Abstract
Objective
Analysis and comparison of country-level data from the VISIONARY study, examining treatment outcomes with the topical fixed-dose combination of preservative-free tafluprost (0.0015%) and timolol (0.5%) (PF tafluprost/timolol FC) in adults with open-angle glaucoma (OAG) and ocular hypertension (OHT) who were insufficiently treated with or unable to tolerate either beta-blocker or prostaglandin analogue (PGA) topical monotherapy.
Methods
A European, prospective, observational study was conducted in 11 countries. Adults with OAG/OHT were switched to the PF tafluprost/timolol FC from either PGA or beta-blocker topical monotherapy. Statistical analysis examined changes in mean standard deviation (SD) intraocular pressure (IOP) from baseline at Week 4, Week 12 and Month 6. Data were documented for each eye separately at baseline and during follow up visits, with the eye reported to have the higher IOP (mmHg), as measured using Goldmann applanation tonometry, being selected for analysis (study eye). Country-level subanalysis examined outcomes by prior monotherapy, diagnosis and timing of dosing for those countries recruiting ≥20 patients (Country-level Subanalysis Population). Two-sided paired t-test was used to assess significance regarding mean IOP reduction from baseline and a compound symmetry covariance model was used in cross-country comparisons regarding variation in IOP change from baseline. Treatment-related adverse events (AEs) were evaluated.
Results
Mean (SD) age among patients recruited to the VISIONARY study ranged between 63.9 (11.8) and 72.4 (10.6) years across all countries. The majority of participants (>50%) were female in each country. The Country-level Subanalysis Population included 551 eyes. Mean (SD) IOP was significantly reduced from baseline in each country at Week 4, Week 12 and Month 6 (p < .0001). Mean IOP reduction at Month 6 ranged from 5.0 mmHg (22.6%, Hungary) to 7.8 mmHg (31.8%, Latvia) and varied significantly between countries (p < .001). The greatest reductions were in Latvia and Russia, where baseline IOP was highest. Country-level IOP reductions were significant irrespective of prior monotherapy, diagnosis or dosing time (p < .0001). Most treatment-related AEs occurred in the UK (26 events, 73% mild). One serious AE was reported (Russia, status asthmaticus). Tolerability with PF tafluprost/timolol FC therapy was rated as good/very good by most patients (85.7–100%) in all countries.
Conclusion
Subanalysis of VISIONARY study data revealed significant IOP reductions following a switch to the PF tafluprost/timolol FC from either PGA or beta-blocker topical monotherapy. Cross-country variation was likely due to baseline IOP differences. Within country, outcomes were consistent regardless of diagnosis, dosing or prior monotherapy. Treatment was generally well tolerated.
Introduction
Reduction of intraocular pressure (IOP) is critical in slowing progression of disease and preserving vision in glaucomaCitation1–6. Treatment across Europe typically follows the European Glaucoma Society (EGS) guidelines, where reimbursement arrangements allowCitation7,Citation8. Topical combination therapies, usually comprising a prostaglandin analogue (PGA) and beta-receptor blocker (beta-blocker), have been extensively used in glaucoma care in Europe when monotherapies fail to lower IOP sufficientlyCitation8–11. Fixed-dose combination (FC) formulations simplify the treatment regimen and reduce the number of daily instillations required, and preservative-free formulations may improve tolerability by avoiding ocular exposure to potentially toxic preservative agents, including the commonly used benzalkonium chloride (BAK)Citation8,Citation10–17. Over the past decade, the preservative-free (PF) fixed-dose combination of tafluprost 0.0015% and timolol 0.5% (PF tafluprost/timolol FC) has demonstrated efficacy and tolerability in the treatment of open-angle glaucoma (OAG) and ocular hypertension (OHT) in randomized controlled trials (RCTs) and observational studiesCitation17–26.
Most recently, the VISIONARY studyCitation25,Citation26 assessed, in a real-world setting, the IOP-lowering efficacy and tolerability of the PF tafluprost/timolol FC in patients with OAG or OHT who had demonstrated, as the main reason to change therapy, insufficient IOP control or poor local tolerance with PGA or beta-blocker monotherapyCitation25. This large, multicenter study examined systematically registered clinical data from participants attending 66 ophthalmology clinics across 11 European countries over a 6-month periodCitation25. Patients enrolled on the study had previously been treated with preserved or PF formulations of topical beta-blocker (27.9%) or latanoprost (34.9%), bimatoprost (11.1%), tafluprost (15.1%) and travoprost (10.9%)Citation26. For the VISIONARY study population, the switch to the PF tafluprost/timolol FC was associated with significant IOP reductions compared with baseline pressures under monotherapy of 5.7 mmHg (24.9%) at Month 6 (p < .0001) alongside a favorable tolerability profileCitation25. Mean (standard deviation [SD]) IOP reductions from baseline, after 6 months of treatment with PF tafluprost/timolol FC, for prior PGA users ranged between 4.6 (4.39) mmHg (20.5%; p < .001) and 6.1 (4.25) mmHg (25.9%; p < .0001) in the prior bimatoprost and latanoprost (PF and preserved formulations) subgroups, respectivelyCitation25,Citation26. The maximal IOP reduction was rapidly observed, from Week 4, and maintained over the 6-month study period while key signs and symptoms of ocular surface health improvedCitation25,Citation26. Since the VISIONARY study comprised various European patient populations from Sweden to Italy and Ireland to Russia, it is of scientific and practical importance to analyze and compare the individual country results separately. Here, we report a further analysis of the VISIONARY study data, examining country-level efficacy and safety data. This analysis provides an opportunity to explore the areas of commonality and contrast between the countries participating in the VISIONARY study regarding the treatment outcomes achieved and the types of patients selected for PF tafluprost/timolol FC therapy in routine clinical practice.
