Abstract
Objective
This study used the latest available data cuts from the CARTITUDE-1 and KarMMa clinical trials to update previously published matching-adjusted indirect treatment comparisons (MAICs) assessing the comparative efficacy of ciltacabtagene autoleucel (cilta-cel) versus the FDA-approved idecabtagene vicleucel (ide-cel) dose range of 300 to 450 × 106 CAR-positive T-cells in the treatment of patients with relapsed or refractory multiple myeloma (RRMM) who were previously treated with a proteasome inhibitor, an immunomodulatory drug, and an anti-CD38 monoclonal antibody (i.e. triple-class exposed).
Methods
MAICs were performed with the latest available individual patient data for cilta-cel (CARTITUDE-1) and published summary-level data for ide-cel (KarMMa). The analyses included treated patients from CARTITUDE-1 who satisfied the eligibility criteria for KarMMa. The MAIC adjusted for unbalanced baseline covariates of prognostic significance identified in the literature and by clinical expertise. Comparative efficacy was assessed for overall response rate (ORR), complete response or better (≥CR) rate, duration of response (DoR), progression-free survival (PFS), and overall survival (OS).
Results
Cilta-cel was associated with statistically significantly improved ORR (odds ratio [OR]: 94.93 [95% confidence interval [CI]: 21.86, 412.25; p < .0001]; relative risk [RR]: 1.34), ≥CR rate (OR: 5.65 [95% CI: 2.51, 12.69; p < .0001]; RR: 2.23), DoR (hazard ratio [HR]: 0.52 [95% CI: 0.30, 0.88; p = .0152]), PFS, (HR: 0.38 [95% CI: 0.24, 0.62; p < .0001]), and OS (HR: 0.43 [95% CI: 0.22, 0.88; p = .0200]) compared with ide-cel.
Conclusions
These analyses demonstrate improved efficacy with cilta-cel versus ide-cel for all outcomes over longer follow-up and highlight its therapeutic potential in triple-class exposed RRMM patients.
Introduction
Multiple myeloma (MM) is a highly heterogenous cancer characterized by an excess of monoclonal (M) proteins and rapid growth of malignant plasma cellsCitation1. Despite many advancements in therapy with improved responses and survival, MM remains an incurable malignancy and most patients develop relapsed or refractory myeloma (RRMM). To address the need for new, efficacious treatment options, two novel chimeric antigen receptor T-cell (CAR-T) products have been introduced for the treatment of patients with RRMM.
Ciltacabtagene autoleucel (cilta-cel; JNJ-68284528) and idecabtagene vicleucel (ide-cel; bb2121) are two B-cell maturation antigen (BCMA)-targeted CAR-T therapies that have been approved by the Food and Drug Administration (FDA) and European Medicine Agency (EMA) in the treatment of RRMMCitation1–4. Ide-cel is a second-generation autologous CAR-T therapy with an extracellular anti-BCMA single-chain variable fragment, a 4-1BB costimulatory domain and CD3ζ signaling domain. Cilta-cel is unique in that it has two BMCA-specific single-binding domains, a 4-1BB costimulatory domain, and a CD3ζ signaling cytoplasmic domain. Both therapies have been evaluated in the treatment of patients who have previously received a proteasome inhibitor (PI), an immunomodulatory drug (IMiD), and a monoclonal antibody (MoAB) (i.e. triple-class exposed), with cilta-cel being evaluated in the CARTITUDE-1 trialCitation5 and ide-cel being evaluated in the KarMMa trialCitation6. However, these treatments have not been compared directly in any prospective clinical trial.
Previously, results of matching-adjusted indirect treatment comparisons (MAICs) assessing the comparative efficacy of cilta-cel versus ide-cel (FDA-approved dose range of 300 to 450 × 106 CAR-positive T-cells) were published that demonstrated improved efficacy with cilta-cel versus ide-cel.Citation7 However, new data cuts have since become available for both CARTITUDE-1 and KarMMa. Herein, we present updated MAIC results of cilta-cel versus ide-cel using longer-term follow-up data.
