Abstract
Introduction
Misdiagnosis of bipolar I disorder (BP-I) as major depressive disorder (MDD) leads to increased healthcare resource utilization and costs. The cost-effectiveness of the Rapid Mood Screener (RMS), a tool to identify BP-I in patients with depressive symptoms, was assessed in patients diagnosed with MDD presenting with depressive episodes.
Methods
A decision-tree model of a hypothetical cohort of 1000 patients in a US health plan was used to estimate the number of correct diagnoses and overall total, direct healthcare costs over a 3-year timeframe for RMS-screened versus unscreened patients. Model inputs included the prevalence of BP-I in patients diagnosed with MDD, RMS sensitivity/specificity, and the cost of misdiagnosing BP-I as MDD.
Results
Screening with the RMS resulted in 171, 159, and 143 additional correct BP-I or MDD diagnoses at Years 1, 2, and 3, respectively. Total healthcare plan cost savings were $1279 per patient in Year 1. Cumulative cost savings per patient for RMS screening versus no RMS screening were $2307 over 2 years and $3011 over 3 years. Scenario analyses showed that the RMS would remain cost-saving assuming a lower prevalence of BP-I (20% or 10%) versus the base case (24.3%).
Conclusion
The RMS is a cost-effective tool to identify BP-I in patients who would otherwise be misdiagnosed with MDD. Screening with the RMS resulted in cost-savings over 3 years, with model results remaining robust even with lower prevalence of BP-I and reduced RMS sensitivity assumptions.
Introduction
Bipolar I disorder (BP-I) is a serious psychiatric illness characterized by manic, hypomanic, and depressive episodes and symptoms, which are accompanied by significant functional impairment, reduced quality of life for patients, and substantial economic implicationsCitation1–3. The current annual total cost of BP-I in the United States, including direct healthcare costs, direct non-healthcare costs, and indirect costs, is estimated to be approximately $200 billionCitation4,Citation5. Although mania is the hallmark symptom of BP-I, depression is the most common presenting mood episodeCitation6,Citation7, and the leading cause of morbidity and mortalityCitation8. Differentiating bipolar depression from major depressive disorder (MDD) is a challenging issue for clinicians. Some patients being treated for depression may actually have BP-I and many struggle for years before being properly diagnosedCitation9–11. As seen in a recent database analysis, misdiagnosis adds to the already substantial cost of BP-I since patients who were first misdiagnosed with MDD use significantly more healthcare resources and incur significantly higher healthcare costs than patients whose initial diagnosis of BP-I was correctCitation12. Adding to the complexity, patients with correctly diagnosed MDD can also transition to bipolar disorder over the course of their illness, with one long-term prospective study reporting that 1% of patients per year changed from a diagnosis of MDD to BP-I over 20 yearsCitation13 and other reports estimating even higher change ratesCitation14.
While some evidence exists that structural and functional MRI may be able to help distinguish between BP-I and unipolar depressionCitation15, routine clinical screening remains a prudent strategy for all patients who present with depressive symptoms or those who are diagnosed with MDD who develop significant affective symptoms over timeCitation8. The Rapid Mood Screener (RMS) is a self-administered screening tool that was developed to differentiate BP-I from MDD in patients with depressive symptoms (full version available at https://doi.org/10.1080/03007995.2020.1860358)Citation16. The pragmatic 6-item screening tool, which was validated in an observational study of patients with BP-I, consists of 3 items that screen for hallmark manic symptoms and 3 items that evaluate depressive characteristics that are more likely to indicate BP-I than MDD (e.g. earlier age of depression onset, prior negative response to antidepressant treatment, multiple depressive episodes). In the validation study, when 4 or more RMS items were endorsed (“yes”), sensitivity (the true positive rate) was 88% and specificity (the true negative rate) was 80%Citation16; as such, this threshold is considered a positive screen for BP-I. Although a screening tool does not provide a diagnosis, a positive screen alerts the clinician that diagnostic evaluation is needed to confirm the diagnosis. The RMS can be completed in less than 2 min during or outside of a clinical visit (e.g. online, via electronic medical record system, waiting room), making the freely available RMS a patient-friendly screener that can be easily integrated into clinical practice.
To evaluate the cost-effectiveness of RMS screening for patients diagnosed with MDD, we conducted a decision-tree analysis using RMS test characteristics and model inputs based on real-world data to compare screening with the RMS versus no screening.
