Abstract
Objective
Original brand extended-release (ER) oxycodone tablets (OC) for oral use were reformulated (ORF) with abuse-deterrent properties (ADP) against inhalation and injection routes in August 2010. This product transition provided an opportunity to compare “before and after” reformulation abuse trends. Our goal was to assess the change in abuse of brand oxycodone ER from before and after introduction of ORF.
Methods
Change in self-reported non-oral “OxyContin” abuse in the previous 30 days during two years pre- and four years post-reformulation was assessed among adults evaluated for substance use and treatment planning using the Addiction Severity Index-Multimedia Version (ASI-MV). Comparator opioids were used to provide a frame of reference for changes in abuse due to competing population-level opioid abuse interventions and other factors unrelated to the reformulation. A proportion (PR) and abuse report dispensing ratio (ARDR) are reported because a single measure of abuse has not been identified that can optimally describe opioid abuse or changes in opioid abuse.
Results
Interrupted time-series analyses indicated an immediate decline in non-oral abuse measures post-reformulation (PR = −52.1%; ARDR = −32.2%). Significant decreases from pre- to post-reformulation in non-oral abuse overall were observed (PR [95% CI] = −30.7% [-46.9%, −9.5%]; ARDR = −29.3% [-37.5%, −20.1%]). Comparator opioids did not demonstrate similar trends over the period.
Conclusions
Methodology applied in this study suitably assessed the effectiveness of an ADP product. Among individuals assessed for substance use, a differential decline in non-oral abuse of brand ER oxycodone was observed since introduction of ORF.
Introduction
An extended-release (ER) oral tablet formulation of oxycodone hydrochloride (OxyContin) is approved for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which alternative treatment options are inadequateCitation1. When taken as intended (tablet swallowed intact), OxyContin is designed to provide delivery of oxycodone over 12 h. Oxycodone is classified as a Schedule II drug under the Controlled Substances Act due to its potential for abuse, as well as for psychological and physical dependence. Original OxyContin became a target of abuse after it was recognized that breaking, crushing, or chewing could rapidly release oxycodone from the extended-release matrix of the tablet, at which point it was commonly manipulated for abuse via non-oral routesCitation2,Citation3.
Non-oral administration routes of opioid abuse (i.e. snorting and injecting) have serious adverse health consequences and are an important public health concernCitation5–9. Abuse deterrent formulations of opioids aim to make abuse more difficult and have been considered to play a role in tackling the opioid abuse and misuse epidemicCitation4. To make the tablets more difficult to abuse by the intranasal and intravenous routes, original brand ER oxycodone (OC) was reformulated (ORF) in August 2010. Its physicochemical abuse-deterrent properties (ADP) were designed to make manipulation difficultCitation10–12, while also retaining clinical effectivenessCitation13. The tablet was designed to hinder the release of more oxycodone in a shorter period of time than intended, as OC was an extended-release formulation of oxycodone. ORF was not intended to address abuse by swallowing the tablet or multiple tablets whole, similar to most abuse deterrent formulations of opioid medicationsCitation14. The reformulation of OxyContin was not expected to reduce abuse of other products or reduce overall abuse of opioids or non-opioid drugs. Abuse-deterrent formulations (ADFs) of prescription opioid medications are but one part of a multi-faceted approach to mitigate the opioid crisis, and FDA continues to encourage the development of such formulationsCitation15. ADFs do not, however, reduce the risk of addictionCitation15.
ORF was introduced with no change to the tradename or reference to abuse deterrence in the labelCitation13. Shipment of OC to wholesalers ceased in August 2010 and shipment of ORF commenced a few days laterCitation13. By December 2010, 91% of brand ER oxycodone prescriptions were filled with ORF [IQVIA National Prescription Audit]. The abrupt and complete change in product formulation enabled a pre/post comparison to evaluate the effects of the formulation changeCitation13. The National Addictions Vigilance Intervention and Prevention Program (NAVIPPRO) system collects data from clinical sites using the Addiction Severity Index-Multimedia Version (ASI-MV) assessment tool which is a practical dataset to assess benefits of interventions aimed to reduce opioid abuseCitation16.
This study was one in a series of four formal postmarketing requirements (PMR) by the U.S. Food and Drug Administration (FDA) designed in consultation with the FDA, including three observational surveillance studies and one retrospective claims study assessing overdose and overdose death risk changes in a patient populationCitation17. Each study had its limitations, largely because they each were observational studies in which the data were originally collected for different purposes. As is typical of these types of studies, when considered together, the design limitations of a single study may be mitigated when its findings are supported by the design strengths and findings of other studiesCitation18. More reliable answers to research questions are obtained by integrating results from several different approaches, each with different and unrelated key sources of potential bias.