Methods
Data sources
Details of the CARTITUDE-1 and KarMMa clinical trials have been previously published in the original publication,Citation7 however, the current MAICs used updated data cuts from both studies (). The treated population of CARTITUDE-1 consisted of 97 patients who received infusions of cilta-cel (median [range] dose: 0.71 × 106 [0.51–0.95 × 106] CAR-T cells/kg) after lymphodepletionCitation8. The data cut-off for CARTITUDE-1 was January 2022 and the median follow-up duration was 27.7 months. The comparator population of interest for the present study consisted of 124 patients from KarMMa who underwent leukapheresis for the 300 × 106 and 450 × 106 CAR + T cells dose cohorts and received an initial infusion of ide-cel after lymphodepletionCitation9. Although an additional four patients were treated in the 150 × 106 CAR-T cells cohortCitation9, this dose level has not been approved by the FDA and was therefore not included in the main analysis. The latest available data were used for the FDA-approved dose cohort (300 to 450 × 106 CAR-T cells; N = 124) of KarMMa depending on the outcome (). For overall response rate (ORR) and complete response or better (≥CR) rate, the data cut-off was 21 December 2020 for which the median follow-up was 24.8 monthsCitation10–12. For duration of response (DoR) and progression-free survival (PFS), the data cut-off was 7 April 2020 with median follow-up duration of 15.4 monthsCitation13. For overall survival (OS), the data cut-off was January 2020, with median follow-up of 13.3 monthsCitation9. Given that DoR, PFS, and OS were not available for the FDA-approved doses from the latest published data cut for KarMMaCitation10–12, sensitivity analyses were performed for these outcomes using the latest available data (median follow-up 24.8 months) from the “all doses” cohort (150 to 450 × 106 CAR-T cells; N = 128)Citation10–12.
Table 1. Availability of data from CARTITUDE-1 and KarMMa by outcome.
Outcomes
Five efficacy outcomes were assessed in this analysis: ORR, ≥CR rate, DoR, PFS, and OS. For detailed outcome definitions, please refer to the original publicationCitation7.
Statistical analyses
Matching-adjusted indirect comparison
For details on the MAIC methods please refer to the original publicationCitation7. In summary, individual patient data from CARTITUDE-1 and published summary-level data from KarMMa were used to estimate the relative effect of cilta-cel versus ide-celCitation9–15. To adjust for imbalances in patient characteristics of prognostic significance, infused patients of CARTITUDE-1 who satisfied the eligibility criteria of KarMMa were reweighted to match the distribution of prognostic factors in KarMMa. To reflect the impact of weighting, the effective sample size (ESS) was calculatedCitation16.
Comparative efficacy for cilta-cel versus ide-cel was estimated for ORR and ≥ CR rate with odds ratios (ORs) and 95% confidence intervals (CIs) using weighted logistic regression. Relative risks (RRs) were also calculated as the ratio of response rates for cilta-cel versus ide-cel. For DoR, PFS, and OS, hazard ratios (HRs) and 95% CIs were estimated using a weighted Cox proportional hazards modelCitation17. All analyses were conducted based on the methods developed by Signorovitch et alCitation18. and were in line with the National Institute of Health and Care Excellence (NICE) Evidence Synthesis Technical Support Document SeriesCitation19.
Identification and rank ordering of prognostic factors
As in the original publication, the base case scenario adjusted for refractory status, cytogenetic profile, revised International Staging System (R-ISS) stage, and all plasmacytomasCitation7. For details on the identification and ranking of prognostic factors, please refer to the original publicationCitation7.
Additional sensitivity analyses were performed, which adjusted for all commonly available rank-ordered variables (adding number of prior LOTs, time since MM diagnosis, age, prior stem cell transplant, ECOG status, and sex).