Methods
VISIONARY study design and visit schedule
The VISIONARY study was a 6-month, observational, multicenter, European, prospective clinical studyCitation25. The VISIONARY study was registered under the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) European Union electronic Register of Post-Authorisation Studies (EU PAS Register) (EU PAS register number EUPAS22204) and was compliant with the principles of the Declaration of Helsinki. All patients provided written informed consent and the institutional review board (IRB) or independent ethics committee (IEC) at each VISIONARY study center approved the protocol.
Prospective data were collected from consecutive patients attending ophthalmology appointments at 66 clinics in Denmark, Hungary, Ireland, Italy, Latvia, Netherlands, Norway, Russia, Spain, Sweden and the United Kingdom (UK) between 10th April 2017 and 9th January 2019. Data regarding baseline demographics and the mean change in IOP from baseline at Month 6 were reported for all countries included in the VISIONARY study Full Analysis Set (FAS). However, country-level statistical subanalysis was conducted only for countries that enrolled 20 patients or more in the VISIONARY study (Country-level Subanalysis Population).
Patients were mandated to attend VISIONARY study visits at baseline and Month 6 only, although data were recorded at interim Week 4 and Week 12 visits for those choosing to attend. Baseline measures were recorded within 7 days prior to the switch from either a topical PGA or beta-blocker monotherapy to PF tafluprost/timolol FC (Santen Oy, Finland). Data were documented for each eye separately at baseline and during follow up visits, with the eye reported to have the higher IOP (mmHg), as measured using Goldmann applanation tonometry (GAT), being selected for analysis (study eye).
Inclusion and exclusion criteria and study treatment
The VISIONARY study included males/females, aged ≥18 years, who were diagnosed with OAG or OHT and currently treated with either PGA or beta-blocker monotherapy. Reasons for initiating PF tafluprost/timolol FC therapy, as judged by the investigating clinician according to their usual practice, were insufficient IOP control, poor local tolerance, progression of glaucoma, poor compliance, conversion of OHT or “other reasons”. Clinicians could give more than one reason for switching to the PF tafluprost/timolol FC. Participants were considered likely to benefit from a PF formulation. Patients were not allowed to enter the VISIONARY study if they had undergone ophthalmic surgery within 6 months, had received previous PF tafluprost/timolol FC treatment, were pregnant or breast feeding. Those with any contraindication against tafluprost or timolol treatment according to the approved licensed indication were also excluded from entering the VISIONARY study.
PF tafluprost/timolol FC (one drop daily) was instilled to affected eye(s), either in the morning or in the evening and the time of instillation was recorded.
Efficacy variables and data analysis
The primary endpoint of the VISIONARY study was absolute mean (SD) IOP change from under monotherapy baseline at Month 6, following the initiation of PF tafluprost/timolol FC treatment, measured with GAT. IOP change from baseline was assessed at Month 6 and at interim study visits according to country. Statistical analyses were conducted where data were available for ≥20 participants. Country-level data were analyzed to examine the mean absolute (mmHg) and relative (%) IOP change from baseline at each study visit, the proportion of responders at Month 6 demonstrating IOP reductions from baseline of ≥20%, ≥25%, ≥30% and ≥35%, and the mean IOP change from baseline according to prior monotherapy, baseline IOP, time of study treatment dosing and baseline diagnosis. A cross-country analysis also examined the variation in IOP change across those countries where data were available for ≥20 participants.
Safety analysis
Adverse events (AEs) and treatment-related AEs occurring during the VISIONARY study period were documented at study visits. The intensity/severity of each AE (e.g. signs of ocular hyperemia), was graded using a four-grade scale (none, mild, moderate, severe). In the current analysis, treatment-related AEs are presented by country.
Statistical analysis
ICON Plc (Dublin, Ireland) conducted all statistical analyses on behalf of the VISIONARY study group. Statistical analysis was conducted only where data were available for ≥20 participants in a country or within-country subgroup. Analysis regarding the mean (SD) change in IOP from baseline was conducted only for the Country-level Subanalysis Population, which consisted of those countries in which ≥20 patients were recruited at baseline (Hungary, Italy, Latvia, Russia, Spain and the UK). Data distribution was assessed using Q-Q plots, histograms and the Shapiro-Wilk or the Kolmogorov-Smirnov test, as needed. For normally distributed data, the mean and SD are presented and the two-sided paired t-test was used for the comparisons. Cross-country comparisons regarding the variation in IOP change from baseline at each time point were conducted using a compound symmetry covariance model. For country-level subanalysis, a mixed effects model for repeated measurements was fitted, with a compound symmetry covariance structure, to assess the change in IOP based on the effects of time (study visits), subgroup (countries recruiting ≥20 patients) and the interaction of time and subgroup. The p value used as the cut-off for statistical significance was <.05.