Methods
Model overview
A decision-tree analysis was developed to evaluate outcomes and costs of screening for BP-I using the RMS versus no screening in patients diagnosed with MDD. No screening for bipolar disorder is the current standard of care in primary care practiceCitation17. The model assumed that screening was a one-time occurrence followed by a specialist visit for a positive screening result; all patients were followed up for up to 3 years. A patient’s diagnosis status was based on the underlying prevalence of misdiagnosis, and whether they were screened and had a specialist follow-up visit to confirm their diagnosis. Outcomes of interest were the number of correctly diagnosed patients with BP-I or MDD, and the cost per patient. Analyses were conducted from the perspective of a commercial third-party US payer.
Model structure
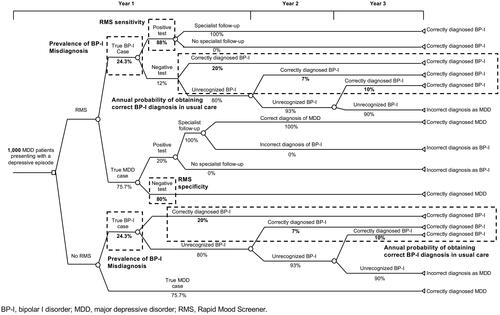
The model was structured based on a prior analysis of screening in BP-I using the Mood Disorder Questionnaire (MDQ)Citation18. Among all patients, there was an underlying true prevalence of BP-I misdiagnosis and correctly diagnosed MDD, and the population was apportioned into those who were screened for BP-I and those who were not (). In the screening branch of the model, patients completed the 6-item RMS. For all patients who screened positive on the RMS (i.e. true and false positives), it was assumed that each had a follow-up visit with a specialist to confirm the screening result, or identify a false positive and confirm an MDD diagnosis. Patients who are correctly diagnosed at any point were assumed to remain in that health state throughout the remainder of the model time horizon.
In the branch of the model representing patients who were not screened, the diagnosis of BP-I or MDD was made at the initial visit based on the probability of a primary correct diagnosis under a usual care condition. No patients in the no-screening arm were expected to be incorrectly diagnosed with BP-I. Patients receiving usual care without screening were assumed to be diagnosed at the rate consistent with standard diagnosing, with some correctly diagnosed cases each year. Correctly diagnosed patients (BP-I or MDD) in any year were assumed to remain in the same health state throughout the remainder of the model time horizon.
Base case model inputs
The model was populated with a hypothetical cohort of 1000 patients aged 18 years or older with a previous diagnosis of MDD (). Model inputs included BP-I prevalence among patients diagnosed with MDD and presenting with a depressive episode, RMS sensitivity and specificity, likelihood of correcting MDD misdiagnoses among patients with BP-I in usual care, and costs associated with screening, specialist referral, and each diagnosis (). The prevalence of BP-I among patients diagnosed with MDD was based on findings from a long-term prospective trial in patients with major mood disordersCitation13. As determined in the validation study, RMS sensitivity was 88% and specificity was 80%Citation16. RMS sensitivity indicates that 88% of patients with true BP-I would screen positive (i.e. true positive) for the disorder, while the remaining 12% of patients with BP-I would have an incorrect negative screen (i.e. false negative). RMS specificity indicates that 80% of patients with a correct MDD diagnosis would screen negative for BP-I on the RMS (i.e. true negative), while 20% of patients with a correct MDD diagnosis would have an incorrect positive screen (i.e. false positive). All positive RMS screens were assumed to lead to specialist follow-up and a correct diagnosis.
Table 1. Summary of epidemiology, RMS, and cost inputs.
Cost inputs included the cost of one-time specialist follow-up and the cost of each diagnostic scenario (i.e. incorrectly diagnosed MDD, correctly diagnosed MDD, correctly diagnosed BP-I, and incorrectly diagnosed with BP-I) (). Given that the RMS is a brief patient-reported questionnaire, administration costs were assumed to be $0. Mean annual costs for BP-I patients with an incorrect MDD diagnosis and for patients with a correct BP-I diagnosis were derived from an analysis of claims dataCitation12, which provided an estimate of mean costs in Year 1 and over approximately 2.5 years. To generate inputs for mean costs in Years 1, 2, and 3, an exponential regression model was constructed, with total costs over the entire claims analysis follow-up as the area under the cost per day curve; the annual cost of the second year was calculated as the area under the curve between Years 1 and 2 and between Years 2 and 3. All costs were expressed in 2021 USD.