Research ethics statement
The CARTITUDE-1 trial protocol was reviewed and approved by an independent ethics committee/institutional review board (IEC/IRB) at all participating sites. All patients participating in the trial provided written informed consent. Similarly, the KarMMa trial protocol was approved by local or independent IRBs or ethics committees at participating sites and all patients provided written informed consent.Citation9 The current analyses were conducted in accordance with a protocol and statistical analysis plan developed prior to the start of data analysis.
Results
Population alignment
The baseline characteristics of the 97 treated patients in CARTITUDE-1 and the 124 patients in KarMMa who underwent infusion for both dose cohorts remained unchanged from the original publicationCitation7. For details of adjustment and the resulting ESS in each population, please refer to Supplementary Table A.1, adapted from the original publicationCitation7.
Overall response rate and ≥ complete response rate
For both the unadjusted and adjusted comparisons, the observed response rates, and the relative treatment effect estimates (RRs and ORs) are summarized in .
Table 2. Response rates and comparative efficacy for cilta-cel versus ide-cel.
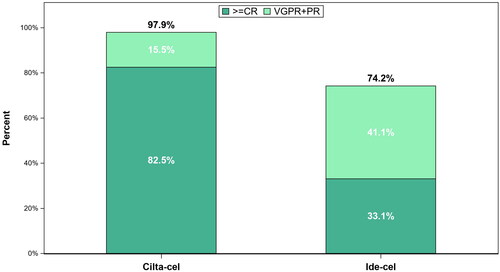
Prior to adjustment, 98% of patients who received cilta-cel responded and 83% achieved ≥ CR (). In comparison, 74% of patients treated with ide-cel responded and 33% achieved ≥ CR (). After base case adjustment, patients treated with cilta-cel were 1.3-fold more likely to respond (OR: 94.93 [95% CI: 21.86, 412.25; p < .0001]; RR: 1.34) and 2.2-fold more likely to achieve ≥ CR (OR: 5.65 [95% CI: 2.51, 12.69; p < .0001]; RR: 2.23) compared to patients treated with ide-cel.
Duration of response
For both the unadjusted and adjusted comparisons, the relative treatment effect estimates (HRs) for DoR are summarized in .
Table 3. Estimated medians and comparative efficacy for cilta-cel versus ide-cel.
The unadjusted HR for DoR for cilta-cel versus ide-cel was 0.35 (95% CI: 0.23, 0.54; p < .0001) (). After base case adjustment, DoR was significantly longer for cilta-cel compared to ide-cel (HR: 0.52 [95% CI: 0.30, 0.88); p = .0152]).
Figure 2. Observed (unadjusted) and adjusted (base case) Kaplan–Meier plots of duration of response. Base case results adjusted for refractory status, cytogenetic profile, revised International Staging System stage, and all plasmacytomas. Abbreviations. CI, confidence interval; ESS, effective sample size; HR, hazard ratio.
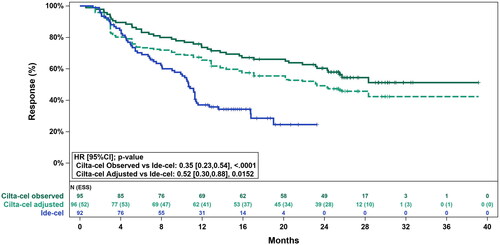
The Grambsch-Therneau testCitation20 for violation of the proportional hazards assumption was non-significant for the base case adjusted analysis (p = .69), indicating that the proportional hazards assumption was not violated.
Progression-free survival and overall survival
For both the unadjusted and adjusted comparisons, the relative treatment effect estimates (HRs) for PFS and OS are summarized in . PFS data were available for all 124 patients in KarMMa who underwent infusion for the 300 × 106 and 450 × 106 CAR + T cells dose cohorts, whereas OS data were only available in the public domain for 123 of these patients (the available data removed one patient who received a non-conforming product that did not meet the product release specifications for ide-cel)Citation14.