A major challenge in measuring population-level changes in abuse is described by FDA. Because there is not a direct measure of the true at-risk or exposed population of persons who are abusers or who are at risk for becoming abusers in the three PMR surveillance studies of the four total studies, suitable proxy measures were neededCitation19. The general uncertainty about which denominator to which to refer these data is acknowledged by FDACitation19, with subsequent guidance recommending two estimates of population riskCitation14.
The goal of this observational surveillance study was to assess the change in previous 30-day non-oral abuse of brand oxycodone ER from before (pre-) to after (post-) introduction of ORF among adults evaluated with the ASI-MV.
Methods
Study population
The NAVIPPRO is a national surveillance system designed for prescription drug abuse signal detection and verification used to inform prevention and intervention programsCitation20. NAVIPPRO collects data from enrolled substance abuse treatment centers and other sites nationally using the ASI-MV clinical toolCitation20,Citation21. The ASI-MV is a validated, electronic, self-administered clinical tool used to collect information from individuals assessed for substance use and treatment planning including data on overall abuse of specific drugs (legal and illegal), abuse by routes of administration in the past 30 days and other respondent attributes.
The ASI-MV is based on a standardized clinical instrument, the ASI, originally created and tested for reliability and validity as a paper version in 1985Citation22. The ASI was transferred to a multimedia platform and tested for validity in 2001Citation21 and has been found to be a more reliable method of collecting information than an interviewer administered ASI assessment. The ASI-MV was further tested against an interviewer administered survey in 2009 and concluded that the ASI-MV had improved self-disclosureCitation23.
Study period
A two-year baseline period (pre-) compared to a four-year post-reformulation period was evaluated, excluding a six-month transition period. The baseline period, 1 July 2008 through 30 June 2010, provides a relatively stable estimate of OC prescriptions, without the large fluctuations in brand versus generic ER oxycodone prescriptions observed in early 2008 after reinstatement of the brand ER oxycodone patent [IQVIA National Prescription Audit]. The follow-up period, 1 January 2011 to 31 December 2014, provides an estimate of sustained effect over a period prior to many interventions to reduce the prescribing, overdose, abuse, addiction and diversion of opioidsCitation24. The transition period, 1 July 2010 through 31 December 2010, was excluded from analyses as it represents the period of transition after introduction of ORF to the market and the initial decrease in supply and availability of OC.
Study design
This is an observational surveillance study of ASI-MV assessment data among adults aged 18 to 90 years.
Study analytic sample
The ASI-MV network is dynamic in nature as new sites can be added and individual sites within the ASI-MV network contribute data on varying schedules, with varying sample size. When evaluating trends over time, including all ASI-MV sites could result in an inconsistent sampling fraction across study periodsCitation25. A consistent sampling fraction over the study period is important to assess change in abuse over timeCitation25. Therefore, the analytic sample included only those sites contributing at least one assessment during each quarter of the pre- and post-reformulation periods. Sensitivity analyses included a less-restrictive set of sites, only ASI-MV sites contributing at least one assessment each year during the pre- and post-reformulation periods. By relaxing the restrictions on the set of sites to generate a dataset with a constant sampling fraction, more data from the original dataset was retained. Furthermore, only the respondent three-digit ZIP Codes contributing at least five ASI-MV assessments from the start of the baseline period (1 July 2008) through the four-year follow-up period are included. By restricting data to sites which contributed a minimum number of assessments during the study period, the possibility of sporadic and possibly outlying reports was reduced.
A total of 34 sites provided data for the analyses, represented by 10 states. With the less restrictive site definition for sensitivity analyses, sites which contributed at least one assessment/year, a total of 175 sites provided data for the sensitivity analyses, represented by 23 states.
Repeat assessments, defined as assessments completed by individuals who had taken an ASI-MV assessment more than once within a 30-day period, were removed from all analyses. However, assessments completed by the same individual outside the 30-day window were allowed and counted independently. They accounted for 10.2% of assessments included in the analytic sample.
Note that individuals who reported past 30-day abuse of a product could select use of multiple routes of abuse as part of the ASI-MV assessment.
