Abstract
Objective
To describe the clinical characteristics of varicella patients seeking medical consultation and the use of antimicrobials for their management in Thailand in the absence of universal varicella vaccination (UVV).
Methods
A multicenter, retrospective chart review of 260 children and adults with a primary diagnosis of varicella was conducted at one private and three public hospitals in Bangkok, Thailand. Charts of varicella patients (inpatient or outpatient) were randomly selected over a 5-year period. Key outcomes included clinical complications and the use of antibiotics, antivirals, and other medications.
Results
Charts of 200 children (mean age 5.7 years, range 0.3–16 years) and 60 adults (mean age 27.9 years, range 18–50 years) were reviewed. Fourteen patients (including 8 children) were hospitalized. Five percent of the children and none of the adults were immunocompromised. At least 1 varicella-related complication was reported by 7.3% (7% of children, 8.3% of adults, p = .778) of all patients, including 57.1% (62.5% of children, 50% of adults) of inpatients (p < .001, compared with outpatients). Skin/soft tissue infection (47.7%) and dehydration (47.4%) were the most common complications. Antivirals (mainly oral acyclovir) were prescribed to 46.5% of patients (31.5% of children, 96.7% of adults, p < .001). Antibiotics were prescribed to 20.8% of patients (19% of children, 26.7% of adults, p = .199). Topical, oral, and intravenous antibiotics were prescribed to 12.3%, 8.5%, and 1.2% of patients, respectively. Antimicrobial prescriptions were higher among adults (p < .001) and immunocompromised patients (p = .025). Apart from antimicrobials, acetaminophen (62.3%) and oral antihistamines (51.5%) were the most prescribed.
Conclusion
A considerable number of varicella patients, both children and adults, seeking medical consultation in Thai hospitals are prescribed antibiotics and antivirals, with one-fifth of patients being prescribed an antibiotic and almost half prescribed an antiviral. The study may be of interest to policymakers in Thailand and other Asia-Pacific countries considering UVV implementation.
Introduction
Primary infection with the varicella-zoster virus (VZV) causes varicella, a highly contagious diseaseCitation1. Symptoms of infection include fever, malaise, and an itchy rash. While the disease is generally mild and self-limiting, severe complications like skin and soft tissue infections, neurological complications, and pneumonia may occur, but are rarely fatalCitation2. Complications are more likely among infants (<1 year), adults, and immunocompromised patientsCitation1,Citation2. Varicella infection usually confers lifelong immunity; however, reactivation of latent VZV in a previously infected individual in later life may result in herpes zoster (shingles)Citation1,Citation2.
Varicella is associated with considerable healthcare resource utilization, including the prescription of antibiotics and antivirals. A multinational, medical chart review study (MARVEL) of pediatric varicella patients (1–14 years of age, diagnosed between 2009 and 2016) was conducted in five middle-income countries: Hungary, Poland, Argentina, Mexico, and Peru. The pooled analysis of 401 outpatients and 386 inpatients showed that, in the absence of universal varicella vaccination (UVV), antibiotics were prescribed to 12.7% of outpatients and 68.9% of inpatientsCitation3,Citation4. High-income countries without UVV programs like France, England, and Belgium show different prescription patterns, with antibiotics prescribed to 25.1%−27.3% of varicella patients (mainly children) in a primary care settingCitation5–7. Antibiotics were prescribed to 42.6% and 83.2% of the children hospitalized for varicella in an Italian and Brazilian study, respectivelyCitation8,Citation9.
Safe and highly effective vaccines are available against varicella, with UVV programs reporting a significant decline in varicella morbidity and mortality in both developed and low- and middle-income countriesCitation1,Citation2,Citation10–24. UVV is also associated with a decline in the use of antibiotics and antivirals for varicella management, as estimated by a recent United States modeling studyCitation25. Preventing the unnecessary use of antibiotics and antivirals is especially important considering the growing global public health burden of antimicrobial resistance, and in Thailand specificallyCitation26–28.
A systematic literature review on the burden of varicella in Asia found the annual varicella incidence to be 100–2530 per 100,000 in countries without UVV, and 17.8–323 per 100,000 in countries with UVVCitation29. Thailand does not have varicella immunization in its national immunization programCitation13. The incidence of varicella in Thailand reported to the Ministry of Health was 70.88 per 100,000 in 2019 [30]. However, since varicella is not a notifiable disease in Thailand, and many patients with milder cases do not seek medical care, the true burden of varicella is likely to be higher. Like other tropical countries, Thailand reports considerable susceptibility to varicella among adolescents and adultsCitation1,Citation30–32. Though the reason for this difference in epidemiology compared to temperate countries is unclear, it is hypothesized to be due to either the climate (particularly temperature), population density, virus properties, risk of exposure, or a mixture of these factorsCitation1,Citation30–32.