Results
VISIONARY study population demographics
Baseline demographics for the total VISIONARY study population are shown in . Mean (SD) age across all countries ranged between 63.9 (11.8) years in Russia and 72.4 (10.6) years in Ireland. The majority of participants (>50%) were female in each country. As ophthalmologists in Ireland used rebound tonometry (iCare Finland Oy) for 14 of the 17 patients included there (which was outside of the protocol stipulation that GAT should be used for IOP measurement), these iCare data were excluded from the FAS for the VISIONARY study which subsequently comprised 577 participants in totalCitation25. As stated in the statistical methods, country-level subanalysis examining IOP data was only conducted for countries that enrolled 20 patients or more. The Country-level Subanalysis Population therefore included data from ophthalmology clinics in Hungary, Italy, Latvia, Russia, Spain and the UK only (551 eyes).
Table 1. Baseline demographics (total VISIONARY study population).
Of the 577 patients in the VISIONARY study FAS, 27.7% (160 patients) were from Italy, 16.6% (96 patients) from Hungary, 15.9% (92 patients) from Spain, 15.1% (87 patients) from Russia, 11.3% (65 patients) from the UK, 8.8% (51 patients) from Latvia, 0.5% (3 patients) from Ireland, 2.3% (13 patients) from Norway, 1.4% (8 patients) from Denmark, and 0.2% (1 patient) from the Netherlands and 0.2% (1 patient) from Sweden.
In the Country-level Subanalysis Population (countries recruiting ≥20 patients), mean baseline IOP ranged from 19.6 mmHg in Italy to 24.0 mmHg in Latvia. The majority of participants from Italy, Hungary, Spain, Russia, the UK, and Latvia were diagnosed with POAG, followed by OHT. With the exception of Latvia, where the majority of patients were previously treated with beta-blocker monotherapy (60.8%), most participants (>60%) in each country had received prior PGA monotherapy. Insufficient IOP control was the main reason for a change in therapy across all countries in the Country-level Subanalysis Population (64.4–98.0%). Poor local tolerance was the second most common reason for treatment switch in Italy, Hungary, Russia, the UK and Latvia. Progression of glaucoma was the second reason for switch in Spain.
Intraocular pressure change from under monotherapy baseline
shows the change in mean (SD) IOP according to country at each study visit for the Country-level Subanalysis Population (countries recruiting ≥20 patients). Mean (SD) IOP reduction from baseline was statistically significant at each study visit for all of the countries included in the statistical analysis (p < .0001). At Month 6, the reduction in mean (SD) IOP from baseline was 7.8 (3.40) mmHg (31.8%) in Latvia, 7.1 (5.10) mmHg (28.1%) in Russia, 5.8 (3.95) mmHg (24.9%) in the UK, 5.2 (4.04) mmHg (22.3%) in Spain, 5.1 (3.70) mmHg (24.1%) in Italy and 5.0 (3.77) mmHg (22.6%) in Hungary. Cross-country analysis including data from Latvia, Russia, the UK, Spain, Italy and Hungary showed the variation in IOP reduction at each study visit to be statistically significant between countries (p < .001).
Table 2. Change in mean intraocular pressure from baseline according to country (Country-level Subanalysis Population – countries recruiting ≥20 patients).
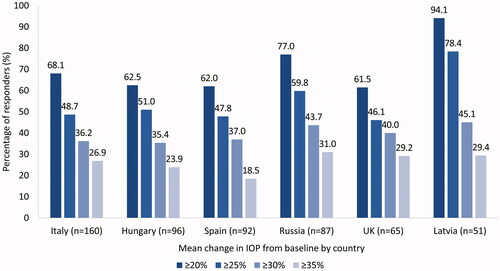
shows country-level data for the proportion of responders at Month 6 according to different IOP data cut-off values. The proportion of patients demonstrating an IOP reduction ≥20% from under baseline monotherapy ranged from 61.5% in the UK to 94.1% in Latvia. The proportion achieving a reduction in IOP ≥25% from under monotherapy ranged from 46.1% in the UK to 78.4% in Latvia. Between 35.4% (Hungary) and 45.1% (Latvia) showed IOP reductions from baseline that were ≥30%, while between 18.5% (Spain) and 31.0% (Russia) demonstrated reductions from baseline of ≥35%.
Figure 1. Percentage of responders according to different intraocular pressure reduction cut-off values at Month 6, according to country (Country-level Subanalysis Population -countries recruiting ≥20 patients). Abbreviations. IOP, intraocular pressure.
shows the mean (SD) change in IOP by country according to prior monotherapy. Significant IOP reductions were demonstrated at each study visit for prior PGA users in all countries included in the statistical analysis (p < .0001). At Month 6, mean (SD) IOP reductions among prior PGA users ranged between 3.7 (3.30) mmHg (17.6%) in Hungary and 7.9 (3.48) mmHg (31.8%) in Latvia. Prior beta-blocker users in Italy, Hungary, Russia, and Latvia also demonstrated significant IOP reductions at Month 6 (p < .0001), ranging between 5.6 (3.88) mmHg (26.8%) in Italy and 9.5 (4.61) mmHg (36.0%) in Russia.
Table 3. Mean (SD) intraocular pressure change according to baseline monotherapy, by country (Country-level Subanalysis Population – countries recruiting ≥20 patients).
