Abstract
Objective
Surveillance for hepatocellular carcinoma (HCC) is known to be underutilized; however, neither the variation of surveillance adherence by cirrhosis etiology nor the patient-side economic burden of surveillance are well understood. To identify potential barriers to HCC surveillance, we assessed utilization patterns and costs among US patients with cirrhosis monitored in routine clinical practice.
Methods
We conducted a retrospective study of insured adult patients with cirrhosis using national administrative claims data from January 2013 through June 2019. Time up-to-date with recommended surveillance, correlates of surveillance receipt, and surveillance-associated costs were assessed during a ≥ 6-month follow-up.
Results
Among 15,543 patients with cirrhosis (mean [SD] age 64.0 [11.1] years, 50.7% male), 45.8% and 58.7% had received any abdominal imaging at 6 and 12 months, respectively. Patients were up-to-date with recommended surveillance for only 31% of a median 1.3-year follow-up. Those with viral hepatitis were more likely to receive surveillance than those with other etiologies (hazard ratio [HR] 1.55, 95% CI 1.11–2.17, p = .010 for patients without a baseline gastroenterologist [GI] visit and 2.69, 95% CI 1.77–4.09, p < .001 for patients with a GI visit, relative to those with nonalcoholic fatty liver disease and no GI visit). For all etiologies except NAFLD, the HR (95% CI) for surveillance receipt was higher among patients with vs without a baseline GI visit (alcohol-related, 1.164 [1.002–1.351] vs 0.880 [0.796–0.972]; viral hepatitis, 2.688 [1.765–4.093] vs 1.553 [1.111–2.171]; Other, 0.612 [0.519–0.722] vs 0.549 [0.470–0.641]). Mean total and patient-paid daily surveillance-related costs ranged from $540 and $113, respectively (ultrasound) to $1580 and $300, respectively (magnetic resonance imaging), and mean estimated patient productivity costs were $730–$2514 annually.
Conclusion
HCC surveillance was underutilized and was lowest among patients with nonviral etiologies and those who had not seen a gastroenterologist. Surveillance-related out-of-pocket expenses and lost productivity were substantial. The development of surveillance strategies that reduce patient burden, such as those using blood-based biomarkers, may help improve surveillance adherence and effectiveness.
Introduction
Hepatocellular carcinoma (HCC) is the fastest growing cause of cancer deaths in the US, with an estimated 30,000 attributable deaths in 2020 aloneCitation1. While HCC has the seventh highest incidence among cancer types globally, it ranks fourth in cancer deaths and second in years of life lost, with a 5-year survival rate of only 10.6%Citation2,Citation3.
Treatments available for intermediate- or advanced-stage HCC, including embolization, ablation, and systemic chemotherapies such as multikinase inhibitors and immune checkpoint inhibitorsCitation4,Citation5, are generally palliative. However, when HCC is diagnosed at an early stage, patients may be eligible for curative treatment such as surgical resection, local ablation, or liver transplantation, with expected 5-year survival rates up to 50%–80%Citation4. Accordingly, professional society guidelines recommend semi-annual surveillance with abdominal ultrasound with or without alpha fetoprotein (AFP) for high-risk patients, including those with cirrhosisCitation4,Citation6. Unfortunately, the sensitivity of ultrasound can be particularly poor in patients with obesity, advanced liver fibrosis, or nonalcoholic fatty liver disease, and this strategy misses over one-third of early-stage HCCCitation7,Citation8. Alternative imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) are gaining use in clinical practice; however, ultrasound remains the primary HCC surveillance method, owing to advantages such as non-invasiveness, low cost, and lack of radiation exposureCitation9.
Unfortunately, despite evidence that HCC surveillance promotes early detection and improves survival, it is severely underusedCitation10–14. A recent systematic review and meta-analysis of 29 studies including nearly 120,000 patients with cirrhosis revealed that only 24% received surveillanceCitation15. This widespread lack of adherence to surveillance guidelines has had damaging effects, as two-thirds of individuals with HCC are diagnosed at an advanced stage when the prognosis is poorCitation16. As a result, the real-world effectiveness of HCC surveillance is substantially lower than may be expected on the basis of clinical trial resultsCitation17.
It is not clear if adherence to HCC surveillance varies among patients with cirrhosis of different etiologies, since epidemiology and patient characteristics can vary considerably depending on the underlying liver disease. Understanding the direct and indirect costs of HCC surveillance can also inform efforts to implement currently available surveillance strategies and develop new mechanisms. This study was conducted to assess patterns of HCC surveillance utilization and costs in a nationwide cohort of insured US patients with cirrhosis monitored in routine clinical practice.
