Introduction
Acute inflammation is a necessary part of successful healing after musculoskeletal insult. Disruption or prolongation of this process can lead to suboptimal outcomesCitation1. Blocking pro-inflammation pathways by means of non-steroidal anti-inflammatory drugs (NSAIDs) is routinely used to manage pain and inflammationCitation2. Such treatment continues to be advocated by authors in this journalCitation3. While often symptomatically beneficial and of potential value in breaking the pain cycle and helping clinicians clarify the relative importance of inflammation in a patient’s symptoms, their use may encourage harmful pain-masking, interfere with tissue healing (homeostasis), promote chronicity and predispose to re-injuryCitation4–6. Furthermore, potentially serious side effects of NSAIDs are well establishedCitation7. There is currently much interest in the potential of moving from treatments which inhibit inflammation to those promoting resolution, a new area of scientific research termed “resolution pharmacology”Citation8. For patients who have suffered injury or are experiencing acute or acute on chronic osteoarthritis symptoms is there currently enough evidence to justify a shift in therapeutic approach towards pro-resolution strategies?
Overview of homeostasis
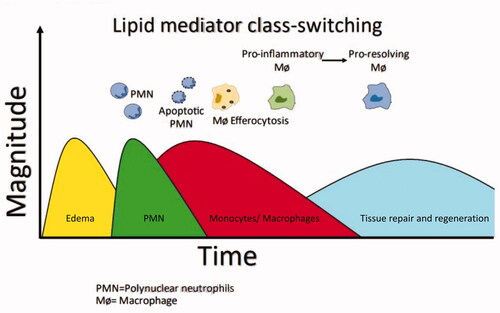
It was previously thought that inflammation was actively induced to defend the host, which then passively declined with cessation of the inducer. It is now known that tissue homeostasis, healing and restoration of function involve both a pro-inflammation phase and a resolution phase. Recent research suggests a complex, tightly regulated process, with both phases being initiated after injury or infection and then proceeding in parallel, rather than as a linear series of stepsCitation1. It has been suggested that the “beginning programs the end”, with inflammatory cells involved in the active phase of inflammation undergoing a functional repolarization and then contributing to the onset of resolutionCitation8.
Many mediators that promote the inflammatory phase can simultaneously initiate a program for active resolutionCitation9. Cellular events following injury are summarized in . On sensing tissue damage, pro-inflammatory macrophages release mediators, notably cytokines, leukotrienes and prostaglandins (e.g. prostaglandin [PG] E2). These induce increased blood flow, microvascular permeability and edema. There is rapid polymorphonuclear neutrophil (PMN) ingress across capillaries to engulf and degrade pathogens, followed by the slower passage of monocytes, accompanied by the classical features of swelling, pain, redness and fever. Resolution is initiated during the inflammation stage by apoptotic PMNs releasing specialized pro-resolving mediators (SPMs), which inhibit further migrationCitation10. Involved in this lipid mediator class switch are cytokines, resolvins, protectins, lipoxins, maresins and annexin-A1 (AnxA1). Macrophages then ingest the apoptotic neutrophils (efferocytosis), transforming them into resolution-phase macrophages. This macrophage phenotype switch from pro-inflammation to pro-resolution contributes towards post-resolution immune tolerance and prevention of autoimmunity. Similarly, a neutrophil phenotype switch results in reprogramming to an anti-inflammatory type, promoting neutrophil reverse migration. All these elements are thought to contribute to tissue homeostasis and a return to normal function. Interestingly, a further phase occurring following resolution of inflammation has recently been identified, consisting of a third wave of leukocytesCitation10. Identification of these interrelated stages of healing and associated mediators provides researchers with new therapeutic opportunities.
Indications, actions and risks of NSAIDs
NSAIDs are widely recommended for the short-term treatment of pain and osteoarthritis (OA) symptoms, with many people self-medicating with over-the-counter productsCitation11.
