Abstract
Objective
The treatment of moderate-to-severe plaque psoriasis has seen significant improvements in recent years with the advent of biologic drugs. The aim of this study was to assess the cost-effectiveness of anti-IL17 drugs and other biologic therapies used to treat moderate-to-severe plaque psoriasis in France and Germany over a one-year time horizon.
Methods
We developed a cost per responder model for biologic drugs used in psoriasis treatment. The model included anti-IL17s (brodalumab, secukinumab, ixekizumab and bimekizumab), anti-TNFs (adalimumab, etanercept, certolizumab and infliximab), an anti-IL12/23 (ustekinumab), and anti-IL23s (risankizumab, guselkumab and tildrakizumab). Efficacy estimates were collected through a systematic literature review of network meta-analyses on long-term Psoriasis Area and Severity Index (PASI) measures. Dose recommendations and country-specific prices were used to calculate drug costs. Biosimilar drug prices were used when available as a substitute for the originator drugs.
Results
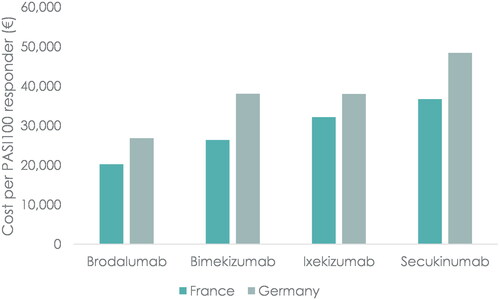
After one year, brodalumab had the lowest cost per PASI100-responder in both France (€20,220) and Germany (€26,807) across all available biologic treatments. Among the anti-IL17s, brodalumab had a 23% lower cost per PASI100-responder vs. the nearest comparator in France (bimekizumab, €26,369), and 30% lower vs. nearest comparator in Germany (ixekizumab, €38,027). Brodalumab also had the lowest cost per PASI75- and PASI90-responder among the anti-IL17s in both France and Germany after one year. Adalimumab had the lowest cost per PASI100-responder among the anti-TNFs in both France (€23,418) and Germany (€38,264). Among the anti-IL-23s, risankizumab had the lowest cost per PASI100-responder in both France (€20,969) and Germany (€26,994).
Conclusion
Driven by its lower costs and high response rates, brodalumab was the most cost-effective treatment option for moderate-to-severe plaque psoriasis over a one-year time-horizon within the anti-IL17 class and when compared to all other biologics in France and Germany.
1. Background
Psoriasis is a chronic immune-mediated disease caused by inflammation of the skin with involvement of proinflammatory cytokines such as TNF-α, IL-17 and IL23Citation1,Citation2. The most frequent phenotype is chronic plaque psoriasis (psoriasis vulgaris), which is characterized by sharply demarcated scaly plaques on the body surface, typically symmetrical, affecting the torso, extensor surfaces of the extremities, and the scalpCitation1,Citation3–5. These plaques can coalesce and cover large areas of the body. Patients suffering from plaque psoriasis may also experience pain, pruritus and bleeding in the affected areasCitation1.
Plaque psoriasis accounts for approximately 85–90% of all cases of psoriasis and affects about 2% of the population in Western Europe, including France and GermanyCitation6–8. Relatively few studies have assessed the incidence of psoriasis, but evidence suggests that western Europe has the highest incidence of psoriasis in the world with an age standardized incidence rate of 204.5 per 100,000 peopleCitation8.
Psoriasis is associated with a number of comorbidities such as cardiovascular diseases, type 2 diabetes, obesity, inflammatory bowel disease and psoriatic arthritisCitation1,Citation9,Citation10. In addition, patients with plaque psoriasis also experience a significant negative impact on their health-related quality of life (HRQoL) with pruritus being a significant contributor to reduced HRQoLCitation11. In relation to this, patients with psoriasis often go through difficulties with body image and self-esteem as well as feelings of stigma and embarrassment concerning their body’s appearance, with disease severity being an important factor in psychological distressCitation12–14.
Approximately 20% of patients with plaque psoriasis suffer from moderate-to-severe psoriasisCitation15. Commonly, moderate-to-severe plaque psoriasis is defined by the rule of ten; a body surface area (BSA) >10% or psoriasis area and severity index (PASI) >10 and/or dermatology life quality index (DLQI) >10Citation16. The PASI is a composite index with scores ranging from 0 to 72, with higher values indicating a more severe or more extensive conditionCitation17. PASI100 and PASI90 are commonly used to assess treatment effectiveness in patients with plaque psoriasis, indicating a 100% and 90% reduction in the PASI score, respectively, compared to their baseline. In addition, the PASI score is often used as an outcome measure in clinical trials for evaluating the efficacy of psoriasis treatments and in cost-effectiveness analyses of psoriasis medicationsCitation17.