A chart review study conducted in Thailand found that among 101 children (age range: 1 month–18 years) hospitalized for varicella, 73.3% reported some complications, with the most common being skin and soft tissue infections (50.5%), pneumonia (12.7%), and neurological complications (6.4%)Citation33. Thirty-five (34.7%) of these 101 children had some underlying predisposing conditions for severe varicella. To our best knowledge, no study has been conducted in Thailand to describe the clinical characteristics of varicella patients seeking treatment in an outpatient setting and no such study has been conducted among adults. There is also limited literature available on the use of antimicrobials in varicella management in Thailand. Apisarnthanarak et al. assessed an outbreak of varicella among Thai health workers where 10 of the 140 exposed healthcare workers were treated with acyclovirCitation34. Our study is the first study that comprehensively assesses the use of antimicrobials for varicella management in both inpatient and outpatient settings as well as in children and adults in Thailand.
The objective of this study was to describe the clinical characteristics of varicella patients seeking care in either outpatient or inpatient settings in Thailand, specifically focusing on varicella-related complications and the use of antibiotics and antivirals in varicella management.
Methods
Study design
This was a multicenter, retrospective chart review study to evaluate the clinical burden of illness associated with varicella among the unvaccinated population.
Study setting
The study was conducted at four hospitals in Bangkok, Thailand, one private (Mission Hospital) and three public (Siriraj, Ramathibodi, and Hospital for Tropical Diseases). Almost a quarter (22.2%) of the total Thai population live in the Bangkok Metropolitan regionCitation35. All the hospitals provide primary and secondary care and have both outpatient and inpatient facilities. The three public hospitals are also referral centers and provide highly specialized tertiary care. Prior to data collection, the study was approved by the Central Research Ethics Committee (CREC) for Thailand and the local ethics committees of each hospital. Informed consent was not required as this was a retrospective medical chart review involving de-identified data, which were aggregated for analysis.
Study population
Pediatric (0–17 years) and adult (18+ years) patients with a primary diagnosis of varicella between January 1, 2014, and December 31, 2018, who were seen in either outpatient or inpatient settings were targeted for study inclusion. Exclusion criteria included a diagnosis of herpes zoster, a documented second case of varicella, or prior varicella vaccination. Charts of 260 varicella patients from four hospitals (Mission Hospital [n = 30], Siriraj [n = 100], Ramathibodi [n = 80], and Hospital for Tropical Diseases [n = 50]) were randomly selected over a 5-year period (2014–2018) based on month and year of varicella diagnosis. To generate the randomized month, the index period and the expected patient count per physician were used. If the physician did not have a patient chart within the selected random month, the physician used the following month. Outpatients were defined as patients who visited only the outpatient clinic or emergency department of the hospital without being admitted, while inpatients were varicella patients admitted to the hospital. A convenience sample of 260 was deemed to be adequate at the 95% confidence level, based on a priori precision estimates. Soft quotas were used to ensure three-quarters were pediatric and roughly 5% were inpatients, with patients from both public and private hospitals.
Data collection
Data were extracted from paper-based medical records for the entire observation period of each patient, i.e. the period from the date of first diagnosis of varicella until disease resolution, or the last contact date if the date of the resolution was unavailable. Participating physicians were responsible for completing a case report form (CRF) for each patient using data from medical charts and physician notes. The CRFs were developed by the study team based on the MARVEL studyCitation3. Charts were examined for the presence of varicella-related complications such as skin/soft tissue infection and dehydration, among others, and any conditions leading to immunocompromised status as well as prescriptions (see footnote of for details). Medication use was captured during data collection using the CRFs and included generic drug name, dose, route, and frequency. Medications were grouped by generic drug names and by drug classes and validated by clinical experts (e.g. cephalosporins, penicillins, antihistamines). Multiple medications and medication switches were also captured.
Table 1. Patient demographic and disease characteristics.
Key outcomes of interest include those related to varicella-related clinical complications and the use of antibiotics, antivirals, and other medications. Quality checks of the data were conducted and queried as needed prior to the finalization of the dataset for analysis.