Their primary mode of action is believed to be the inhibition of the arachidonic acid cascade, notably the cyclooxygenase 1 and 2 (COX) isoenzymes induced by injury. This inhibition interferes with production of inflammatory mediators, including vasoactive amines, eicosanoids, cytokines, chemokines and cell adhesion molecules, leading to a reduction in inflammation and pain. Inhibiting PGI2, PGE2 and thromboxane A2 appears to be central to their actionsCitation12. A systematic review and network meta-analysis by da Costa et al. concluded that oral etoricoxib 60 mg/day and diclofenac 150 mg/day seem to be the most effective NSAIDs for pain and improving function in patients with osteoarthritisCitation11.
Although NSAIDs are generally beneficial for mild-to-moderate pain, some people are intolerant of them or reluctant users, and around 2% are hypersensitiveCitation13. Importantly, NSAIDs are associated with varying degrees of gastrointestinal, cardiovascular and renal complicationsCitation14. Many healthcare professionals, however, are unaware of the importance of dose, route of administration, treatment duration, patient age and drug interactions. They may consequently fail to counsel regarding risks and benefitsCitation15. It is not generally appreciated, for example, that widely available NSAIDs such as ibuprofen and diclofenac are associated with a threefold increase in stroke risk. Similarly, diclofenac increases cardiovascular deaths fourfold while doubling all-cause deaths. Prescribing a cyclooxygenase-2 (COX-2) selective inhibitor does reduce the risk of gastrointestinal disturbances, but for some their use has been overshadowed by experiences with drugs such as rofecoxib (sold as VioxxFootnotei), which led to significant cardiovascular eventsCitation12.
A further issue is homeostasis. It has been suggested that actions of NSAIDs on inflammatory mediators may interfere with bone and tendon repair, leading to failed healing at the site of injury and an increased risk of re-injuryCitation4. In a study on rabbits reported in 2018 by Sauerschnig et al., selective COX-2 inhibitors caused impaired tendon-to-bone healing, weakened mechanical stability and decreased PGE2 content of the synovial fluidCitation16. In a survey study, Franco et al. examined the relationship between NSAID use and recovery after Achilles tendon rupture in 361 patientsCitation17. NSAID users reported significantly lower Achilles Tendon Total Rupture Scores than non-NSAID users indicating a worse recovery. There was no difference in time to normal walking or incidence of re-rupture. It is noteworthy that Perry et al.Citation6 have suggested an association between regular use of NSAIDs and radiographic progression of knee OA.
In contrast to the above, systematic reviews around the same time by Duchman et al., Ghosh et al. and Constantinescu et al. concluded that there was insufficient evidence for or against NSAIDs following acute injury and usage should be decided on a case-by-case basisCitation4,Citation18,Citation19. Drug selection may, however, be important in the light of the evidence from Sauerschnig et al. and others that selective COX-2 inhibitors can negatively affect soft tissue healing after surgical repair in both animals and humansCitation18,Citation19.
How we currently treat pain could be wrong. A recent multicenter clinical study suggests that the management of acute inflammation using NSAIDs may be counterproductive for long-term outcomes among low back pain (LBP) sufferers. Parisien et al. performed transcriptome analysis in peripheral immune cells of 98 patients with acute LBPCitation20. Resolution of pain after 3 months was associated with thousands of dynamic transcriptional changes but none in those with persistent pain. Transient neutrophil-driven up-regulation of the inflammatory response appears to protect against the development of chronic pain. In a mouse model, treatment with a steroid or NSAID (but not other drugs) was associated with persistent pain despite early improvement and a depletion of neutrophils. Delayed pain resolution could be prevented by the injection of neutrophils or proteins normally released by neutrophils. An examination of pain trajectories in human subjects registered with the UK Biobank identified an increased risk of pain persistence among subjects taking NSAIDs.
