Abstract
Objective
To provide real-word evidence of patients with SCD initiating crizanlizumab, their use of other SCD treatments, and crizanlizumab treatment patterns.
Methods
Using IQVIA’s US-based, Longitudinal Patient-Centric Pharmacy and Medical Claims Databases patients with a diagnosis of SCD between November 1, 2018, and April 30, 2021, and ≥1 claim for crizanlizumab (date of first claim = index date) between November 1, 2019, and January 31, 2021 who were ≥16 years of age, and had ≥12 months of pre-index data were selected for analysis. Two cohorts were identified based on available follow-up time (3- and 6-month cohorts). Patient characteristics were reported along with pre- and post-index SCD treatments and crizanlizumab treatment patterns (e.g. total doses received, gap-days between doses, days on therapy, discontinuation, and restarts).
Results
540 patients met the base inclusion criteria (345 in the 3-month cohort and 262 in the 6-month cohort. Most patients (64%) were female with a mean (SD) age of 35 (12) years overall. Concomitant hydroxyurea use was observed in 19–39% of patients, while concomitant L-glutamine use was observed for 4–8% of patients. 85% of 3-month cohort patients received at least two doses of crizanlizumab, while 66% of the 6-month cohort received at least 4 doses of crizanlizumab. The median number of gap days between doses was 1 or 2.
Conclusions
66% of patients who receive crizanlizumab receive at least 4 doses within 6-months. The low median number of gap days suggests high adherence.
Keywords:
Introduction
Sickle cell disease (SCD) is an inherited genetic blood disorder that progresses into a systemic disease characterized by hemolytic anemia and cycles of microvascular vaso-occlusion that affects approximately 100,000 AmericansCitation1,Citation2. Vaso occlusion is the hallmark of SCD and can lead to serious acute and chronic complications including vaso-occlusive crises (VOC), which are recurrent painful episodes associated with morbidity and mortality. VOCs are the main cause of emergency room (ER) visits (>70%) and hospital admissions (95%) in adult patients with SCD, with prior VOC hospitalization associated with increased rates of complications (e.g. leg ulcers, p = 0.001) and death (p < .001)Citation3–7. VOCs also led to significant costs, with total health care costs generally rising with age ($872–$2,562 per patient-month in the 0–9 and 60–64 year age groups, respectively) and estimated incremental economic burden of SCD estimated at $2.98 billion per year in the US (inflation-adjusted dollar in 2015)Citation8,Citation9.
Treatment options for SCD have been limited historically, with blood transfusions and pain management serving as the mainstays of care until 1998 when hydroxyureaFootnotei gained an SCD indication as the first FDA-approved SCD treatment targeting the pathogenesis of the disease. L-glutamineFootnoteii was subsequently approved in 2017 for an SCD indication, followed by crizanlizumabFootnoteiii and voxelotorFootnoteiv in November of 2019Citation10. The FDA-approved indication for crizanlizumab is to “reduce the frequency of vaso-occlusive crises in adults and pediatric patients aged 16 years and older with sickle cell disease”Citation11.
Given its approval in 2019, published evidence on the real-world effectiveness of crizanlizumab is not readily available. There is a need for a better understanding of crizanlizumab use in clinical practice and real-world outcomes of treated patients to inform the emerging scientific literature. Availability of traditional real-world data (e.g. adjudicated administrative medical claims) was limited at the time of this study due to data lags from the adjudication process. This study used claims data to provide a real-word description of patients with SCD initiating crizanlizumab, their use of other SCD treatments, and crizanlizumab treatment patterns.
Methods
Study population and data sources
This was a retrospective descriptive analysis of health care claims data using the IQVIA open-source medical and pharmacy claims databases. The medical claims database is derived from professional fee claims and provides unadjudicated patient-level diagnoses and procedures for visits to US office-based physicians, ambulatory, and general health care sites including age, gender, and geographically representative commercial, Medicare, Medicaid, and cash claims amounting to >1 billion claims per year and >860,000 providers per month. The pharmacy claims database contains longitudinal data collected directly from pharmacy suppliers for adjudicated dispensed prescriptions sourced from retail (92% coverage), mail (62% coverage of traditional and specialty mail order), and long-term care (76% coverage) amounting to over 150 million unique patients and over 1 million unique prescribers, with data updated monthly.
