36 months) follow-up period, divided by the date of the last somatropin prescription during the specific follow-up period plus days’ supply of that prescription, minus the date of the index somatropin prescription. Patients were classified as having good adherence (MPR ≥0.80) or suboptimal adherence (MPR <0.80), commonly used as a threshold for adherence studies, which equates to about 1 missed injection/week; there is clinical rationale for this threshold, as prior research has supported that this approximate adherence threshold is associated with poorer growth outcomesCitation8,Citation14. In addition, sensitivity analyses were performed among cohorts of patients with 24 and 36 months of eligibility after the index date. Discontinuation was defined as the first observation of a gap of >60 days between prescription fills for somatropin during the follow-up period. The date of the first observed gap was defined as discontinuation date plus days’ supply of the prescription preceding the gap, and sensitivity analyses were conducted using alternate gap criteria (15-, 30-, 45-, and 90-day gaps). Time to discontinuation was defined as the number of days from the index date to the discontinuation date.
Other measures included patient demographic and clinical characteristics, including age (as of index date), US geographic region (Northeast, South, North Central, West, and Other), sex, and race/ethnicity (Black, White, Hispanic, Asian, and Other). The Quan–Charlson comorbidity index (CCI) was calculated based on diagnosis codes associated with medical claims during the study baseline periodCitation15,Citation16. Finally, the total number of concomitant prescription medications filled for each patient during the study baseline period was calculated using pharmacy claims data.
Statistical analysis
Descriptive analyses were performed for all study variables, including means and standard deviations for continuous variables, and frequency counts and percentages for categorical variables. To evaluate adherence, unadjusted as well as adjusted logistic regression models were fitted for baseline characteristics associated with having suboptimal adherence to somatropin (MPR <0.80) for 12, 24, and 36 months of continuous follow-up. Odds ratios (OR) with 95% confidence intervals (CI) and p-values were calculated for each of the characteristics. Sensitivity analyses were performed which evaluated suboptimal adherence at 12 and 24 months among the 24-month follow-up cohort, and at 12, 24, and 36 months among the 36-month follow-up cohort. To evaluate discontinuation, unadjusted as well as adjusted Cox regression models were fitted for baseline characteristics associated with time to discontinuation of somatropin, and hazard ratios (HR) with associated 95% CIs and p values were generated for patient baseline characteristics. Variables included for adjustment in multivariable models were age, sex, geographic region, race/ethnicity, CCI, and number of concomitant medications at baseline.
Results
Study population
Overall, 40,064 patients were identified in the CMS Medicaid database with medical and prescription drug benefit eligibility and at least 2 new somatropin prescriptions filled between July 2014 and December 2018 (). After application of age and diagnosis criteria, and excluding other causes of short stature, 8450 individuals (21%) remained. After determination of available follow-up based on continuous eligibility post-index date, 4 non-exclusive cohorts were constructed: 3 months follow-up (n = 5064), 12 months follow-up (n = 3623), 24 months follow-up (n = 1004), and 36 months follow-up (n = 90). Demographic and clinical characteristics of the study population are included in . Mean patient age for all cohorts was 10–11 years, and about 7 in 10 patients were male. Overall, about 40% were White non-Hispanic, 30% were Hispanic, 7% Black, and 2% Asian. Almost half of patients were prescribed 3 or more concomitant medications at baseline; only about 1 in 4 were not prescribed other medications at baseline.
Figure 1. Sample attrition for Medicaid pediatric growth hormone deficiency cohort. 1Eligible patients were grouped into non-exclusive cohorts based on length of available follow-up, determined by index date and continuous enrollment. For example, all patients in the 36-month continuous enrollment cohort, by definition, are also included in the 3-, 12-, and 24-month continuous enrollment cohorts.
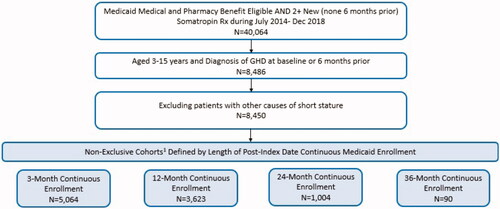
Table 1. Medicaid pGHD patient demographic and baseline clinical characteristics.
