Abstract
Objective
Evaluate the long-term safety and efficacy of vortioxetine in the management of major depressive disorder (MDD) in two open-label one-year studies, including a post-hoc analysis of its effects on symptoms related to anhedonia.
Methods
Both studies were 52-week, open-label, flexible-dose extension studies to evaluate the safety and efficacy of vortioxetine in adult patients with MDD following prior double-blind studies. Patients in the first study (NCT00761306) were flexibly treated with vortioxetine 5 or 10 mg/day (N = 74), and patients in the second study (NCT01323478) received vortioxetine 15 or 20 mg/day (N = 71).
Results
The safety and tolerability profile of vortioxetine was similar between the two studies; treatment-emergent adverse events with the highest incidence were nausea, dizziness, headache, and nasopharyngitis. Across both studies, improvements achieved during the preceding double-blind studies period were maintained, and additional improvements were observed with open-label treatment. Patients showed a mean ± SD reduction (improvement) in Montgomery and Åsberg Depression Rating Scale (MADRS) total score from open-label baseline to Week 52 of 4.3 ± 9.2 points in the 5–10 mg study, and 10.9 ± 10.0 in the 15–20 mg study. Post-hoc MMRM analyses of MADRS anhedonia factor scores also showed continued improvements over long-term treatment; patients showed a mean ± SE reduction from an open-label baseline to Week 52 of 3.10 ± 0.57 points in the 5–10 mg study, and 5.62 ± 0.60 in the 15–20 mg study.
Conclusions
Data from both studies confirm the safety and efficacy of flexibly dosed vortioxetine over 52 weeks of treatment and demonstrate that MADRS anhedonia factor scores continue to improve with long-term maintenance treatment.
Introduction
Major depressive disorder (MDD) is often a long-term illness, and long-term maintenance therapy is recommended for patients who have responded to acute treatment to prevent relapse and recurrenceCitation1. While there are several classes of antidepressants to treat MDD, many patients fail to fully respond to treatment or stay chronically illCitation2.
Vortioxetine is a multimodal antidepressant with a pharmacological profile that is distinct from other currently available antidepressants. It acts as an inhibitor of the serotonin (5-HT) transporter as well as modulating the activity of multiple 5-HT receptor subtypes, and also mediates its therapeutic effects via the norepinephrine, dopamine, amino acids, histamine, and cholinergic systemsCitation3–5. Currently approved at doses between 5 mg and 20 mg, the efficacy, safety and tolerability of vortioxetine versus placebo in managing depressive symptoms has been established in a large clinical development program including more than 12,000 patients with MDDCitation6–19. In the long-term, maintenance treatment with vortioxetine has been shown to maintain and further improve the efficacy established during acute treatment with vortioxetine. In a pooled analysis of five studies, the total response rate was 75% and the total remission rate was 61% at one yearCitation20. Importantly, efficacy was consistent across patient subgroups regardless of gender, age, initial level of depressive or anxiety symptoms, number of previous major depressive episodes (MDEs), or duration of the current MDE. Long-term maintenance treatment with vortioxetine has also been proven effective in preventing relapse of MDD and is well toleratedCitation21.
Anhedonia, referring to a diminished interest or pleasure in activities, is reported in up to 75% of patients with major depressive disorder (MDD)Citation22. Encompassing several distinct and overlapping dimensions (e.g. reward valuation, reward response, and reward learning), anhedonia is often considered one of the most promising endophenotypes of depressionCitation23. When present, it is associated with higher rates of social withdrawal and impairment, appetite loss, and reactivity of moodCitation24. Although anhedonic symptoms often improve to varying extents with antidepressant treatment, the persistence of residual anhedonia is often a key driver of suboptimal responseCitation25–27. Recent pooled analyses of the short-term studies have suggested that vortioxetine may be particularly effective in managing the symptoms of anhedonia—showing a superior effect on anhedonia compared with agomelatineCitation28. In that study, effects on anhedonia were evaluated using the Montgomery and Åsberg Depression Rating Scale (MADRS) anhedonia factor scoreCitation29. We report here the safety and efficacy of vortioxetine in two open-label one-year extension studies, including a post-hoc analysis of its longer-term effects on MADRS anhedonia factor scores.