Statistical analysis
Descriptive statistics were used to summarize study variables. No imputation of missing data was conducted. Categorical variables were reported as counts and percentages of patients. Continuous variables were reported using mean, standard deviation (SD), median, and minimum and maximum values. All statistical analyses were performed using SAS® software version 9.4 (SAS Institute Inc., Cary, NC, USA). Demographics, complications, and medication use were compared between groups (e.g. adults and pediatrics, age groups, etc.) using chi-square test, Fischer’s exact test, and Wilcoxon rank sum test, as appropriate; p values of .05 or lower were considered to be statistically significant.
Results
Patient characteristics
We reviewed the medical charts of 260 eligible patients, of whom 200 (77%) were children, and 60 (23%) were adults. reports patient demographic and disease characteristics. The mean age at varicella diagnosis was 5.7 years (range 0.3–16 years) for pediatric patients and 27.9 years (range 18–50 years) for adult patients. Most of the patients were male (59.5% of children, 51.7% of adults), resided in the Central (Bangkok) Region of Thailand (86.0% of children, 71.7% of adults), and were treated in a public hospital (90.0% of children, 83.3% of adults). While 28.5% of pediatric patients were in school either full-time or part-time, 61.7% of adult patients were employed full-time (). A total of 14 (5.4%) patients (8 children and 6 adults) were hospitalized. Ten children (5.0% of children) and none of the adults were immunocompromised, while 73 patients (28.1%) had at least one pre-existing medical condition (29.5% of children, 23.3% of adults). Half the patients (50.4%) had fewer than 50 skin lesions, indicating milder disease; and 20.4% had 51–299 lesions. There were no patients with more than 300 lesions. Allergic rhinitis (5%) and allergy (5%) were the most common pre-existing medical conditions among pediatric and adult patients, respectively (Supplemental Figure S1).
Clinical complications
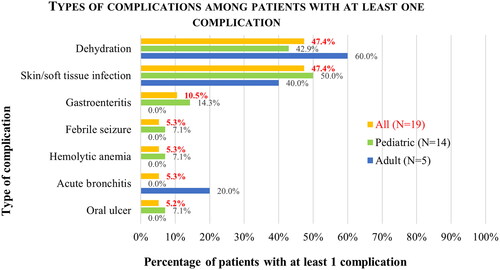
Varicella-related complications were reported in 19 patients (7.3%), including 7.0% of all pediatric patients and 8.3% of adult patients (; see for description and breakdown of complications). Complications were much higher among inpatients, with 57.1% of inpatients (62.5% of hospitalized children, 50.0% of hospitalized adults) and only 4.5% of outpatients reporting complications (p < .001) (Supplemental Table 1). Among patients who had at least one complication (N = 19), the most common complications were skin/soft tissue infection and dehydration (each 47.4%), followed by gastroenteritis (10.5%).
Medication use
Overall, 20.8% of patients were prescribed at least one antibiotic (). Oral antibiotics were prescribed to 8.5% of patients, intravenous (IV) antibiotics to 1.2%, and topical antibiotics to 12.3%. Antibiotic use was higher among adults compared to children, both overall (26.7% versus 19.0%; p = .199) and for each route of antibiotic administration (oral: 10.0% versus 8.0%, p = .625; IV: 1.7% versus 1.0%, p = .546; and topical: 16.7% versus 11.0%, p = .241); however, p values were not significant. The mean number of antibiotics per patient was comparable (1.1 antibiotic per patient for children and adults, p = .905). The most common classes of antibiotics prescribed were pseudomonic acid (mainly topical mupirocin) and penicillin (Supplemental Figure S2). A higher proportion of adults aged 30 or older (35.0%) were prescribed at least one antibiotic to manage varicella, compared to younger age groups (<5 years: 17.8%; 5 to <10 years: 12.8%; 10 to <18 years: 25.9%; and 18 to <30 years: 22.5%, p = .238); however, p values were not significant (). Antibiotics were prescribed to 17.7% of pediatric and 20.4% of adult outpatients compared to 50.0% of pediatric and 83.3% of adult inpatients (Supplemental Table 1).