In recent years, the number of highly effective biologic drugs has increased greatly and revolutionised the treatment for patients with moderate-to-severe plaque psoriasisCitation18–20. Hence, achieving a complete clearance of psoriasis (PASI100) is now both possible and desirable. In addition to this, fewer side effects have been observed in patients using biologic drugs compared to traditional systemic small molecule therapiesCitation1.
In Europe, several biologic drugs are available for the treatment of plaque psoriasis: anti-TNFs (etanercept, infliximab, adalimumab and certolizumab), anti-IL12/23s (ustekinumab), anti-IL17s (secukinumab, ixekizumab and brodalumab) and anti-IL23s (tildrakizumab, guselkumab and risankizumab)Citation1,Citation21–23. Recently, a new anti-IL17 biologic drug called bimekizumab has also been approved by the European Medicines Agency (EMA) and represents the latest anti-IL17 treatment for patients with moderate-to-severe plaque psoriasisCitation24. In Europe, some of the first launches of bimekizumab were in France and Germany.
The vast selection of both new and old biologic drugs, including a wide range of biosimilars, highlights clinicians and decision-makers’ need for meaningful insights on the cost-effectiveness of the current biologic treatments in plaque psoriasis due to the substantial costs associated with drug acquisition. As the efficacy of biologics used in the treatment of plaque psoriasis varies greatlyCitation25, the PASI response must be accounted for to properly compare the cost-effectiveness.
To our knowledge, there are no published cost-effectiveness analyses that encompass all the anti-IL17s in the current landscape of biologics used to treat moderate-to-severe psoriasis. The objective of this study was to estimate the cost per responder of all currently licensed anti-IL17s in two major European countries, France and Germany, as well as other biologics used to treat moderate-to-severe psoriasis, over a one-year time horizon.
2. Methods
2.1. Systematic literature review
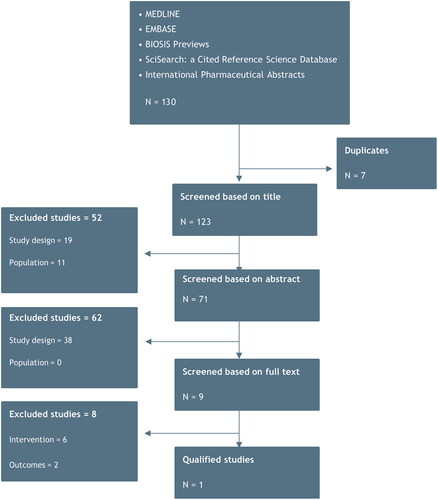
A systematic literature review (SLR) was conducted to identify long-term efficacy estimates (PASI response after one year of treatment) of biological drugs used in plaque psoriasis. The focus was to include all licensed anti-IL17s, but the strategy also allowed for inclusion of other biologics with comparable efficacy data. The systematic review was performed following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelinesCitation26. The electronic platform PROQUEST, which searches multiple platforms simultaneously, including Medline, Embase, BIOSIS previews, SciSearch and International Pharmaceutical Abstracts, was used for this systematic review. Details of the applied search string are presented in Table S1. The search was conducted on 3 November 2022 and yielded 130 hits.
Inclusion and exclusion criteria were defined using the PICO (population, intervention, comparator and outcomes) reporting system. The systematic literature review included studies with patients who had a confirmed diagnosis of moderate or severe plaque psoriasis and were treated with anti-IL17 biologic therapy as monotherapy. Additionally, studies had to be systematic literature reviews (SLR) or network meta-analyses (NMA) presenting long-term efficacy data (>48 weeks). The specified eligibility criteria are presented in Table S2.
Two independent reviewers (NN and CDJ) assessed all identified studies in a blinded three-step process. Studies were included in accordance with the PICO criteria, and any case of disagreement about the eligibility of a study was resolved through discussion between the two reviewers.
After de-duplication, 123 studies were screened based on title, which excluded 52 studies. In the second phase, eligible studies were screened based on abstract. In this phase, 62 studies were excluded. Finally, the remaining nine studies were full-text screened, at which level eight studies were excluded, leaving one qualified study by Armstrong et al.Citation27, from which long-term efficacy estimates of biological drugs of interest could be extracted and used as inputs in the cost per responder model. illustrates the different stages of the SLR in a PRISMA flow diagram.