Patients with a diagnosis of SCD between November 1, 2018 and April 30, 2021 were identified using International Classification of Diseases, Tenth Revision (ICD-10) codes (patients with ≥1 claim during the study period with ICD-10 D57.xx, except D57.3). Among these patients, those who initiated crizanlizumab between November 1, 2019 and January 31, 2021 (index period) were selected into the treatment cohort using Healthcare Common Procedure Coding System (HCPCS) codes (J0791 or C9053 [crizanlizumab administration] OR J3590 [unspecified biologic administration] on the same date as a diagnostic claim for SCD). The indexing timeframe was based on the crizanlizumab approval date (November 2019) and allowed for a 1-year lookback period and a minimum of 3 months (3 m cohort) of follow-up. A subset analysis of the 3 m cohort with 6-months of available (6 m cohort) follow-up was performed. Stability and eligibility rules were applied to help ensure complete capture of claims throughout pre- and post-index periods. The index date was the date of the first crizanlizumab administration. Patients 16 years and older on the index date with the linkage between and stability and eligibility within the medical and pharmacy claims datasets for the pre-index lookback and post-index follow-up periods were included. In medical claims, patients were considered eligible if they had at least 1 outpatient visit during the 12 months prior to the index date, and stable if all claims for the patient were sourced from physicians meeting reporting benchmarks for each month of the study period (e.g. 100% of claims captured in the specified time period). In pharmacy claims, patients were considered eligible if they had at least 1 prescription claim during 12 months prior to the index date, and stable if all claims for the patient were sourced from pharmacies whose weekly volume of claims fell above the median volume of claims in the prior 10 weeks for that same pharmacy. Post-index stability and eligibility were also required (3 months for the 3 m cohort and 6 months for the 6 m cohort).
Covariates
Patient demographic and clinical characteristics were extracted from the linked database from the pre-index period. Age was reported as a continuous variable as well as categorically by age groups, while patient sex, geographical region, and index crizanlizumab claim payer type were reported categorically. When multiple payer types were observed, the following hierarchy was used: Medicare, Commercial, Medicaid, and Unknown. Diagnoses were identified by ICD-10 diagnosis codes, crizanlizumab by HCPCS code, other drug treatments by NDC codes, and blood transfusions by ICD-10, CPT, and HCPCS procedure codes. Patient comorbidities were evaluated by the proportion of patients with claims for select comorbidities, as well as a Charlson Comorbidity Index (CCI) score which was reported as a continuous variable as well as categorically by score category. History of hydroxyurea, L-glutamine, and voxelotor use were reported by the proportion of patients with claims for each treatment in the pre-index period. SCD genotype observed in the pre-index period was reported categorically.
SCD treatments observed after initiating crizanlizumab were evaluated in the 3-month and 6-month post-index periods for the 3 m cohort and 6 m cohort, respectively. Treatments evaluated were hydroxyurea, L-glutamine, and voxelotor, and reported as the proportion of patients with claims for each treatment in the post-index period, as well as a continuous variable for the number of claims for each treatment in the post-index period.
Crizanlizumab treatment patterns were reported as the total number of doses received (categorical), gap days (d) between doses (measured as days between the end of the days of supply of one dose to the next administered dose, where days supply was set per the United States package insert to 14 d for the initial crizanlizumab dose, 28 d for each subsequent doseCitation11; the gap days calculations excluded patients with crizanlizumab discontinuation), the proportion of patients with fewer administrations than should have been given relative to the dosing schedule and follow-up period, days on therapy (continuous), the proportion of patients with crizanlizumab discontinuation using a ≥ 30-d gap to define discontinuation for the 3 m cohort and ≥60-d gap for the 6 m cohort, and proportion of patients restarting crizanlizumab after discontinuation. In cases where the criteria for discontinuation cannot be met because a gap in therapy is truncated by the end of follow-up, the end of therapy was defined as the end of days supply.