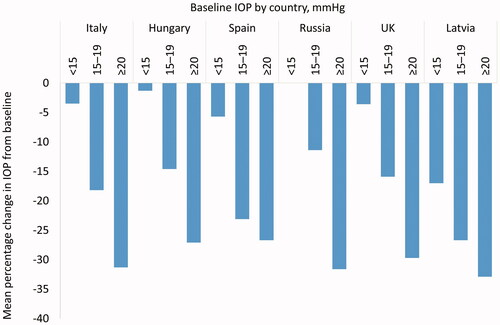
shows country-level data regarding the mean percentage change in IOP from under monotherapy according to the pressure recorded at the baseline visit (<15 mmHg, 15–20 mmHg and ≥20 mmHg). The highest mean reductions were observed among those with baseline IOP ≥20 mmHg, ranging between 26.7% in Spain and 32.9% in Latvia at Month 6. Those with a baseline pressure of 15–19 mmHg achieved reductions in IOP ranging from 11.4% in Russian to 26.7% in Latvia at Month 6. Patients with a baseline IOP under monotherapy <15 mmHg demonstrated IOP reductions that ranged between 1.3% in Hungary and 17.0% in Latvia at Month 6.
Figure 2. Mean percentage change in IOP at Month 6 according to baseline pressure and country, at each VISIONARY study visit (Country-level Subanalysis Population – countries recruiting ≥20 patients). Abbreviations. IOP, intraocular pressure.
shows the mean (SD) change in IOP according to the time of day that the study drug was administered in each country. The majority of participants instilled the PF tafluprost/timolol FC treatment in the evening, rather than the morning. This was true in all countries reporting data on the time of dosing; Italy, Hungary, Latvia, Russia, Spain and the UK. At Month 6, evening instillation was conducted by 128 participants in Italy, 91 in Hungary, 89 in Spain, 63 in Russia, 45 in Latvia and 31 in the UK. Significant mean reductions in IOP were observed at Month 6 with evening dosing in each of these countries (p < .0001). Statistical analysis was not possible at Month 6 for patients dosing PF tafluprost/timolol FC in the morning since insufficient patient data were available for this subgroup in each of the countries.
Table 4. Change in mean intraocular pressure from baseline according to timing of PF tafluprost/timolol FC dosing, by country (Country-level Subanalysis Population – countries recruiting ≥20 patients).
shows the mean (SD) IOP change at Month 6 from under baseline monotherapy according to the diagnostic group and by country. At Month 6, participants with POAG demonstrated statistically significant reductions in mean (SD) IOP from baseline in each country (p < .0001). Reductions in mean (SD) IOP at Month 6 ranged between 4.7 (3.62) mmHg (22.5%) in Italy and 7.9 (3.46) mmHg (32.0%) in Latvia (p < .0001). Italy and Spain were the only countries with sufficient patients diagnosed with OHT to allow statistical analysis to be conducted and respective Month 6 IOP reductions were 6.5 (3.35) mmHg (30.3%) and 6.3 (4.33) mmHg (25.8%). Mean IOP reductions among patients with OHT in Italy and Spain were statistically significant at Month 6 compared with baseline (p < .0001).
Table 5. Change in mean intraocular pressure at Month 6 from baseline according to diagnosis, by country (Country-level Subanalysis Population – countries recruiting ≥20 patients).
Safety and tolerability
Treatment-related AEs, reported in each country, for the FAS are shown in . Overall, 42 treatment-related AEs were reported by participants in Italy, Hungary, Russia, Spain, the UK and Denmark The UK reported the most treatment-related AEs (26 events), although the majority (73.1%) were mild in intensity in this country. Only one serious treatment-related AE was reported, described by the investigator as moderate in intensity, which occurred in Russia (status asthmaticus).
Table 6. Reported treatment-related adverse events according to country (Full Analysis Set).
Similarly to data published for the VISIONARY study FAS, the Country-level Subanalysis Population rated tolerability as good or very good in Hungary (93.8%), Italy (91.7%), Latvia (100%), Russia (85.7%), Spain (91.0%) and the UK (90.0%)Citation25,Citation26.
Discussion
In multicenter prospective clinical investigations, data obtained on participants from several countries are typically pooled for analysis. This approach provides strong evidence but cannot provide information on the potential between-country differences. Those differences are of particular importance in real-life investigations, like the VISIONARY study, in which both population-related and practice-related aspects may influence outcomes. To overcome such limitations relating to the recently published VISIONARY studyCitation25, the current analysis evaluated and compared the IOP-lowering effect of the PF tafluprost/timolol FC between the participating countries. The occurrence of treatment-related AEs was also examined for each participating countryCitation25.
First, and most importantly, the country-level data presented in the current Country-level Subanalysis confirmed those reported for the VISIONARY FAS dataset, demonstrating consistent, statistically and clinically significant IOP reduction after switching OAG and OHT patients from beta-blocker or PGA monotherapy to PF tafluprost/timolol FC therapyCitation26. Favorable responder rates were reported in each country. The IOP reduction was already present at the Week 4 visit in each country and persisted with no long-term drift during the 6-month VISIONARY study period. The individual country data also reaffirmed the IOP-lowering efficacy and tolerability outcomes published in RCTs and real-world studies concerning PF tafluprost/timolol FC treatment in OAG and OHTCitation18–25.