Figure 2. Prescription of antibiotics and antivirals among varicella patients, by mode of administration. Patients could be prescribed more than one type of medication (topical/oral/IV), and hence the sum may be more than 100%; IV: intravenous. *Comparison of pediatric versus adult patients showed that the proportions prescribed any antivirals, oral antivirals, and topical antivirals were significantly different at p < .05. Results were not significantly different for the other outcomes shown.
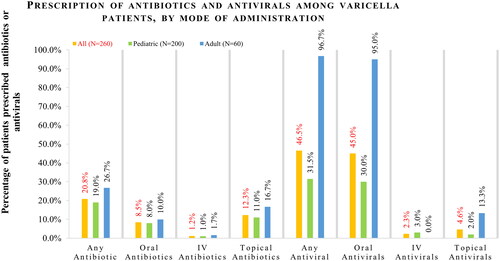
Figure 3. Prescription of antibiotics and antivirals among varicella patients, by age group. *Comparison across all age groups was significant at p < .05 for patients prescribed ≥ 1 antimicrobial and for patients prescribed ≥ 1 antiviral (p < .001 for both).
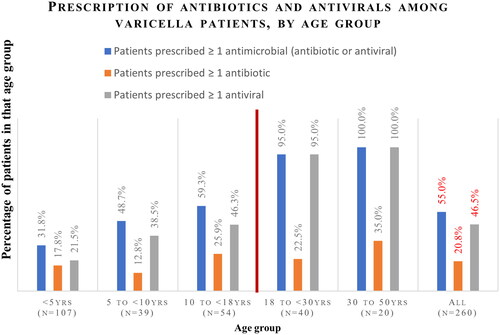
More patients were prescribed antivirals than antibiotics. Overall, 46.5% of patients were prescribed at least one antiviral medication (31.5% of pediatric patients, 96.7% of adult patients, p < .001) (). Oral antivirals were prescribed to 45.0% of patients, IV antivirals to 2.3%, and topical antivirals to 4.6%. Acyclovir was the most common antiviral, prescribed to 99.2% of patients who were prescribed at least one antiviral. The mean number of antivirals per patient was comparable for both pediatric and adult patients (mean 1.1 antiviral prescriptions per patient prescribed ≥1 antiviral prescription, p = .87). As shown in Supplemental Table 1, 29.2% of pediatric outpatients and 96.3% of adult outpatients were prescribed antivirals compared to 87.5% of pediatric and 100% of adult inpatients.
The proportion of patients with at least one antimicrobial prescription (antibiotic or antiviral) increased with age, from 31.8% of children aged <5 years to 100.0% of adults aged 30 or older (). Antibiotic prescriptions were more common among patients with complications compared to those without complications (≥ 1 antimicrobial: 68.4% versus 53.9%, p = .222; ≥ 1 antibiotic: 47.4% versus 18.7%, p = .003; ≥ 1 antiviral: 63.2% versus 45.2%, p = .131). Similarly, antimicrobial prescriptions were also higher among immunocompromised patients compared with those who were immunocompetent (≥ 1 antimicrobial: 90.0% versus 53.6%, p = .025; ≥ 1 antibiotic: 30.0% versus 20.4%, p = .438; ≥ 1 antiviral: 80.0% versus 45.2%, p = .048).
Apart from antibiotics and antivirals, acetaminophen (62.3%) and oral antihistamines (51.5%) were the most prescribed medications, followed by anti-itch creams, xylocaine, and medications used to ensure adequate hydration (oral rehydration solution, IV fluids, and normal saline, etc.) (Supplemental Figure S3).
Discussion
This multicenter, retrospective chart review study comprehensively describes the clinical characteristics of pediatric and adult varicella patients seeking medical care in hospitals in Thailand and the use of antimicrobials in their management. Also, there is substantial use of antibiotics and antivirals among patients, with one-fifth (20.8%) of all patients being prescribed at least one antibiotic and almost half (46.5%) prescribed at least one antiviral.
Our study shows that 7.3% of patients experienced some type of complication. Complications were reported among 7.0% of pediatric and 8.3% of adult patients. Skin/soft tissue infections (47.4%) and dehydration (47.4%) were the two most common complications reported among both children and adults. These were also the two most common complications among children with varicella in Hungary and Poland in the MARVEL studyCitation36,Citation37. The MARVEL study was a multinational, medical chart review study conducted in 5 middle-income countries among children <14 years, with an almost equal split between inpatients and outpatients in each countryCitation3,Citation4. However, unlike in MARVEL and other studies looking only at hospitalized patients, severe complications like pneumonia, sepsis, or cerebellitis were not reported in our study, probably since these complications necessitate hospitalization, and our sample was 95% outpatientCitation3,Citation4,Citation8,Citation9,Citation33.