Adherence and discontinuation
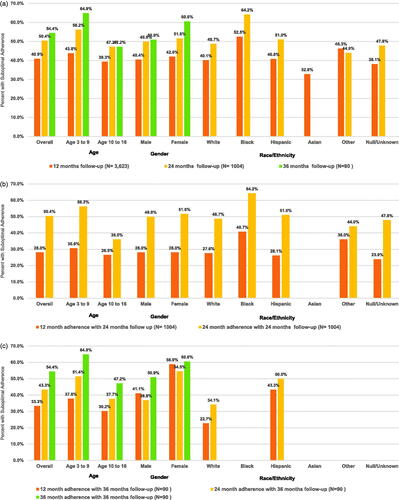
Mean (95% CI) adherence, as measured using medication possession ratio (MPR), among those with 12, 24, 36 months of follow-up was 0.79 (0.78–0.80), 0.75 (0.73–0.76), and 0.74 (0.70–0.79), respectively (data not shown). Overall, among the 12-, 24-, and 36-month follow-up cohorts, 40.9%, 50.4%, and 54.4% of pGHD patients, respectively, had suboptimal somatropin adherence (). Suboptimal adherence was higher among children aged 3-9 years compared to older children, and this difference was more pronounced among cohorts with longer follow-up. Suboptimal adherence was most pronounced among Black children. At 12 months, suboptimal adherence was observed among 52.5% of Black, 40.8% of Hispanic, 40.1% of White, and 32.8% of Asian children; at 24 months, suboptimal adherence was observed among 64.2% of Black, 51.0% of Hispanic, and 48.7% of White children. Insufficient sample size prohibited estimation of 24-month adherence among Asian children. Sensitivity analysis demonstrated that among the 24-month follow-up cohort, overall, 28.0% had suboptimal adherence at 12 months and 50.4% at 24 months (); among the 36-month follow-up cohort, 33.3% had suboptimal adherence at 12 months, 43.3% at 24 months, and 54.4% at 36 months ().
Figure 2. Suboptimal somatropin adherence (MPR <0.80) over 3 years among a Medicaid population diagnoses with pGHD. (a) Proportion of patients* with suboptimal somatropin adherence (MPR <0.80) among 3 pGHD cohorts with 1, 2, and 3 years of follow-up. ( b) Sensitivity analysis proportion of patients* with suboptimal somatropin adherence (MPR <0.80) at 1 and 2 years of follow-up among children with pGHD and 2 years of continuous follow-up. (c) Sensitivity analysis proportion of patients* with suboptimal somatropin adherence (MPR <0.80) at 1, 2, and 3 years of follow-up among children with pGHD and 3 years of continuous follow-up. *For categories with n < 11, descriptive statistics were unable to be presented due to Medicaid privacy regulations. Abbreviations. MPR, medication possession ratio; pGHD, pediatric growth hormone deficiency.
Unadjusted logistic regression results are included in . Within the 12- and 24-month follow-up cohorts, significant predictors of suboptimal adherence included age, census region, and race/ethnicity. In the 12-month follow-up cohort, adjusted odds of suboptimal adherence for age 10-16 years (vs. 3–9 referent) were 0.83 (95% CI 0.72–0.96), and Black (vs White ref) were 1.67 (95% CI 1.27–2.16). Among the 24-month follow-up cohort, the adjusted odds of suboptimal adherence for age 10–16 (vs. 3–9) were 0.70 (95% CI 0.54–0.91); Black (vs. White ref) 1.89 (95% CI 1.15–3.08). Due to small sample size and insufficient power, no significant predictors were identified in the 36-month follow-up cohort. Significant predictors identified in the unadjusted logistic regressions persisted after adjustment for covariates.
Table 2. Unadjusted logistic regression for baseline characteristics associated with suboptimal adherence to somatropin (MPR <0.80) among Medicaid pGHD cohorts with 1–3 years of follow-up.
Discontinuation was evaluated among all study patients with at least 3 months of continuous follow-up; among these individuals (n = 5064), 49.2% discontinued somatropin therapy. Among those who discontinued therapy, mean time to discontinuation was 263.3 days (SD 213.1). In unadjusted Cox regression models, Black race was associated with increased risk of discontinuation, while Northeast census region (Maine, New Hampshire, Vermont, Massachusetts, Rhode Island, Connecticut, New York, New Jersey, and Pennsylvania) was associated with decreased risk (). After adjustment, both factors remained significant predictors of time to discontinuation.
Table 3. Unadjusted and adjusted Cox regression model results for baseline characteristics associated with time to discontinuation of somatropin among children with pGHD and ≥3 months of follow-up.