An individual was considered to have abused brand ER oxycodone in the post-reformulation period if that person selected “Old OxyContin” or “OxyContin Reformulated” as a product abused in the past 30 days in the assessment. The ASI-MV collects real-world patient reported behaviors from clinical settings. Abuse reports by individuals within the ASI-MV clinical tool are counted and used as the numerator for measures of abuse evaluated in this study. The ASI-MV System does not have information about diagnoses of opioid use disorder, as the ASI-MV is an assessment instrument, not a diagnostic instrument. Therefore, the term “abuse” is used here to refer to a report by an individual rather than a medical diagnosis for that individual.
Study comparators
Comparators were used to assess whether changes in outcomes following the introduction of ORF were specific to ORF, accounting for secular trends or other opioid abuse interventions that might affect opioid abuse rates in general. Characteristics of opioid products can vary and therefore careful consideration is warranted when selecting appropriate comparators and interpreting relative resultsCitation14. The drugs, as set forth by FDACitation14,Citation19, used for comparison to brand ER oxycodone were ER morphine, immediate release (IR) hydrocodone combination products, and other Schedule II opioids (excluding brand ER oxycodone and methadone). Other Schedule II opioids included analgesic tablets that contained ER oxymorphone, ER hydromorphone, or IR oxycodone. It excluded methadone, transdermal fentanyl, and ER tapentadol (because it entered the market after the reformulation of OxyContin).
Descriptive analysis
Population characteristics of non-oral abuse and abuse by inhaling and injecting were evaluated for brand ER oxycodone and comparator opioids. Characteristics included age, gender, race, self-reported pain, the expected treatment modality (the treatment setting anticipated by the clinician prior to assessment completion; e.g. inpatient, outpatient), history of injection abuse of prescription opioids, and the number of prescription opioid products abused in the past 30 days.
Measures of abuse
Since opioid abuse is a multidimensional conceptCitation19, three measures of abuse were estimated in this study and implemented in five statistical models. For the abuse risk measures, abuse count for studied opioids were used for the numerator, while the number of assessments (proportion [PR]) and dosage units dispensed (abuse report dispensing ratio [ARDR]) in a region were used as the denominators. Abuse count, adjusted for dosage units dispensed, was used for the third measure of abuse.
Statistical models
Five generalized linear models were employed to evaluate and test for differences between the pre- and post-reformulation periods on estimates of past 30-day non-oral abuse of brand ER oxycodone and comparator opioids (). Model 1 uses Poisson regression of abuse counts with a log-link function and the number of assessments is used as an offset. Similarly, Model 2 uses Poisson regression of abuse counts with a log-link function and dosage units dispensed within the respondents’ three-digit ZIP Code is used as an offset. Model 2a adds the number of assessments as a covariate to Model 2. Model 3 uses Poisson regression of abuse counts with a log-link function but instead of an offset, uses dosage units dispensed as a covariate. Model 3a adds the number of assessments as a covariate to Model 3. The regression structure used a generalized estimating equation (GEE) to estimate the parameters of a generalized linear model with a possible unknown correlation between outcomes.
Table 1. Summary of statistical regression models.
The statistical models used aggregate-level (area-level based on respondent three-digit ZIP Code) counts of self-reported abuse. Note that the repeated subject component of the Poisson regression models was excluded in instances in which convergence issues arose due to sparse data. When the model did not converge with the repeated statement, the model was re-run without it. With the dataset aggregated to the respondent three-digit ZIP Code level by calendar quarter, the proposed research question(s) while accounting for temporal correlation of respondent three-digit ZIP Code level drug-specific probabilities of abuse could be assessed.
Figures of the measures of abuse-time were generated. Percent change from baseline and associated 95% confidence intervals (CIs) were calculated. The statistical model estimates were used to estimate ratios of risk ratios (RORR) and 95% confidence intervals for brand ER oxycodone versus comparator opioids. All analyses performed used the general linear modeling procedure (GENMOD) in SAS 9.4. LSMESTIMATE statements of the GENMOD procedure were used to estimate and test parameters of interest (SAS 9.4, Cary, NC, USA).
For the interrupted time series analysis, applied to each of these models, calendar quarters were ordered and represented by an ordinal variable with unit increments per quarter during the period from July 2008 to December 2014, with the six-month transition period excluded. Log-linear regression was conducted for the pre- and post-reformulation periods separately. Then, the pre- and post-period slopes were compared, and the immediate shift was evaluated as the percent difference between the model-fitted last quarter of the pre-reformulation period to the first quarter of the post-reformulation period.