Statistical analyses
This was a descriptive study and involved no comparative analyses. For continuous variables, means, standard deviations (SD), and medians with interquartile ranges (IQR) were generated. For categorical variables, frequencies and percentages were presented. The results were analyzed by cohort and stratified by age group. Baseline measures were evaluated over the 12-months prior to the index date (pre-index period), while post-index measures were evaluated from the index date to the end of follow-up per cohort (post-index period; 3 months for the 3 m cohort, 6 months for the 6 m cohort). All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
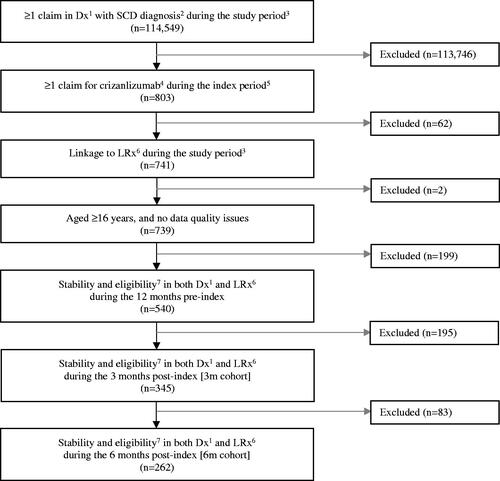
In total, 540 patients met the index and pre-index criteria, while 345 patients met the post-index criteria for the 3 m cohort, and 262 of these patients also met the post-index criteria for the 6 m cohort (). Patient demographic and clinical characteristics for the 345 patients who met the post-index criteria for the 3 m cohort and for the 262 patients who met the post-index criteria for the 6 m cohort are provided in Supplementary Tables S1 and S2, respectively.
Figure 1. Patient attrition overall and among patients with > 3 months (3 m cohort) and > 6 months (6 m cohort) of follow-up post crizanlizumab initiation. 1Dx = IQVIA Patient Centric Medical Claims database. 2SCD diagnosis = ICD-10 D57.xx, except D57.3. 3Study period = 01 November 2018–30 April 2021. 4Crizanlizumab = J0791 (crizanlizumab) OR C9053 (crizanlizumab) OR J3590 (unspecified biologic) on the same day as the claim for SCD (ICD-10 D57.xx, except D57.3). 5Index period = 01 November 2019–31 January 2021. 6LRx = IQVIA Longitudinal Prescription Claims database. 7Stability and eligibility = In Dx: At least 1 outpatient visit during the specified period AND provider stability during the specified period; In LRx: At least 1 prescription claim during the specified period AND pharmacy stability (inclusion of data from pharmacies that consistently report data monthly) during the specified period.
Patient characteristics
presents patient demographic and clinical characteristics overall and by age group. Overall, the mean (SD) age was 35 (12) years, 64% were female, and commercial insurance was the most frequently observed payer type (48%) followed by Medicaid (31%) and Medicare (21%). Stratified by age group, while commercial insurance remained the most commonly observed coverage type, Medicaid was observed as the second most common payer in the younger age categories while Medicare was the second most common payer in the 55+ year age group. Instances of comorbid conditions appeared to increase with age, as reflected in the mean (SD) CCI scores by age group: 0.93 (1.23) for the 16–34 year age category, 1.47 (1.82) for the 35–54 year age group, and 2.91 (2.51) for the 55+ year age group; as well as in the relative proportions of patients with a CCI score of 0 across age categories: 47% for the 16–34 year age group, 41% for the 35–54 year age group, and 19% for the 55+ year age group. The most observed comorbid conditions across age categories were chronic pain/fibromyalgia and osteoarthritis. Chronic conditions such as hypertension (14% 16–34 year, 38% 35–54 year, 65% 55+ year), diabetes mellitus (2% 16–34 year, 10% 35–54 year, 28% 55+ year), dyslipidemia (3% 16–34 year, 7% 35–54 year, 23% 55+ year), and peripheral vascular disease (4% 16–34 year, 7% 35–54 year, 23% 55+ year) appeared to increase in frequency with age, while certain other conditions such as asthma (28% 16–34 year, 15% 35–54 year, 12% 55+ year), alcohol/drug abuse (28% 16–34 year, 31% 35–54 year, 12% 55+ year), and depression (21% 16–34 year, 20% 35–54 year, 7% 55+ year) appeared to decrease in frequency with age. For SCD-related comorbidities associated with organ damage, the most observed were acute chest syndrome (ACS, 25%), acute kidney injury (AKI, 13%), and pulmonary hypertension (10%). In the 12-month pre-index period, patients were most likely to have used hydroxyurea across age groups (56%), followed by L-glutamine (13%). For the data sources used in this study, data for voxelotor appeared, at least in part, to be blocked by the manufacturer. The most common SCD genotype observed in the pre-index period was Hb SS across age groups (98%), followed by Hb SC (51%).