Current approaches to the non-surgical management of musculoskeletal disease
The management of musculoskeletal disease is complicated by an incomplete understanding of the pathophysiological processes involved and the mechanism of action of many current therapies. Where information is available, this is often of low gradeCitation4.
If prolonged use of NSAIDs is associated with side effects and can interfere with homeostasis, could a different approach alone or in combination be more successful? Rest, ice, compression and elevation (known as “RICE”) are traditional strategies following limb injury. They can be supplemented by stretching, range-of-movement and isometric exercises plus a stability and fitness maintenance programCitation21.
Acetaminophen, better known as paracetamol, is useful to treat mild-to-moderate acute/acute on chronic pain or fever but has no anti-inflammatory action. It is believed to exert its effect centrally by inhibiting COX-2 and cannabinoid receptors in the brain. Although generally regarded as safe within recommended doses, a NICE review suggests that paracetamol is of “reduced effectiveness” in osteoarthritisCitation22,Citation23. Higher up the pain ladder, opioids have a powerful central analgesic and sedative effect but are associated with side effects such as drowsiness and constipationCitation24. With long-term use there is a risk of addiction, and opioids may be less effective on their own for chronic pain with or without musculoskeletal injury than non-opioid strategiesCitation25. Where neuropathic pain is a feature, anti-depressants (e.g. amitriptyline) and anti-epileptics (e.g. gabapentin, pregabalin) may be of value but are also associated with significant side effectsCitation26.
Corticosteroid tablets or injections are widely used in non-resolving musculoskeletal and rheumatoid diseases. Their rapid anti-inflammatory and resultant analgesic effects are mediated by activation of glucocorticoid receptors and metabolic downregulation in inflammatory cells. Corticosteroids are known to delay tissue healing and cause local tissue atrophy and, with prolonged use, can lead to osteoporosis, hypertension, diabetes, weight gain and susceptibility to infection. Osteoarthritis of the knee and hip are common problems for which occasional intra-articular corticosteroid injections are considered safe and effective for short-term pain relief and to improve function. There are concerns, however, that repeated injections may result in chondrotoxicity and accelerated cartilage lossCitation27.
For patients with rheumatoid diseases, long-term treatment with disease-modifying anti-rheumatic drugs, such as methotrexate, hydroxychloroquine and sulphasalazine, helps relieve pain and inflammation by general suppression of the immune system. If the treatment fails to control symptoms or induces complications, a switch to biologics and biosimilars that reduce inflammation by targeting specific cells and proteins may be indicated, e.g. adalimumab and etanercept. As might be expected from immunosuppressants, side effects are common and potentially life-threatening, and some of the newer agents are expensiveCitation28.
From the above discussion, it will be seen that current management of pain, swelling and loss of function of musculoskeletal origin is only variably effective with the potential for serious side effects.
What is pro-resolution therapy?
There is currently much interest in the potential for treating diseases considered to have an inflammatory component by means of a pro-resolution approachCitation9. These diseases range from inflammatory bowel disease and rheumatoid arthritis to Alzheimer’s disease. Already, some 1100 patents have been registered for potential pro-resolution drugs, with at least 13 major pharmaceutical companies devoting resources to this field.
Pro-resolution involves enhancing or promoting factors essential for removal of the inciting stimulus. Other elements include dampening pro-inflammatory signaling and stimulating leukocyte clearance. Central to this are SPMs, which have been shown in animal experiments at low doses to act on specific immune receptors as well nociceptors to limit pain and inflammation in a time-dependent mannerCitation9.
An ever-expanding list of SPMs that could serve as therapeutic targets is being recognized, including AnxA1, lipoxin A4 (LXA4), resolvins (e.g. resolvin [Rv] D1, RvD2, RvD3, RvE1, RvE2), maresins (e.g. maresin-1 [MaR1]), protectins, melanocortin peptides and their target receptors, notably formyl peptide receptor 2 (FPR2/ALX)Citation10. Activation of messenger AMP (cAMP) appears to have a central role in resolution.