The unadjusted HR for cilta-cel versus ide-cel was 0.27 (95% CI: 0.18, 0.41; p < .0001) for PFS () and 0.31 (95% CI: 0.18, 0.52; p < .0001) for OS (). After base case adjustment, cilta-cel was statistically significantly longer compared to ide-cel for PFS (HR: 0.38 [95% CI: 0.24, 0.62; p < .0001]) and for OS (HR: 0.43 [95% CI: 0.22, 0.88; p = .0200]).
Figure 3. Observed (unadjusted) and adjusted (base case) Kaplan–Meier plots of progression-free survival. Base case results adjusted for refractory status, cytogenetic profile, revised International Staging System stage, and all plasmacytomas. Abbreviations. CI, confidence interval; ESS, effective sample size; HR, hazard ratio.
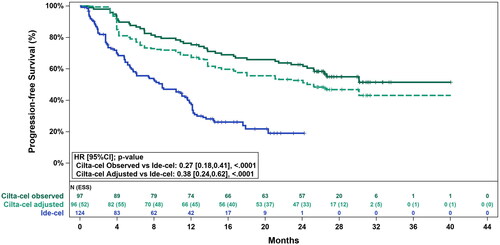
Figure 4. Observed (unadjusted) and adjusted (base case) Kaplan–Meier plots of overall survival. Base case results adjusted for refractory status, cytogenetic profile, revised International Staging System stage, and all plasmacytomas. Abbreviations. CI, confidence interval; ESS, effective sample size; HR, hazard ratio.
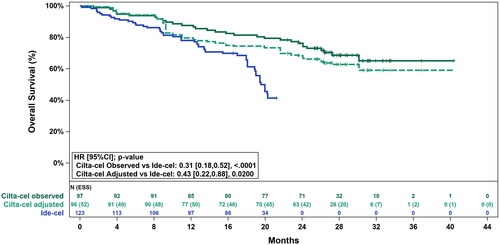
The Grambsch-Therneau testCitation20 for violation of the proportional hazards assumption was non-significant for both outcomes in the base case adjusted analysis (PFS: p = .63; OS: p = .07), indicating that the proportional hazard assumption was not violated.
Sensitivity analyses
The sensitivity analyses of the time-to-event outcomes using the “all doses” cohort from the latest data cut for KarMMa showed superior outcomes for cilta-cel versus ide-cel over longer follow-up and were consistent with the base case comparative estimates presented in for the FDA-approved doses. Comparative efficacy estimates for the “all doses” comparison were as follows: DoR: HR = 0.51 (95% CI: 0.32, 0.83; p = .0063); PFS: HR = 0.38 (95% CI: 0.25, 0.60; p < .0001); OS: HR = 0.58 (95% CI: 0.34, 1.00; p = .049).
The sensitivity analyses that incrementally adjusted for additional baseline characteristics (for details, please refer to the original publicationCitation7) showed superior outcomes for cilta-cel versus ide-cel. Comparative efficacy estimates for the all variables adjusted comparison were as follows: ORR: OR = 70.53 (95% CI: 12.23, 406.82; p < .0001); RR = 1.34; ≥CR rate: OR = 4.47 (95% CI: 1.95, 10.26; p = .0004); RR = 2.08; DoR: HR= 0.59 (95% CI: 0.34, 1.02; p = .0610); PFS: HR = 0.43 (95% CI: 0.26, 0.71; p = .0010); and OS: HR = 0.49 (95% CI: 0.23, 1.04; p = .0638).
Discussion
Despite an ever-changing treatment landscape, with several new drugs being approved in the past decadeCitation2,Citation21–25, MM remains a difficult to treat and incurable disease. Patients who relapse after receiving IMiDs, PIs, and anti-CD38 MoABs (i.e. triple-class exposed patients) have a particularly high unmet need for new safe and effective treatments. Given that head-to-head clinical trials evaluating the efficacy and safety of cilta-cel and ide-cel have not been conducted, MAICs are a valid alternative to estimate the relative treatment effect. Previous analyses have been published and showed improved efficacy with cilta-cel compared with ide-cel; however, follow-up data were limited to 18 months for cilta-cel and 13.3 months for ide-celCitation7. Since the publication of previous MAIC, longer-term follow-up data >2 years from CARTITUDE-1 and KarMMa became available, allowing an updated assessment of the comparative efficacy of cilta-cel and ide-cel to better inform healthcare decisions for physicians, patients, and policy makers.