Discussion
In this Medicaid population, a substantial proportion of children with pGHD had suboptimal adherence to prescribed daily somatropin injections. The proportion of patients with suboptimal adherence to somatropin was related to cohort length of follow-up: the proportion with suboptimal adherence in the Medicaid database was 40.9%, 50.4%, 54.4% at months 12, 24, and 36, respectively. Furthermore, almost 1 in 2 (49.2%) patients followed for ≥3 months discontinued prescribed somatropin therapy. We also found that lower age, race/ethnicity (Black as compared to non-Hispanic White), and geographic region were associated with greater odds of suboptimal adherence, and the risk of discontinuation was associated with Black race and geographic region.
Our finding of suboptimal somatropin adherence in a Medicaid population was more than twice that of what has been reported for commercially insured children (19.6%) at 12 months, and 60% higher at 36 months. A recent retrospective claims analysis estimated that about 20 and 34% of commercially insured children with pGHD were suboptimally adherent at 12 and 36 months, respectivelyCitation17. In addition to higher rates of suboptimal adherence in our Medicaid population, discontinuation rates were higher (49.2%) than what was previously reported for commercially insured children (42.2%) followed for at least 3 months, and median time to discontinuation was 206 days in the current study compared to 442 days among commercially insured children. A recent study of the economic burden of pGHD, found suboptimal adherence (proportion of days covered <0.80) for 81.6% of Medicaid patients and 67.7% of commercially insured patients (mean follow-up 3–4 years)Citation18. This study also found high levels of discontinuation with 49.1% of Medicaid patients and 24.3% of commercially insured patients discontinuing GH treatment before the age of 13Citation18. A survey of patients treated with GH found the main factors associated with poor treatment adherence were discomfort with injections, dissatisfaction with treatment outcomes, inadequate contact with healthcare providers and misperceptions about the potential consequences of missed GH dosesCitation19.
In the current study, among a Medicaid population, younger children (3–9 years) had greater odds of suboptimal adherence than older children (11–16 years). One potential explanation may be increased adolescent self-consciousness about height, which may lead to greater motivation to adhere to their treatment regimen. For example, a retrospective study of Medicaid enrollees with acne vulgaris found that adolescents may be more likely than children to be adherent to medication for acne, although the difference was not significantCitation20.
Black children were more likely to have suboptimal adherence and to discontinue therapy than White children. Prior research has indicated lower quality of health care for children and adolescents enrolled in Medicaid compared to their commercial counterpartsCitation21,Citation22. Landon et al.Citation21 found that based on Health Plan Employer and Data Information Set (HEDIS) quality of care indicators, Medicaid enrollees had lower rates for clinical access indicators than members of commercial plansCitation15. While both studies found worse adherence among Black children as compared to White, overall higher rates of suboptimal adherence in the Medicaid population are not solely explainable based on measured patient demographic characteristics. In the current study of Medicaid enrollees, suboptimal adherence was observed among 40.1% of White, 52.5% of Black, and 40.8% of Hispanic children, compared to 16.8% of White, 18.9% of Black, and 20.7% of commercially insured childrenCitation17. We also found that children from the Northeastern US were less likely to discontinue GH treatment; this may reflect regional differences in access to health care or be related to state-level differences in Medicaid health plans. Additional research is warranted to understand structural, policy, and other barriers to GH treatment specific to the Medicaid population.
Several other studies have evaluated the effects of socioeconomic status on adherence to prescribed GH therapy outside of the US. In a study of GH treatment in New Zealand, Cutfield et al.Citation8 found that Maori ethnicity was associated with lower GH adherence. Aydin et al.Citation10 studied 217 pediatric GH patients in Turkey, and while the authors found that male gender was associated with better adherence, socioeconomic status was not associated with adherence. Farfel et al.Citation23 studied 2263 pediatric GH patients from an Israeli health maintenance organization (HMO), and observed poor adherence associated with younger age of treatment initiation, but no effect of socioeconomic status or gender. Hughes et al.Citation24, in an Australian GH program evaluation, found that among patients who ended GH therapy, patients from a lower socioeconomic background more likely to have early cessation of GH therapy. De Pedro et al.Citation25, in a study of 158 children treated with GH in Argentina, found that maternal educational level predicted adherence, with a higher proportion of children with “good adherence” (≥92%) among mothers with a college education as compared to those with moderate to poor adherence (<92%). Our study provides a valuable addition to this literature by evaluating GH adherence in a socioeconomically disadvantaged population within the US healthcare system. In a 2011 literature review, key drivers of poor GH adherence identified both psychological/emotional and social problems and stressed the interconnected nature of these factors to socioeconomic issues such as poverty, low education levels, and lack of social supportCitation26. While socioeconomic status may impact GH adherence across study settings, structural and policy related barriers to adherence may differ substantially based on characteristics unique to each setting and its healthcare system. Since poor adherence to prescribed GH regimens is associated with decreased final height, our study’s findings suggest that children with pGHD who are socioeconomically disadvantaged may be less likely to achieve maximum adult height potential, possibly impacting quality of life in the longer term. While there is a lack of long-term data on such outcomes in pGHD, short-term treatment with GH has been associated with improved social, emotional, and physical quality of lifeCitation27. Provider-targeted initiatives to recognize at-risk children and parents for outreach and education related to the implications of GH adherence on clinical outcomes may be warranted. Furthermore, GH regimens with fewer doses and more convenient dosing requirements, such as LAGH, could help to improve adherence and outcomes among socioeconomically disadvantaged children.