Analyses
The total numbers of correctly and incorrectly diagnosed patients were estimated with the model. Costs were computed per patient per year overall and by diagnosis (i.e. correctly diagnosed MDD, correctly diagnosed BP-I, incorrectly diagnosed MDD). Total costs over 3 years and annually for Years 1, 2, and 3 were compared between patients screened with the RMS and those who were not screened. One-way sensitivity analyses were conducted to determine the impact of varying probabilities and cost inputs in the model. With the exception of BP-I prevalence, lower and upper boundaries used in sensitivity analysis were ±10% from base-case values; the prevalence of BP-I, a key model parameter, was varied from 20% to 25% based on a clinically plausible range. Finally, because a lower prevalence of BP-I among patients diagnosed with MDD impacts the cost of specialist referral due to potential false positive, a scenario analysis was conducted where the prevalence of BP-I was reduced to 10%, while all other model inputs were held constant. The model was programmed in Microsoft Excel.
Results
Base case results
In a health plan of 1000 patients diagnosed with MDD, 977 were correctly diagnosed using the RMS and 806 were correctly diagnosed without screening in Year 1, indicating that 171 additional patients were correctly diagnosed through screening (). In the second year, 159 additional patients were correctly diagnosed through screening with the RMS; in the third year, 143 additional patients were correctly diagnosed through screening.
Table 2. RMS screening versus no screening: number of correct diagnoses.
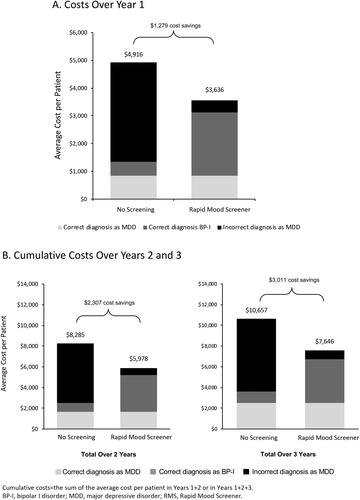
Compared with no screening, RMS screening resulted in a cost savings of $1279 per patient in the first year (). At the end of years 2 and 3, screening with the RMS resulted in cumulative savings of $2307 and $3011 per patient, respectively (). The cost of BP-I incorrectly diagnosed as MDD accounted for the majority of costs in the no-screening group ($3565 of the total $4916 per patient over 3 years) (). Of the total per-patient cost in the no-screening group over Years 2 and 3, incorrect MDD diagnosis accounted for $5743 of $8285, and $7030 of $10,657, respectively ().
One-way sensitivity and scenario analyses
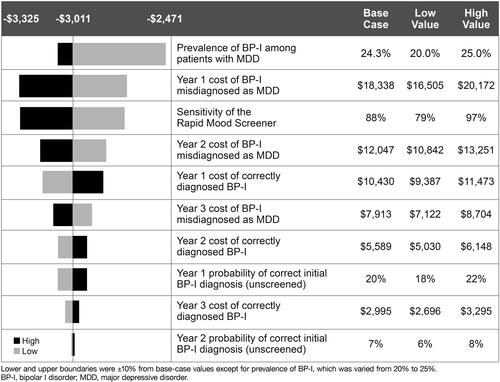
In the one-way sensitivity analysis, screening with the RMS remained cost-saving under varied input assumptions (). The most influential model input was the prevalence of BP-I among patients diagnosed with MDD. In a scenario analysis where the prevalence of BP-I was reduced to 10% and all other model inputs were held constant, cumulative 3-year costs were $5138 per person in the RMS screening group and $6353 per person in the no-screening group; total cost savings at BP-I prevalence of 10% was $1215 per person.
Discussion
Base case model results indicated that more patients with BP-I received a correct diagnosis with RMS screening compared with no screening over the 3-year timeframe of the model, resulting in cost savings. The largest difference was observed in the first year, with 171 more patients correctly diagnosed with screening versus no screening (977 vs 806). Over time, as more patients who were initially misdiagnosed received a correct diagnosis, the number of additional correct diagnoses decreased but still remained substantial, with 159 more patients correctly diagnosed at the end of Year 2 with RMS screening versus no screening, and 143 more patients correctly diagnosed at the end of Year 3. Cost savings of screening with the RMS were also greatest in the first year ($1279 per person), with cumulative savings of $2307 per person over 2 years and $3011 per person over 3 years. One-way sensitivity analysis and evaluation across other plausible scenarios indicated that utilizing the RMS remained cost-saving over a wide range of assumptions. Broadly speaking, our results confirmed earlier findings that screening for bipolar disorder among patients with depressive symptoms is cost-saving to the health systemCitation18.