The results from this updated analysis using longer-term follow-up data showed that cilta-cel continues to provide superior efficacy compared with ide-cel beyond the follow-up period included in the original MAIC publication. While the updated estimates were consistent with those in the original publication, the use of more mature data resulted in more precise confidence intervals around these estimates. A summary of the base case results from both the original publication and the present analyses is presented in Supplementary Table B.1. The base case analyses adjusting for the most clinically important factors (i.e. refractory status, cytogenetic profile, R-ISS stage, and all plasmacytomas) showed statistically significant results in favor of cilta-cel compared with ide-cel for all outcomes (ORR, ≥CR rate, DoR, PFS, and OS).
Patients treated with cilta-cel were 1.3-fold more likely to respond (ORR) and 2.2-fold more likely to achieve a ≥ CR than patients treated with ide-cel. The observed ≥ CR rate for CARTITUDE-1 in the present analysis (82.5%), notably exceeded the observed rate for patients in the 300 × 106 and 450 × 106 CAR + T cells dose cohorts in KarMMa (33.1%). These results were consistent with those published previously, with cilta-cel demonstrating an increased likelihood of a deeper response compared with ide-cel.
Furthermore, cilta-cel reduced the risk of disease progression or death by 48% in responders (DoR) and 62% in all patients (PFS) compared with ide-cel. These findings were consistent with the sensitivity analyses and the previously published MAIC.Citation7 After a median follow-up duration of 27.7 months, base case adjusted median DoR and median PFS for cilta-cel were 23.3 months and 25.2 months. These estimates exceed the median DoR of 10.7 months and the median PFS of 8.9 months observed for patients in the FDA-approved dose (300 × 106 and 450 × 106 CAR + T cells) cohorts in KarMMa. It should be noted that at the time of the present analyses, no DoR or PFS data were available for the FDA-approved dose cohorts from the latest published data cut for KarMMa (data cut-off was 21 December 2020 with median duration of follow-up of 24.8 months). Therefore, sensitivity analyses using the latest published data for KarMMa in the “all doses” cohort were performed and results were consistently in favor of cilta-cel. However, it should be noted that the median DoR and PFS for the “all doses” cohort of KarMMa from the latest published data cut (DoR: 11.0 [95% CI: 8.1, 12.3]; PFS: 8.8 [95% CI: 5.7, 11.6] months) are unlikely to differ significantly from those of the FDA-approved dose cohorts as only 4 of the 128 patients fall outside the FDA-approved dose range.
In the evaluation of OS, the current analysis found that cilta-cel significantly reduced the risk of death by 57% (p = .0200) compared with ide-cel. These results were consistent with those published previously, however the use of longer follow-up data provided a statistically significant result in favor of cilta-cel. It should be noted that at the time of the present analyses, no new OS data were available for the FDA-approved dose cohorts from KarMMa. In the absence of new data for the FDA-approved doses, sensitivity analyses using the latest published data for KarMMa (data cut-off was 21 December 2020 with median duration of follow-up of 24.8 months) in the “all doses” cohort were performed and results were consistent in favor of cilta-cel. However, it should be noted that the median OS for the “all doses” cohort of KarMMa from the latest published data cut (24.3 [95% CI: 19.9, 31.4] months) is unlikely to differ significantly from that of the FDA-approved dose cohorts as only 4 of the 128 patients fall outside the FDA-approved dose range.