The primary analysis (anti-IL17s) and secondary analysis (all other biologics) were based on the efficacy estimates found in the SLR. However, efficacy estimates on the biologics tildrakizumab, certolizumab and infliximab, which are also used in the treatment of plaque psoriasis, were not part of the NMA by Armstrong et al.Citation27 and thus could not be included in the secondary analysis that contained other biologics outside of the anti-IL17 treatment class. To accommodate this, we performed a scenario analysis using the PASI100 efficacy estimates on tildrakizumab (33.4%), certolizumab (27.6%) and infliximab (30.9%) from Thaci et al. (tildrakizumab)Citation28 and Yasmeen et al. (certolizumab and infliximab)Citation25.
In addition to the aforementioned scenario analysis, we performed three scenario analyses to assess the robustness of the primary analysis using PASI75, PASI90 and short-term PASI100 efficacy data (10–16 weeks of treatment). Armstrong et al.Citation27 was used to inform on PASI75 and PASI90, while another NMA by Armstrong et al.Citation29 was used to inform on the efficacy measures for short-term PASI100. In the scenario analysis with short-term PASI100, we used a 16-week time horizon to align with the short-term efficacy data. All efficacy estimates are presented in .
Table 1. Efficacy of biologics.
2.2. Model framework
We developed a simple health economic model with a French and German payer perspective to investigate the cost-effectiveness of currently available biologics used in the treatment of moderate-to-severe plaque psoriasis. In the primary analysis, cost-effectiveness was evaluated based on the currently licensed anti-IL17 biologic drug with the lowest cost per PASI100-responder after one year of treatment. As a secondary analysis, the cost per PASI100-responder for other biologics available in France and Germany was also explored.
The model simulated the course of treatment for biologics used in treatment of plaque psoriasis over a one-year period beginning with induction treatment and later maintenance treatment. Over this period, the model accumulated the cost per patient for the different biologics included in the model. Costs related to administration of drugs, monitoring and adverse events were not included in the analysis.
In the model, the cost-effectiveness estimates were calculated by dividing the total cost of treatment by the percentage of patients who respond to the treatment, as defined by a specific PASI criteria. Thus, the model calculated the cost per patient who achieved a PASI response, i.e. the cost per responder. The cost per responder analysis is a metric used to evaluate the cost-effectiveness of different drugs in treating a specific condition. The cost per responder metric allows for a comparison of the costs associated with different drugs for the same condition and helps to determine which drug provides the best value for the investment.
2.3. Dosing and pricing of biologics
In the model, the cost of each treatment was based on the pharmaceutical retail prices in France (from the National Health Insurance Agency) extracted on 1 February 2023 and the manufacturer prices in Germany (from ABDATA Pharma-Daten-Service) extracted on 15 March 2023Citation30,Citation31. The vial and pack size were chosen to facilitate the lowest possible cost of treatment. When biosimilar substitution was possible, the cheapest alternative was used.
The calculation of treatment costs was based on the dosing recommendations of each drug in the summaries of product characteristics (SmPC) by the EMACitation32–43. As the model simulated the course of treatment according to the SmPCs, both the induction and maintenance phases of the biologic treatments were included in the analysis ().
Table 2. Posology and cost of biologics.
Dose adjustment is frequent in clinical practice and may occur due to suboptimal treatment. Dose adjustment of biologic treatments in patients with plaque psoriasis has been described by Egeberg et al. in a real-world evidence studyCitation44. Egeberg et al. investigated the average weighted dosages for adalimumab, etanercept, secukinumab and ustekinumab and found that these were 13%, 23%, 8% and 3% above the recommended levels, respectivelyCitation44. Dose adjustment was not included in the primary analysis of the present study, as data for all four anti-IL17s included in the analysis were not available in the study by Egeberg et al.Citation44. A study by Torres et al.Citation45 found that the proportion of patients who were dose-adjusted varied greatly across treatments: 7.4% of patients treated with secukinumab were dose-adjusted, 6.4% for ixekizumab and 2.4% for brodalumabCitation45. To evaluate the significance of dose adjustment in the primary analysis, we included a scenario analysis with this parameter by estimating a dose adjustment factor for the three remaining anti-IL17s (ixekizumab, brodalumab and bimekizumab) that were not included in Egeberg et al.Citation44. As secukinumab was the only anti-IL17 that was reported by Egeberg et al.Citation44 we used the relative difference between secukinumab and the remaining anti-IL17s in Torres et al.Citation45 to estimate the dose adjustment factor for ixekizumab and brodalumab. As bimekizumab was not included in Torres et al.Citation45, we used the average value from secukinumab, ixekizumab and brodalumab to estimate the dose adjustment factor for this drug. Thus, the dose adjustment factors used in this scenario analysis were 8% for secukinumab, 6.9% for ixekizumab, 2.2% for brodalumab and 5.7% for bimekizumab.