Table 1. Patient demographic and clinical characteristics by age group for all patients initiating crizanlizumab during the study index period.
Other SCD treatments
presents results by cohort (not mutually exclusive) and age groups (mutually exclusive within each cohort). These tables describe both pre- and post-index use of hydroxyurea, L-glutamine, and voxelotor, with the pre-index utilization outcomes providing context for post-index utilization outcomes. The pre-index window of the evaluation was set equal to the post-index window of evaluation in these analyses, while the pre-index window of evaluation for was the full 12-month pre-index period. Post-index hydroxyurea use across cohorts and age groups ranged from 19 to 39% of patients while concomitant L-glutamine use was observed for 4–8% of patients. When comparing post-index use within age groups for each cohort, the greatest proportion of patients had concomitant hydroxyurea use (e.g. 32% of the 3 m cohort, age group 16–34 years), followed by L-glutamine (e.g. 4% of the 3 m cohort, age group 16–34 years). Examined across age groups, concomitant use of hydroxyurea appeared to decline with age while concomitant use of L-glutamine increased with age, regardless of available follow-up time.
Table 2. Hydroxyurea, L-glutamine, and voxelotor use, pre-and post-index, by cohort (3 m vs. 6 m) overall and by age group.
Crizanlizumab treatment patterns
presents the total number of crizanlizumab doses received and gaps between doses, by cohort (not mutually exclusive). Looking at the total doses received, of the 345 patients with 3 months post-index follow-up (3 m cohort), 85% of patients received at least two doses of crizanlizumab. Of the 262 patients with 6 months post-index follow-up (6 m cohort), 66% of patients received at least 4 doses of crizanlizumab. Per cohort, the average number of gap days between doses appeared to decrease between each subsequent dose with a mean (SD) of 7 (8) days in the 3 m cohort and 10 (13) days in the 6 m cohort between the end of the days supply of dose one and the administration of dose two. Gap days between doses decreased by dose 3 to dose 4 to a mean (SD) of 2 (4) days in the 3 m cohort and 8 (12) days in the 6 m cohort. The median number of gap days between doses was one or two days in both cohorts ().
Figure 2. Average gap days between doses among patients with > 3 months (3 m cohort) and > 6 months (6 m cohort) of follow-up post crizanlizumab initiation1,2. *Note that for the 3 m cohort, patients are not expected to receive more than 4 doses during the 3-months of follow-up. 1Gap days measured as days between the end of the days of supply of one dose to the next administered dose, where days supply was set to 14 d for the initial crizanlizumab dose, 28 d for each subsequent dose. 2Gap days were calculated among patients who did not discontinue crizanlizumab (discontinuation defined by a ≥30-d gap for the 3-month cohort and ≥60-d gap for the 6-month cohort from the end of days of supply) over the follow-up period (3-months post-index for 3-month cohort, 6-months post-index for 6-month cohort). In cases where the criteria for discontinuation cannot be met because a gap in therapy is truncated by the end of follow-up, the end of therapy was defined as the end of days supply.