Notable strengths of these analyses have been discussed previouslyCitation7 and remain applicable herein. However, an additional strength of these updated analyses is the use of the most contemporary data cut available for each outcome from both CARTITUDE-1 and KarMMa. The use of the most mature data available for the comparators lends additional credibility to the findings. Furthermore, although IPD was not available for KarMMa, reliable reconstruction of the observed IPD was possible through the simulation of IPD from published Kaplan–Meier plots for DoR, PFS, and OS using the validated Guyot methodCitation26–28. The Kaplan–Meier curves developed from simulated IPD were nearly identical to the published Kaplan–Meier curves, with potential slight discrepancies in the exact timings of censoring, which are expected to have only a minimal impact on the estimated HRs.
As in any comparison using non-randomized data, the potential for residual confounding cannot be disregarded. Furthermore, some clinically relevant prognostic factors were not collected in CARTITUDE-1 (e.g. 1q abnormalities) or reported in KarMMa (for details, please refer to the discussion in the original publicationCitation7) however, the most important factors, as identified in close consultation with multiple independent clinical experts, were accounted for. It should also be noted that while 45.2% of 73 patients who received bridging therapy in CARTITUDE-1 had a transient decrease in tumor burden (defined as change in serum M-protein, urine M-protein, or difference between involved and uninvolved free light chain) prior to cilta-cel infusion, a decrease in tumor burden does not equate to being a responder per IMWG criteria. No subjects in CARTITUDE-1 achieved CR while on bridging therapyCitation8. In the KarMMa study, the 4% of patients who received bridging therapy and had a confirmed partial or very good partial response as per investigator assessment also did not achieve a CRCitation9. Therefore, there is no indication that patients in CARTITUDE-1 had a different disease biology than those in KarMMa. Furthermore, as per trial protocol, bridging therapy must have been previously received in a prior line of therapy and therefore all patients who received bridging therapy had either progressed or been non-responsive to those same agents prior to enrollment in CARTITUDE-1.
Since the focus of the present study was to update previously published comparative efficacy analyses using the latest published data with longer follow-up from both studies, safety outcomes were not assessedCitation7. Future studies may be conducted including real-world data to address this data gap.
These updated analyses using the latest published data with longer follow-up from both studies were consistent with previously published comparative efficacy analyses in favor of cilta-cel compared with ide-cel in the treatment of triple-class exposed patients with RRMM, specifically in terms of response rates and survival outcomes. Future studies using real-world data may be used to fill this evidence gap and validate the findings from the current analyses.
Conclusion
The results of this updated MAIC analysis using longer follow-up data showed that the superior efficacy of cilta-cel compared with ide-cel was maintained for all outcomes studied (ORR, ≥CR rate, DoR, PFS, and OS). Overall, the results indicate that cilta-cel offers substantial clinical benefits for patients with triple-class exposed RRMM, greater than those provided by ide-cel.
Transparency
Declaration of funding
This work was supported by Janssen Pharmaceuticals and Legend Biotech.
Declaration of financial/other relationships
TM is a consultant for GlaxoSmithKline and receives research funding from Janssen, Amgen, and Sanofi. SZU receives grant support from Pharmacyclics, receives grant support and consulting fees from Amgen, Bristol-Myers Squibb, Celgene, Sanofi, Seattle Genetics, Janssen, Takeda, SkylineDx, Merck, and GlaxoSmithKline, and consulting fees from AbbVie, Karyopharm, and Mundipharma. JMS, TR, WD, TY, AB, SVS, JD, and SV are employed by Janssen and have restricted stock units and/or stock options. CCJ is employed by Janssen and is a consultant physician at the Memorial Sloan Kettering Cancer Center. LP and AG are employed by Legend Biotech, Piscataway, NJ, USA. MB and IAS are employed by EVERSANA, Burlington, ON, Canada, and AH is employed by EVERSANA, Chicago, IL, USA, which was contracted by Janssen to work on this project. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were responsible for study conception and design. JD, SVS were responsible for acquisition and analysis. All authors were responsible for drafting or reviewing the manuscript and approving the final version. All authors were responsible for interpretation of data and revising it critically.