Model assumption assessments were conducted for each model to determine whether the relationships in the variables met the model assumptions by evaluating a residual versus linear predictor plot. Model goodness-of-fit was assessed using Akaike information criteria (AIC), AIC corrected (AICc) and Bayesian information criteria (BIC) statistics, residual plots and observed versus predicted plots.
The proportion (PR, Model 1) and the abuse report dispensing ratio adjusted for the total number of assessments (ARDR, Model 2a) are presented. Additional analyses and sensitivity analyses were conducted and submitted to the FDACitation26. Many of the additional analyses and sensitivity analyses were conducted as a matrix with multiple combinations of attributes applied. While there were minor differences in the results for each of the other analyses and sensitivity analyses compared to the principal analyses presented here, none affected the interpretations of the principal analyses.
Ethics
This study uses anonymized, de-identified data collected during the course of ongoing clinical assessment at treatment facilities in the NAVIPPRO Network. As such, it is exempt from requirements for institutional review board review.
Informed consent
Not applicable due to our study design (non-human subject research).
Results
A total of 66,897 assessments were included in this study. The study sample in the pre-/post-reformulation periods included 989/2866 reports of non-oral brand ER oxycodone abuse, 90/641 of ER morphine, 820/4009 of IR hydrocodone combination products, and 1924/6101 of other Schedule II opioids. The age, ethnicity, and gender distribution of respondents was similar across all brand ER oxycodone and primary comparator groups; the male population proportion was slightly greater than the female population in all groups (). Most subjects were age 21 to 34 years, white, had an expected treatment modality of residential/inpatient or outpatient/non-methadone treatment, and reported abusing approximately 6 to 8 different prescription opioids in the past 30 days. Among brand ER oxycodone and comparator opioid assessments, the expected treatment modality was most frequently residential/inpatient and outpatient/non-methadone treatment facilities. Among total assessments, the treatment modality frequency of residential inpatient was less than half of that reported in the opioid abuse reports groups and DUI/DWI modality was higher (21.5% versus 0.9% to 2.4%). Individuals who reported ER morphine abuse had a higher proportion of history of injection of prescription opioids (75.9%) compared to abusers of brand ER oxycodone or other comparator opioids (range: 31.3% to 54.1%).
Table 2. Population characteristics of individuals who reported opioid abuse via non-oral routes among ASI-MV sites contributing quarterly assessment data.
Modeled brand ER oxycodone PR of non-oral abuse (Model 1; ) indicates a decline in non-oral abuse during the post-reformulation period as compared to the pre-reformulation period. The interrupted time series analysis, shown in , also demonstrates a decline in non-oral abuse, with a 52.1% decrease in the last quarter of the pre-period model to the first quarter of the post-period (immediate shift; ) for the PR. The average PR pre-to-post decline was −30.7% (95% CI = −46.9, −9.5; ). The PR results from the comparator opioids, together with the modeled abuse measure-time profiles, were significantly different from the trends observed for brand ER oxycodone over the post-reformulation period. Specifically, brand ER oxycodone had the earliest as well as the largest reduction in abuse comparatively across all study groups.
Figure 1. Non-oral abuse-time and interrupted time series modeled data (selected models) for Oxycontin and opioid comparators for (A) modeled brand ER oxycodone and comparator groups PR of non-oral abuse reports, (B) interrupted time series models of brand ER oxycodone and comparator groups PR of non-oral abuse reports, (C) modeled brand ER oxycodone and comparator groups ARDR of non-oral abuse reports, and (D) interrupted time series models of brand ER oxycodone and comparator groups ARDR of non-oral abuse reports. Abbreviations. ARDR, abuse report dispensing ratio; ER, extended release; IR, immediate release; PR, proportion.
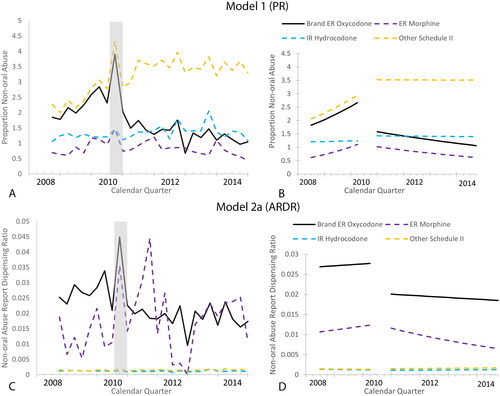
Table 3. Interrupted time series selected models’ assessment of immediate shift in ASI-MV reports of non-oral abuse.