Methods
Study design
Both studies were long-term (52 week), open-label, flexible-dose extension studies to evaluate the safety, tolerability, and efficacy of vortioxetine in adult patients with MDD. The first study examined the effect of vortioxetine given at dose of 5 or 10 mg per day in patients who completed an earlier six-week venlafaxine-referenced study (followed by a two week, double-blind, down-tapering period)Citation6 and the second study examined the effect of vortioxetine given at dose of 15 or 20 mg per day in patients who completed an earlier eight-week duloxetine-referenced studyCitation11. Both open-label studies began with one week of fixed-dose treatment with vortioxetine 10 mg/day before patients entered a 52-week flexible dose period (where patients received vortioxetine 5–10 mg and 15–20 mg, respectively).
Both studies received protocol approval from the institutional review board or ethics committee of each site in accordance with Good Clinical Practice guidelines, and the principles of the Declaration of Helsinki. All participants provided written informed consent prior to enrollment. The studies were registered at clinical trials.gov with the number NCT00761306 for the 5–10 mg study and NCT01323478 for the 15–20 mg study.
Study participants and treatment
For both studies, adult men and women (aged 18–65 years in the 5–10 mg study and 18–75 years in the 15–20 mg study) were included if they had completed the prior short-term studiesCitation6,Citation11. For both studies, patients had to have a primary diagnosis of recurrent MDD (duration of current episode ≥3 months), at the beginning of each of the respective short-term lead-in studies and at the start of each open-label extension study. Key exclusion criteria for the double-blind studies were prior resistance to two prior antidepressant treatments of ≥6 weeks duration, comorbid psychiatric (including any psychotic disorder) or neurologic illness, and those at serious risk of suicideCitation6,Citation11. Patients in the 5–10 mg open-label study had a MADRS total score ≥30 at baseline of the prior six-week double-blind studyCitation6, while patients in the 15–20 mg open-label study had to have a MADRS score ≥26 in the prior eight-week double-blind studyCitation11.
Assessments and endpoints
Study participants were assessed at baseline, which was defined as the date open-label vortioxetine treatment (10 mg for the first week in both studies) was initiated and at Weeks 1, 2, 4, 8, 12, 16, 20, 24, 28, 36, 44, and 52 or study withdrawal. The primary objective of both studies was to assess the tolerability and safety of long-term vortioxetine treatment as assessed through the number of patients continuing in the study and via reporting of treatment-emergent adverse events (TEAEs) as well as vital signs and weight, ECGs, clinical laboratory values and physical examination. Exploratory efficacy analyses (common to both open-label studies) assessed the change from open-label baseline to Week 52 in MADRS total and anhedonia subscores as well as the percent patients in remission (MADRS total score ≤10) and responding to treatment (reduction of ≥50% in MADRS total score versus baseline of the preceding double-blind study). Overall quality of life was assessed using the EuroQol (EQ5D) Utility IndexCitation30. In addition, the 15–20 mg study assessed patient functioning using Sheehan Disability Scale (SDS) total and function subdomain scores (work/school, social life, and family life/home responsibilities)Citation31.
Statistics
The ‘Safety Set’ included all patients who were enrolled and received at least one dose of open-label study medication. Antidepressant effect was measured in the full analysis set (FAS) which included patients in the safety analysis set who had at least one post-baseline efficacy measurement. Safety, MADRS total score and SDS scores are summarized using descriptive statistics performed with the observed case.
In addition, change from baseline in MADRS total score and anhedonia scores were analyzed post-hoc using a Mixed Model for Repeated Measurements (MMRM) adjusting for study site, week, baseline score, and the interaction between baseline score and week. Effects on anhedonia were analyzed according to the methods used by Cao et al.Citation29 using the MADRS five-item anhedonia factor score, which was based on the following MADRS items: 1 (apparent sadness), 2 (reported sadness), 6 (concentration difficulties), 7 (lassitude), and 8 (inability to feel). Changes in anhedonia factor scores have been shown to highly correlate with the Snaith-Hamilton Pleasure Scale (SHAPS)Citation29. For the 15–20 mg study, the relationship between potential effects on anhedonia and functioning was investigated at each visit using Pearson correlation (SAS version 9.4 or later).
Results
Patient disposition and baseline characteristics
A total of 74 patients entered the 5–10 mg open-label study, of whom 54 completed and 20 withdrew from the study (adverse events, n = 5; lack of efficacy, n = 4; other n = 11). For these patients, the mean ± SD MADRS score had reduced from 33.7 ± 2.8 (severe MDD) at double-blind baseline to 10.7 ± 8.4 at open-label baseline. The 15–20 mg study enrolled of 71 patients, of whom 47 completed and 24 withdrew from the study (adverse events, n = 7; lack of efficacy, n = 4; other n = 13). For these patients, the mean ± SD MADRS score had reduced from 31.1 ± 3.3 (moderate–severe MDD) at double-blind baseline to 16.2 ± 10.0 at open-label baseline.