One challenge for scientists is to build a stable analog that is not broken down before it can act at the site of injury. Addition of a methyl group to LXA4 to form a more stable 15(R/S)-methyl-LXA4 has, for example, been found to be of value in infantile eczema. Similarly, the lipoxin analog BLXA4-ME may have application in oral gingivitis, while the RvE1 analog, RX-10045 has been researched in chronic dry eye syndromeCitation29. These early results hint at the potential for future pro-resolution drugs.
It is interesting to note that aspirin, an NSAID, appears to act differently to other class members by not only blocking the biosynthesis of prostaglandins, but also at low dose stimulating the production of specific pro-resolving mediators such as resolvins and lipoxinsCitation30. These effects are currently being investigated across different therapeutic areas, notably cancer treatmentCitation31.
Pro-resolution drugs
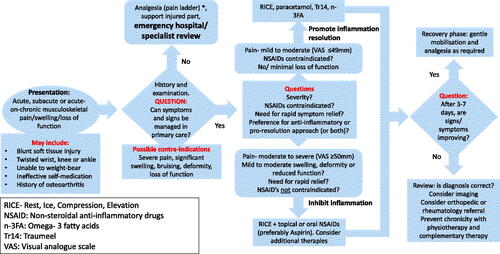
It is disappointing that 20 years of resolution pharmacology has so far failed to successfully bring one new drug to the marketCitation32. Small studies in humans involving nutritional supplements and “natural” medicines are showing promise; we consider these sufficiently mature to be of clinical value. We consequently suggest a revised therapeutic algorithm in primary care for acute, sub-acute or acute on chronic pain/swelling of presumed musculoskeletal origin (). This is particularly relevant in patients with mild-to-moderate symptoms where there is a desire to avoid or complement the effects of NSAIDs. It will be seen that all traditional elements are present such as the pain ladder and the value of a clinical review at 3–7 days as dictated by the natural cycle of inflammation.
Figure 2. How considering inflammation resolution might alter the management of musculoskeletal injury. *The WHO “Pain Ladder” classifies pain in terms of severity and recommends a series of increasingly potent analgesic treatment steps according to responseCitation20.
Because SPMs are lipid mediators biosynthesized from omega-3 fatty acids (n-3FA) there is interest in the effects of supplementation. Ingesting n-3FA can increase endogenous SPM levels and prevent pain and inflammation in diseases such as rheumatoid arthritis and diabetic neuropathy. Benefits have also been seen in coronary heart disease, Alzheimer’s disease and chronic kidney diseaseCitation29.
Ginseng, the root of Panax ginseng, continues to be widely used for therapeutic purposes in Asia. Ginsenosides are the main active constituents and have been shown to negatively regulate expression of pro-inflammatory cytokines (interleukin [IL]-1β, IL-6 and tumor necrosis factor [TNF]-α) and enzymes (COX-2 and inducible nitric oxide synthase) in M1-polarized macrophages and microglia. They have also been found to exhibit a pro-resolution effect by inducing M2 polarization of macrophages and microglia, which in turn contribute to suppression of inflammation and promotion of inflammation resolutionCitation33. While its mechanism of action remains to be fully elucidated, several small studies suggest possible clinical benefits. For example, Caldwell et al. in a mixed population, three-arm study found that Korean ginseng reduced the magnitude of pain and soreness following resistance exercise compared to placebo treatmentCitation34. Similarly, Lin et al. in a placebo-controlled trial found that American ginseng attenuated exercise-induced muscle damage via the modulation of lipid peroxidation and inflammatory adaptation in a small group of active male college studentsCitation35.