Prevalence estimates for bipolar disorder in adult populations or in patients diagnosed with MDD are inconsistent in the literatureCitation19, suggesting that cost models should take this variability into account in order to achieve a comprehensive result. In our RMS evaluation, one-way sensitivity analysis showed that the prevalence of BP-I among patients with MDD had the most impact on the cost-effectiveness of RMS screening versus no screening. Namely, cost savings were still observed when the BP-I prevalence was reduced to 10%, with cumulative savings of $1215 per patient seen over three years. Other influential factors affecting cost in the one-way sensitivity analysis were the first-year cost of patients with BP-I who were misdiagnosed as MDD and the sensitivity of the RMS, with similar magnitudes of impact noted for each. Collectively, our results suggest that shifting even a small number of patients into the correct diagnosis saves money given that the cost of an incorrect MDD diagnosis far exceeds the cost of a correct BP-I diagnosis and there is little cost associated with screening.
While cost savings is one important aspect of using the RMS, it is also important for clinicians to be aware that it is a practical tool to use in a clinical practice setting. The RMS is a short and easy-to-use screener that can be administered by the clinician or patient, with easy scoring that makes the result immediately informative. Containing clearly worded items that screen for both manic and depressive symptoms, the RMS can assist a clinician in differentiating bipolar I disorder from MDD while helping to uncover difficult-to-identify manic symptoms. Appropriate in either a primary care or psychiatric setting, the RMS is not a diagnostic tool, but it can help a clinician determine when a full diagnostic work-up for bipolar I disorder is necessary. Clinical utility in addition to demonstrated cost savings combine to make the RMS a valuable and pragmatic addition to everyday clinical practice.
The strengths of our analysis included a validation process that followed established best practices to ensure the validity of the model and the RMS cost-effectiveness analyses. Internal validity was established through a robust quality control check that minimized the likelihood of programming errors, and validation steps included input data verification. External validity was tested by validation against a published model that evaluated the MDQCitation18, which is a widely recognized bipolar disorder screening toolCitation20, to ensure that similar results were generated if similar inputs were used across both models. Our analysis was subject to limitations related to model inputs, which were taken from the published literature and internal claims analysis and did not take into consideration costs associated with clinical variables (i.e. severity) or indirect medical costs (i.e. reduced absenteeism). Additionally, the annual diagnosis rates used in our analysis were based off results from a 2003 study; however, this was the most appropriate reference we could use in our analysis due to its study design. While it is possible that the true rate of correct diagnosis may be somewhat higher than what was observed in this older studyCitation9, our sensitivity analysis demonstrated cost savings even if we increased the rate of correct diagnosis to 80%. The RMS model also did not account for patient attrition, which was omitted for simplicity and the limited impact it would have given the short time frame of analysis. Further, patients with an MDD diagnosis and those referred for specialist follow-up were assumed to be correctly diagnosed, which may have underestimated the number of incorrect diagnoses. Finally, the results of our analysis may not be generalizable since costs for treating BP-I and MDD vary widely across payer types and our analysis was from the perspective of a commercial third-party payer in the US.
Conclusion
Although no screening is the current standard of care in bipolar disorder, a decision-tree analysis comparing the cost-effectiveness of screening for BP-I with the RMS versus no screening found that RMS screening resulted in more correctly diagnosed patients and total health plan cost savings among a hypothetical cohort of 1000 patients with MDD over a 3-year time horizon. Model outputs were most impacted by the prevalence of BP-I among patients diagnosed with MDD and the first-year cost of misdiagnosis in patients with BP-I. Relative to no screening, screening with the RMS remained cost-saving over 3 years regardless of assuming a true BP-I prevalence that was higher (base case, 24.3%) or lower (scenario analysis, 10%). Model outcomes indicate that one-time screening with the RMS may be a cost-effective way to identify patients with BP-I who would otherwise be misdiagnosed with MDD, suggesting that screening could lead to better treatment and patient health outcomes in this complex and frequently misdiagnosed illness.
Transparency
Author contributions
All authors participated in the writing, editing, and critical revision for intellectual content, and approval of the final version of this manuscript. All authors met ICMJE authorship criteria and agree to be accountable for all aspects of the work. Neither honoraria nor payments were made for authorship.
Acknowledgements
Writing and editorial assistance were provided to the authors by Carol Brown, MS, of Prescott Medical Communications Group (Chicago, IL), which was funded by AbbVie.
Declaration of funding
This manuscript was supported by funding from AbbVie. The authors had full control of the content and approved the final version.