Methods
Study design
A retrospective observational study was conducted using administrative claims data from the Optum Research DatabaseCitation18 from 01 January 2013 through 30 June 2019 (study period, eFigure 1). The Optum Research Database is geographically diverse across the US and contains deidentified medical and pharmacy claims data and linked enrollment information for individuals enrolled in US health plans. Medical claims include diagnosis and procedure codes from the International Classification of Diseases, 9th and 10th Revisions, Clinical Modification (ICD-9-CM and ICD-10-CM); Current Procedural Terminology or Healthcare Common Procedure Coding System codes; site of service codes; and paid amounts. Pharmacy claims include drug name, National Drug Code, dosage form, drug strength, fill date, and financial information for plan-paid outpatient pharmacy services. No identifiable protected health information was accessed in the conduct of this study; therefore, institutional review board approval or waiver of approval was not required.
Patient selection
The study included adult patients (≥18 years) enrolled in commercial or Medicare Advantage with Part D health plans with ≥2 non-diagnostic claims for cirrhosis (see eTable 1 for codes) at least 30 days apart from 01 January 2014 through 31 December 2018 (identification period; eFigure 1). Patients were excluded from the study if they had a history of liver cancer (≥2 non-diagnostic claims with liver cancer diagnosis codes ≥30 days apart within a 365-day period)Citation19, liver transplantation, or decompensated cirrhosis (see eTable 1 for codes).
The date of the first qualifying claim for cirrhosis was designated as the index date. The 12 months pre-index were designated as the baseline period. All patients were observed for ≥6 months post-index, and follow-up ended at the earliest of health plan disenrollment, liver cancer diagnosis, liver transplantation, new hepatic decompensation, or study end (eFigure 1; see eTable 1 for codes). Included patients were required to have continuous enrollment with medical and pharmacy benefits during baseline and follow-up.
Liver disease etiology was classified as hepatitis B, hepatitis C, alcohol-related, nonalcoholic fatty liver disease (NAFLD; metabolic-associated fatty liver disease nomenclature may also apply to some patients in this groupCitation20), or Other on the basis of ICD-10-CM codes (see eTable 2 for criteria). Etiologies other than NAFLD and Other were not mutually exclusive. Patients were categorized as surveillance-naïve if they had no evidence of baseline HCC surveillance (abdominal ultrasound, serum des-gamma-carboxy-prothrombin, MRI, CT scan, or serum AFP).
Study variables
Patient demographic and clinical characteristics were measured during the baseline period. Comorbidities were assessed via Quan-Charlson comorbidity scoreCitation21 and Clinical Classifications Software from the Agency for Healthcare Research and QualityCitation22. Healthcare provider specialty was captured from claims with diagnosis codes for cirrhosis during the follow-up period.
HCC surveillance events
Abdominal ultrasounds, MRI scans, CT scans, and AFP tests during follow-up were considered to be HCC surveillance events. AFP tests within 14 days of ultrasound were considered to accompany the ultrasound. As a sensitivity analysis, AFP tests occurring within 60 days of ultrasound were also captured. Surveillance events that included any abdominal imaging were considered as complete, whereas AFP alone was incomplete.
Proportion of days covered
The proportion of follow-up time during which patients were up-to-date with recommended HCC surveillance was assessed using proportion of days covered (PDC), calculated as (days covered)/(days of follow-up)Citation23. Any abdominal imaging was considered to provide 6 months of days covered. PDC was analyzed separately for all patients and for patients with any evidence of follow-up surveillance.
Cost outcomes
Cost outcomes were analyzed during the first surveillance episode, defined as the first follow-up surveillance event plus all other surveillance events within the next 60 days. To focus on outpatient (planned) surveillance, costs were calculated only among patients without an inpatient stay or emergency room visit during follow-up. For each type of complete HCC surveillance event, total daily costs were calculated as the sum of all healthcare costs on the day surveillance occurred. Health plan–paid and patient-paid mean and median daily costs during the first surveillance episode were calculated for each type of surveillance event. For patients with ultrasound plus AFP, costs on the day of the AFP test were added to the costs on the day of the ultrasound if the tests occurred on different days. All combinations of surveillance types occurring on the same day were assessed; however, data are shown only for ultrasound plus AFP, as few other combinations were observed (15 other combinations, totaling only 1.2% of surveillance days, with no single combination exceeding 0.3%).
Yearly patient productivity costs due to surveillance-related healthcare encounters were estimated by assuming 4 working hours lost per outpatient visit to account for travel and appointment time, multiplied by the estimated average wage derived from US Bureau of Labor Statistics (BLS) dataCitation24 and the federal minimum wageCitation25. Costs were adjusted to 2018 US dollars.