3. Results
3.1. Primary analysis
In the primary analysis, the cost-effectiveness of the four anti-IL17s (secukinumab, ixekizumab, brodalumab and bimekizumab) was assessed ().
Table 3. Primary analysis: cost per patient and cost per responder after one year of treatment (€).
Brodalumab had the lowest treatment cost in both France and Germany with a cost per patient of €11,344 and €15,039, respectively. In France, the cost per patient for the remaining anti-IL17s were €15,109 for bimekizumab, €15,172 for secukinumab and €15,367 for ixekizumab. In Germany, the cost per patient for the remaining anti-IL17s were €18,177 for ixekizumab, €19,998 for secukinumab and €21,810 for bimekizumab. These results indicate that relatively small differences in the cost per patient exist among ixekizumab, secukinumab and bimekizumab, while the cost of treatment with brodalumab is significantly lower compared to the other anti-IL17s.
The cost per responder results showed that brodalumab was the cost-effective treatment option among the anti-IL17s with a cost per PASI100-responder of €20,220 in France and €26,807 in Germany after one year of treatment. Thus, comparing to the nearest anti-IL17 comparator, the cost per PASI100 responder was 23% lower in France (vs. bimekizumab, €26,369) and 30% lower in Germany (vs. ixekizumab, €38,027). In France, the cost per responder for the remaining anti-IL17s were €32,149 for ixekizumab and €36,735 for secukinumab. In Germany, the cost per responder for the remaining anti-IL17s were €38,063 for bimekizumab and €48,422 for secukinumab. The cost-effectiveness of brodalumab was driven by the relatively lower cost per patient compared to the remaining anti-IL17s, while at the same time being highly effective in achieving a PASI100 response (). The results from the primary analysis are illustrated in .
3.2. Secondary analysis
In this analysis, all biologics included in Armstrong et al.Citation27 were compared. In France, brodalumab (anti-IL17) was the most cost-effective biologic, with a cost per PASI100-responder of €20,220, when compared to all biologics included in the analysis. Adalimumab was most cost-effective among the anti-TNFs with a cost per PASI100-responder of €23,418. Ustekinumab was the only anti-IL12/23 and had a cost per PASI100-responder of €35,666. Risankizumab was most cost-effective among the anti-IL23s with a cost per PASI100-responder of €20,969.
In Germany, brodalumab was also the most cost-effective treatment when comparing overall to all other biologics, with a cost per PASI100-responder of €26,807. Among the anti-TNFs, adalimumab was the most cost-effective treatment with a cost per PASI100-responder of €38,264, while risankizumab was the most cost-effective among the anti-IL23s with a cost per PASI100-responder of €26,994. Ustekinumab (anti-IL12/23) had a cost per PASI100-responder of €72,078.
presents the cost per patient and cost per PASI100-responder results from the secondary analysis in France and Germany. When considering the cost per patient analysis, it is apparent that the anti-TNFs were cheaper compared to the anti-IL17s, anti-IL23s and anti-IL12/23s. Thus, adalimumab and etanercept had the lowest cost per patient in both France and Germany; likely due to the presence of biosimilar competition, which is not present in other drug classes. Among the anti-IL17s, brodalumab had the lowest cost per patient in both France and Germany. Guselkumab had the lowest cost per patient among the anti-IL23s in both France and Germany.
Table 4. Secondary analysis: cost per patient and cost per responder after one year of treatment (€).
Despite having the lowest cost per patient, the anti-TNFs did not perform as well as the remaining biologics when considering the cost per PASI100-responder. This was caused by the PASI100 response rates for the anti-TNFs being significantly lower compared to the anti-IL17s, anti-IL23s and anti-IL12/23s.
3.3. Scenario analyses
In total, four scenario analyses were performed on the primary analysis for both France and Germany and included changing the definition of a responder (PASI75 and PASI90), using short-term efficacy data (PASI100) and including dose adjustment. We also undertook a scenario analysis on the secondary analysis where tildrakizumab, certolizumab and infliximab were included, using efficacy measures from Thaci et al.Citation28 and Yasmeen et al.Citation25.
3.3.1. Cost per PASI75-responder and cost per PASI90-responder
When changing the responder definition to PASI75 and PASI90, brodalumab had the lowest cost per responder compared to the other available anti-IL17 treatments in both France and Germany over a one-year time horizon, thus being consistent with the primary analysis. In France, the cost per responder for brodalumab were €12,731 and €14,432 for PASI75 and PASI90, respectively. In Germany, the cost per responder for brodalumab were €16,879 and €19,134 for PASI75 and PASI90, respectively. The lower cost per responder for PASI75 and PASI90 compared to PASI100 was due to the less stringent responder definition allowing more patients to classify as responders ().