Declaration of financial/other relationships
R.S. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC); speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, AbbVie, Atai Life Sciences. R.S. McIntyre is the CEO of Braxia Scientific Corp. L. Bloudek and J. Timmons are employees of Curta, Inc, which was funded by AbbVie to perform the analysis. P. Gillard was an employee of AbbVie at the time of the study. A. Harrington was an employee of AbbVie at the time of the study and may hold stock. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data availability statement
AbbVie is committed to responsible data sharing. This includes access to anonymized, individual and/or study-level data (analysis data sets), as well as other information (e.g. protocols and Study Reports), as long as the studies are not part of an ongoing or planned regulatory submission and consistent with the informed consent conditions for the study. This includes requests for clinical trial data for unlicensed products and indications. Study data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). For more information on the process, or to submit a request, contact the authors.
References
- Miller S, Dell’Osso B, Ketter TA. The prevalence and burden of bipolar depression. J Affect Disord. 2014;169(Suppl 1):S3–S11.
- Shippee ND, Shah ND, Williams MD, et al. Differences in demographic composition and in work, social, and functional limitations among the populations with unipolar depression and bipolar disorder: results from a nationally representative sample. Health Qual Life Outcomes. 2011;9:90.
- Sole B, Bonnin CM, Torrent C, et al. Neurocognitive impairment across the bipolar spectrum. CNS Neurosci Ther. 2012;18(3):194–200.
- Bessonova L, Ogden K, Doane MJ, et al. The economic burden of bipolar disorder in the United States: a systematic literature review. Clinicoecon Outcomes Res. 2020;12:481–497.
- Cloutier M, Greene M, Guerin A, et al. The economic burden of bipolar I disorder in the United States in 2015. J Affect Disord. 2018;226:45–51.
- Baldessarini RJ, Tondo L, Visioli C. First-episode types in bipolar disorder: predictive associations with later illness. Acta Psychiatr Scand. 2014;129(5):383–392.
- Mitchell PB, Goodwin GM, Johnson GF, et al. Diagnostic guidelines for bipolar depression: a probabilistic approach. Bipolar Disord. 2008;10(1 Pt 2):144–152.
- McIntyre RS, Berk M, Brietzke E, et al. Bipolar disorders. Lancet. 2020;396(10265):1841–1856.
- Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003;64(2):161–174.
- Hu J, Mansur R, McIntyre RS. Mixed specifier for bipolar mania and depression: highlights of DSM-5 changes and implications for diagnosis and treatment in primary care. Prim Care Companion CNS Disord. 2014;16(2):PCC.13r01599.
- Singh T, Rajput M. Misdiagnosis of bipolar disorder. Psychiatry. 2006; Oct3(10):57–63.
- McIntyre R, Laliberté F, Germain G, et al. The real-world health resource use and costs of misdiagnosing bipolar I disorder. J Affect Disord. 2022;316:26–33.
- Angst J, Sellaro R, Stassen HH, et al. Diagnostic conversion from depression to bipolar disorders: results of a long-term prospective study of hospital admissions. J Affect Disord. 2005;84(2–3):149–157.
- Kessing LV, Willer I, Andersen PK, et al. Rate and predictors of conversion from unipolar to bipolar disorder: a systematic review and meta-analysis. Bipolar Disord. 2017;19(5):324–335.
- Han K, De Berardis D, Fornaro M, et al. Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:20–27.
- McIntyre RS, Patel MD, Masand PS, et al. The rapid mood screener (RMS): a novel and pragmatic screener for bipolar I disorder. Curr Med Res Opin. 2021;37(1):135–144.
- Thase ME, Stahl SE, McIntyre RS, et al. Healthcare provider perspectives on bipolar I disorder screening and the Rapid Mood Screener (RMS), a pragmatic, new tool. Poster presented during the Neuroscience Education Institute (NEI) 2020 Virtual Scientific Poster Session. 2020.
- Menzin J, Sussman M, Tafesse E, et al. A model of the economic impact of a bipolar disorder screening program in primary care. J Clin Psychiatry. 2009;70(9):1230–1236.
- Rowland TA, Marwaha S. Epidemiology and risk factors for bipolar disorder. Ther Adv Psychopharmacol. 2018;8(9):251–269.
- Hirschfeld RM, Holzer C, Calabrese JR, et al. Validity of the mood disorder questionnaire: a general population study. Am J Psychiatry. 2003;160(1):178–180.
- Centers for Medicare and Medicaid Services. CMS physician fee schedule. 2021 [cited 2021 Mar 29]. Available from: https://www.cms.gov/medicare/physician-fee-schedule/