Statistical analysis
Study variables were analyzed descriptively. Time to follow-up surveillance and the censoring-adjusted proportion of patients receiving follow-up surveillance were evaluated using Kaplan-Meier analysis. Proportional hazards models were used to evaluate the effect of baseline provider specialty on receipt of surveillance among surveillance-naïve patients, including interaction analysis of baseline provider specialty and liver disease etiology. An ordinary least squares model was used to evaluate the effect of baseline provider specialty on PDC among patients who had no surveillance during the baseline period but had at least 1 follow-up surveillance event. All multivariable models were adjusted for liver disease etiology, age group, sex, geographic region, presence of high-deductible health plan, Charlson comorbidity score category, and select comorbidities; the ordinary least squares model was also adjusted for follow-up length. All results were stratified by liver disease etiology. Comparative statistical testing among cirrhosis subgroups was not performed because these groups were not mutually exclusive. Statistical analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC). Statistical significance was defined as a 2-tailed p-value ≤ .05.
Results
Study sample
Of 70,350 potential patients with cirrhosis, 15,543 met study inclusion criteria (mean [SD] age 64.0 [11.1] years, 50.7% male, 58.2% Medicare insurance) (eFigure 2 and ). The most common cirrhosis etiologies were NAFLD (38.2%), HCV (29.1%), and alcohol-related (27.3%) (). About half of patients (52.3%) had evidence of HCC surveillance during the baseline period, with ultrasound (41.4%) and serum AFP (24.9%) being the most commonly used modalities. The proportion of patients with prior surveillance was lowest (43.4%) among those with alcohol-related cirrhosis and highest (67.6%) among those with HBV infection ().
Table 1. Patient demographic and clinical characteristics.
Mean (SD) follow-up time was 1.7 (1.4) years (median 1.3 years). More than half of patients (58.3%) received health care from a gastroenterologist (GI) during follow-up, with the highest proportion among patients with HBV (71.6%) or HCV (70.5%) infection and a lower proportion among those with alcohol-related cirrhosis (56.5%) ().
Study outcomes
HCC surveillance events
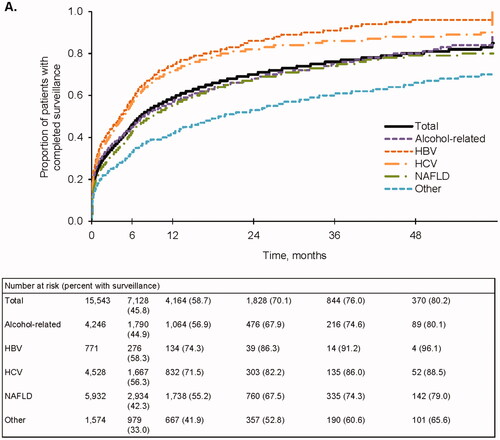
Overall, the proportions of patients who received any abdominal imaging during follow-up were 45.8% and 58.7% at 6 and 12 months, respectively (). Completion of abdominal imaging was higher at 6 and 12 months among patients with HBV (58.3% and 74.3%, respectively) and HCV infection (56.3% and 71.5%, respectively). In contrast, receipt of any abdominal imaging at 6 and 12 months was lower for nonviral etiologies (alcohol-related, 44.9% and 56.9%; NAFLD, 42.3% and 55.2%; Other, 33.0% and 41.9%, respectively).