Table 5. Scenario analyses (€).
The scenario analysis also showed that the cost-effectiveness results for the remaining anti-IL17s were consistent with the primary analysis. This indicated that the results of the primary analysis were robust to changes in the definition of a responder.
3.3.2. Cost per PASI100-responder using short-term efficacy
Using short-term (10–16 weeks) PASI100 efficacy measures to define responders did not change the outcome of the primary analysis, as brodalumab was still the most cost-effective anti-IL17 treatment in both France and Germany with a cost per PASI100-responder of €8633 and €11,445, respectively (). Despite having a response rate of 57.8% compared to brodalumab (43.8%), bimekizumab remained less cost-effective, as the induction phase posology for bimekizumab was associated with a higher loading dose compared to brodalumab and thus yielded a higher cost per patient.
3.3.3. Inclusion of dose adjustment
In this scenario analysis, Egeberg et al. and Torres et al. were used to estimate a dose adjustment factor for the anti-IL17s included in the primary analysisCitation44,Citation45. Despite increased costs associated with each biologic, the results of this scenario analysis remained consistent with the primary analysis. We found that the cost per responder for brodalumab was only marginally affected by the inclusion of dose adjustment. This was caused by the estimated dose adjustment factor for brodalumab being relatively low (2.2%) compared to the other anti-IL17s (secukinumab 8%, ixekizumab 6.9% and bimekizumab 5.7%). This scenario should be interpreted with caution, since the dose adjustment factor was based on assumptions and not real-life data for all of the individual treatments.
3.3.4. Inclusion of tildrakizumab, certolizumab and infliximab
In this scenario analysis, we compared the cost-effectiveness of tildrakizumab, certolizumab and infliximab (efficacy estimates based on Thaci et al.Citation28 and Yasmeen et al.Citation25) to the results from the secondary analysis. Thus, this scenario analysis compared the full assortment of biologics available in the treatment of plaque psoriasis.
We found that the cost per responder for tildrakizumab (anti-IL23) was €35,964 in France and €41,796 in Germany and thus substantially less cost-effective compared to brodalumab (the most cost-effective treatment overall) which had a cost per PASI100-responder of €20,220 in France and €26,807 in Germany. It was also less cost-effective compared to risankizumab in both France and Germany (most cost-effective anti-IL23 treatment) which had a cost per PASI100-responder of €20,969 and €26,994, respectively.
Similar results were observed for certolizumab and infliximab (anti-TNFs), which were also less cost-effective compared to the most cost-effective anti-TNF, adalimumab, in both France and Germany ().
4. Discussion
To our knowledge, this is the first study to assess the cost-effectiveness of all licensed anti-IL17s available in France and Germany, including the newly approved anti-IL17 drug, bimekizumab.
We found that brodalumab was the most cost-effective treatment overall in both France and Germany. The cost-effectiveness of brodalumab was caused by it being highly effective in achieving a PASI100 response along with relatively low acquisition costs due to fewer induction dosages compared to other anti-IL17 ().
Scenario analyses reinforced the results of the primary analysis. Indeed, using a less stringent definition of responders (PASI75 or PASI90) resulted in a lower cost per responder, as more patients were considered responders; however, no changes were observed in relation to the order of cost-effective in both France and Germany. Including dose adjustment in the analysis showed that brodalumab was still the most cost-effective biologic treatment; however, this scenario analysis should be interpreted with caution as more robust evidence of dose adjustment for all anti-IL17s is needed to confirm the effects of this parameter on the cost-effectiveness. Evaluating all 12 available biologic drugs at once, i.e. comparing the cost-effectiveness of tildrakizumab, certolizumab and infliximab to the results from the secondary analysis, did not alter the overall conclusion in neither France nor Germany.
The results of this analysis align with similar published literature; however, no studies have included the full assortment of biologics used in plaque psoriasis, including bimekizumab, making a direct comparison difficult.
Augustin et al.Citation46 investigated the cost per PASI90-responder for adalimumab, apremilast, brodalumab, etanercept, guselkumab, infliximab, ixekizumab, secukinumab, tildrakizumab and ustekinumab in Germany. Similar to the findings in the present study, brodalumab had the lowest cost per PASI90-responder overallCitation46.