While our study has several strengths, including the size of the database used and geographic representation across the US, several limitations are important to consider. This study utilized administrative claims data for patient encounters, and since claims data incorporates diagnosis information using ICD9/ICD10 codes for billing purposes, it does not measure validated clinical diagnoses. However, to minimize misclassification, we limited our study to patients with ≥2 somatropin prescriptions and a GHD diagnosis, and we excluded patients who may be prescribed somatropin for other indications. Claims data are collected for service reimbursement, and as such, use of administrative data for research purposes may be subject to errors or omissions. While the data from the TAF are adjudicated and adjusted to control for severe data quality issues, remaining issues may impact this study. Firstly, data in the T-MSIS/TAF vary significantly due to state-level differences in administration of the Medicaid program. Additionally, data coverage varies year over year, due to states transitioning to the T-MSIS file structure at different times. In 2014 and 2015, only a subset of states provided T-MSIS data to CMS, and 2016 was the first year that all 50 states began participating in the T-MSIS/TAF. Overall data quality and usability across states improved from 2016 to 2018, especially in the more populous states. We suspect that having fewer states participating in 2014 and 2015 is one of the primary causes for the low sample sizes observed in the 36-month cohort, and these factors may impact ability to follow-up patients longitudinally over several years in the Medicaid data. Specifically, we hypothesize that the relatively poor 2016 data quality is one of the causes for the notable drop in cohort size from 12 months to 24 months. Patients may have had changes to insurance (i.e. from Medicaid to Commercial coverage) which may have contributed to this drop in cohort size over time since we required continuous enrollment; disruptions in Medicaid coverage are common, as patients may drop in and out of Medicaid coverageCitation28. Another limitation of the Medicaid data is that only two diagnosis codes are available per claim for non-inpatient claims. While we cannot be sure of the impact of this on sample size, we suspect that this may have impacted the initial step of identifying patients with documented GHD diagnosis in claims data. Finally, our findings suggest that almost half (49.2%) of children in this study discontinued therapy with somatropin. While we conducted sensitivity analyses using different thresholds for a gap which qualified as a discontinuation, some children may have restarted somatropin therapy after a prolonged gap in therapy, and our analysis did not assess this possibility.
Conclusions
Within a Medicaid population of children with GHD, the proportion with suboptimal adherence to somatropin grew over time, and the risk of suboptimal adherence was associated with younger age and Black race/ethnicity. Risk of discontinuation of somatropin varied by geographic region but was consistently higher among Black children (as compared to White children). Strategies that facilitate adherence to somatropin may support improved clinical outcomes in these vulnerable groups and could support the achievement of final height outcomes that are more consistent with those seen among White and Asian cohorts. Improving adherence to prescribed GH regimen among this at-risk population, and specifically among sociodemographic subgroups at highest risk, is warranted to improve clinical outcomes.
Transparency
Declaration of funding
This study was sponsored by Pfizer.
Declaration of financial/other relationships
JL, YC, JMJA, AG and MPW are employees of Pfizer and may hold stock/stock options in Pfizer. PJ is an employee of Genesis Research and was a paid consultant by Pfizer in connection with this research and development of this manuscript. SP and MK are employees of Clarify Health Solutions and were paid consultants by Pfizer in connection with this research and development of this manuscript. LS has served as a consultant to Novo Nordisk, Opko Health Inc., and Pfizer, and served as an investigator in clinical trials funded by Novo Nordisk, Opko Health Inc., and Lumos Diagnostics. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
Writing and editorial assistance was provided by Jenifer Wogen and Amy Glenwright of Genesis Research, LLC, Hoboken, NJ.
References
- Bao XL, Shi YF, Du YC, et al. Prevalence of growth hormone deficiency of children in Beijing. Chin Med J. 1992;105(5):401–405.
- Lindsay R, Feldkamp M, Harris D, et al. Utah growth study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994;125(1):29–35.