TraumeelFootnoteii is a natural medicine consisting of 14 components formulated some 60 years ago. It is registered as a homeopathic preparation, although it contains measurable ingredients and exhibits demonstrable biological effects. It has, for example, been shown to inhibit secretion of the cytokines IL-1β, TNF-α and IL-8 in a resting state, as well as in activated immune cellsCitation36. Compared with an NSAID, Traumeel has been shown to modulate different inflammatory processesCitation37. Diclofenac, for example, inhibits COX-2 and hence PGE2 production. By comparison, Traumeel does not inhibit COX-2 induction, thus allowing normal activation of COX/5-lipoxygenase-dependent resolution pathways in the early phase of inflammation.
Using a mouse peritonitis model, Jordan et al. demonstrated that Traumeel promotes biosynthesis of SPMs and the recruitment of innate leukocytes and macrophages essential for the clearance of apoptotic PMNs and cellular debris by efferocytosis. The result is a shortening of the resolution index, i.e. it promotes faster inflammation resolutionCitation38.
These finding are supported by St Laurent et al., who demonstrated that Traumeel, but not diclofenac, reduced mRNA levels in the leukotriene synthetic pathway of a mouse wound model. A suggested mechanism is activation of the components of nuclear factor erythroid 2-related factor 2/Kelch-like ECH-associated protein 1 (Nrf2/KEAP1). This may explain some of its anti-inflammatory and pro-resolving propertiesCitation37. Further, the product caused a transcriptomic signature consistent with alterations in the types of cells that are present in the wound. This appears to reflect the elevated regulatory T cells found in Traumeel-treated woundsCitation37.
Another interesting finding from this work is that the modes of action of diclofenac and Traumeel appear to differ depending on the stage of inflammation. While in the initial stages there is an approximate 50% overlap, the processes they act on then appear to diverge. St Laurent et al. found that shared molecular pathways involving the extracellular matrix, innate immunity, cell migration and inflammation were influenced by diclofenac immediately after injury. By comparison, administration of Traumeel exerted an effect at later stages of wound repairCitation37.
Other preliminary preclinical data also indicate that Traumeel modulates signaling pathways and behavior of multiple immune cell types, which promote inflammation resolution.
Randomized clinical trials and observational studies generally confirm the efficacy, effectiveness and safety of Traumeel for the treatment of various musculoskeletal diseases, including acute ankle sprains, tendinopathy, epicondylitis and knee osteoarthritis (in combination with Zeel TFootnoteiii)Citation21,Citation39,Citation40. It appears to be at least as effective as oral NSAIDs in the acute settingCitation41–43. Further evidence for a possible pro-resolution effect in humans comes from Muders et al., who found that it can limit exercise-induced muscle damage by modulating certain inflammation-regulating signalsCitation44. By comparison, Markworth et al. found that ibuprofen blocked SPM production, suggesting that ibuprofen might delay natural resolutionCitation45. While we may not fully understand how Traumeel works, or which components are most active, the available evidence is similar or of better quality than that used to justify some other therapies in musculoskeletal diseases. It should be noted that not all findings for Traumeel have been positive and one must always consider the potential for a placebo responseCitation46. In a study looking at pain after elective hallux valgus surgery, Singer et al. failed to demonstrate superiority compared to placebo in minimizing pain or analgesic consumption during the 14-day post-operative periodCitation47. However, a transient reduction in the daily maximum post-operative pain score was observed on the day of surgery in the Traumeel group which the authors suggest may be clinically important (treatment-time interaction test p = .04). It is worth pointing out that considerations regarding a placebo effect become less relevant with increasing time after injury as the importance of more objective criteria such as chronicity and re-injury take their place.
Conclusions
NSAIDs and corticosteroids are firmly fixed in the therapeutic armamentarium of most clinicians. They are efficacious drugs but are contraindicated across a range of comorbidities. Many patients would prefer an alternative approach, once given a balanced explanation of risks and benefits. Where the healthcare professional and/or patient is seeking a pro-resolution approach to treatment with possible benefits in terms of healing and reducing chronicity, we suggest modification of the current generally practiced treatment regimen ().