Generally, brodalumab is regarded as the most cost-effective biologic drug used in the treatment of plaque psoriasisCitation46–48. Egeberg et al.Citation47 investigated the cost-effectiveness of a variety of treatment sequences in Spain and included most biologic drugs that are currently available (certolizumab, ustekinumab, brodalumab, secukinumab, ixekizumab, guselkumab and risankizumab). The study found that using brodalumab as first-line treatment led to the most cost-effective treatment sequence, thus aligning with the findings of the current studyCitation47. A study by Al Sawah et al.Citation49 investigated the cost per PASI100-responder for anti-IL17s (ixekizumab and secukinumab), anti-IL12/23 (ustekinumab) and anti-TNFs (adalimumab and etanercept) in the US. The study found that ixekizumab was cost-effective in the class of anti-IL17s and adalimumab was cost-effective in the class of anti-TNFs. As brodalumab and bimekizumab were not included in the study by Al Sawah et al.Citation49, these findings align with the current study when disregarding the results for brodalumab and bimekizumab. Another study by Ravasio et al.Citation50 also investigated the cost per PASI100-responder for anti-IL17s (ixekizumab and secukinumab) anti-IL12/23 (ustekinumab) and anti-TNFs (adalimumab and etanercept) in Italy. The findings of this study were similar to the findings of Al Sawah et al.Citation49 and the current study when disregarding the results for brodalumab and bimekizumab.
4.1. Limitations
The strength of our study was the SLR used to inform the model on the efficacy of the different biologics used in the treatment of plaque psoriasis. This ensured a robust foundation for the analysis and ensured that current available evidence related to the PASI efficacy measures was accounted for.
However, our study had some limitations. Firstly, the NMA used to inform on efficacy, Armstrong et al.Citation27, did not include tildrakizumab, certolizumab and infliximab, thus limiting the secondary analysis to include etanercept, adalimumab, secukinumab, ixekizumab, brodalumab, bimekizumab, ustekinumab, guselkumab and risankizumab. We accounted for this limitation by applying other sources on efficacy for tildrakizumab, certolizumab and infliximab in a scenario analysis. This scenario analysis must be interpreted with caution, as the efficacy measures for tildrakizumab, certolizumab and infliximab did not originate from the same NMA as the remaining biologics. This generates a potential for methodological differences, including variations and heterogeneity in population characteristics, between the studies from which the efficacy measures originated.
Secondly, treatment discontinuation was not included in the present study. In practice, a proportion of patients who initiate treatment with biologics will discontinue treatment due to lack of efficacy and/or adverse events. As evidence suggests that most patients remain on treatment for more than one yearCitation51,Citation52, this limitation may have a marginal effect on the results. As such, Torres et al. found that discontinuation from ustekinumab, secukinumab, ixekizumab, brodalumab, guselkumab and risankizumab was between 5% and 15% after one year of treatmentCitation52.
Thirdly, a limitation of our study was that costs related to administration of drugs, monitoring and adverse events were not included in the analysis. The significance of these costs have previously been investigated in a study by Augustin et al.Citation46, who found that these costs comprise approximately 2% of the total cost for psoriasis patients treated with biologic drugs. In addition to this, only minor differences between the treatments existedCitation46. Thus, it seems likely that excluding administration of drugs, monitoring and adverse events had little or no effect on the results.
Given the nature of cost-effectiveness analyses, the results in the present study were heavily influenced by the treatment costs. In the coming years, introduction of biosimilar products to the market is expected and may alter the market dynamics in the field of biologics. This has the potential to impact the cost-effectiveness of biologics used in the treatment of plaque psoriasis and thereby also the results of the present study.
5. Conclusion
The cost per responder analysis demonstrated that brodalumab was the most cost-effective option within the anti-IL17 class and when compared to all biologics available in the treatment of plaque psoriasis in France and Germany over a one-year time horizon.
Based on this analysis, we found that the cost-effectiveness of biologics used in the treatment of moderate-to-severe plaque psoriasis varies greatly and must be considered to optimize both the outcomes for patients and the resource utilization in healthcare sectors.
Transparency
Author contributions
All authors participated in the concept, design, analysis, writing and revision of the manuscript.
Supplemental Material
Download MS Word (15.5 KB)Acknowledgements
The authors wish to thank Christian Dall Johansen (BSc) for the literature review support.
Declaration of funding
This study was funded by LEO Pharma.
Declaration of financial/other relationships
François Skowron received consulting fees and payment from AbbVie, Almirall, LEO Pharma, Eli Lilly and Sanofi. Nanna Nyholm is an employee at LEO Pharma. Anne Danø and Henrik Schnack are employees at EY, working as a paid vendor for LEO Pharma, and have assisted in the development of this study as well as medical writing support. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. IJMS. 2019;20(6):1475.
- Pellegrini C, Orlandi A, Costanza G, et al. Expression of IL-23/Th17-related cytokines in basal cell carcinoma and in the response to medical treatments. Shiku H, editor. PLOS One. 2017;12(8):e0183415.