Second, the cross-country analysis revealed differences between the participating countries regarding IOP results, demonstrating the clinical importance of our current analysis for ophthalmologists working in different European regions. The IOP reduction from baseline was statistically and clinically significant in all countries, but the variation in IOP change between countries was found to be statistically significant. Some consistency was seen in IOP-lowering outcomes in neighboring geographical locations (e.g. Latvia and Russia), which may be related to similarities of the populations. To explain the between-country differences in IOP reduction, baseline IOP under monotherapy needs to be investigated. It has been shown that baseline IOP has a considerable impact on IOP reduction achieved with topical medicationCitation8,Citation15,Citation20. Thus, countries reporting higher mean baseline pressures achieved greater IOP reductions, and countries reporting lower baseline mean IOP values achieved smaller reductions, although pressure reductions in all countries were still statistically and clinically significant. The current results represent important information for ophthalmologists treating glaucoma patients in different European countries. When switching a patient from PGA or beta-blocker monotherapy to PF tafluprost/timolol FC, the expected additional IOP reduction is likely influenced by the baseline IOP level typical for that country. In other words, a relatively smaller additional IOP reduction can be expected in most cases in countries where the typical IOP under monotherapy is low. In such countries, individual decision making considering the degree of glaucomatous structural damage and visual field deterioration is needed to determine if the expected further IOP reduction is sufficient to reach the individual target IOP. These insights are emphasized by the effect of the clinically important baseline under monotherapy IOP ranges investigated by us. At Month 6, the pressure reduction for eyes with baseline IOP ≥20 mmHg ranged between 26.7% (Spain) and 32.9% (Latvia), which is clinically highly favorable for all countries. For eyes with baseline IOP under monotherapy of between 15 and 19 mmHg, the additional IOP reduction varied between 11.4% (Russian) and 26.7% (Latvia); and for eyes with baseline IOP <15 mmHg it ranged between 1.3% (Hungary) and 17.0% (Latvia). The additional IOP reduction for these baseline IOP categories was considerably varied across different countries, which underlines the importance of considering the country characteristics and individual decision making in routine clinical practice. It is possible that the average starting IOP may have been slightly higher in Latvia for each of the baseline IOP subgroups (<15 mmHg, 15–19 mmHg and ≥20 mmHg) and that this might have resulted in greater changes in pressure in each subgroup. Further analysis would be required to understand whether the high proportion of prior beta-blocker use in Latvia might have driven some of the differences seen in IOP reduction here compared with other countries. However, subanalysis according to prior monotherapy revealed that the IOP change from baseline at Month 6 was similar among previous PGA users (7.9 mmHg) and beta-blocker users (7.8 mmHg) in Latvia and both subgroups demonstrated IOP reductions from baseline of 31.8% at Month 6.
It is also possible that variations in treatment compliance in each country may have led to differences in IOP-lowering efficacy as better adherence with treatment should support improved outcomes. Again, variations in local practice (e.g. availability of patient support mechanisms/education, appointment length) may link to levels of adherence and treatment effectiveness. Treatment compliance was generally reported to be good/very good in the VISIONARY study, but further subanalysis of these data at a country level might be warranted in future workCitation25,Citation26.
Third, in each of those six countries where data were available for ≥20 patients, the responder rates for the >20%, >25%, >30% and >35% IOP reduction categories were all favorable. Countries with relatively low baseline IOP (19.6–22.0 mmHg; Italy, Hungary, Spain and the UK) showed similar rates for the ≥20%, ≥25% and ≥30% IOP reduction categories, respectively (e.g. 61.5–68.1% responder rates for ≥20% pressure reduction) but variable rates (18.5–29.2%) for the ≥35% IOP reduction category. Those two countries (Russia and Latvia) in which the mean baseline IOP under monotherapy was relatively high (23.8 mmHg and 24.0 mmHg, respectively) showed similarly high results in all categories (e.g. 77.0% and 94.1% responders for ≥20%; and 31.0% and 29.4% for ≥35% IOP reduction, respectively). These results suggest that ophthalmologists working in different European geographic areas may expect somewhat different probability of reaching the targeted IOP response. These data suggest a trend in IOP reduction patterns at the country-level, but additional subanalysis would be required to understand whether these differences hold true at the individual patient level.
Fourth, we wished to analyze the additional IOP reduction provided by the PF tafluprost/timolol FC according to diagnostic categories. However, only patients with POAG represented a population that was large enough for statistical analysis in several countries. The POAG subgroup achieved significant IOP reduction from baseline in each country. It is noteworthy that POAG patients showed considerably different baseline IOP under monotherapy in the different countries. Therefore it is not unexpected that the additional IOP reduction from baseline varied in this diagnostic subgroup, ranging between 22.5% (Italy) and 32.0% (Latvia) additional IOP-lowering.
Fifth, we investigated the influence of baseline monotherapy type (PGA or beta-blocker) and study medication instillation time on the additional IOP reduction reported in the different countries. PGAs were the predominant monotherapy used at baseline in Italy, Spain, Hungary, Russia and the UK, whereas 60.8% of patients in Latvia were treated with a beta-blocker. PF tafluprost/timolol FC provided statistically and clinically significant IOP-lowering efficacy for eyes on any monotherapy type in each individual country. In contrast to the full dataset analysis of the VISIONARY study, in which no difference was found in the IOP reduction between eyes treated with the PF tafluprost/timolol FC in the morning and in the evening, no individual country included a sufficient number of patients instilling medication in the morning to allow statistical analysisCitation25. Therefore the effect of instillation time on the additional IOP reduction could not be compared between the countries.