Supplementation with n-3FA, or use of a ginsenoside or Traumeel, are alternatives for which there is growing clinical evidence. They appear to be free from any significant side effects and can be used alone or in combination with other treatments. A theoretical advantage of a multitarget agent such as Traumeel is that, because it has effects across the signaling network, balancing signaling inhibition with promotion by targeting synergistic pathways, it may allow smaller pharmacological doses of co-prescribed medication to be administered and thereby minimize side effects. Chief disadvantages are slower onset of pain control and the likely need to self-fund these products. Being classed as a supplement, natural or homeopathic treatment in most markets may prevent them being taken seriously by the medical and scientific community. This is a shame, as many clinicians, the sporting community and veterinary practices attest to their effectiveness.
Transparency
Declaration of funding
Since a previous publication on this topic in CMRO, new data on inflammation resolution have become available which may affect the management of acute musculoskeletal injury. At a Scientific Advisory Board (SAB) meeting in November 2019, the present authors decided that this new information warranted a revised document commenting on how treatments other than NSAIDs may benefit some patients. Further, participants in the SAB recommended that such a document deserved wide dissemination in a peer-reviewed journal.
Heel GmbH funded the SAB but did not influence the decision-making process or article contents. Stgilesmedical GmbH (www.stgmed.com), an independent medical writing company, supported the authors in developing this manuscript and associated figures.
Declaration of financial/other relationships
All authors have disclosed that they have received payments from Heel as Advisory Board members.
B.W. has disclosed that he has received lecture fees from Heel. K.R. has disclosed that he has acted as an advisor for Biotechnos, CSL Behring, TRB-Cimedika, Bionoltra, Fidia and Romfarma; and as a speaker for Schering, CSL Bering, Takeda, Novartis and Fermatron.
No additional potential conflict of interest was reported by C.S., L.V.B. or A.M. CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the conception and design of this commentary article, the drafting of the paper or revising it critically for intellectual content; and the final approval of the version to be published. Further, all authors agree to be accountable for all aspects of the work.
Acknowledgements
The authors thank Dr Steven Walker, Franziska Koenig-Paratore and Daniel Roberts at Stgilesmedical GmbH, Berlin Germany (www.stgmed.com) for writing support, which was sponsored by Biologische Heilmittel Heel GmbH, Baden-Baden, Germany. Editing was by Petra Roberts. We acknowledge support by the Open Access Publication Fund of Humboldt-Universität zu Berlin.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Notes
i Vioxx. Merck & Co., Inc. Rahway, New Jersey, USA. Rofecoxib was voluntarily withdrawn from the market in 2004.
ii Traumeel, Biologische Heilmittel Heel GmbH, Baden-Baden, Germany
iii Zeel T, Biologische Heilmittel Heel GmbH, Baden-Baden, Germany
References
- Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15(8):551–567.
- Machado GC, Abdel-Shaheed C, Underwood M, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for musculoskeletal pain. BMJ. 2021;2021:n104.
- McMahon SB, Dargan P, Lanas A, et al. The burden of musculoskeletal pain and the role of topical non-steroidal anti-inflammatory drugs (NSAIDs) in its treatment. Ten underpinning statements from a global pain faculty. Curr Med Res Opin. 2021;37(2):287–292.
- Duchman KR, Lemmex DB, Patel SH, et al. The effect of non-steroidal anti-inflammatory drugs on tendon-to-bone healing: a systematic review with subgroup meta-analysis. Iowa Orthop J. 2019;39(1):107–119.
- Speed C, Wolfarth B. Challenges of pain masking in the management of soft tissue disorders: optimizing patient outcomes with a multi-targeted approach. Curr Med Res Opin. 2014;30(5):953–959.
- Perry TA, Wang X, Nevitt M, et al. Association between current medication use and progression of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Rheumatology. 2021;60(10):4624–4632.
- Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020;180:114147.
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197.