Aside from the differences in baseline severity, both open-label studies enrolled a similar population of patients living with MDD. Both studies included a majority of women and all, but one, enrolled patients were white ().
Table 1. Demographic and clinical characteristics.
Safety
Taken overall, the safety profile of vortioxetine was similar between the two studies evaluating doses of 5–10 mg and 15–20 mg, respectively. As shown in , the TEAEs with the highest incidence were nausea, dizziness, headache, and nasopharyngitis, which were mainly mild or moderate in severity. Serious AEs were reported in only one patient in each study; one patient had thyroiditis in the first study, and one patient treated with vortioxetine had cholelithiasis in the second study. No deaths were reported during either study periods.
Table 2. TEAEs in the study period.
Five patients (7%) in the 5–10 mg study and seven patients (10%) in the 15–20 mg study withdrew due to TEAEs. TEAEs leading to withdrawal from treatment with vortioxetine 5–10 mg were: depressed mood, gastroenteritis, major depression, nausea, and increased weight. TEAEs leading to withdrawal from treatment with vortioxetine 15–20 mg were: acute cholecystitis, cholelithiasis, depression, headache, insomnia, nausea, pregnancy, and pruritus. Mean weight increased by 2.2 kg from open-label baseline to Week 52 in the study of vortioxetine 5–10 mg and by 1.5 kg in the 15–20 mg study. Increased weight was reported as a TEAE in 14 (18.9%) patients in the 5–10 mg study and 3 (4.2%) patients in the 15–20 mg study. There were no suicide-related TEAEs in either study.
There were no clinically relevant changes in the mean laboratory values, vital signs, or ECG parameter values, and the proportion of patients with potentially clinically significant values were overall low.
Descriptive efficacy
Across both open-label extension studies, improvements achieved during the preceding double-blind studies period were maintained, and additional improvements were observed during the open-label study (). For patients in the 5–10 mg open-label extension study, mean ± SD MADRS total scores decreased from 10.7 ± 8.4 at open-label baseline to 5.3 ± 6.6 at Week 52. Similarly, patients in the 15–20 mg open-label extension study, showed reductions in mean ± SD MADRS total scores from 16.2 ± 10.0 at open-label baseline to 5.0 ± 5.9 at Week 52.
Table 3. Descriptive efficacy (FAS).
The long-term efficacy of vortioxetine therapy was also demonstrated in terms of patients who were defined as treatment responders (patients with a ≥ 50% decrease in MADRS total score from baseline) or in remission (MADRS total score ≤10) (). For the total 5–10 mg study population, the proportion of patients who were classified as treatment responders (as compared to baseline of the double-blind lead in) increased from 76% at open-label baseline to 82% at Week 52 and the proportion of patients in remission increased from 58% to 82%. Similarly, for patients in the 15–20 mg study population, the proportion of treatment responders increased from 48% at open-label baseline to 94% at Week 52 and the proportion of patients in remission increased from 32% to 81%.
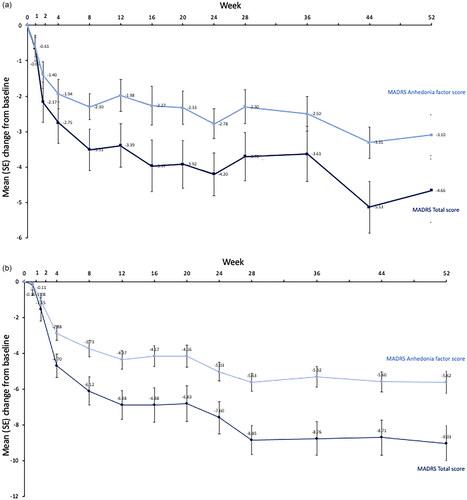
MMRM analyses on change from baseline in MADRS total and anhedonia scores showed that patients on average improved over time (). Patients showed a mean ± SE reduction (improvement) in MADRS total scores from an open-label baseline to Week 52 of 4.66 ± 0.89 points in the 5–10 mg study, and 9.03 ± 0.99 in the 15–20 mg study. Analyses of anhedonia items showed a similar pattern of continued improvements; patients showed a mean ± SE reduction from an open-label baseline to Week 52 of 3.10 ± 0.57 points in the 5–10 mg study, and 5.62 ± 0.60 in the 15–20 mg study.