Table 4. Pre- to post-period Poisson regression selected model results for ASI-MV reports of non-oral abuse.
The non-oral ARDR (Model 2a; ) also indicates a decline in reports of non-oral abuse during the post-reformulation period as compared to the pre-reformulation period, however it was not as pronounced as the PR (Model 1). The interrupted time series analysis, shown in , also demonstrates the decline for the non-oral brand ER oxycodone ARDR, with a 32.2% decrease in the last quarter of the pre-period model to the first quarter of the post-period (immediate shift; ). The average pre-to-post ARDR decline was −29.3% (95% CI = −37.5, −20.1; ). The ARDR results from the comparator opioids were different from the trend observed for brand ER oxycodone over the post-reformulation period; ER morphine was the only comparator that showed a statistically significant decrease in non-oral abuse (although not until 2012, ) while a statistically significant increase in abuse was discovered in the Other Schedule II comparator group.
Sensitivity analyses
A narrower 95% confidence was observed for brand ER oxycodone percent change estimates for the sites contributing at least one assessment per year, as expected with the additional data. However, the width of the confidence intervals varied for the comparator opioids. While the brand ER oxycodone point estimates for the percent change were generally similar according to site selection method, the estimates varied for comparator opioids. The observed variation in point estimates and occasional decreases in the precision of the estimates support the assertion that the variation in sites may impact non-oral abuse rates. The value in restricting the data set to the sites which provided consistent reporting, is the provision of estimates from a similar source population and appropriate data sources. Therefore, the main analyses which restrict the data to the sites which contributed data for at least 1 assessment/quarter in each of the quarters during the pre- and post-reformulation periods, was considered to be a more appropriate estimate of the change in abuse measures in this population.
Discussion
We measured changes in non-oral abuse before and after introduction of the reformulated brand ER oxycodone tablets, employing five analytical models, applied with and without interrupted time series approaches. These methods, along with sensitivity analyses, provide evidence to support effectiveness of reformulated brand ER oxycodone on reduction of non-oral abuse when evaluated against comparator opioids within a sentinel population of adults evaluated for substance use problems and treatment planning using the ASI-MV assessment.
As a condition of the U.S. FDA approval of the reformulation of OxyContin in 2010, Purdue conducted a variety of postmarketing epidemiology studies to monitor and quantify the impact of the reformulation on abuse of OxyContin and its consequences. As evaluating the effects of abuse-deterrent formulations was a new and unprecedented area of investigation, numerous studies were conducted across a range of populations, data sources, outcomes and time horizons. The study described in this paper was one in a series of what FDA ultimately defined as the four formal postmarketing requirements (PMR), issued by FDA in 2016, and designed in consultation with FDA, including three observational surveillance studies and one retrospective claims study assessing overdose and overdose death risk changes in a patient populationCitation17. In 2020, FDA held a public Advisory Committee meeting where the committees discussed the results of the required PMRCitation26.
Since there is not consensus for one measure of abuseCitation19, three measures were selected. Each measure has strengths and limitations and together provide a more complete assessment of changes in abuseCitation13. The first measure is the proportion of non-oral abuse counts per number of assessments, a method that accounts for the size of a population from which the opioid abuse cases arose. The second measure is non-oral abuse report dispensing ratio per dosage units dispensed in the region from which the cases arose, a drug availability denominator which is intended to quantify abuse for the available product in the community. The third measure is a non-oral abuse count adjusting for dosage units dispensed, a measure similar to the second measure, but employing statistical methods that allow the model more flexibility to estimate percent change with fewer assumptions about the relationship between abuse counts and dosage units dispensed. The ARDR and adjusted abuse count measures of abuse each had a variation for which the number of assessments was also adjusted.
Several Poisson regression modeling approaches were employed to assess changes in the selected measures of non-oral abuse and abuse by route pre- and post-reformulation with ADP. Model 1 used the number of assessments as an offset; Model 2 used the dosage units dispensed, a utilization measure, as an offset; Model 2a used the dosage units dispensed, a utilization measure, as an offset and adjusted for the number of assessments; Model 3 used dosage units dispensed as a continuous covariate; Model 3a used dosage units dispensed and number of assessments as covariates.