- Sugimoto MA, Vago JP, Perretti M, et al. Mediators of the resolution of the inflammatory response. Trends Immunol. 2019;40(3):212–227.
- Chiang N, Serhan CN. Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem. 2020;64(3):443–462.
- da Costa BR, Pereira TV, Saadat P, et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ. 2021;375:n2321.
- Curtis E, Fuggle N, Shaw S, et al. Safety of cyclooxygenase-2 inhibitors in osteoarthritis: outcomes of a systematic review and meta-analysis. Drugs Aging. 2019;36(Suppl 1):25–44.
- Wöhrl S. NSAID hypersensitivity – recommendations for diagnostic work up and patient management. Allergo J Int. 2018;27(4):114–121.
- Ussai S, Miceli L, Pisa FE, et al. Impact of potential inappropriate NSAIDs use in chronic pain. Drug Des Devel Ther. 2015;9:2073–2077.
- Arain A, Rasheed M, Sallam N, et al. Patient’s knowledge and use of oral non-steroidal anti-inflammatory drugs in a rheumatology clinic. Kans J Med. 2019;12(4):132–135.
- Sauerschnig M, Stolberg-Stolberg J, Schmidt C, et al. Effect of COX-2 inhibition on tendon-to-bone healing and PGE2 concentration after anterior cruciate ligament reconstruction. Eur J Med Res. 2018;23(1):1.
- Franco V, Grotts J, Lin JC, et al. Non-steroidal anti-Inflammatory drug use and recovery after Achilles tendon rupture. Muscle Ligaments Tendons J. 2019;09(03):314–321.
- Ghosh N, Kolade OO, Shontz E, et al. Nonsteroidal anti-inflammatory drugs (NSAIDs) and their effect on musculoskeletal soft-tissue healing: a scoping review. JBJS Rev. 2019;7(12):e4.
- Constantinescu DS, Campbell MP, Moatshe G, et al. Effects of perioperative nonsteroidal anti-inflammatory drug administration on soft tissue healing: a systematic review of clinical outcomes after sports medicine orthopaedic surgery procedures. Orthop J Sports Med. 2019;7(4):2325967119838873.
- Parisien M, Lima LV, Dagostino C, et al. Acute inflammatory response via neutrophil activation protects against the development of chronic pain. Sci Transl Med. 2022;14(644):eabj9954.
- Bisciotti GN, Volpi P, Amato M, et al. Italian consensus conference on guidelines for conservative treatment on lower limb muscle injuries in athlete. BMJ Open Sport Exerc Med. 2018;4(1):e000323.
- Conaghan PG, Arden N, Avouac B, et al. Safety of paracetamol in osteoarthritis: what does the literature say? Drugs Aging. 2019;36(Suppl 1):7–14.
- NIfHaC. Osteoarthritis: care and management clinical guidelines. London: NICE; 2014.
- Ahmadi A, Bazargan-Hejazi S, Heidari Zadie Z, et al. Pain management in trauma: a review study. J Inj Violence Res. 2016;8(2):89–98.
- Ashaye T, Hounsome N, Carnes D, et al. Opioid prescribing for chronic musculoskeletal pain in UK primary care: results from a cohort analysis of the COPERS trial. BMJ Open. 2018;8(6):e019491.
- Cavalli E, Mammana S, Nicoletti F, et al. The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol. 2019;33:1–83.
- Nichols AW. Complications associated with the use of corticosteroids in the treatment of athletic injuries. Clin J Sport Med. 2005;15(5):370–375.
- Smolen JS, Goncalves J, Quinn M, et al. Era of biosimilars in rheumatology: reshaping the healthcare environment. RMD Open. 2019;5(1):e000900.
- Fattori V, Zaninelli TH, Rasquel-Oliveira FS, et al. Specialized pro-resolving lipid mediators: a new class of non-immunosuppressive and non-opioid analgesic drugs. Pharmacol Res. 2020;151:104549.