Finally, the safety data showed treatment-related AEs to be typically mild in intensity and present at the site of instillation in each country. The variation seen between countries regarding the number of reported AEs may reflect local practices or systems regarding collection of safety data or culturally determined sensitivity to subjective symptoms. While three or fewer treatment-related AEs were reported among each of the 160, 87 and 92 patient datasets respectively collected in Italy, Russia and Spain, 26 treatment-related AEs were recorded among the 65 patients included in the VISIONARY study from the UK. Knowledge of this between-country difference can be useful for clinicians treating glaucoma patients in routine practice. In European regions where minor local reactions are not typically considered or reported as AEs by patients and ophthalmologists, those real-life data from the UK and/or from the total VISIONARY study dataset may be considered to overestimate the significance of AEs related to switching to PF tafluprost/timolol FC. The status asthmaticus case reported in Russia is indicative of a potential prescribing error since the licensed indication for PF tafluprost/timolol FC therapy states that it is contraindicated for use in people with established (or a history of) bronchial asthma.
Conclusion
Country-level analysis of the VISIONARY study reaffirmed the IOP-lowering efficacy of the PF tafluprost/timolol FC on eyes with OAG and OHT in several European countries, separately. Before the study period and at the baseline visit, patients were either inadequately controlled on a PGA or beta-blocker monotherapy or unable to tolerate these medications in each of the participating countries. Clinically and statistically significant IOP reductions were demonstrated throughout the 6-month study period in each population. Despite country-level variations in baseline pressure, IOP under monotherapy was a strong predictor of treatment outcome with PF tafluprost/timolol FC and the additional IOP reduction remained clinically significant for each country. The analysis revealed some variations in PF tafluprost/tafluprost FC prescribing and clinical practice, while safety data indicated that treatment was generally well tolerated in each European study population.
VISIONARY study group Principal Investigators
Austria: Christoph Faschinger (LKH – Universitaetsklinikum Graz); Sweden: Enping Chen (St Eriks Eye Clinic, Stockholm); Hungary: Gábor Holló (Semmelweis University, Budapest), Gabor Nemeth (Borsod-Abaúj-Zemplén Megyei Kórház Szemészeti Miskolc), Gyorgy Bator (Markusovszky Egyetemi Oktató Kórház, Szombathely), Alexis Tsorbatzoglou (Szabolcs-Szatmár-Bereg County Hospital and University Teaching Hospital, Nyíregyháza), Tamas Acs (Bács-Kiskun Megyei Kórház, Kecskemét), Maria Ferencz (Szent Imre Egyetemi Oktatkórház, Budapest), Zoltán Sohajda (Kenézy Gyula Kórház és Rendelőintéze, Debrecen), Jeno Toth (Fejér Megyei Szent György Egyetemi Oktató Kórház, Székesfehérvár), Veronika Volner (Uzsoki Utcai Kórház, Budapest), Gabor Vogt (Magyar Honvédség Egészségügyi Központ, Budapest), Zsolt Biro (PTE- Szemészeti Klinika, Pécs), Andrea Facskó (Szegedi Tudomá nyegyetem Szent-Györgyi Albert Klinikai Központ, Szent-Győrgy), János Nemes (Megyei Flór Ferenc Kórház, Kistarcsa), Andras Berta (University of Debrecen, Debrecen), Ilona Elek (Bugát Pál Kórház, Győngyős); Ireland: Eugene Ng (The Whitfield Clinic Butlerstown, Waterford); Italy: Francesco Oddone (IRCSS-Fondazione Bietti, Roma), Gemma Rossi (IRCCS-Fondazione Policlinico San Matteo, Pavia), Luca Rossetti (Ospedaliera San Paolo, Milan), Michele Vetrugno (Università di Bari, Bari), Michele Iester (Eye Clinic, DiNOGMI, University of Genoa, Ospedale Policlinico San Martino, Genoa, Genoa), Giorgio Marchini (Ospedale Civile Maggiore Borgo Trento, Verona), Vincenzo Scorcia (Università degli studi Magna Graecia, Catanzaro), Giovanni Staurenghi (Ospedale Luigi Sacco, Milano), Carlo Cagini (Università degli Studi di Perugia, Perugia), Tommaso Salgarello (Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma), Paolo Bettin (IRCCS Ospedale San Raffaele, Milano), Michele Figus (Ospedale Cisanello, Pisa), Gian Luca Scuderi (Ospedale Sant’Andrea, Roma), Stefano De Cilla (Azienda Ospedaliera Universitaria Maggiore della Carita Presidio Ospedaliero San Rocco, Novara); Latvia: Iveta Grundmane (Grund-opt Ltd, Valmiera), Nora Linavska (LENS-L Ltd, Liepaja), Lasma Volksone (Lavolks Ltd, Riga), Guna Laganovska (P. Stradiņš Clinical University Hospital, Riga), Kristine Baumane (Riga East Clinical University Hospital, Riga); Netherlands: Hans Lemij (Rotterdam Ophthalmic Institute, Rotterdam); Norway: Kjell Gunnar Gundersen (Dr Kjell Gunnar Gundersen MD, Haugesund); Russia: Marina Zimina (LLC Vzglyad, Leningradskaya), Valery Erichev (Federal State Budgetary Scientific Institution Scientific, Research Institute of Eye Diseases, Moscow), Elmira