While the dosage units dispensed as a denominator for the ARDR may be appropriate for other purposes, it has limitations and complexities in interpretability in a population of individuals who abuse opioids. Limitations include diversion of the dosage units dispensed and related factors. Many individuals who abuse opioids are thought to abuse drugs that are both available and cost the least, irrespective of the type of opioidCitation24. Therefore, price and availability are important considerations for abuse over which specific opioid is abused, which limits the utility of a utilization denominator. Variability in dose of each tablet and pharmacokinetics (IR vs ER) further complicate interpretability. Additionally, care should be taken in evaluating models associated with dosage units dispensed as they could be direct causation or mediation variables. In other words, if it is observed that a significant fraction of the change in abuse can be explained by a decrease in demand among individuals who abuse the product, then measuring the reformulation effect using this model may lead to errors.
Overall, following the introduction of reformulated brand ER oxycodone, self-reported non-oral brand ER oxycodone abuse was significantly reduced among those assessed for substance use problems and treatment planning. This reduction was distinguished from changes observed in comparator opioids. This aligns with the expected effect of an abuse deterrent opioid formulation which employs physicochemical barriers to deter manipulation of the product for non-oral routes of abuse. This study also illustrates convergent validity with other published studies from a variety of data sources and summarized by Dart et al. Citation24.
Modeled brand ER oxycodone PR and ARDR of non-oral abuse reports indicated a decline in non-oral abuse during the post-reformulation period as compared to the pre-reformulation period. The interrupted time series analysis demonstrates a decline in the immediate shift. The immediate decline further supports a reformulation effect based upon the temporal changes. The average PR and ARDR pre-to-post declines were −30.7% and −29.3, respectively, which supports a sustained effect of the reformulation beyond the immediate shift.
Comparator opioids (ER morphine, IR hydrocodone products, and other Schedule II opioids) were evaluated to provide a frame of reference for declines in abuse due to the effects of numerous other opioid abuse interventions and other confounding factors versus the effects due to the brand ER oxycodone reformulation. The PR and ARDR results from the comparator opioids were different from the trend observed for brand ER oxycodone over the post-reformulation period. The differences in the pre-post reformulation changes in non-oral abuse for the comparator opioids relative to brand ER oxycodone separate the effectiveness of the reformulation of brand ER oxycodone from other population-based interventions.
Strengths and limitations
A strength of the study is the broad surveillance system which captures data from a hard-to-reach population of individuals who abuse prescription opioids. The centers utilizing the ASI-MV in standard clinical practice are distributed throughout the US. The reliability testing and validation of the assessment tool provides assurance in the data collected. Another strength is the variety and granularity of information collected from the individuals assessed for substance use and treatment planning, including route of abuse.
The variety of statistical approaches applied along with the internal consistency in the results in the study provides another strength of this study. Furthermore, the many sensitivity analyses having results consistent with the principal analyses were also a strength, demonstrating the robustness of the results. To disentangle the effect of reformulation from other initiatives aimed to curb prescription opioid abuse, comparator opioids were studied for secular trends. The results from the diverse statistical approaches and comparator groups reinforce the interpretations from the primary analysis, supporting a reformulation effect of reduced non-oral abuse.
While the results provide evidence that the reformulation of brand ER oxycodone was effective at reducing non-oral abuse, a number of limitations in this study were present. First, the ability to generalize results from adults evaluated with the ASI-MV for substance use problems and treatment planning to the entire US population of individuals who abuse prescription opioids may not be completely appropriate. The data for this study were all self-reported data which has limitations particularly for the population of individuals who abuse opioids, which may be reluctant to provide information for fear of legal implications or to admit having a substance abuse problem. However, a previous study indicated that use of the ASI-MV improved self-disclosure over in-person ASI interviews with a clinicianCitation23. Another limitation is the inclusion of repeat assessments outside a 30-day window which could bias the results toward the reports of abuse of the repeat individuals.
As with any data source using self-reported data, misclassification could impact the results. However, the ASI-MV assessment includes photographs of medications and popular slang terms to minimize recall bias. This study also assessed reports of OC or ORF, minimizing any misclassification between the original and reformulated branded ER oxycodone.
For assessments completed by the same individual over time (outside of a 30-day window), the probability of an individual abusing certain opioid products would likely be correlated with the probability of the same individual abusing the same or other opioid products during same period when reassessed at other points in time. However, such within-subject correlation cannot be addressed when the data are aggregated to the respondent 3-digit ZIP Code level.
Due to the limitations in the study design, this study does not allow for direct evaluation of causality, hence the breadth of sensitivity analyses used to identify differential changes that cannot be explained by secular trends or other opioid-wide interventions.