- Serhan CN, Fredman G, Yang R, et al. Novel proresolving aspirin-triggered DHA pathway. Chem Biol. 2011;18(8):976–987.
- Gilligan MM, Gartung A, Sulciner ML, et al. Aspirin-triggered proresolving mediators stimulate resolution in cancer. Proc Natl Acad Sci USA. 2019;116(13):6292–6297.
- Park J, Langmead CJ, Riddy DM. New advances in targeting the resolution of inflammation: implications for specialized pro-resolving mediator GPCR drug discovery. ACS Pharmacol Transl Sci. 2020;3(1):88–106.
- Im DS. Pro-resolving effect of ginsenosides as an anti-inflammatory mechanism of panax ginseng. Biomolecules. 2020;10(3):444.
- Caldwell LK, DuPont WH, Beeler MK, et al. The effects of a Korean ginseng, GINST15, on perceptual effort, psychomotor performance, and physical performance in men and women. J Sports Sci Med. 2018;17(1):92–100.
- Lin C-H, Lin Y-A, Chen S-L, et al. American ginseng attenuates eccentric exercise-induced muscle damage via the modulation of lipid peroxidation and inflammatory adaptation in males. Nutrients. 2021;14(1):78.
- Porozov S, Cahalon L, Weiser M, et al. Inhibition of IL-1beta and TNF-alpha secretion from resting and activated human immunocytes by the homeopathic medication Traumeel S. Clin Dev Immunol. 2004;11(2):143–149.
- St Laurent G, Toma I, Seilheimer B, et al. RNAseq analysis of treatment-dependent signaling changes during inflammation in a mouse cutaneous wound healing model. BMC Genomics. 2021;22(1):854.
- Jordan PM, van Goethem E, Müller AM, et al. The natural combination medicine Traumeel (Tr14) improves resolution of inflammation by promoting the biosynthesis of specialized pro-resolving mediators. Pharmaceuticals. 2021;14(11):1123.
- Zenner S, Metelmann H. Therapieerfahrungen mit traumeel S salbe: Ergebnisse einer multizentrischen anwendungsbeobachtung an 3422 patienten. Biologische Medizin. 1992;21(5):341–349.
- Lozada CJ, Del Rio E, Reitberg DP, et al. A double-blind, randomized, saline-controlled study of the efficacy and safety of co-administered intra-articular injections of Tr14 and Ze14 for treatment of painful osteoarthritis of the knee: the MOZArT trial. Eur J Integr Med. 2017;13:54–63.
- Schneider C. Traumeel – an emerging option to nonsteroidal anti-inflammatory drugs in the management of acute musculoskeletal injuries. Int J Gen Med. 2011;4:225–234.
- González de Vega C, Speed C, Wolfarth B, et al. Traumeel vs. diclofenac for reducing pain and improving ankle mobility after acute ankle sprain: a multicentre, randomised, blinded, controlled and non-inferiority trial. Int J Clin Pract. 2013;67(10):979–989.
- Birnesser H, Oberbaum M, Klein P, et al. The homeopathic preparation Traumeel compared with NSAIDs for symptomatic treatment of epicondylitis. J Musculoskelet Res. 2004;08(02n03):119–128.
- Muders K, Pilat C, Deuster V, et al. Effects of Traumeel (Tr14) on exercise-induced muscle damage response in healthy subjects: a double-blind RCT. Mediators Inflamm. 2016;2016:1–18.
- Markworth JF, Vella L, Lingard BS, et al. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am J Physiol Regul Integr Comp Physiol. 2013;305(11):R1281–R1296.
- Saps M, Youssef N, Miranda A, et al. Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology. 2009;137(4):1261–1269.
- Singer SR, Amit-Kohn M, Weiss S, et al. Traumeel S for pain relief following hallux valgus surgery: a randomized controlled trial. BMC Clin Pharmacol. 2010;10:9.