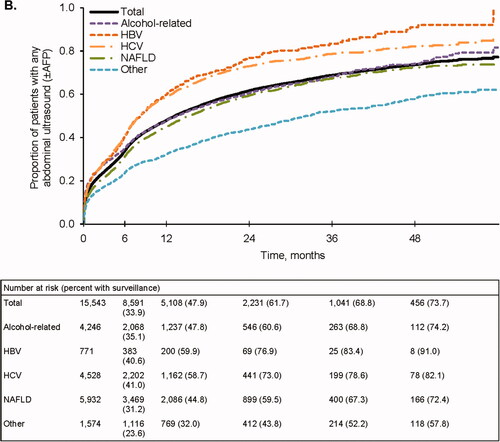
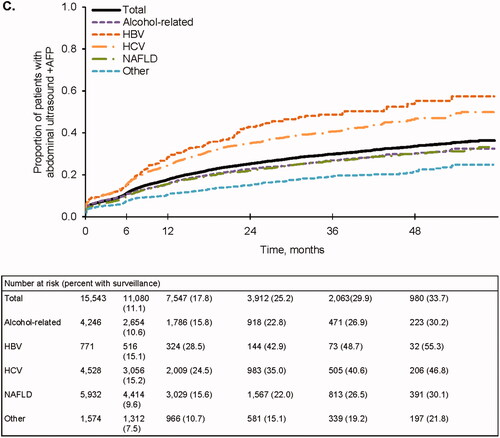
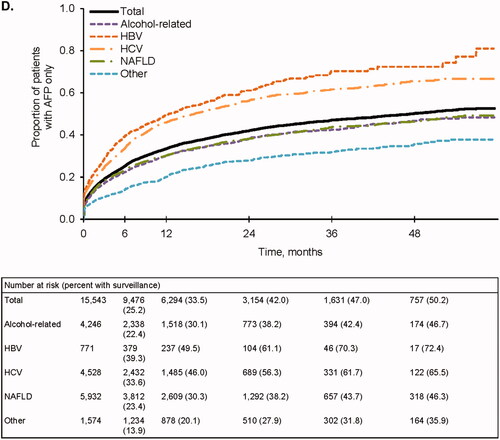
Figure 1. Follow-up surveillance events. (A) Completed surveillance events. Events that included any abdominal imaging were considered to be complete. (B) Any abdominal ultrasound (±AFP). (C) Abdominal ultrasound + AFP. (D) AFP only. AFP, alpha fetoprotein; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease.
Results were similar when considering ultrasound-based surveillance with or without AFP. Only 33.9% and 47.9% of patients completed an abdominal ultrasound at 6 and 12 months, respectively (), and few received an ultrasound with AFP (11.1% and 17.8% at 6 and 12 months, respectively) (). Ultrasound receipt at 6 and 12 months was higher among patients with HBV (40.6% and 59.9%, respectively) and HCV (41.0% and 58.7%, respectively) (). Patients with viral-related cirrhosis also had higher receipt of ultrasound with AFP at both time points (). In contrast, receipt of any abdominal ultrasound with or without AFP was lowest in those with nonviral etiologies (). A relatively large proportion of patients received AFP alone: 25.2% at 6 months and 33.5% at 12 months (). In a sensitivity analysis that increased the time permitted between ultrasounds and AFP tests from 14 days to 60 days, the overall proportion of patients receiving AFP alone remained substantial: 18.2% and 25.5% at 6 and 12 months, respectively.
Proportion of days covered
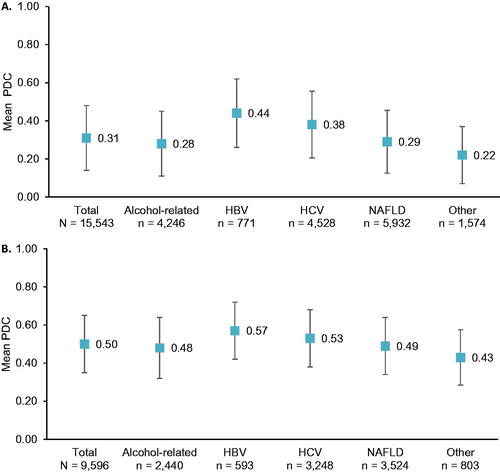
On average, patients were up-to-date with biannual imaging-based HCC surveillance for only 31% of the follow-up period (PDC 0.31, SD 0.34); this percentage was highest for the HBV and HCV subgroups (). Among individuals with ≥1 surveillance event during follow-up (n = 9596), the mean percentage of time up-to-date increased to 50% (PDC 0.50, SD 0.30) (), with similar PDCs across subgroups ().
Figure 2. Follow-up proportion of days covered. Error bars represent 1 standard deviation. (A) PDC among all patients (n = 15,543). (B) PDC among patients with follow-up surveillance (n = 9596). HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; PDC, proportion of days covered.
Factors associated with HCC surveillance
In multivariable-adjusted analyses among surveillance-naïve patients, those with HBV- and/or HCV-related cirrhosis vs other etiologies were more likely to receive surveillance during follow-up, as were those who had received GI care during the baseline period. Using patients with NAFLD and no GI visit as the reference group, the hazard ratio (HR) (95% CI) for surveillance receipt among GI and non-GI patients with viral cirrhosis was 1.55 (1.11–2.17) and 2.69 (1.77–4.09), respectively (). On the other hand, patients with alcohol-related cirrhosis were less likely to have surveillance receipt (HR 0.88, 95% CI 0.80–0.97 and HR 1.16, 95% CI 1.00–1.35 for non-GI and GI patients, respectively) (). The effect of receiving gastroenterology subspecialty care on PDC was not significant (estimate [95% CI] 1.435 [−0.470 to 3.339], p = .14) (eTable 3).