As expected, complication rates were significantly lower among outpatients (4.5%) compared to inpatients (57.1%) in our study, with 4.7% of pediatric outpatients and 62.5% of pediatric inpatients reporting at least one complication. This is lower than the complication rate reported among children in a primary care/outpatient setting by other studies: 12.2% (5 European and Latin American countries - MARVEL study), 12.6% (Belgium) and 15.3% (France)Citation4,Citation6,Citation7. Additionally, 78.8% of pediatric inpatients in the 5 countries (pooled MARVEL study), 71.3% in Italy, 73% in Thailand, and >88% in Brazil reported at least one complicationCitation4,Citation8,Citation9,Citation33. It is likely that patients hospitalized across various studies have different disease severity and risk factors than those treated as outpatients and hence a higher rate of complications. The definition of complications may also differ across the studies.
Antivirals were prescribed to a significantly larger proportion of adult patients compared to pediatric patients (96.7% versus 31.5%, p value < .001), which is consistent with treatment recommendations for adults who are at high risk of developing severe varicellaCitation1. The CDC guidelines recommend antiviral medications to treat varicella in otherwise healthy people older than 12 years of age, as well as pregnant women, people with chronic skin or lung disease or receiving long-term salicylate/steroid therapy, and those with a weakened immune systemCitation38. Antivirals were prescribed to 29.2% of the pediatric outpatients in our study, comparable to 33.9% of pediatric outpatients in the pooled analysis from the multi-country MARVEL studyCitation3,Citation4. However, a much lower proportion of children were treated with antivirals in a primary care setting in developed countries like England, France, and Belgium (1.5%, 2.2%, and 2.7%, respectively), even though these studies assessed antiviral use for any reason during a 3-month follow-up period following varicella diagnosisCitation5–7. Though our results show that the percentage of patients prescribed at least one antiviral increased with age, this trend was not consistent for those prescribed at least one antibiotic.
Antibiotic use reported among children in our study was slightly but not significantly lower than among adults (19.0% versus 26.7%). Additionally, 17.7% of pediatric outpatients were prescribed antibiotics, which is slightly higher than the pooled MARVEL study (12.7%) and lower than children in primary care settings in France (25.1%), Belgium (27.3%), and England (25.9%)Citation6,Citation7. This may again be due to the longer follow-up period in these European studies. It is possible that not all antimicrobial use reported in the three months following varicella infection in these studies is directly attributable to varicella, leading to an overestimation of varicella-related antimicrobial use. Though the small sample size limits the strength of the conclusions regarding inpatients in our study, 50% of pediatric inpatients were prescribed antibiotics compared to 42.6% in Italy, 68.9% in the 5 MARVEL countries (pooled), and 83.2% in BrazilCitation3,Citation4,Citation8,Citation9. These differences may also be due to differences in prescription patterns, disease severity, and healthcare systems for varicella management between studies.
The most common classes of antibiotics prescribed in our study of primarily pediatric and adult outpatients were pseudomonic acid (mainly topical mupirocin) and penicillin. Clindamycin was the most common antibiotic in the MARVEL study, and amoxicillin (penicillin) was the most common antibiotic prescribed to French, Belgian, and English children in a primary care settingCitation4. The difference in antibiotics prescribed across these studies could be due to differences in prescribing preferences and treatment guidelines among countries as well as the study sample.
Compared to temperate countries, where >90% of varicella infections occur before adolescence, in tropical countries like Thailand, varicella infection is typically acquired at an older ageCitation1. For example, seroprevalence data from Thailand showed that only 27% of healthy children (≤12 years) in 2014 had antibodies against varicella compared to almost 90% of those over 30 years and 100% of those over 50 yearsCitation39. The reason for the late onset of varicella in not definitively known, though the climate (particularly temperature), population density, virus properties, and risk of exposure are some probable causesCitation1,Citation31,Citation32,Citation39. The late onset of varicella coupled with the higher antibiotic and antiviral usage in older age groups is likely to increase the use of antimicrobials for varicella management in Thailand compared to other countries.