Figure 1. Mean (SE) change from baseline in MADRS total and MADRS anhedonia factor scores (a) 5–10 mg study and (b) 15–20 mg study.
Analyses of EQ5D data showed that quality of life was either maintained (5–10 mg study) or continued to improve (15–20 mg study), following improvements in the prior controlled studies (). In the 15–20 mg study, all the mean SDS total and single-item scores decreased (improved) from open-label baseline to Week 52 (FAS, OC). In the total population, the mean SDS total score decreased from 13.2 at baseline to 4.9 at Week 52, and the mean scores of the single-items work, social life, and family life decreased to within the range of 1.4 to 1.7. Strong positive correlations (R = 0.66–0.82) were observed between the change from baseline in MADRS Anhedonia factor score and change from baseline SDS total score at all timepoints.
Discussion
Taken together, both open-label studies confirm the existing literature that treatment with vortioxetine flexibly dosed at either 5–10 mg or 15–20 mg was generally safe and well tolerated in patients with MDD. The safety profile was consistent with what is expected for vortioxetine, and a low proportion of patients discontinued treatment due to adverse events. Exploratory efficacy analyses further showed that patients treated with daily doses of vortioxetine had sustained improvement in depressive state compared with the baselines of the prior double-blind baseline studiesCitation6,Citation11 throughout one year of open-label treatment. By the end of both studies, both the number of MADRS responders and those in remission (MADRS ≤ 10) had increased. Importantly, we show that the longer-term benefits of maintenance treatment with vortioxetine on overall symptoms track with improvements in anhedonia factor scores and that this was positively correlated (R = 0.66–0.82) with continued improvements in functioning as assessed by the SDS.
The safety and tolerability of vortioxetine in these two studies, is in line with that previously reported in short-term placebo controlled studies, in similar long-term observational studiesCitation20 and in clinical practiceCitation32. The majority of AEs were characterized as mild or moderate, and the long-term AE profile of vortioxetine was similar to that observed during the short-term lead-in efficacy studiesCitation6,Citation11. Although there was an apparent difference in the reporting of increased weight as an adverse event (18.9% in the 5–10 mg study vs 4.2% in the 15–20 mg study), the mean weight change was similar between the two studies (2.2 kg in the 5–10 mg study and 1.5 kg in the 15–20 mg study) and most were events considered ‘mild’. In a systematic review of five other open-label studies, around 6% of patients reported weight gain (5.7% with 5–10 mg dosing and 5.9% with 15–20 mg dosing)Citation33. Otherwise, some of the tolerability issues that can cause non-adherence with other antidepressants, such as sexual dysfunction, was reassuringly low.
Overall, the exploratory efficacy analyses from these two studies demonstrates that long-term treatment of MDD with vortioxetine can be beneficial for patients having been treated acutely with this drug. There is now general acceptance of the goal to treat depressed patients to wellness or functional remissionCitation34. In a recent pooled-analysis of six to eight week randomized control trials, McIntyre et al. showed significant dose-dependent effects anhedonia and functioning compared with placebo over the range of 5–20 mg/day, with higher doses (10, 15, and 20 mg) shown to be associated with greater clinical responseCitation28. Moreover, using path analysis, they showed that the beneficial effects of vortioxetine on functioning in the placebo-controlled studies were mostly driven by the improvement in MADRS anhedonia factors, suggesting a potential mediation effect for anhedonia on other outcomes. Our findings confirm and extend these observations by showing a continued effect of vortioxetine treatment on anhedonia over 52 weeks, with EQ5D results supporting improvement of overall quality of life and SDS results from the 15–20 mg study also showing functional improvement that correlates with improvement with anhedonia.
Both extension studies were primarily designed to assess the long-term safety of vortioxetine in MDD. In common with all open-label studies, any efficacy conclusions from our findings are exploratory and are limited by the lack of a control, making it difficult to assess causality of any changes in outcome measures. Also in common with most other safety extension studies of registration trials, the population sample was limited in terms of comorbid psychiatric illness, concomitant treatments and risk of suicidalityCitation6,Citation11 and may not be generalizable for patients with more complex, refractory, comorbid and severe forms of depression. Other limitations include the post-hoc nature of the anhedonia factor analyses. Prospective controlled studies specifically designed to assess the long-term efficacy against anhedonic symptoms would add rigor to the growing evidence base for this agent. While the efficacy of vortioxetine against specific anhedonia scales (such as the SHAPS) would have been of interest, these data were not collected in the two open-label studies and our analyses were therefore limited to MADRS anhedonia factor scores previously used in other analysesCitation28,Citation29. For the purposes of this report, the two studies were kept separate because the populations were non-comparable at baseline (i.e. different MADRS scores at baseline). While analysis of short-term studies has previously indicated a dose-response for vortioxetine on anhedoniaCitation28, the differences in population characteristics between the two open-label studies preclude us from drawing any similar conclusions.