Table 2. Proportional hazards multivariable model of time to surveillance receipt among surveillance-naïve patients.
Cost outcomes
Total and patient-paid mean daily costs of outpatient surveillance for the overall patient population were highest for MRI only ($1580 and $300, respectively) and lowest for ultrasound only ($540 and $113, respectively) (). In light of the skewed distribution of costs, total median daily costs were lower than mean daily costs but remained highest for MRI only ($1065) and lowest for the US only ($214). Daily surveillance costs did not vary substantially by liver disease etiology.
Table 3. Costs for completed outpatient surveillance.
Overall, the estimated mean (SD) yearly patient productivity costs of outpatient surveillance using BLS wage data and the federal minimum wage were $1296 ($4852) and $376 ($1408), respectively (). Productivity costs were highest for patients with alcohol-related cirrhosis: $2480 ($8190) using BLS wage data and $720 ($2377) using the federal minimum wage. Median patient productivity costs were lower than mean costs but remained highest for alcohol-related cirrhosis ($456 and $132 using BLS wage data and the federal minimum wage, respectively).
Discussion
Although current guidelines from professional societies recommend HCC surveillance every 6 months for high-risk patients, only about one-third of patients in this study of individuals with continuous health insurance coverage had received an abdominal ultrasound (the primary HCC surveillance mechanism) after 6 months of follow-up, and less than half of patients had received any abdominal imaging at all. Moreover, a substantial number of patients received AFP testing alone, which is not a recommended HCC surveillance strategy.
Receipt of guideline-recommended surveillance was generally highest among patients in the HBV and HCV subgroups. This could be due in part to referral bias, as patients diagnosed with a viral infection and potentially receiving drug therapy may be more motivated to follow through with planned surveillance. The HBV and HCV subgroups also had the highest percentages of patients who saw a gastroenterologist during follow-up: approximately 71%, compared with 53%–57% in the remaining subgroups. This is in line with prior studies that observed better HCC surveillance among patients receiving care from a gastroenterologist or hepatologistCitation11–13,Citation26. Taken together, these findings suggest that patients with cirrhosis may benefit from being referred to and monitored by a specialist, which may also help improve adherence to other recommended care such as endoscopy for esophageal varices surveillanceCitation27.
However, connection with a specialist may not be possible for all patients, and many individuals with cirrhosis are not currently managed by gastroenterologists or hepatologistsCitation28. Instead, a substantial portion of such care is handled by primary care providers (PCPs), a practice that may become even more common as the prevalence of cirrhosis continues to riseCitation29. Improving PCP awareness of the standard of care for cirrhosis is critical to increasing HCC surveillance utilization among patients without access to a specialist, as prior studies have shown substantial gaps in PCP understanding of the natural history of HCC and the availability of recommended surveillance methodsCitation30–32. Interventions designed to help PCPs identify patients eligible for HCC surveillance, including educational programs as well as clinical reminders implemented via electronic medical records, have been shown to significantly increase surveillance receipt compared with usual careCitation33–36. Interventions that target patients directly have also been suggested. In a multicenter randomized controlled trial, an outreach program in which eligible patients received letters and phone reminders recommending that they undergo HCC surveillance resulted in significantly higher rates of adherence to HCC surveillance recommendationsCitation37.
The present analysis also reveals additional patient-side barriers to HCC surveillance. Although the majority of surveillance-related costs were paid by health plans, patients’ out-of-pocket expenses remained high, particularly for MRI and CT. Productivity costs due to time spent on outpatient surveillance visits were also substantial. These findings are congruent with survey data indicating that patients with cirrhosis commonly cite concerns about the costs of surveillance and related transportation as barriers to adherenceCitation11,Citation30,Citation31,Citation38. These patient-reported barriers are associated with lower rates of surveillance in clinical practice and may help inform interventions to increase HCC surveillance utilizationCitation38.
The low surveillance rates and high patient-paid costs observed in this study may have been affected by insurance company denials of authorization and/or payment, which has been cited by providers as a barrierCitation39. Although the role of insurance denials in HCC surveillance receipt is not well studied, patient insurance status has been shown to have an impact; for example, coverage through a preferred provider organization is positively associated with surveillance, while coverage through a health maintenance organization is negatively associatedCitation40,Citation41. These findings suggest that the effect of insurance limitations on surveillance receipt merits further investigation. Interventions designed to help patients navigate the management of their chronic disease, which have shown promise for helping those with chronic HCV obtain prior authorization approval for antiviral treatmentCitation42, may also be worth considering.