- Yang E, Beck K, Sanchez I, et al. The impact of genital psoriasis on quality of life: a systematic review. Psoriasis. 2018;8:41–47.
- Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385.
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1301–1315.
- Ortonne J, Chimenti S, Luger T, et al. Scalp psoriasis: European consensus on grading and treatment algorithm: scalp psoriasis: European consensus. J Eur Acad Dermatol Venereol. 2009;23(12):1435–1444.
- Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
- Damiani G, Bragazzi NL, Karimkhani Aksut C, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med. 2021;8:743180.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 Pt 1):556–562.
- Gisondi P, Bellinato F, Girolomoni G, et al. Pathogenesis of chronic plaque psoriasis and its intersection with cardio-metabolic comorbidities. Front Pharmacol. 2020;11:117.
- Damiani G, Cazzaniga S, Conic RRZ, et al. Pruritus characteristics in a large italian cohort of psoriatic patients. J Eur Acad Dermatol Venereol. 2019;33(7):1316–1324.
- Bhosle MJ, Kulkarni A, Feldman SR, et al. Quality of life in patients with psoriasis. Health Qual Life Outcomes. 2006;4(1):35.
- Tribó M, Turroja M, Castaño-Vinyals G, et al. Patients with moderate to severe psoriasis associate with higher risk of depression and anxiety symptoms: results of a multivariate study of 300 Spanish individuals with psoriasis. Acta Derm Venereol. 2019;99(4):417–422.
- Kowalewska B, Jankowiak B, Cybulski M, et al. Effect of disease severity on the quality of life and sense of stigmatization in psoriatics. Clin Cosmet Investig Dermatol. 2021;14:107–121.
- Goff KL, Karimkhani C, Boyers LN, et al. The global burden of psoriatic skin disease. Br J Dermatol. 2015;172(6):1665–1668.
- Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10.
- Leonardi C, See K, Gallo G, et al. Psoriasis severity assessment combining physician and patient reported outcomes: the optimal psoriasis assessment tool. Dermatol Ther. 2021;11(4):1249–1263.
- Kragballe K, van de Kerkhof PCM, Gordon KB. Unmet needs in the treatment of psoriasis. Eur J Dermatol. 2014;24(5):523–532.
- Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane skin group, editor. Cochrane Database Syst Rev. 2020;1(1):CD011535. 10.1002/14651858.CD011535.pub2
- Sawyer LM, Cornic L, Levin LÅ, et al. Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. J Eur Acad Dermatol Venereol. 2019;33(2):355–366.
- Rønholt K, Iversen L. Old and new biological therapies for psoriasis. IJMS. 2017;18(11):2297.
- Amatore F, Villani A ‐, Tauber M, et al. French guidelines on the use of systemic treatments for moderate‐to‐severe psoriasis in adults. J Eur Acad Dermatol Venereol. 2019;33(3):464–483.
- Nast A, Altenburg A, Augustin M, et al. German S3‐guideline on the treatment of psoriasis vulgaris, adapted from EuroGuiDerm – part 1: treatment goals and treatment recommendations. J Dtsch Dermatol Ges. 2021;19(6):934.
- Ruggiero A, Potestio L, Camela Snr E, et al. Bimekizumab for the treatment of psoriasis: a review of the current knowledge. Psoriasis. 2022;12:127–137.
- Yasmeen N, Sawyer LM, Malottki K, et al. Targeted therapies for patients with moderate-to-severe psoriasis: a systematic review and network meta-analysis of PASI response at 1 year. J Dermatolog Treat. 2022;33(1):204–218.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Armstrong AW, Soliman AM, Betts KA, et al. Long-term benefit–risk profiles of treatments for moderate-to-severe plaque psoriasis: a network meta-analysis. Dermatol Ther. 2022;12(1):167–184.
- Thaci D, Piaserico S, Warren RB, et al. Five‐year efficacy and safety of tildrakizumab in patients with moderate‐to‐severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2). Br J Dermatol. 2021;185(2):323–334.
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther. 2022;12(8):1777–1792.
- ABDATA Pharma-Daten-Service. Pharmaceutical manufacturer prices in Germany. 2023.
- National Health Insurance Agency (AMELI). Pharmaceutical retail prices in France. 2023.