The introduction of UVV programs has led to significant declines in varicella cases and hospitalizationsCitation1,Citation2,Citation10–24. In Taiwan, following the implementation of a nationwide, one-dose varicella vaccination program for children aged 12–18 months in 2004, the incidence of varicella in children decreased by 65%, according to a review of records before and after the program was started (2000–2008)Citation19. Similarly, UVV has led to substantial reductions in varicella cases, hospitalizations, and deaths in other countries, including the United States, Italy, Brazil, Israel, and GermanyCitation1,Citation2,Citation10–24,Citation40.
A modeling study in the United States estimated that UVV implementation led to a 95% decrease in antiviral and antibiotic prescription and costs associated with varicella annually among childrenCitation25. Preventing the unnecessary use of antibiotics and antivirals is especially important in the context of the global burden of antimicrobial resistanceCitation41. The World Health Organization (WHO) considers the South-East Asia Region, to which Thailand belongs, to be the most at-risk region for this problem, and combatting antimicrobial resistance is one of six priority programs in WHO’s country strategy for ThailandCitation27. UVV could be a useful strategy to decrease varicella-related complications and associated antimicrobial prescriptions that would avert varicella-related antimicrobial useCitation42–44.
Limitations
The limitations of this study include those inherent to retrospective chart reviews. Inaccurate or incomplete information in patient records will impact data quality. The study captured only individuals who sought care for varicella, which would mainly consist of patients sick enough to require medical attention. The data collected are representative only of varicella patients who sought care from four hospitals in the Bangkok Metropolitan Area, limiting the generalizability of results nationwide. Additionally, the study included only 14 inpatients. Although the study originally sought to recruit 300 patients (∼20%–30% inpatients), investigators at the four sites had to stop data extraction at 260 patients (5% inpatients), due to the increased patient load during the COVID-19 pandemic. However, this proportion of varicella patients requiring inpatient care is in line with real-world estimates from Taiwan (1.41%–6%)Citation29,Citation45,Citation46. Assessing the appropriateness of the treatment and bacterial resistance profile for each patient was out of the scope for the study.
Conclusion
A multicenter chart review study showed that a considerable number of varicella patients seeking medical care in outpatient or inpatient settings at Thai hospitals are prescribed antibiotics and antivirals. Approximately one-fifth of all patients were prescribed at least one antibiotic and almost half were prescribed at least one antiviral for varicella management. Antivirals were prescribed to 96.7% of adults compared to 31.5% of children. Results from this study provide clinical perspectives that could inform the development of public health and immunization policies related to varicella and varicella vaccination. The study may be of interest to policymakers in Thailand and similar countries considering UVV to reduce disease burden and antimicrobial use.
Transparency
Author contributions
SS, MP, IS, and KC were involved in the study design and oversight. KC, SK, SC, and SL were involved in the data collection. NK, JS, SS, MP, KC, and IS were involved in data analysis. SS, JS, and MP were involved in drafting the manuscript. All authors were involved in the interpretation of the data, revised the paper critically for intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Previous presentation
Data from this manuscript were previously presented in part at the World Society for Pediatric Infectious Diseases Congress (WSPID 2022, virtual poster) and the Asian Congress of Pediatric Infectious Diseases (ACPID 2022, Seoul).
Supplemental Material
Download PDF (117.9 KB)Acknowledgements
The authors thank the personnel at all four sites and the RTI-HS team for their gracious assistance with site recruitment and data collection. We also thank Jyoti Aggarwal and Jenna Bhaloo (for oversight of data collection), Bianca Jackson (for analytic and medical writing support), Narumon Werayingyong (for assistance with CREC and IRB approvals), Deepa Patel (for study management), and Christina DuVernay (for editorial assistance).
Declaration of funding
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Declaration of financial/other relationships
SS and MP are employees of Merck Sharp & Dohme (MSD) LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and own stock in Merck & Co., Inc., Rahway, NJ, USA. IS is an employee of MSD Thailand and owns stock in Merck & Co., Inc., Rahway, NJ, USA. NK was and JS is an employee of OPEN Health, and JS owns stock in OPEN Health, which received funding from MSD for this study. KC, SK, SL and SC are employees of their respective institutions, which received research funding to conduct the study. They have no other financial relationships to report apart from an honorarium received by KC for consultation on this study. All authors have no other conflicts of interest to disclose. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Varicella and herpes zoster vaccines: WHO position paper. Wkly Epidemiol Rec. 2014;89(25):265–287.
- Centers for Disease Control and Prevention. Varicella. In: Epidemiology and prevention of vaccine-preventable diseases. 14th ed. Washington (DC): Public Health Foundation; 2021.