Adbulaeva (State Autonomous Institution of Healthcare Republican Clinical Ophthalmological Hospital of the Ministry of Healthcare of the Republic of Tatarstan, Republic of Tatarstan), Elena Karlova (State Budgetary Institution of Healthcare Samara Regional Clinical Ophthalmological Hospital, Samara), Ekaterina Zakharova (RKOB Sverdlova, Yakutsk), Irina Panova (S. Fyodorov Eye Microsurgery Federal State Institution, Saint Petersburg), Boris Malyugin (Sv. Fyodorov’s Eye Microsurgery Complex, Moscow); Spain: Iñaki Rodríguez-Agirretxe (H.U. Donostia, Guipuzcoa), Fernando Lopez-Lopez (Instituto Oftalmologico Gomez-Ulla, Galicia), Antonio Moreno Valladares (Hospital Ntra. Sra. del Perpetuo Socorro, Albacete), Javier Benitez del Castillo (Hospital Universitario de Jerez, Cadiz), Rafael Gimenez (Hospital Reina Sofia, Cordoba), Maria Parrilla Vallejo (Hospital La Macarena, Seville), Jose Javier Garcia-Medina (Hospital General Universitario Morales Meseguer, Murcia), Alfonso Anton Lopez (Institut Catalá de Retina, Barcelona), Sergio Torregrosa (Hospital Punta de Europa, Cadiz), Jorge Loscos (Hospital German Trias I Pujol, Barcelona); Denmark: Miriam Kolko (Rigshospitalet Valdemar Hansensvej, Glostrup); UK: Ejaz Ansari (Maidstone Hospital Hermitage, Maidstone, Kent), David Broadway (Norfolk and Norwich University Hospital, Norwich, Norfolk), Katharine Claridge (Royal Cornwall Hospital, Truro, Cornwall), Simon Ruben (Southend University Hospital NHS Foundation Trust, Westcliff-On-Sea, Essex), James Kirwan (Queen Alexandra Hospital, Portsmouth Hospitals NHS Trust, Portsmouth, Hampshire), Anca Nita (Royal Victoria Infirmary, The Newcastle Upon Tyne Hospitals NHS Foundation Trust, Newcastle Upon Tyne), Michael Smith (Royal Devon & Exeter NHS Foundation Trust, Exeter, Devon), Areeb Moosavi (Milton Keynes University Hospital NHS Foundation Trust, Milton Keynes), Anthony JW King (Nottingham University Hospitals NHS Trust, Nottingham), Matthew Kinsella (Buckinghamshire Healthcare NHS Trust, Stoke Mandeville Hospital, Buckinghamshire).
Transparency
Declaration of funding
Funding was provided by Santen SA, Geneva, Switzerland, for the VISIONARY study, statistical analysis, medical writing services and Rapid Service Fees. The contribution of IRCCS Fondazione Bietti to this work was supported by the Italian Ministry of Health and by Fondazione Roma.
Declaration of financial/other relationships
Gábor Holló has received consultancy and lecturing fees from Santen and Aerie. Fernando Lopez-Lopez has no relevant disclosures. James Kirwan has received speaker fees from Santen, Aspire and Thea during the past 5 years. Marina Zimina has no relevant financial disclosures. Claudia Fassari is an employee at Santen SA. Francesco Oddone has received consultancy fees from Santen, Allergan, Sooft, Omikron Italia and Centervue. and Novartis. A reviewer on this manuscript has disclosed that their institution has received financial support from PSI Foundation. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. All authors had full access to all of the data in this study and take complete responsibility for the accuracy of the data analysis.
Each author was a Principal Investigator on the VISIONARY study and was responsible for the reporting of patient data, along with those authors listed in the VISIONARY study group (below), or they were instrumental in the study design and analysis. Each of the main authors on this paper reviewed and commented on the data reported herein and all authors approved the final manuscript.
Clinical trials registry
The study was registered under the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) European Union electronic Register of Post-Authorisation Studies (EU PAS Register) (EU PAS register number: EUPAS22204).
Ethics statement
The study complied with the principles of the Declaration of Helsinki of 1964 as revised in 2013. All patients included were required to provide written informed consent prior to their enrolment. The VISIONARY study protocol was approved by the Institutional Review Board (IRB) or Independent Ethics Committee (IEC) at each center/institution and written informed consent was obtained from subjects prior to enrolment. Each of the VISIONARY study centers/institutions are listed in the VISIONARY Study Group section (above) alongside the relevant Principal Investigator.
Acknowledgements
Medical writing, editorial and other assistance
Feride Sahin and Gabriela Saborio provided input concerning the design and implementation of the study as well as the analyses and reporting of data on behalf of Santen. Claire Lea also provided input and guidance on the reporting of scientific data on behalf of Santen. Medical writing services were provided on behalf of the authors by Rebecca Down at Copperfox Communications Limited.
Thanking patient participants
The authors thank the participants for their valuable contribution to the VISIONARY study.