- European Medicines Agency. Taltz: summary of product characteristics [Internet]. 2020 [cited 2022 Dec 11]. Available from: https://www.ema.europa.eu/en/documents/product-information/taltz-epar-product-information_en.pdf
- European Medicines Agency. Bimzelx®: summary of product characteristics [Internet]. 2022 [cited 2022 Dec 11]. Available from: https://www.ema.europa.eu/en/documents/product-information/bimzelx-epar-product-information_en.pdf
- European Medicines Agency. Cosentyx®: summary of product characteristics [Internet]. 2022 [cited 2022 Dec 11]. Available from: https://www.ema.europa.eu/en/documents/product-information/cosentyx-epar-product-information_en.pdf
- European Medicines Agency. Enbrel®: summary of product characteristics [Internet]. 2022 [cited 2022 Dec 11]. Available from: https://www.ema.europa.eu/en/documents/product-information/enbrel-epar-product-information_en.pdf
- European Medicines Agency. Hulio®: summary of product characteristics [Internet]. 2022 [cited 2022 Dec 11]. Available from: https://www.ema.europa.eu/en/documents/product-information/hulio-epar-product-information_en.pdf
- European Medicines Agency. Kyntheum®: summary of product characteristics [Internet]. 2022 [cited 2022 Dec 11]. Available from: https://www.ema.europa.eu/en/documents/product-information/kyntheum-epar-product-information_en.pdf
- European Medicines Agency. SkyriziTM: summary of product characteristics [Internet]. 2022 [cited 2022 Dec 11]. Available from: https://www.ema.europa.eu/en/documents/product-information/skyrizi-epar-product-information_en.pdf
- European Medicines Agency. Stelara®: summary of product characteristics [Internet]. 2022 [cited 2022 Dec 11]. Available from: https://www.ema.europa.eu/en/documents/product-information/stelara-epar-product-information_en.pdf
- European Medicines Agency. Tremfya®: summary of product characteristics [Internet]. 2022 [cited 2022 Dec 11]. Available from: https://www.ema.europa.eu/en/documents/product-information/tremfya-epar-product-information_en.pdf
- European Medicines Agency. Cimzia®: summary of product characteristics [Internet]. 2022 [cited 2022 Dec 13]. Available from: https://www.ema.europa.eu/en/documents/product-information/cimzia-epar-product-information_en.pdf
- European Medicines Agency. Ilumetri®: summary of product characteristics [Internet]. 2022 [cited 2022 Dec 13]. Available from: https://www.ema.europa.eu/en/documents/product-information/ilumetri-epar-product-information_en.pdf
- European Medicines Agency. Zessly®: summary of product characteristics [Internet]. 2022 [cited 2022 Dec 13]. Available from: https://www.ema.europa.eu/en/documents/product-information/zessly-epar-product-information_en.pdf
- Egeberg A, Freilich J, Stelmaszuk MN, et al. Real-world dose adjustments of biologic treatments in psoriasis and their economic impact – a Swedish National Population Study. Clin Exp Dermatol. 2022;47(11):1968–1975.
- Torres T, Puig L, Vender R, et al. Drug survival of interleukin (IL)‑17 and IL‑23 inhibitors for the treatment of psoriasis: a retrospective multi‑country, multicentric cohort study. Am J Clin Dermatol. 2022;23(6):891–904.
- Augustin M, Wirth D, Mahlich J, et al. Cost per responder analysis of guselkumab versus targeted therapies in the treatment of moderate to severe plaque psoriasis in Germany. J Dermatolog Treat. 2022;33(2):976–982.
- Egeberg A, Danø A, Pedersen MH, et al. Modeling the optimal sequence of biologic therapies in plaque psoriasis in Spain. J Med Econ. 2021;24(1):1134–1142.
- Feldman SR, Rastogi S, Lin J. Effect of prior biologic use on cost-effectiveness of brodalumab vs. Ustekinumab for treatment of moderate-to-severe psoriasis in the United States. Dermatol Ther. 2018;8(3):441–453.
- Al Sawah S, Foster SA, Burge R, et al. Cost per additional responder for ixekizumab and other FDA-approved biologics in moderate-to-severe plaque psoriasis. J Med Econ. 2017;20(12):1224–1230.
- Ravasio R, Antonelli S, Maiorino A, et al. Cost per responder for ixekizumab and other biologic drugs approved for the treatment of moderate-to-severe plaque psoriasis in Italy. Glob Reg Health Technol Assess. 2019;2019(8):228424031882228.
- Sbidian E, Mezzarobba M, Weill A, et al. Persistence of treatment with biologics for patients with psoriasis: a real‐world analysis of 16 545 biologic‐naïve patients from the french national health insurance database (SNIIRAM). Br J Dermatol. 2019;180(1):86–93.
- Torres T, Puig L, Vender R, et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for psoriasis treatment: a retrospective multi-country, multicentric cohort study. Am J Clin Dermatol. 2021;22(4):567–579.