- Wolfson LJ, Castillo ME, Giglio N, et al. Varicella healthcare resource utilization in middle income countries: a pooled analysis of the multi-country MARVEL study in Latin America & Europe. Hum Vaccin Immunother. 2019;15(4):932–941.
- Wolfson LJ, Castillo ME, Giglio N, et al. The use of antibiotics in the treatment of pediatric varicella patients: real-world evidence from the multi-country MARVEL study in Latin America & Europe. BMC Public Health. 2019;19(1):826.
- Kujawski S, Casey C, Haas H, et al. Antibiotic use and economic burden of varicella infection among pediatric patients: a retrospective cohort analysis of real-world data in France (congress presentation; abstract accepted). Lisbon, Portugal: European Society for Paediatric Infectious Diseases (ESPID); 2023.
- Kujawski SA, Banks V, Casey CS, et al. Burden of antibiotic use among pediatric patients with varicella infection: a retrospective cohort analysis of real-world data in England (congress presentation). Athens, Greece: European Society for Paediatric Infectious Diseases (ESPID); 2022.
- Vandenhaute J, Tsakeu E, Chevalier P, et al. Assessing the use of antibiotics and the burden of varicella in Belgium using a retrospective GP database analysis. BMC Infect Dis. 2021;21(1):1150.
- Bozzola E, Marchesani S, Ficari A, et al. Assessing the use of antibiotics in pediatric patients hospitalized for varicella. Ital J Pediatr. 2022;48(1):196.
- Diniz LMO, Maia MMM, Oliveira YV, et al. Study of complications of varicella-zoster virus infection in hospitalized children at a reference hospital for infectious disease treatment. Hosp Pediatr. 2018;8(7):419–425.
- Wutzler P, Bonanni P, Burgess M, et al. Varicella vaccination – the global experience. Expert Rev Vaccines. 2017;16(8):833–843.
- Marin M, Lopez AS, Melgar M, et al. Decline in severe varicella disease during the United States varicella vaccination program: hospitalizations and deaths, 1990–2019. J Infect Dis. 2022;226(Suppl 4): s407–s415.
- Marin M, Marti M, Kambhampati A, et al. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics. 2016;137(3):e20153741.
- Varela FH, Pinto LA, Scotta MC. Global impact of varicella vaccination programs. Hum Vaccin Immunother. 2019;15(3):645–657.
- Giglio N, Lasalvia P, Pawaskar M, et al. Trends in varicella burden of disease following introduction of routine childhood varicella vaccination in Argentina: a 12-year time series analysis. Vaccines. 2022;10(7):1151.
- Pawaskar M, Gil-Rojas Y, Parellada CI, et al. The impact of universal varicella vaccination on the clinical burden of varicella in Colombia: a national database analysis, 2008–2019. Vaccine. 2022;40(35):5095–5102.
- Kupek E, Tritany EF. Impact of vaccination against varicella on the reduction of the disease incidence in children and adolescents from Florianópolis, Brazil. J Pediatr. 2009;85(4):365–368.
- Quian J, Rüttimann R, Romero C, et al. Impact of universal varicella vaccination on 1-year-olds in Uruguay: 1997–2005. Arch Dis Child. 2008;93(10):845–850.
- Elbaz M, Paret G, Yohai AB, et al. Immunisation led to a major reduction in paediatric patients hospitalised because of the varicella infection in Israel. Acta Paediatr. 2016;105(4):e161–e166.
- Chao DY, Chien YZ, Yeh YP, et al. The incidence of varicella and herpes zoster in Taiwan during a period of increasing varicella vaccine coverage, 2000–2008. Epidemiol Infect. 2012;140(6):1131–1140.
- Spoulou V, Alain S, Gabutti G, et al. Implementing universal varicella vaccination in Europe: the path forward. Pediatr Infect Dis J. 2019;38(2):181–188.
- Bechini A, Boccalini S, Baldo V, et al. Impact of universal vaccination against varicella in Italy. Hum Vaccin Immunother. 2015;11(1):63–71.
- Piazza MF, Amicizia D, Paganino C, et al. Has clinical and epidemiological varicella burden changed over time in children? Overview on hospitalizations, comorbidities and costs from 2010 to 2017 in Italy. Vaccines. 2021;9(12):1485.