Conclusions
Taken together the two studies add to the strong evidence base that vortioxetine is generally safe, well tolerated, and efficacious in the long-term management of MDDCitation3,Citation20,Citation33. Furthermore, our open-label findings suggest that the patients’ depressive symptoms related to anhedonia continued to improve with long-term (maintenance) vortioxetine treatment. These symptoms can be difficult to treat but important to long-term functional outcomes for people living with MDD.
Transparency
Author contributions
S. Nitschky Schmidt was the statistician responsible for this pooled analysis. All authors were involved in the interpretation of the data. GW. Mattingly and EH Reines contributed to the first draft of the paper equally, and O Necking, S. Nitschky Schmidt, and H. Ren revised it critically for intellectual content. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Acknowledgements
The authors wish to thank all participants in the studies, as well as the investigators and sites involved. Medical writing assistance was provided by Anita Chadha-Patel of ACP Clinical Communications (Hertfordshire, UK) and was supported by H Lundbeck A/S.
Declaration of funding
This study was funded by H Lundbeck A/S.
Declaration of financial/other relationships
GW. Mattingly has received consultant fees or honoraria from AbbVie, Acadia, Alkermes, Avanir, Axsome, Boehringer, Eisai, Emalex, Ironshore, Intra-Cellular, Janssen, Lundbeck, Medgenics, Neos, Neurocrine, NLS-1 Pharma AG, Otsuka, Redax, Rhodes, Roche, Sage, Shire, Sunovion, Supernus, Takeda, Teva, and Trispharma. O. Necking, S. Nitschky Schmidt, and E.H. Reines are full-time employees of H. Lundbeck A/S. H. Ren was a full-time employee of H. Lundbeck A/S at the time of manuscript preparation. A reviewer on this manuscript has disclosed that they have received manuscript or speaker’s fees from Astellas, Eisai, Eli Lilly, Elsevier Japan, Janssen Pharmaceuticals, Kyowa Yakuhin, Lundbeck Japan, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD, Nihon Medi-Physics, Novartis, Otsuka Pharmaceutical, Shionogi, Shire, Sumitomo Pharma, Takeda Pharmaceutical, Tsumura, Viatris, Wiley Japan, and Yoshitomi Yakuhin, and research grants from Eisai, Mochida Pharmaceutical, Meiji Seika Pharma, Shionogi and Sumitomo Pharma. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article. The authors may be contacted for further data sharing.
References
- Bauer M, Severus E, Köhler S, et al. World federation of societies of biological psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders. part 2: maintenance treatment of major depressive disorder-update 2015. World J Biol Psychiatry. 2015;16(2):76–95.
- Kalin NH. Advances in understanding and treating mood disorders. Am J Psychiatry. 2020;177(8):647–650.
- Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther. 2015;145:43–57.
- Gonda X, Sharma SR, Tarazi FI. Vortioxetine: a novel antidepressant for the treatment of major depressive disorder. Expert Opin Drug Discov. 2019;14(1):81–89.
- McIntyre RS. The role of new antidepressants in clinical practice in Canada: a brief review of vortioxetine, levomilnacipran ER, and vilazodone. Neuropsychiatr Dis Treat. 2017;13:2913–2919.
- Alvarez E, Perez V, Dragheim M, et al. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15(5):589–600.
- Baldwin DS, Loft H, Dragheim M. A randomised, double-blind, placebo controlled, duloxetine-referenced, fixed-dose study of three dosages of Lu AA21004 in acute treatment of major depressive disorder (MDD). Eur Neuropsychopharmacol. 2012;22(7):482–491.
- Henigsberg N, Mahableshwarkar AR, Jacobsen P, et al. A randomized, double-blind, placebo-controlled 8-week trial of the efficacy and tolerability of multiple doses of Lu AA21004 in adults with major depressive disorder. J Clin Psychiatry. 2012;73(7):953–959.
- Jain R, Mahableshwarkar AR, Jacobsen PL, et al. A randomized, double-blind, placebo-controlled 6-wk trial of the efficacy and tolerability of 5 mg vortioxetine in adults with major depressive disorder. Int J Neuropsychopharmacol. 2013;16(2):313–321.