Among possible approaches to improving HCC surveillance effectiveness, the development of new blood-based biomarkers with sufficient sensitivity and specificity for surveillance is considered to have high potentialCitation17. The present findings support this idea, as the high proportion of patients who received AFP testing only—despite it not being a guideline-recommended HCC surveillance mechanism—may reflect patient and provider comfort with blood-based screening tests, which tend to be low-cost and require minimal time commitment and preparation relative to imaging-based screeningCitation43. Recently, a novel multimarker blood test was shown to have higher sensitivity for detecting early-stage HCC than ultrasound alone and similar sensitivity to ultrasound plus AFP, while maintaining high specificityCitation44,Citation45. These results bode well for a future in which blood-based testing is used to facilitate patient access to effective and relatively low-cost HCC screening while holding the false positive rate at a level similar to or lower than that of currently recommended modalities.
Study limitations
This study has several limitations. First, the presence of a diagnosis code on a claim is not proof of disease, as codes may have been entered incorrectly or included as rule-out diagnoses. Patient misidentification was minimized by requiring ≥2 separate non-diagnostic claims for cirrhosis during the identification period; however, this may have caused surveillance to be overestimated, as patients with only 1 cirrhosis code were excluded. Second, while blood tests are generally less time-intensive than imaging-based screening, patient productivity cost calculations in this study estimated 4 work hours lost per encounter for all surveillance methods to help account for factors such as travel time and work hours lost by individuals providing transportation. In addition, it was not possible for this study to distinguish the ancillary costs of other services that occurred on the same day as the AFP laboratory test (e.g. phlebotomy services). Together, these factors may have led to the overestimation of AFP testing costs, although the extent of this overestimation may be variable. While the present study was unable to separately detail costs attributable to the 2 Current Procedural Terminology codes used to identify AFP testing (one for serum AFP, another for total AFP), publicly available reimbursement data show divergent costs that vary by code and geographic region; for example, Medicare reimbursements for serum AFP vs total AFP were $17 vs $64, and costs for serum AFP in some areas ranged from $22 to $212Citation46,Citation47. Third, variations in HCC surveillance receipt by treating institution were not examined in this analysis; however, existing evidence, as well as our own clinical experience, suggests that patient- and provider-level barriers to surveillance are substantially more impactful than barriers at the hospital levelCitation15,Citation48. Finally, because this analysis was conducted in a US population with commercial or Medicare insurance, study results may not be generalizable to other populations (e.g. uninsured patients or those outside the US).
Conclusion
HCC surveillance was underutilized among patients with cirrhosis of all etiologies but was poorest among those with nonviral liver diseases and those not receiving GI care. The economic burden carried by patients due to both out-of-pocket expenses for surveillance and productivity lost to surveillance-related healthcare encounters was substantial. In addition to better awareness of HCC surveillance guidelines among non-specialists, the development of surveillance methods that reduce patient burden may help mitigate existing gaps.
Transparency
Declaration of funding
This work was funded by Exact Sciences Corporation.
Declaration of financial/other relationships
Burak Ozbay is an employee of and owns stock in Exact Sciences Corporation. Nicole Engel-Nitz and Tim Bancroft are employees of Optum, which was contracted by Exact Sciences Corporation to conduct this study. Mindie Nguyen has received grants from B.K. Kee Foundation, Enanta Pharmaceuticals Inc, Gilead Sciences Inc, Glycotest, National Cancer Institute, Pfizer, and Vir Biotechnology; and has served as a consultant for Bayer, Eisai Co Ltd, Eli Lilly and Company, Exact Sciences Corporation, Gilead Sciences, Intercept Pharmaceuticals, Janssen, Laboratory of Advanced Medicine, Novartis, and Spring Bank Pharmaceuticals. Lewis Roberts has received grants from ARIAD Pharmaceuticals, Bayer, BTG International Inc, Exact Sciences Corporation, Gilead Sciences Inc, and Glycotest; has received royalties from Five Prime Therapeutics; has received payment for presentations or educational events from AstraZeneca Pharmaceuticals LP, Clinical Care Options, Envision Communications, Genentech Inc, The Lynx Group LLC, MJH Life Sciences, and Pontifax Venture Capital; and has served as a consultant or on advisory boards for AstraZeneca Pharmaceuticals LP, Bayer, Clinical Care Options, Exact Sciences Corporation, Genentech Inc, Gilead Sciences Inc, GRAIL, Tavec Inc, MJH Life Sciences, Pontifax Venture Capital, and QED Therapeutics. Amit Singal has served as a consultant or on advisory boards for Bayer, Exact Sciences Corporation, Glycotest, GRAIL, Roche, and Wako Diagnostics. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Mindie Nguyen, Lewis Roberts, Nicole Engel-Nitz, Burak Ozbay, and Amit Singal conceptualized and designed the study. Nicole Engel-Nitz and Tim Bancroft analyzed the data. All authors interpreted the data, critically revised the manuscript for important intellectual content, and approved the final version for publication.