Updated_KarMMa_pub_supplemental_appendix_2022_10_11_clean.docx
Download MS Word (129.1 KB)Acknowledgements
The CARTITUDE-1 study and these analyses were funded by Janssen Research & Development, LLC, and Legend Biotech, Inc. Medical writing support was provided by EVERSANA and funded by Janssen Global Services, LLC. The authors acknowledge the contribution of Eloquent Scientific Solutions for the editorial support.
Data availability statement
Requests for access to the CARTITUDE-1 trial study data may be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu. The data sharing policy of Janssen Pharmaceutical Companies is available at https://www.janssen.com/clinical-trials/transparency. For KarMMa study, a data sharing statement is available with the full text of the primary publication at NEJM.org.Citation12
References
- Janssen US. FDA approves CARVYKTI™ (ciltacabtagene autoleucel), Janssen’s first cell therapy, a BCMA-directed CAR-T immunotherapy for the treatment of patients with relapsed or refractory multiple myeloma [updated 2022 Feb 28; 2022 Mar 1]. Available from: https://www.janssen.com/us-fda-approves-carvykti-ciltacabtagene-autoleucel-janssens-first-cell-therapy-bcma-directed-car-t
- Bristol Myers Squibb. U.S. Food and Drug Administration approves Bristol Myers Squibb’s and bluebird bio’s Abecma (idecabtagene vicleucel), the first anti-BCMA CAR T cell therapy for relapsed or refractory multiple myeloma; 2021 [updated 2021 Mar 26; 2021 May 14]. Available from: https://news.bms.com/news/corporate-financial/2021/U.S.-Food-and-Drug-Administration-Approves-Bristol-Myers-Squibbs-and-bluebird-bios-Abecma-idecabtagene-vicleucel-the-First-Anti-BCMA-CAR-T-Cell-Therapy-for-Relapsed-or-Refractory-Multiple-Myeloma/default.aspx
- EMA. First cell-based gene therapy to treat adult patients with multiple myeloma [updated 2021 Jun 25; 2022 Jul 19]. Available from: https://www.ema.europa.eu/en/news/first-cell-based-gene-therapy-treat-adult-patients-multiple-myeloma
- Janssen. European Commission grants conditional approval of CARVYKTI® (ciltacabtagene autoleucel), Janssen’s first cell therapy, for the treatment of patients with relapsed and refractory multiple myeloma [updated 2022 May 26; 2022 Jul 19]. Available from: https://www.jnj.com/european-commission-grants-conditional-approval-of-carvykti-ciltacabtagene-autoleucel-janssens-first-cell-therapy-for-the-treatment-of-patients-with-relapsed-and-refractory-multiple-myeloma
- ClinicalTrials.gov. A study of JNJ-68284528, a chimeric antigen receptor T cell (CAR-T) therapy directed against B-cell maturation antigen (BCMA) in participants with relapsed or refractory multiple myeloma (CARTITUDE-1) [updated 2020 Dec 31; 2021 May 14]. Available from: https://clinicaltrials.gov/ct2/show/NCT03548207?term=NCT03548207&draw=2&rank=1
- ClinicalTrials.gov. Efficacy and safety study of bb2121 in subjects with relapsed and refractory multiple myeloma (KarMMa) [updated 2020 Jun 16; 2021 May 14]. Available from: https://clinicaltrials.gov/ct2/show/NCT03361748?term=NCT03361748&draw=2&rank=1
- Martin T, Usmani SZ, Schecter JM, et al. Matching-adjusted indirect comparison of efficacy outcomes for ciltacabtagene autoleucel in CARTITUDE-1 versus idecabtagene vicleucel in KarMMa for the treatment of patients with relapsed or refractory multiple myeloma. Curr Med Res Opin. 2021;37(10):1779–1788.
- Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a BCMA-directed CAR T-cell therapy in patients with relapsed/refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–324.
- Munshi NC, Anderson LD, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716.