Despite these limitations, many of which are commonly present in epidemiologic studies, the ASI-MV provides a large dataset employing a validated assessment instrument with consistent and dedicated surveillance management. The variety of statistical methods and robustness of the results further provide confidence in the results.
Importantly, while a decline in non-oral abuse of reformulated OxyContin was observed, abuse via inhalation and injection routes, as well as the oral route, is still possible. Furthermore, the reformulation of OxyContin was not expected to reduce abuse of other products or reduce overall abuse of opioids or non-opioid drugs. ADFs of prescription opioid medications are but one part of a multi-faceted approach to mitigate the opioid crisis. In addition, the abuse-deterrent properties of reformulated OxyContin do not change the risk of addiction. All prescription opioids, including those with FDA-recognized ADP, carry risks of addiction, abuse, and misuse, which can lead to overdose and death.
Conclusions
The Poisson regression modeling applied to the ASI-MV surveillance data in this study assessed the effectiveness of an ADP product. Among individuals assessed for substance use and treatment planning, a decline in non-oral abuse of brand ER oxycodone was observed immediately after the six-month transition period and the decline was sustained through the four years of the follow-up period. The significant reductions in non-oral abuse of brand ER oxycodone were not found in comparator opioid products, thereby distinguishing the effectiveness of ORF from competing population-based opioid abuse interventions or other confounding factors.
Transparency
Author contributions
Study conception/design: JLG, TDG, VR. Analysis/interpretation of data: RDR, JLG, TDG, VR. Draft or revision of the manuscript: RDR, JLG, TDG, VR. All authors have provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Acknowledgements
The authors thank the Purdue Pharma L.P. Epidemiology Advisory Board for their insights and feedback into the study design and Alec Walker for his valuable contributions to the interpretation of the research. We also acknowledge former Purdue Pharma L.P. researchers who had a role in early study conceptualization and design aspects, and Stacy Baldridge, Jennifer Giordano, and Nelson Sessler of Purdue Pharma L.P. for their insightful feedback on the research. We thank Holly Richendrfer, PhD, of Evidera for her editorial support.
Declaration of funding
This work was supported by Purdue Pharma L.P., Stamford, CT. Uprise Health/Inflexxion, Irvine, CA received funding from Purdue Pharma L.P. to conduct this study and for the development of this manuscript. RDR was an employee of Purdue Pharma L.P. at the time of the study. JLG and TDG are employees of Inflexxion. VR is an employee of Ephicacy Consulting Group, Iselin, New Jersey and collaborated as an external consultant to Inflexxion for this study. Editorial support provided by Holly Richendrfer, PhD of Evidera was funded by Purdue Pharma L.P.
Declaration of financial/other relationships
JLG and TDG are employees of, and VR is a consultant to, Inflexxion. Inflexxion contracts with government agencies and multiple pharmaceutical companies that market some of the products included in the study groups evaluated for this article. RDR was an employee of Purdue Pharma L.P., the sponsor of OxyContin, at the time of the study. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Data availability statement
Source data are publicly available from the FDA and can be found at: https://www.fda.gov/advisory-committees/advisory-committee-calendar/september-10-11-2020-joint-meeting-drug-safety-and-risk-management-advisory-committee-and-anesthetic.
References
- OxyContin (oxycodone hydrochloride) Extended-Release Tablets. Full prescribing information. Purdue Pharma L.P; [accessed 2022 Aug 2]. Available from: http://app.purduepharma.com/xmlpublishing/pi.aspx?id=o
- Hays LR. A profile of OxyContin addiction. J Addict Dis. 2004;23(4):1–9.
- Sees KL, Di Marino ME, Ruediger NK, et al. Non-medical use of OxyContin tablets in the United States. J Pain Palliat Care Pharmacother. 2005;19:13–23.
- Pitt AL, Humphreys K, Brandeau ML. Modeling health benefits and harms of public policy responses to the US opioid epidemic. Am J Public Health. 2018;108(10):1394–1400.
- Greene D. Total necrosis of the intranasal structures and soft palate as a result of nasal inhalation of crushed OxyContin. Ear Nose Throat J. 2005;84(8):512.
- Jewers WM, Rawal YB, Allen CM, et al. Palatal perforation associated with intranasal prescription narcotic abuse. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(5):594–597.
- Strang J, Bearn J, Farrell M, et al. Route of drug use and its implications for drug effect, risk of dependence and health consequences. Drug Alcohol Rev. 1998;17(2):197–211.