Supplement_18Apr2022.docx
Download MS Word (65.2 KB)Acknowledgements
The authors thank Susan Peckous, MA (Optum) for project management; Feng Cao, PhD, Randy Gerdes, and Lynn Wacha (all of Optum) for programming the analytic dataset; Lee Brekke, PhD (Optum) for consultation on statistical techniques; and Bret Gitar, MS and Kevin Sundquist, MS (both of Optum) for verification of the analysis. Medical writing services were provided by Yvette Edmonds, PhD (Optum) and contracted by Exact Sciences Corporation.
References
- National Cancer Institute. Cancer stat facts: Liver and intrahepatic bile duct cancer. 2020. [cited 2020 July]. Available at: https://seer.cancer.gov/statfacts/html/livibd.html.
- Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017. JAMA Oncol. 2019;5(12):1749–1769.
- Noone A, Howlader N, Krapcho M, et al. Seer cancer statistics review, 1975–2017. 2020. [cited 2020 July]. Available at: https://seer.cancer.gov/csr/1975_2017/.
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380.
- Abd El Aziz MA, Facciorusso A, Nayfeh T, et al. Immune checkpoint inhibitors for unresectable hepatocellular carcinoma. Vaccines. 2020;8(4):616.
- Covey AM. Hepatocellular carcinoma: updates to screening and diagnosis. J Natl Compr Canc Netw. 2018;16(5S):663–665.
- Simmons O, Fetzer DT, Yokoo T, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45(1):169–177.
- Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154(6):1706–1718.e1701.
- Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72(2):250–261.
- Singal AG, Yopp ACS, Packer M, et al. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861–867.
- Goldberg DS, Taddei TH, Serper M, et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology. 2017;65(3):864–874.
- Ahmed Mohammed HA, Yang JD, Giama NH, et al. Factors influencing surveillance for hepatocellular carcinoma in patients with liver cirrhosis. Liver Cancer. 2017;6(2):126–136.
- Singal AG, Tiro J, Li X, et al. Hepatocellular carcinoma surveillance among patients with cirrhosis in a population-based integrated health care delivery system. J Clin Gastroenterol. 2017;51(7):650–655.
- Tran SA, Le A, Zhao C, et al. Rate of hepatocellular carcinoma surveillance remains low for a large, real-life cohort of patients with hepatitis C cirrhosis. BMJ Open Gastroenterol. 2018;5(1):e000192.
- Wolf E, Rich NE, Marrero JA, et al. Utilization of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta-analysis. Hepatology. 2021;73(2):713–725.
- Zhao C, Xing F, Yeo YH, et al. Only one-third of hepatocellular carcinoma cases are diagnosed via screening or surveillance: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2020;32(3):406–419.
- Onyirioha K, Mittal S, Singal AG. Is hepatocellular carcinoma surveillance in high-risk populations effective? Hepat Oncol. 2020;7(3):HEP25.
- Optum, Inc. Optum claims data. 2021. [cited 2021 July 27]. Available from: https://www.optum.com/business/solutions/life-sciences/real-world-data/claims-data.html.
- Whyte JL, Engel-Nitz NM, Teitelbaum A, et al. An evaluation of algorithms for identifying metastatic breast, lung, or colorectal cancer in administrative claims data. Med Care. 2015;53(7):e49-57–e57.
- Eslam M, Sanyal AJ, George J. International consensus P. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1991.
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- Clinical Classifications Software (CCS) for ICD-9-CM/ICD-10-CM. Agency for healthcare research and quality. Rockville (MD); 2020. Available from: https://www.hcup-us.ahrq.gov/tools_software.jsp
- Goldberg DS, Valderrama A, Kamalakar R, et al. Hepatocellular carcinoma surveillance among cirrhotic patients with commercial health insurance. J Clin Gastroenterol. 2016;50(3):258–265.
- US Department of Labor, Bureau of Labor Statistics. National occupational employment and wage estimates. [cited April 2017]. Available from: https://www.bls.gov/oes/current/oes_nat.htm#00-0000.
- US Department of Labor. Minimum wage; 2017. Available from: https://www.dol.gov/agencies/whd/minimum-wage.
- Gaba RC, Kallwitz ER, Parvinian A, et al. Imaging surveillance and multidisciplinary review improves curative therapy access and survival in HCC patients. Ann Hepatol. 2013;12(5):766–773.