- Mahableshwarkar AR, Jacobsen PL, Chen Y. A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Curr Med Res Opin. 2013;29(3):217–226.
- Boulenger JP, Loft H, Olsen CK. Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(3):138–149.
- McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557–1567.
- Mahableshwarkar AR, Jacobsen PL, Chen Y, et al. A randomized, double-blind, duloxetine-referenced study comparing efficacy and tolerability of 2 fixed doses of vortioxetine in the acute treatment of adults with MDD. Psychopharmacology. 2015;232(12):2061–2070.
- Jacobsen PL, Mahableshwarkar AR, Serenko M, et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of vortioxetine 10 mg and 20 mg in adults with major depressive disorder. J Clin Psychiatry. 2015;76(5):575–582.
- Mahableshwarkar AR, Jacobsen PL, Serenko M, et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of 2 doses of vortioxetine in adults with major depressive disorder. J Clin Psychiatry. 2015;76(5):583–591.
- Nishimura A, Aritomi Y, Sasai K, et al. Randomized, double-blind, placebo-controlled 8-week trial of the efficacy, safety, and tolerability of 5, 10, and 20 mg/day vortioxetine in adults with major depressive disorder. Psychiatry Clin Neurosci. 2018;72(2):64–72.
- Inoue T, Nishimura A, Sasai K, et al. Randomized, 8-week, double-blind, placebo-controlled trial of vortioxetine in Japanese adults with major depressive disorder, followed by a 52-week open-label extension trial. Psychiatry Clin Neurosci. 2018;72(2):103–115.
- Inoue T, Sasai K, Kitagawa T, et al. Randomized, double-blind, placebo-controlled study to assess the efficacy and safety of vortioxetine in Japanese patients with major depressive disorder. Psychiatry Clin Neurosci. 2020;74(2):140–148.
- Iovieno N, Papakostas GI, Feeney A, et al. Vortioxetine versus placebo for major depressive disorder: a comprehensive analysis of the clinical trial dataset. J Clin Psychiatry. 2021;82(4):20r13682.
- Vieta E, Loft H, Florea I. Effectiveness of long-term vortioxetine treatment of patients with major depressive disorder. Eur Neuropsychopharmacol. 2017;27(9):877–884.
- Boulenger JP, Loft H, Florea I. A randomized clinical study of Lu AA21004 in the prevention of relapse in patients with major depressive disorder. J Psychopharmacol. 2012;26(11):1408–1416.
- Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton pleasure scale (SHAPS). J Affect Disord. 2007;99(1-3):83–89.
- Hasler G, Drevets WC, Manji HK, et al. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781.
- Buckner JD, Joiner TE Jr, Pettit JW, et al. Implications of the DSM's emphasis on sadness and anhedonia in major depressive disorder. Psychiatry Res. 2008;159(1-2):25–30.
- Nierenberg AA. Residual symptoms in depression: prevalence and impact. J Clin Psychiatry. 2015;76(11):e1480.
- Uher R, Perlis RH, Henigsberg N, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med. 2012;42(5):967–980.
- Cao B, Zhu J, Zuckerman H, et al. Pharmacological interventions targeting anhedonia in patients with major depressive disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:109–117.
- McIntyre RS, Loft H, Christensen MC. Efficacy of vortioxetine on anhedonia: results from a pooled analysis of Short-Term studies in patients with major depressive disorder. Neuropsychiatr Dis Treat. 2021;17:575–585.
- Cao B, Park C, Subramaniapillai M, et al. The efficacy of vortioxetine on anhedonia in patients with major depressive disorder. Front Psychiatry. 2019;10:17.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736.
- Sheehan KH, Sheehan DV. Assessing treatment effects in clinical trials with the discan metric of the Sheehan Disability Scale. Int Clin Psychopharmacol. 2008;23(2):70–83.
- Mattingly G, Christensen M, Simonsen K, et al. Effectiveness of vortioxetine in real-world clinical practice: interim results from the relieve study. Eur Psychiatry. 2021;64(S1):S341–S341.
- Baldwin DS, Chrones L, Florea I, et al. The safety and tolerability of vortioxetine: analysis of data from randomized placebo-controlled trials and open-label extension studies. J Psychopharmacol. 2016;30(3):242–252.
- Oluboka OJ, Katzman MA, Habert J, et al. Functional recovery in major depressive disorder: providing early optimal treatment for the individual patient. Int J Neuropsychopharmacol. 2018;21(2):128–144.