- Yewell J, Haydon R, Archer S, et al. Complications of intranasal prescription narcotic abuse. Ann Otol Rhinol Laryngol. 2002;111(2):174–177.
- Green JL, Bucher Bartelson B, Le Lait MC, et al. Medical outcomes associated with prescription opioid abuse via oral and non-oral routes of administration. Drug Alcohol Depend. 2017;175:140–145.
- Perrino PJ, Colucci SV, Apseloff G, et al. Pharmacokinetics, tolerability, and safety of intranasal administration of reformulated OxyContin(®) tablets compared with original OxyContin (®) tablets in healthy adults. Clin Drug Investig. 2013;33(6):441–449.
- Sellers EM, Perrino PJ, Colucci SV, et al. Attractiveness of reformulated OxyContin(R) tablets: assessing comparative preferences and tampering potential. J Psychopharmacol. 2013;27(9):808–816.
- Harris SC, Perrino PJ, Smith I, et al. Abuse potential, pharmacokinetics, pharmacodynamics, and safety of intranasally administered crushed oxycodone HCl abuse-deterrent controlled-release tablets in recreational opioid users. J Clin Pharmacol. 2014;54(4):468–477.
- US Food & Drug Administration. 2020 Joint meeting of the drug safety and risk management advisory committee and the anesthetic and analgesic drug products advisory committee meeting announcement; 2020 Sept 10–11 [accessed 2022 Aug 2]. Available from: https://www.fda.gov/advisory-committees/advisory-committee-calendar/september-10-11-2020-joint-meeting-drug-safety-and-risk-management-advisory-committee-and-anesthetic
- US Food and Drug Administration. Abuse-deterrent opioids – evaluation and labeling. guidance for industry; 2015 April [accessed 2022 Aug 2]. Available from: https://www.fda.gov/media/84819/download
- US Food and Drug Administration. Abuse-deterrent opioid analgesics; 2021 [accessed 2023 Jan 20]. Available from: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/abuse-deterrent-opioid-analgesics
- Butler SF, Cassidy TA, Chilcoat H, et al. Abuse rates and routes of administration of reformulated extended-release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. J Pain. 2013;14(4):351–358.
- Beachler DC, Hall K, Garg R, et al. An evaluation of the effect of the OxyContin reformulation on unintentional fatal and nonfatal overdose. Clin J Pain. 2022;38(6):396–404.
- Roland CL, Setnik B, Brown DA. Assessing the impact of abuse-deterrent opioids (ADOs): identifying epidemiologic factors related to new entrants with low population exposure. Postgrad Med. 2017;129(1):12–21.
- Secora AM, Dormitzer CM, Staffa JA, et al. Measures to quantify the abuse of prescription opioids: a review of data sources and metrics. Pharmacoepidemiol Drug Saf. 2014;23(12):1227–1237.
- Butler SF, Budman SH, Licari A, et al. National addictions vigilance intervention and prevention program (NAVIPPRO): a real-time, product-specific, public health surveillance system for monitoring prescription drug abuse. Pharmacoepidemiol Drug Saf. 2008;17(12):1142–1154.
- Butler SF, Budman SH, Goldman RJ, et al. Initial validation of a computer-administered addiction severity index: the ASI-MV. Psychol Addict Behav. 2001;15(1):4–12.
- McLellan AT, Luborsky L, Cacciola J, et al. New data from the addiction severity index. J Nerv Ment Dis. 1985;173(7):412–423.
- Butler SF, Villapiano A, Malinow A. The effect of computer-mediated administration on self-disclosure of problems on the addiction severity index. J Addict Med. 2009;3(4):194–203.
- Dart RC, Iwanicki JL, Dasgupta N, et al. Do abuse deterrent opioid formulations work? J Opioid Manag. 2017;13(6):365–378.
- By K, McAninch JK, Keeton SL, et al. Important statistical considerations in the evaluation of post-market studies to assess whether opioids with abuse-deterrent properties result in reduced abuse in the community. Pharmacoepidemiol Drug Saf. 2018;27(5):473–478.
- US Food & Drug Administration. Joint meeting of the drug safety and risk management advisory committee and the anesthetic and analgesic drug products advisory committee. FDA briefing document. Oxycontin abuse deterrent formulation (ADF); 2020 Sept 10–11 [accessed 2022 Aug 2]. Available from: https://www.fda.gov/media/141914/download