- Rona RJ, Tanner JM. Aetiology of idiopathic growth hormone deficiency in England and Wales. Arch Dis Child. 1977;52(3):197–208.
- Vimpani GV, Vimpani AF, Lidgard GP, et al. Prevalence of severe growth hormone deficiency. Br Med J. 1977;2(6084):427–430.
- Komlos J, Breitfelder A. Differences in the physical growth of US-born black and white children and adolescents ages 2–19, born 1942–2002. Ann Hum Biol. 2008;35(1):11–21.
- August GP, Lippe BM, Blethen SL, et al. Growth hormone treatment in the United States: demographic and diagnostic features of 2331 children. J Pediatr. 1990;116(6):899–903.
- Grimberg A, Lindberg A, Wajnrajch M, et al. Racial/ethnic disparities in US pediatric growth hormone treatment. Horm Res Paediatr. 2018;90(2):102–108.
- Cutfield WS, Derraik JG, Gunn AJ, et al. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLOS One. 2011;6(1):e16223.
- Kapoor RR, Burke SA, Sparrow SE, et al. Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93(2):147–148.
- Aydın BK, Aycan Z, Sıklar Z, et al. Adherence to growth hormone therapy: results of a multicenter study. Endocr Pract. 2014;20(1):46–51.
- Jin J, Sklar GE, Min Sen Oh V, et al. Factors affecting therapeutic compliance: a review from the patient's perspective. Ther Clin Risk Manag. 2008;4(1):269–286.
- Loftus J, Yaworsky A, Roland CL, et al. Systematic review of patient experience with a less frequent injection schedule for growth hormone deficiency. Acad Manag Care Pharm. Virtual 2021.
- McNamara M, Turner-Bowker DM, Westhead H, et al. Factors driving patient preferences for growth hormone deficiency (GHD) injection regimen and injection device features: a discrete choice experiment. Patient Prefer Adherence. 2020;14:781–793.
- Loftus J, Miller BS, Parzynski CS, et al. Association of daily growth hormone injection adherence and height among children with growth hormone deficiency. Endocr Pract. 2022. DOI:https://doi.org/10.1016/j.eprac.2022.02.013
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- Loftus J, Chen Y, Alvir JMJ, et al. Sub-optimal adherence with daily growth hormones increases with each year of treatment in a US commercial claims database. J Endocr Soc. 2021;5(Suppl_1):A686–A686.
- Kaplowitz P, Manjelievskaia J, Lopez-Gonzalez L, et al. Economic burden of growth hormone deficiency in a US pediatric population. JMCP. 2021;27(8):1118–1128.
- Rosenfeld RG, Bakker B. Compliance and persistence in pediatric and adult patients receiving growth hormone therapy. Endocr Pract. 2008;14(2):143–154.
- Hester C, Park C, Chung J, et al. Medication adherence in children and adolescents with acne vulgaris in Medicaid: a retrospective study analysis. Pediatr Dermatol. 2016;33(1):49–55.
- Landon BE, Schneider EC, Normand SL, et al. Quality of care in Medicaid managed care and commercial health plans. JAMA. 2007;298(14):1674–1681.
- Thompson JW, Ryan KW, Pinidiya SD, et al. Quality of care for children in commercial and medicaid managed care. JAMA. 2003;290(11):1486–1493.
- Farfel A, Shalitin S, Morag N, et al. Long-term adherence to growth hormone therapy in a large health maintenance organization cohort. Growth Horm IGF Res. 2019;44:1–5.
- Hughes IP, Choong C, Rath S, et al. Early cessation and non-response are important and possibly related problems in growth hormone therapy: an OZGROW analysis. Growth Horm IGF Res. 2016;29:63–70.
- De Pedro S, Murillo M, Salinas I, et al. Variability in adherence to rhGH treatment: Socioeconomic causes and effect on children’s growth. Growth Horm IGF Res. 2016;26:32–35.
- Haverkamp F, Gasteyger C. A review of biopsychosocial strategies to prevent and overcome early-recognized poor adherence in growth hormone therapy of children. J Med Econ. 2011;14(4):448–457.
- González Briceño LG, Viaud M, Beltrand J, et al. Improved general and height-specific quality of life in children with short stature after 1 year on growth hormone. J Clin Endocrinol Metab. 2019;104(6):2103–2111.
- Sugar S, Peters C, De Lew N, et al. Medicaid churning and continuity of care: Evidence and policy considerations before and after the COVID-19 pandemic. US Department of Health and Human Services; 2021. Available from: https://aspe.hhs.gov/system/files/pdf/265366/medicaid-churning-ib.pdf