- Kanwal F, Tapper EB, Ho C, et al. Development of quality measures in cirrhosis by the practice metrics committee of the American Association for the study of liver diseases. Hepatology. 2019;69(4):1787–1797.
- Mellinger JL, Moser S, Welsh DE, et al. Access to subspecialty care and survival among patients with liver disease. Am J Gastroenterol. 2016;111(6):838–844.
- Beste LA, Leipertz SL, Green PK, et al. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471–1482 e1475. quiz e1417-1478.
- Beste LA, Harp BK, Blais RK, et al. Primary care providers report challenges to cirrhosis management and specialty care coordination. Dig Dis Sci. 2015;60(9):2628–2635.
- Dalton-Fitzgerald E, Tiro J, Kandunoori P, et al. Practice patterns and attitudes of primary care providers and barriers to surveillance of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(4):791–798 e791.
- Simmons OL, Feng Y, Parikh ND, et al. Primary care provider practice patterns and barriers to hepatocellular carcinoma surveillance. Clin Gastroenterol Hepatol. 2019;17(4):766–773.
- Kennedy NA, Rodgers A, Altus R, et al. Optimisation of hepatocellular carcinoma surveillance in patients with viral hepatitis: a quality improvement study. Intern Med J. 2013;43(7):772–777.
- Rogal SS, Yakovchenko V, Gonzalez R, et al. The hepatic innovation team collaborative: a successful population-based approach to hepatocellular carcinoma surveillance. Cancers. 2021;13(9):2251.
- Del Poggio P, Olmi S, Ciccarese F, et al. A training program for primary care physicians improves the effectiveness of ultrasound surveillance of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2015;27(9):1103–1108.
- Beste LA, Ioannou GN, Yang Y, et al. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin Gastroenterol Hepatol. 2015;13(1):172–179.
- Singal AG, Reddy S, Radadiya Aka Patel H, et al. Multicenter randomized clinical trial of a mailed outreach strategy for hepatocellular carcinoma surveillance. Clin Gastroenterol Hepatol. 2021. DOI:10.1016/j.cgh.2021.12.014
- Singal AG, Tiro JA, Murphy CC, et al. Patient-reported barriers are associated with receipt of hepatocellular carcinoma surveillance in a multicenter cohort of patients with cirrhosis. Clin Gastroenterol Hepatol. 2021;19(5):987–995.e1.
- Beal EW, Gorji L, Volney J, et al. Provider- and system-level barriers to surveillance for hepatocellular carcinoma among patients with chronic liver disease. Abstract presented at: ASCO Annual Meeting, Chicago, IL; June 3-7, 2022. J Clin Oncol. 2022;40(4_suppl):404–404.
- Yeo YH, Hwang J, Jeong D, et al. Surveillance of patients with cirrhosis remains suboptimal in the United States. J Hepatol. 2021;75(4):856–864.
- Goldberg DS, Valderrama A, Kamalakar R, et al. Hepatocellular carcinoma surveillance rates in commercially insured patients with noncirrhotic chronic hepatitis B. J Viral Hepat. 2015;22(9):727–736.
- Vu TM, Toribio W, Riazi F, et al. Increasing access to hepatitis C virus medications: a program model using patient navigators and specialty pharmacy to obtain prior authorization approval. JMCP. 2018;24(4):329–333.
- Woolen SA, Singal AG, Davenport MS, et al. Patient preferences for hepatocellular carcinoma surveillance parameters. Clin Gastroenterol Hepatol. 2022;20(1):204–215.e6.
- Chalasani NP, Ramasubramanian TS, Bhattacharya A, et al. A novel Blood-Based panel of methylated DNA and protein markers for detection of early-stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2021;19(12):2597–2605 e2594.
- Singal AG, Haaland B, Parikh ND, et al. Comparison of a multitarget blood test to ultrasound and alpha-fetoprotein for hepatocellular carcinoma surveillance: results of a network meta-analysis. Hepatol Commun. 2022. DOI:10.1002/hep4.2045
- Centers for Medicare and Medicaid Services. Clinical laboratory fee schedule. 2021. [cited 2021 Feb 24]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched.
- Maine Health Data Organization and Maine Quality Forum. Compare Maine health costs & quality. 2021. [cited 2021 Feb 24]. Available from: https://www.comparemaine.org/.
- Beal EW, Owen M, McNamara M, et al. Patient-, provider-, and system-level barriers to surveillance for hepatocellular carcinoma in high-risk patients in the USA: a scoping review. J Gastrointest Canc. 2022.