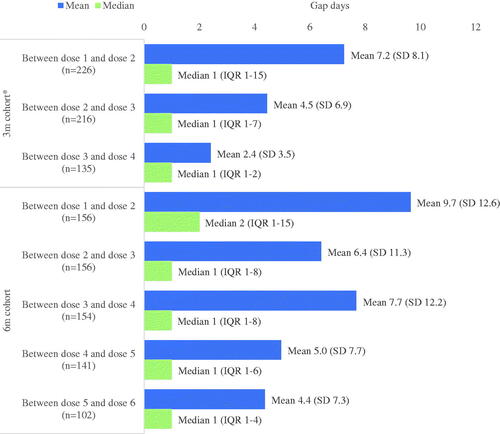
Table 3. Total doses received and gaps between doses by cohort (3 m vs. 6 m) overall.
presents persistence, discontinuation, and restart after discontinuation results by cohort (not mutually exclusive), and age group (mutually exclusive within each cohort). Overall, the mean (SD) time on therapy was 66 (31) days and 123 (63) days for the 3 m and 6 m cohort, respectively. By age group, the youngest category (16–34 years) appeared to have a shorter mean (SD) days on therapy (i.e. 62 d (31) for the 3 m cohort, 118 d (64) for the 6 m cohort) compared to the older age groups (i.e. 70 d (29) for ages 35–54 and 71 d (31) for ages 55+ for the 3 m cohort, 129 d (62) for ages 35–54 and 126 d (62) for ages 55+ for the 6 m cohort). By cohort, 34% of the 3 m and 40% of the 6 m cohort had crizanlizumab discontinuation, while of those that discontinued, 25% and 15% restarted by the end of follow-up for the 3 m and 6 m cohorts, respectively. The youngest age group had the highest proportion of crizanlizumab discontinuation (e.g. for the 3 m cohort 40% of those 16–34 years of age discontinued crizanlizumab, compared to 28% of those 35–54 years of age and 26% of those 55+ years of age) per cohort. For the 3 m cohort, a higher proportion of younger patients also restarted crizanlizumab within the study period (26% of those 16–34 years of age and those 35–54 years of age restarted crizanlizumab, compared to 13% of those 55+ years of age), while for the 6 m cohort, the proportion was similar (15–18%) across age groups.
Table 4. Persistence, discontinuation, and restart after discontinuation, by cohort (3 m vs. 6 m) overall and age group.
Discussion
Using open-source medical and pharmacy claims data, this study examined real-world characteristics of patients initiating crizanlizumab, the use of other SCD treatments after initiation of crizanlizumab, as well as crizanlizumab treatment patterns. To our knowledge, the present study is the first to describe patients utilizing crizanlizumab therapy in a real-world setting. While there remains a paucity of scientific literature regarding crizanlizumab use (particularly within real-word settings), age and gender findings from the present study appear to align with what has been reported thus far with real-world samples of SCD patients (mostly without crizanlizumab use) comprising 49%–67% of females and a mean or median age range of 20–32 years (means from Lobo et al. and Shah et al. respectively) or 17–29 years (medians reported by Lobo et al. and Ataga et al. in the SUSTAIN trial, respectively) vs. a median age of 32 years in the present study)Citation12–14. It is worth noting that the youngest median age of 20 years was reported by Lobo et al. who included patients of all ages, 53% of whom were children aged ≤18 years. The South was the most represented geographic region, a finding not noted in the real-world crizanlizumab literature (i.e. Shah et al.). Rather, Shah and colleagues observed the highest proportion of crizanlizumab patients in the Northeast, an observation most likely due to the Medicaid data source used in their study as two states within in the Northeast (i.e. New York and Pennsylvania) include some of the largest numbers of Medicaid enrollees in the US (6,523,867 and 3,352,159 respectively as of 2022)Citation15. The insurance distribution followed the expectations that most patients would be covered by commercial insurance and older patients would be more likely to utilize Medicare plans. The increase in the proportion of patients reporting chronic conditions was in line with the observed trend that the older age groups appeared to be sicker, with 43% of patients 16–34 reported as having a CCI score of 0 compared to just 19% for those aged 55 years and older. Of note, while this study observed that 7% overall and 23% of patients 55+ years of age with peripheral vascular disease, this may actually represent another vascular process as previous studies have reported peripheral vascular disease to be uncommon in SCD patientsCitation16,Citation17.
For SCD-related comorbidities associated with organ damage, ACS was more commonly observed for younger patients, while both AKI and pulmonary hypertension were more commonly observed for older patients. In the 12-month pre-index period, hydroxyurea and L-glutamine use were observed more commonly among younger patients, though this may reflect these patients being earlier in their treatment journeys; for the older patients, their use of these medications likely predated the study period and thus were not captured. Our findings of overall proportions of patients with SCD having SCD-related comorbidities associated with organ damage was consistent with that seen in the literature as 18% patients in the SUSTAIN trial had a history of acute chest syndrome (vs. 25% found in the present study) and 7% had a history of priapism (4% in the present study)Citation14. The 2020 real-world retrospective study of Medicaid claims conducted by Shah et al. reported ACS among a smaller proportion (3%) of the total sample, perhaps a reflection of differences among baseline measurement periods (6 months pre-index in Shah et al. vs. 12 months pre-index in the present study) or a finding that may be explained by SCD severity in that patients who receive crizanlizumab therapy, such as those in the present study, may have more advanced/active SCD vs. a general population of patients with SCD as was studied by Shah et al.
Hydroxyurea use reported in the present study (57%) was similar to that observed in the SUSTAIN trial (62%) and higher than the results reported by Lobo et al. and Shah et al. (39% and 11% respectively). Underlying reasons for the observed differences in the reported rates of hydroxyurea use are likely similar to those hypothesized for observed differences in ACS rates (i.e. shorter baseline measurement periods [6 months vs. 12 months in the present study] and SCD severity between Lobo et al. Shah et al. and the present study). Notably, hydroxyurea use seems to have decreased post-initiation of crizanlizumab though the results were descriptive in nature (i.e. no hypothesis testing was conducted).
Most of the 3 m cohort (85%) received at least two doses of crizanlizumab, while two-thirds of the 6 m cohort (66%) received at least 4 doses of crizanlizumab. The mean number of gap days decreased between each subsequent dose among both cohorts and the median gap days observed suggests that most of these patients received crizanlizumab doses per the label-recommended schedule. The median time on crizanlizumab therapy was 86 days among the 3 m cohort (i.e. 96% of the 3 m follow-up period) and 154 d for the 6 m cohort (i.e. 86%% of the 6 m follow-up period). This finding coupled with the observation that 48% and 40% of patients in the 3 m and 6 m cohorts had days supply on hand at the end of the study period suggests that many patients remained on therapy for clinically significant periods of time. Discontinuation rates increased as time on therapy increased and decreased overall with age, while restarts among those who discontinued crizanlizumab were highest in the youngest age group for each cohort. Of the 106 patients in the 6 m cohort that discontinued crizanlizumab, 15% (16 patients) restarted crizanlizumab within the 6-month post-index period.
This study had several limitations, including those inherent to the use of claims: a prescription claim indicates only that a prescription has been filled, not that the prescription has been taken; diagnoses may be miscoded in error or as a result of nuances in insurance coverage/reimbursement; care that occurs outside of the systems may not be captured (e.g. SCD therapies received in the inpatient setting that do not result in a CMS-1500 medical claim); patient care that are not included on insurance claims such as reasons for discontinuation are not available. Open-source databases pose additional limitations compared to traditional closed-source claims databases including a lack of an enrollment file to assess continuous enrollment. A proxy measure that relied on health service utilization was used to determine continuous activity with the medical providers and pharmacies contributing to the database, in place of the more traditional measure of continuous enrollment seen with closed claims analyses. Further, healthcare encounters occurring outside of the network of providers that contribute to the medical claims data are not captured. As the medical claims database is primarily generated in the process of outpatient encounters, resource utilization or certain events may be under-reported (e.g. inpatient encounters). The impact of COVID-19 restrictions or other access barriers to crizanlizumab use are also unknown. Despite these limitations, we believe that our study provides important information on the real-world use of crizanlizumab, as these open-source databases are able to provide an earlier look at patient characteristics, use of other SCD medications, and crizanlizumab treatment patterns than are available in more traditional adjudicated claims databases.
Conclusions
Results from this analysis suggest that most patients initiating crizanlizumab are 16–34 years of age and received hydroxyurea prior to crizanlizumab. These real-world results suggest that 66% of patients who receive crizanlizumab receive at least 4 doses within 6-months, patients receiving multiple doses appear to have a reduction in gap-days with each subsequent dose, and that of those who discontinue treatment, 6% restart crizanlizumab within the 6-months post-index, respectively. The low median number of gap days suggests high adherence to the label-recommended dosing schedule. Due to the nature of claims data, the reason for discontinuation or gaps between doses is unknown. The impact of COVID-19 restrictions or other access barriers on crizanlizumab use is unknown. Given the relatively small sample, results may not generalize to all crizanlizumab users.
Transparency
Author contributions
PD, JP, SL, GY, MY, CC, and CM were involved in the conception, design, and interpretation of the data. JH, MY, CC, and CM were involved in the analysis of the data. All authors were involved in the drafting and revising of the article critically for intellectual content, the final approval of the version to be published, and agree to be accountable for all aspects of the work.
IQV_NVS_Criz_2745908__CMRO_20221221_Supplementary_Materials_to_client.docx
Download MS Word (44.7 KB)Acknowledgements
The authors wish to acknowledge Dionne Hines and Rolin Wade for their contribution to study conception and design, Yao Cao for her analysis contribution and Wanjiku Kariuki for her assistance with manuscript submission.
Declaration of funding
This study was funded by Novartis.
Declaration of financial/other relationship
PD served as a consultant for Novartis. JP, SL, and GY are employees of Novartis. MY, CC, CM, and JH are employees of IQVIA, which was contracted by Novartis to perform this study. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Notes
i Droxia
ii Endari
iii Adakveo
iv Oxbryta
References
- Sundd P, Gladwin MT, Novelli EM. Pathophysiology of sickle cell disease. Annu Rev Pathol Mech Dis. 2019;14(1):263–292.
- CDC.gov [Internet]. Data & statistics on sickle cell disease. Washington (DC): Centers for Disease Control and Prevention; [2022 May 2; cited 2023 Mar 3]. Available from: https://www.cdc.gov/ncbddd/sicklecell/data.html
- Shah N, Bhor M, Xie L, et al. Sickle cell disease complications: prevalence and resource utilization. PLOS One. 2019;14(7):e0214355.
- Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79(1):17–25.
- Powars DR, Chan LS, Hiti A, et al. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine. 2005;84(6):363–376.
- Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148(2):94–101.
- Yusuf HR, Atrash HK, Grosse SD, et al. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999–2007. Am J Prev Med. 2010;38(4):S536–S541.
- Huo J, Xiao H, Garg M, et al. The economic burden of sickle cell disease in the United States. Value Health. 2018;21:S108.
- Kauf TL, Coates TD, Huazhi L, et al. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84(6):323–327.
- FDA.gov [Internet]. The FDA encourages new treatments for sickle cell disease; [2018 Jun 18; cited 2022 Jul 11]. Available from: https://www.fda.gov/consumers/consumer-updates/fda-encourages-new-treatments-sickle-cell-disease.
- Adakveo®. Package Insert. 2019. [cited 2023 Mar 3]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761128s000lbl.pdf
- Shah N, Bhor M, Xie L, et al. Medical resource use and costs of treating sickle cell-related vaso-occlusive crisis episodes: a retrospective claims study. J Health Econ Outcomes Res. 2020;7(1):52–68.
- Lobo C, Moura P, Fidlarczyk D, et al. Cost analysis of acute care resource utilization among individuals with sickle cell disease in a middle-income country. BMC Health Serv Res. 2022;22(1):42.
- Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429–439.
- Medicaid.gov. 2019. [cited 2023 Mar 3]. Medicaid and CHIP enrollment data highlights. Available from: https://www.medicaid.gov/medicaid/program-information/medicaid-and-chip-enrollment-data/report-highlights/index.html.
- Elsharawy MA, Moghazy KM, Shawarby MA. Atherosclerosis in sickle cell disease – a review. Int J Angiol. 2009;18(02):62–66.
- Usmani A, Machado RF. Vascular complications of sickle cell disease. Clin Hemorheol Microcirc. 2018;68(2–3):205–221.