Data availability statement
The datasets used during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative normal-tension Glaucoma study group. Am J Ophthalmol. 1998;126:498–505.
- International Council of Ophthalmology. Guidelines for glaucoma care. 2016; [cited 2022 Mar 29]. Available from: http://www.icoph.org/downloads/ICOGlaucomaGuidelines.pdf.
- Janz NK, Wren PA, Lichter PR, et al. The collaborative initial glaucoma treatment study: interim quality of life findings after initial medical or surgical treatment of glaucoma. Ophthalmology. 2001;108(11):1954–1965.
- Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120(10):1268–1279.
- The AGIS Investigators. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS investigators. Am J Ophthalmol. 2000;130:429–440.
- Holló G, Hommer A, Delivery of Glaucoma Care Committee of the European Glaucoma Society. The status of glaucoma diagnostics and care in Europe in 2015: a European survey. Eur J Ophthalmol. 2016;26(3):216–220.
- European Glaucoma Society. Terminology and guidelines for glaucoma 5th Edition. 2020; [cited 2022 Mar 29]. Available from: https://www.eugs.org/eng/egs_guidelines_download.asp.
- Tabet R, Stewart WC, Feldman R, et al. A review of additivity to prostaglandin analogs: fixed and unfixed combinations. Surv Ophthalmol. 2008;53(6):S85–S92.
- Konstas AG, Labbé A, Katsanos A, et al. The treatment of glaucoma using topical preservative-free agents: an evaluation of safety and tolerability. Expert Opin Drug Saf. 2021;20(4):453–466.
- Holló G, Katsanos A, Boboridis KG, et al. Letter to the editor: ‘topical preservative-free ophthalmic treatments an unmet clinical need’. Expert Opin Drug Deliv. 2021;18(4):527–529.
- Aptel F, Cucherat M, Denis P. Efficacy and tolerability of prostaglandin-timolol fixed combinations: a meta-analysis of randomized clinical trials. Eur J Ophthalmol. 2012;22(1):5–18.
- Quaranta L, Biagioli E, Riva I, et al. Prostaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: a systematic review and meta-analysis. J Ocul Pharmacol Ther. 2013;29(4):382–389.
- Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86(4):418–423.
- Holló G, Topouzis F, Fechtner RD. Fixed-combination intraocular pressure-lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert Opin Pharmacother. 2014;15(12):1737–1747.
- Holló G, Katsanos A, Boboridis KG, et al. Preservative-free prostaglandin analogs and prostaglandin/timolol fixed combinations in the treatment of glaucoma: efficacy, safety and potential advantages. Drugs. 2018;78(1):39–64.
- Aguayo Bonniard A, Yeung JY, Chan CC, et al. Ocular surface toxicity from glaucoma topical medications and associated preservatives such as benzalkonium chloride (BAK). Expert Opin Drug Metab Toxicol. 2016;12(11):1279–1289.
- Konstas AG, Katsanos A, Athanasopoulos GP, et al. Preservative-free tafluprost/timolol fixed combination: comparative 24-h efficacy administered morning or evening in open-angle glaucoma patients. Expert Opin Pharmacother. 2018;19(18):1981–1988.
- Holló G, Katsanos A. Safety and tolerability of the tafluprost/timolol fixed combination for the treatment of glaucoma. Expert Opin Drug Saf. 2015;14(4):609–617.
- Holló G, Vuorinen J, Tuominen J, et al. Fixed-dose combination of tafluprost and timolol in the treatment of open-angle glaucoma and ocular hypertension: comparison with other fixed-combination products. Adv Ther. 2014;31(9):932–944.
- Hoy SM. Tafluprost/timolol: a review in open-angle glaucoma or ocular hypertension. Drugs. 2015;75(15):1807–1813.
- Kaarniranta K, Ikaheimo K, Mannermaa E, et al. Pharmacokinetics, efficacy, and safety of the preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% in healthy volunteers: a phase I comparison vs. the corresponding preservative-free monotherapies. Clin Pharmacokinet. 2016;55(4):485–494.
- Pfeiffer N, Traverso CE, Lorenz K, et al. A 6-month study comparing efficacy, safety, and tolerability of the preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% versus each of its individual preservative-free components. Adv Ther. 2014;31(12):1228–1246.
- Pillunat LE, Erb C, Ropo A, et al. Preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% in patients with open-angle glaucoma and ocular hypertension: results of an open-label observational study. Clin Ophthalmol. 2017;11:1051–1064.
- Bourne RRA, Kaarniranta K, Lorenz K, et al. Changes in ocular signs and symptoms in patients switching from bimatoprost-timolol to tafluprost-timolol eye drops: an open-label phase IV study. BMJ Open. 2019;9(4):e024129.
- Oddone F, Tanga L, Kóthy P, et al. Treatment of open-angle glaucoma and ocular hypertension with Preservative-Free tafluprost/timolol fixed-dose combination therapy: the VISIONARY study. Adv Ther. 2020;37(4):1436–1451.
- Oddone F, Kirwan J, Lopez-Lopez F, et al. Switching to preservative-free tafluprost/timolol fixed-dose combination in the treatment of open-angle glaucoma or ocular hypertension: subanalysis of data from the VISIONARY study according to baseline monotherapy treatment. Adv Ther. 2022. DOI:https://doi.org/10.1007/s12325-022-02166-6