- Anderson LD, Munshi NC, Shah N, et al. Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell therapy, in relapsed and refractory multiple myeloma: updated KarMMa results. J Clin Oncol. 2021;39(15_suppl):8016–8016.
- Anderson LD, Shah N, Jagannath S, et al. OAB-027: Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T-cell therapy, for the treatment of patients with relapsed and refractory multiple myeloma (RRMM): updated results from KarMMa. Clin Lymphoma Myeloma Leuk. 2021;21:S17–S18.
- Oriol A, San-Miguel J, Kansagra A, et al. Idecabtagene vicleucel (ide-cel, BB2121), a BCMA-directed CAR T cell therapy, in patients with relapsed and refractory multiple myeloma: Updated KarMMa results. Hemasphere. 2021;5(S2):476.
- EMA. Assessment report: ABEMCA. Amsterdam, The Netherlands: European Medicines Agency; 2021. Available from: https://www.ema.europa.eu/en/documents/assessment-report/abecma-epar-public-assessment-report_en.pdf
- Lin X. US FDA statistical review – ABECMA; 2020 [updated 2021 May 18; 2021 May 28]. Available from: https://www.fda.gov/vaccines-blood-biologics/abecma-idecabtagene-vicleucel
- Celgene Corporation. ABECMA (idecabtagene vicleucel) [package insert]. Summit (NJ): US Food and Drug Administration; 2021. Available from: https://www.fda.gov/vaccines-blood-biologics/abecma-idecabtagene-vicleucel
- Phillippo DM, Ades AE, Dias S, et al. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–211.
- SAS Institute Inc. SAS/STAT®14.3 user’s guide: the PHREG procedure. Cary (NC): SAS Institute Inc; 2017.
- Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947.
- Phillippo DA, Dias S, Palmer S, et al. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submission to NICE. Appendix D: worked example of MAIC and STC [updated 2016 Dec; 2021 May 14]. Available from: http://nicedsu.org.uk/wp-content/uploads/2017/05/Population-adjustment-TSD-FINAL.pdf
- Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526.
- Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(5):548–567.
- FDA.gov. FDA granted accelerated approval to belantamab mafodotin-blmf for multiple myeloma [updated 2020 Aug 6; 2021 May 31]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-granted-accelerated-approval-belantamab-mafodotin-blmf-multiple-myeloma
- Karyopharm Therapeutics Inc. Karyopharm announces FDA approval of XPOVIO™ (selinexor) for the treatment of patients with relapsed or refractory multiple myeloma [updated 2019 Jul 3; 2021 Jun 2]. Available from: https://investors.karyopharm.com/2019-07-03-Karyopharm-Announces-FDA-Approval-of-XPOVIO-TM-selinexor-for-the-Treatment-of-Patients-with-Relapsed-or-Refractory-Multiple-Myeloma
- Oncopeptides. FDA approves oncopeptides’ PEPAXTO® (melphalan flufenamide) for patients with relapsed or refractory multiple myeloma [updated 2021 Feb 26; 2021 Jun 2]. Available from: https://news.cision.com/oncopeptides-ab/r/fda-approves-oncopeptides–pepaxto––melphalan-flufenamide–for-patients-with-relapsed-or-refractor,c.3296082
- GlaxoSmithKline. FDA approves GSK’s BLENREP (belantamab mafodotin-blmf) for the treatment of patients with relapsed or refractory multiple myeloma [updated 2020 Aug 6; 2021 Jun 2]. Available from: https://www.gsk.com/en-gb/media/press-releases/fda-approves-gsk-s-blenrep-belantamab-mafodotin-blmf-for-the-treatment-of-patients-with-relapsed-or-refractory-multiple-myeloma/
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9.
- Saluja R, Cheng S, Santos KA, et al. Estimating hazard ratios from published Kaplan‐Meier survival curves: a methods validation study. Res Syn Meth. 2019;10(3):465–475.
- Latimer N. NICE DSU technical support document 14: undertaking survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data [updated 2013 Mar; 2021 Jun 4]. Available from: http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf