Abstract
Objective
The treatment landscape for the prevention of migraine has rapidly evolved in recent years with the advent of calcitonin gene-related peptide therapy, including erenumab. The objective of this study was to assess patient-reported treatment satisfaction among erenumab users.
Methods
This retrospective, cross-sectional study used data from the 2019 US National Health and Wellness Survey collected during March–July 2019. Respondents self-reporting physician-diagnosed migraine and currently using erenumab were analyzed. Treatment satisfaction was measured on a seven-point Likert scale. Data were further reported by the duration of erenumab treatment. Data on respondents’ socio-demographic characteristics and treatment patterns were also collected.
Results
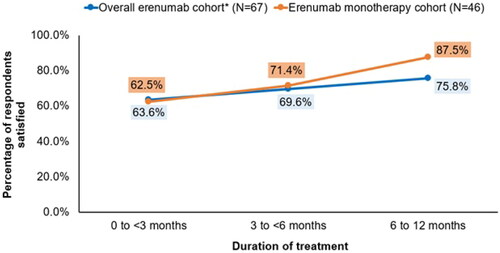
Overall, 67 respondents using erenumab with or without other migraine preventives for up to 1 year were included in the analysis. The mean (standard deviation) age was 46.7 (12.9) years. Most of the respondents were women (86.6%), White (74.6%), and commercially-insured (67.2%). Notably, 40.3% had ≥1 comorbidity per the Charlson Comorbidity Index. Approximately half of the respondents were college graduates and employed (49.3% each). Among the 67 respondents, 46 received erenumab exclusively. Across both cohorts, the percentage of respondents who were satisfied with erenumab treatment was slightly higher among those with a longer treatment duration (overall erenumab cohort: 63.6%, 69.6%, and 75.8% for 0–<3, 3–<6, and 6–12 months, respectively; erenumab monotherapy cohort: 62.5%, 71.4%, and 87.5% for 0–<3, 3–<6, and 6–12 months, respectively). Treatment patterns before switching to erenumab revealed that most respondents had used ≥1 preventive treatment for migraine (80.6%; 54/67), over two-thirds (33/54) of whom had ≥2 treatment failures owing to nonresponse.
Conclusion
Satisfaction was high among long-term erenumab users, indicating that those using erenumab for a longer duration are more satisfied. Furthermore, this study provided insights on the basic socio-demographics, disease characteristics, and health behaviors of erenumab users as well as their treatment patterns before switching to erenumab.
Introduction
Migraine is a debilitating neurological disease that typically affects individuals during their prime working years (25–55 years of age) and is three times more common in women than men.Citation1,Citation2 The impact of migraine symptoms extends beyond physical pain, often leading to poor quality of life among those affected.Citation3,Citation4
The management of migraine is multifaceted and includes the use of acute (specific and nonspecific) and preventive (antiepileptics, beta-blockers, antidepressants, calcium channel antagonists, botulinum neurotoxins [for chronic migraine], serotonin antagonists, and anti–calcitonin gene-related peptide [CGRP] medications) treatments along with patient education and lifestyle modifications.Citation5 Despite this, the burden of migraine persists, partly because of the widespread underuse of and inadequate response and poor adherence to the existing treatments.Citation4,Citation6–14 Moreover, frequent failures (i.e. discontinuations because of lack of efficacy or tolerability) of existing treatments contribute to this burden.Citation13,Citation15
The treatment landscape for the prevention of migraine has rapidly evolved in recent years owing to clinical benefits and favorable safety profiles of CGRP monoclonal antibodies.Citation16,Citation17 Erenumab (erenumab-aooe in the United States [US]; Aimovig®) is the first fully human CGRP receptor monoclonal antibody approved in the US for the preventive treatment of migraine in adults, with a mechanism of action that functions to bind to CGRP receptors to antagonize CGRP receptor function.Citation18 While data from controlled clinical trials and numerous real-world studies have demonstrated the therapeutic efficacy of erenumab in adults with migraine,Citation19–29 there is a paucity of data on patient-reported treatment satisfaction with erenumab.Citation27,Citation29
Treatment satisfaction is an important outcome and can provide valuable insights into patients’ perspectives on their current treatment and their willingness to continue treatment. Therefore, the primary objective of this study was to provide information on patient-reported treatment satisfaction among migraine respondents using erenumab in the US. In addition, this study analyzed the characteristics and treatment patterns of migraine respondents who were using erenumab.
Methods
Study design and data source
This cross-sectional study used data from the 2019 US National Health and Wellness Survey (NHWS) collected between March and July 2019. The NHWS is a large (∼75,000 respondents in the US every year), self-reported, real-world patient database that contains patient-level information obtained via a self-administered, internet-based, opt-in survey of a sample of adults (aged 18 years or older). Potential NHWS respondents are recruited through opt-in online survey panels, using a quota-sampling framework to mimic representativeness in terms of age, sex, and race distribution in the US. The protocol and questionnaire used for the NHWS were reviewed and granted an exemption by the Pearl Institutional Review Board (Indianapolis, IN, USA).
Study population
This study included respondents who self-reported physician-diagnosed migraine and were currently using erenumab with or without other migraine preventives (onabotulinumtoxinA and/or oral migraine prophylactics [divalproex sodium, topiramate, propranolol, and amitriptyline]) concomitantly for up to 1 year.
Outcome measures
The primary outcome measure was patient-reported treatment satisfaction, which was assessed using a seven-point Likert scale (1 = extremely dissatisfied to 7 = extremely satisfied). Based on their responses, the respondents were stratified into those who were dissatisfied (reporting 1 = extremely dissatisfied to 4 = neither dissatisfied nor satisfied) versus those who were satisfied (reporting 5 = somewhat satisfied to 7 = extremely satisfied) with the treatment. Data were reported for the overall cohort as well as stratified by the duration of treatment (<3 months, 3–<6 months, and 6–12 months).
Furthermore, data on socio-demographic characteristics and treatment patterns were collected. Treatment pattern information was obtained from responses to questions about past use of migraine medications, reasons for switching treatment, and the number of preventive medications received and failed before erenumab.
Statistical analysis
Descriptive statistics were used to summarize all study variables. Continuous variables were described using means and standard deviations (SDs), whereas categorical variables were summarized as counts and percentages. Data analyses were performed by Cerner Enviza using SPSS version 23.0 and R statistical software version 4.0.2.
Results
Respondents’ disposition
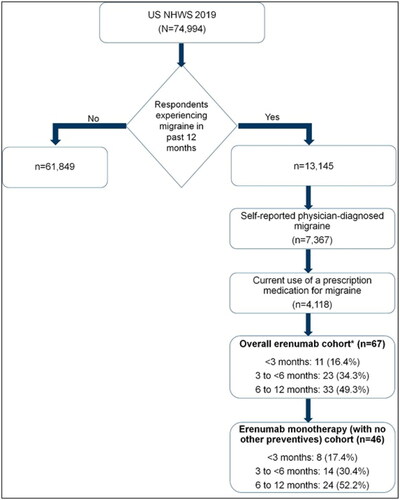
At the time of the survey, 67 respondents reported using erenumab with or without other migraine preventives (hereafter referred to as the overall erenumab cohort) for up to 1 year. Among these, 46 reported using erenumab exclusively with no other concomitant preventives (i.e. onabotulinumtoxinA and/or oral migraine prophylactics [divalproex sodium, topiramate, propranolol, and amitriptyline]; hereafter referred to as the erenumab monotherapy cohort) ().
Figure 1. Selection of study population from the 2019 NHWS in the US. NHWS: National Health and Wellness Survey; US: United States. *Erenumab with or without other migraine preventives (onabotulinumtoxinA and/or oral migraine prophylactics, such as divalproex sodium, topiramate, propranolol, and amitriptyline).
Respondents’ characteristics
presents the socio-demographic characteristics of the respondents using erenumab. The mean (SD) age of the respondents was 46.7 (12.9) years, and 86.6% were women. Respondents were predominantly White (74.6%), commercially insured (67.2%), and married or living with a partner (59.7%). Approximately 42.0% were former or current smokers, 71.6% were occasional drinkers, and 40.3% had at least one comorbid condition per the Charlson Comorbidity Index. Approximately half of the respondents were college graduates and employed (49.3% each). Erenumab users had a longstanding migraine history with a mean (SD) time since diagnosis of 19.2 (13.6) years, and 77.3% reported severe migraines when not using any medication.
Table 1. Characteristics of the migraine respondents using erenumabTable Footnotea.
Treatment satisfaction
Of the 67 respondents in the overall erenumab cohort, 11 (16.4%) received treatment for <3 months, 23 (34.3%) received treatment for 3–<6 months, and the remaining 33 (49.3%) received treatment for 6–12 months. In the erenumab monotherapy cohort (n = 46), 8 (17.4%), 14 (30.4%), and 24 (52.2%) respondents received erenumab exclusively for <3 months, 3–<6 months, and 6–12 months, respectively.
Overall, 71.6% (48/67) of respondents in the overall erenumab cohort were satisfied with their treatment. In the erenumab monotherapy cohort, 78.3% (36/46) of respondents were satisfied with erenumab. Across both cohorts, the percentage of respondents who were satisfied with erenumab treatment was slightly higher among those with a longer treatment duration (). Importantly, erenumab monotherapy users (87.5%) who received treatment for 6–12 months reported the highest treatment satisfaction ().
Treatment patterns
Overall, 61.2% (41/67) of respondents in the overall erenumab cohort had a history of migraine-specific medication use before switching to erenumab. Of these, most respondents reported that erenumab replaced their existing treatment (61.0%; 25/41), whereas the remaining (34.1%; 14/41) had erenumab added to their existing treatment regimen (). The most common reasons for switching to erenumab included physician’s recommendation (72.0%; 18/25) and nonresponse to the previous treatment (60%; 15/25). The other infrequent reasons reported were for reducing side effects (8.0%; 2/25) and lowering costs (4.0%; 1/25).
Table 2. Treatment patterns among migraine respondents before starting erenumabTable Footnotea.
A total of 80.6% (54/67) of respondents in the overall erenumab cohort reported using at least one preventive treatment for migraine before switching to erenumab. Of these patients, 61.1% (33/54) reported treatment failure of two or more prior preventive treatments because of nonresponse ().
Discussion
The current study provides early insights on treatment satisfaction with erenumab in real-world practice. Furthermore, this study highlights the characteristics of erenumab users and treatment patterns before switching to erenumab. The results demonstrated high levels of patient-reported treatment satisfaction (as high as 87.5%) among migraine respondents using erenumab. The respondents surveyed in this study were reflective of the general migraine population in the US, with the majority reporting failure of two or more prior preventive treatments before switching to erenumab.
While treatment guidelines for migraine emphasize the use of preventive medications,Citation30 evidence indicates that the existing preventives are associated with poor adherence, persistence, and discontinuation, and patients with migraine are often dissatisfied with their treatments because of a lack of efficacy and increased side effects.Citation6,Citation7,Citation9,Citation10 These findings indicate that the previously existing migraine preventives did not fully meet the needs of most patients with migraine.
High satisfaction is critical in promoting acceptance of any new preventive treatment and improving health outcomes in patients with migraine.Citation31 Two studies that assessed patient satisfaction with existing preventive medications (topiramate, amitriptyline, propranolol, onabotulinumtoxinA, and nortriptyline) reported suboptimal levels of satisfaction.Citation6,Citation9 The current study, however, assessed patient-reported treatment satisfaction among 67 migraine respondents who used erenumab with or without other preventives within the US NHWS. The results revealed that most respondents who received erenumab (as monotherapy or in combination with other migraine preventives) reported high satisfaction (overall erenumab cohort: 71.6%; erenumab monotherapy cohort: 78.3%). Notably, satisfaction was as high as 87.5% among respondents who received erenumab as monotherapy for 6–12 months, suggesting that participants who received erenumab for a longer duration were more satisfied. The high level of satisfaction with erenumab observed in the current study was consistent with German real-world studies, which reported high satisfaction (≥80%) among patients with highly therapy-refractory migraine who received erenumab.Citation27,Citation29
Although not assessed in this study, it is reasonable to conclude that the high satisfaction with erenumab may be related to better clinical benefits (improvement in migraine-related disability, favorable risk-benefit profile, fewer migraine symptoms, and a better health-related quality of life [HRQoL]) compared with conventional treatments, most of which are repurposed for migraine prevention. These observations corroborate with previous findings of another real-world study in which the majority of patients reported clinical benefits (83%) and high satisfaction (80%) with erenumab treatment.Citation29 Moreover, slightly better satisfaction among respondents who received erenumab exclusively might possibly be due to patients’ perceptions of effectiveness, preference for monotherapy, and fewer adverse effects versus erenumab plus other preventives. However, further research is needed to validate this hypothesis.
Interestingly, the characteristics of respondents enrolled in this study were largely comparable to that of the patient population in clinical trials and real-world studies.Citation21,Citation23,Citation25,Citation26,Citation28,Citation29,Citation32,Citation33 Before switching to erenumab, most respondents in this study had taken at least one prior preventive for migraine (80.6%; 54/67), and over two-thirds (33/54) had experienced ≥2 treatment failures because of nonresponse, which is comparable to the treatment patterns reported by other real-world studies.Citation13,Citation29,Citation34–36 The high levels of satisfaction with erenumab observed in this study contribute to the growing evidence base and extend support to the other real-world studies that have demonstrated the effectiveness of erenumab in patients with migraine irrespective of prior treatment failures.Citation22–24,Citation26,Citation27,Citation29,Citation37
Given the small sample size of erenumab users in the current study, more studies are needed to generate awareness regarding the real-world benefits of erenumab among patients with migraine. Furthermore, prospective longitudinal studies are required to confirm the current findings and investigate the factors that influence treatment satisfaction among erenumab users.
Several caveats of this study merit consideration. The small sample size of this study (owing to the recent introduction of erenumab in the US market combined with the nationally representative sampling technique of all US individuals irrespective of condition/treatment) limits the generalizability of the results. Because this was a cross-sectional survey, the causal and longitudinal relationship of patient-reported treatment satisfaction with erenumab use could not be established. All data (migraine diagnosis, respondent characteristics, prescribed treatments, and satisfaction) were self-reported and were not corroborated with medical records or physicians. Moreover, a potential bias could exist related to the demographics of the survey participants, and therefore, the results need to be interpreted with caution. Although the NHWS used in this study broadly mirrors the US population in terms of age, sex, and race, as with any other patient-reported survey, it likely under-represents people uncomfortable with online survey administration or without access to it, as well as the less healthy older people, institutionalized patients, and those with severe comorbidities and disabilities. A further limitation of this study is that the high costs associated with erenumab may impact the generalizability of results by biasing the erenumab-treated population to individuals with better financial status and/or comprehensive insurance coverage. Finally, this study used a descriptive methodological approach; therefore, inferential studies are needed to confirm the study findings.
Nevertheless, the key strength of this study lies in the NHWS sampling approach, which ensures that the study sample was representative of the general adult population in the US. In addition, this study did address a knowledge gap by providing real-world data on treatment satisfaction with erenumab, which is not typically assessed in controlled clinical trials or available in administrative claims databases.
Conclusion
In summary, this real-world study supports the use of erenumab as a valuable preventive treatment option with high satisfaction among patients with migraine. The magnitude of satisfaction was high among long-term erenumab users, indicating that participants who receive erenumab for a longer duration are more satisfied. Moreover, the results of this study increase our understanding of the characteristics and treatment patterns of erenumab users. As the use of erenumab increases, further longitudinal studies examining erenumab and patient-reported outcomes (satisfaction, migraine symptoms, and HRQoL) and the economic impact of improved migraine management are warranted to confirm the current findings and establish the long-term effectiveness of erenumab.
Transparency
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. PP, LY, SG, JF, MAC, ST, MF, PJ, JT, RS, and PV have made substantial contributions to the analysis or interpretation of data, drafted the work or substantively revised it, approved the submitted version, and agree to be accountable for the work.
Declaration of funding
Funding for this study was provided by Novartis Pharma AG, Basel, Switzerland.
Declaration of financial/other relationships
PP, ST, PJ, and RS are employees of Novartis. JF, PV, MAC, and MF are employees and own stock in Novartis. LY, JT, and SG are employees of Cerner Enviza. A peer reviewer on this manuscript has disclosed that they are currently employed by Lundbeck. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
References
- Hazard E, Munakata J, Bigal ME, et al. The burden of migraine in the United States: current and emerging perspectives on disease management and economic analysis. Value Health. 2009;12(1):55–64.
- Rizzoli P, Mullally WJ. Headache. Am J Med. 2018;131(1):17–24.
- Agosti R. Migraine burden of disease: from the patient’s experience to a Socio-Economic view. Headache. 2018;58:17–32.
- Vo P, Fang J, Bilitou A, et al. Patients’ perspective on the burden of migraine in Europe: a cross-sectional analysis of survey data in France, Germany, Italy, Spain, and the United Kingdom. J Headache Pain. 2018;19(1):82.
- Ong JJY, de Felice M. Migraine treatment: current acute medications and their potential mechanisms of action. Neurotherapeutics. 2018;15(2):274–290.
- Bigal ME, Serrano D, Reed M, et al. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71(8):559–566.
- Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53(4):644–655.
- Bonafede M, Wilson K, Xue F. Long-term treatment patterns of prophylactic and acute migraine medications and incidence of opioid-related adverse events in patients with migraine. Cephalalgia. 2019;39(9):1086–1098.
- Clark M, Schwedt T, Tepper S, et al. Patient satisfaction with prophylactic migraine medications. Headache. 2017;57:133.
- Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20(1):22–33.
- Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia. 2017;37(5):470–485.
- Hepp Z, Dodick DW, Varon SF, et al. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478–488.
- Martelletti P, Schwedt TJ, Lanteri-Minet M, et al. My migraine voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain. 2018;19(1):115.
- Woolley JM, Bonafede MM, Maiese BA, et al. Migraine prophylaxis and acute treatment patterns among commercially insured patients in the United States. Headache. 2017;57(9):1399–1408.
- Korolainen MA, Kurki S, Lassenius MI, et al. Burden of migraine in Finland: health care resource use, sick-leaves and comorbidities in occupational health care. J Headache Pain. 2019;20(1):13.
- Do TP, Guo S, Ashina M. Therapeutic novelties in migraine: new drugs, new hope? J Headache Pain. 2019;20(1):37.
- López‐Bravo A, Oliveros‐Cid A, Sevillano‐Orte L. Treatment satisfaction with calcitonin gene‐related peptide monoclonal antibodies as a new patient‐reported outcome measure: a real‐life experience in migraine. Acta Neuro Scandinavica. 2022;145(6):669–675.
- Aimovig prescribing information. 2018 [cited 2020 Dec 10]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
- Barbanti P, Aurilia C, Egeo G, et al. Erenumab: from scientific evidence to clinical practice—the first Italian real-life data. Neurol Sci. 2019;40(S1):177–179.
- Dodick DW, Ashina M, Brandes JL, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–1037.
- Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123–2132.
- Lambru G, Hill B, Murphy M, et al. A prospective real-world analysis of erenumab in refractory chronic migraine. J Headache Pain. 2020;21(1):61.
- Ornello R, Casalena A, Frattale I, et al. Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain. 2020;21(1):32.
- Raffaelli B, Kalantzis R, Mecklenburg J, et al. Erenumab in chronic migraine patients who previously failed five first-line oral prophylactics and onabotulinumtoxinA: a dual-center retrospective observational study. Front Neurol. 2020;11:417.
- Reuter U, Goadsby PJ, Lanteri-Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. 2018;392(10161):2280–2287.
- Russo A, Silvestro M, di Clemente S, F, et al. Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: a comprehensive real-world experience. J Headache Pain. 2020;21(1):69.
- Scheffler A, Messel O, Wurthmann S, et al. Erenumab in highly therapy-refractory migraine patients: first German real-world evidence. J Headache Pain. 2020;21(1):84.
- Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425–434.
- Straube A, Stude P, Gaul C, et al. Real-world evidence data on the monoclonal antibody erenumab in migraine prevention: perspectives of treating physicians in Germany. J Headache Pain. 2021;22(1):133.
- American Headache Society. The American headache society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1–18.
- Volpicelli Leonard K, Robertson C, Bhowmick A, et al. Perceived treatment satisfaction and effectiveness facilitators among patients with chronic health conditions: a self-reported survey. Interact J Med Res. 2020;9(1):e13029.
- Fang J, Korrer S, Johnson J, et al. Real-world trends in characteristics of migraine patients newly initiated on erenumab in the United States. Eur J Neurology. 2020;27:103–522.
- Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–349.
- Faust E, Pivneva I, Betts K, et al. Treatment patterns, healthcare resource use, and outcomes of patients with chronic and episodic migraines treated with erenumab: a multicenter chart-review study. Headache. 2019;10:293–306.
- Hines D, Shah S, Multani J, et al. Erenumab prescription early view: patient characteristics, treatment patterns and medication adherence in the United States. Headache. 2019;61:199–200.
- Bogdanov A, Chia V, Szekely C, et al. Early use of erenumab in US real world practice. Cephalalgia Rep. 2021;4(4):251581632110204.
- Robbins L. CGRP monoclonal antibodies for chronic migraine: year 1 of clinical use. Pract Pain Manage. 2019;19:58–62.