- Damm O, Witte J, Wetzka S, et al. Epidemiology and economic burden of measles, mumps, pertussis, and varicella in Germany: a systematic review. Int J Public Health. 2016;61(7):847–860.
- Liese JG, Cohen C, Rack A, et al. The effectiveness of varicella vaccination in children in Germany: a case-control study. Pediatr Infect Dis J. 2013;32(9):998–1004.
- Pawaskar M, Fergie J, Harley C, et al. Impact of universal varicella vaccination on the use and cost of antibiotics and antivirals for varicella management in the United States. PLOS One. 2022;17(6):e0269916.
- Sumpradit N, Wongkongkathep S, Malathum K, et al. Thailand’s national strategic plan on antimicrobial resistance: progress and challenges. Bull World Health Organ. 2021;99(9):661–673.
- World Health Organization. Antimicrobial resistance in the South-East Asia. 2022 [cited 2022 Sep 30]. Available from: https://www.who.int/thailand/health-topics/antimicrobial-resistance
- World Health Organization. Antimicrobial resistance. 2022 [cited 2022 Sep 30]. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
- Goh AEN, Choi EH, Chokephaibulkit K, et al. Burden of varicella in the Asia-Pacific region: a systematic literature review. Expert Rev Vaccines. 2019;18(5):475–493.
- Ministry of Public Health-Thailand. Annual Epidemiological Surveillance Report. 2019. p. 91–94.
- Gershon AA, Marin M, Seward JF. 62 – Varicella vaccines. In: Plotkin SA, Orenstein WA, Offit PA, et al., editors. Plotkin’s vaccines. 7th ed. Philadelphia (PA): Elsevier; 2018. p. 1145.e17–1180.e17.
- Lolekha S, Tanthiphabha W, Sornchai P, et al. Effect of climatic factors and population density on varicella zoster virus epidemiology within a tropical country. Am J Trop Med Hyg. 2001;64(3–4):131–136.
- Vandepitte WP, Chanveerachai S, Srisarang S. Clinical characteristics and cost of chickenpox hospitalization in thai children. J Med Assoc Thai. 2014;97:S126–S135.
- Apisarnthanarak A, Kitphati R, Tawatsupha P, et al. Outbreak of varicella-zoster virus infection among Thai healthcare workers. Infect Control Hosp Epidemiol. 2007;28(4):430–434.
- World Population Review. Thailand population. 2023. Available from: https://worldpopulationreview.com/countries/thailand-population
- Meszner Z, Molnar Z, Rampakakis E, et al. Economic burden of varicella in children 1–12 years of age in Hungary, 2011–2015. BMC Infect Dis. 2017;17(1):495.
- Wysocki J, Malecka I, Stryczynska-Kazubska J, et al. Varicella in Poland: economic burden in children 1–12 years of age in Poland, 2010-2015. BMC Public Health. 2018;18(1):410.
- Centers for Disease Control and Prevention. Chickenpox (Varicella). 2023. Available from: https://www.cdc.gov/chickenpox/hcp/index.html#high-risk-people
- Thantithaveewat T, Thananrat T, Preeyaporn V, et al. Seroprevalence of varicella-zoster antibodies in a Thai population. Southeast Asian J Trop Med. 2019;50(1):94–100.
- Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases. 2022 [updated 2018 May 15]. Available from: https://www.cdc.gov/vaccines/pubs/surv-manual/chpt17-varicella.html
- World Health Organization. Ten threats to global health in 2019. 2019. Available from: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
- Buchy P, Ascioglu S, Buisson Y, et al. Impact of vaccines on antimicrobial resistance. Int J Infect Dis. 2020;90:188–196.
- World Health Organization. Antibiotic resistance: why vaccination is important. 2023. Available from: https://www.who.int/news-room/questions-and-answers/item/antibiotic-resistance-why-vaccination-is-important
- Gabutti G. Available evidence and potential for vaccines for reduction in antibiotic prescriptions. Hum Vaccin Immunother. 2022;18(7):2151291.
- Tseng HF, Tan HF, Chang CK. Varicella epidemiology and cost-effectiveness analysis of universal varicella vaccination program in Taiwan. Southeast Asian J Trop Med Public Health. 2005;36(6):1450–1458.
- Lin YH, Huang LM, Chang IS, et al. Disease burden and epidemiological characteristics of varicella in Taiwan from 2000 to 2005. J Microbiol Immunol Infect. 2009;42(1):5–12.