Abstract
Background
Priority setting in health research has been described as essential due to disparities within and between countries and populations. Commercial benefits to the pharmaceutical industry may increase the generation and use of regulatory Real-World Evidence which has recently been reported in the literature. Research must be steered by valuable priorities. This study’s objective is to identify key gaps in the knowledge of triglyceride-induced acute pancreatitis by generating a list of potential research priorities for a Hypertriglyceridemia Patient Registry.
Method
The Jandhyala Method was used to observe the consensus of expert opinion from ten specialist clinicians in the treatment of triglyceride-induced acute pancreatitis across the US and EU.
Results
Ten participants completed the consensus round of the Jandhyala method and generated 38 unique items which they all agreed with. The items were included in the generation of research priorities for a hypertriglyceridemia patient registry and presented a novel application of the Jandhyala method for the development of research questions, in aid of the validation of a core dataset.
Conclusion
The TG-IAP core dataset and research priorities combined can develop a globally harmonized framework where TG-IAP patients can be observed simultaneously using the same set of indicators. This will increase knowledge of the disease and facilitate higher-quality research by addressing issues related to incomplete data sets in observational studies. Furthermore, validation of new tools will be enabled, and diagnosis and monitoring will be improved as well as the detection of changes in disease severity and subsequent disease progression, improving the management of patients with TG-IAP overall. This will inform personalized patient management plans and improve patient outcomes along with their quality of life.
PLAIN LANGUAGE SUMMARY
The differences in healthcare between countries and groups of people will likely affect the type of research needed. This is why people that have experience with specific diseases need to be spoken to, to understand what their concerns are. These types of people could be doctors or patients. When this information is gathered, this could help inform organizations interested in a specific disease on how to help patients in real life situations.
For this study, the researchers worked with ten expert doctors who treat a disease called triglyceride-induced acute pancreatitis (TG-IAP). These doctors were from the United States and the European Union, and they were asked to share their opinions on what the most important research areas are using the Jandhyala method. The doctors generated and agreed on 38 items, all related to the most important research areas for TG-IAP.
The research areas identified can be used with important data collected about patients with TG-IAP to create a study where these patients are monitored in different locations using the same measurements. This study will help people learn more about the disease and improve the quality of research by making sure the most important data is collected. As a result, patients with TG-IAP can have their healthcare improved.
Introduction
Research priority setting is an interpersonal endeavor for deciding which uncertainties need to be resolved through research, and is constructed and applied in a specific context, and population with specific principles, values, and preferencesCitation1.
Priority setting in health research has often been described as a pivotal task. Largely, this is due to the vast disparities which exist both between and within countries and populations. These are paralleled by inequalities in access to health research funds and the associated benefitsCitation2. For example, in low-income settings where health needs are high and resources to address them are limited. The primary goal of prioritizing in health research is, thus, the identification of neglected areas within the healthcare landscape and subsequent investment in research to improve interventions for those sectors and patient populationsCitation2.
Determining research priorities requires measuring their value, whether implicitly or explicitly, as only a measure of value can enable ranking and the allocation of resources to the most important areasCitation3. However, scientific evidence can inform research priorities and aid the allocation of resources to neglected areas.
It is well established that prescribers have control over the rate of adoption of new medicinesCitation4. Thus, including research priorities that consider these specialized individuals is crucial to ensuring timely access to new medicines and reducing the time to maximal adoptionCitation4. This is especially critical in the case of rare diseases and orphan drugs.
Freedman et al.Citation5 identified the unmet needs of rare disease patients with triglyceride-induced acute pancreatitis (TG-IAP) and their treating clinicians and developed a tool (core dataset) to diagnose and monitor these patients. TG-IAP, or acute inflammation of the pancreas, is distinguished by severe abdominal pain and high levels of the pancreatic enzyme caused by hypertriglyceridemiaCitation6, despite it being a significant but ignored factor of acute pancreatitisCitation7. Historically, management strategies focussed on treating hypertriglyceridemia by controlling triglycerides and cholesterol, which remains important due to increases in obesity and inactive lifestyles. However, there is a lack of clinical trials investigating the impact lipid-reducing treatments may have on the course of the disease when used during an episode of TG-IAPCitation5. Observational clinical studies using patient cohorts (registries) may help gather real-world clinical information and data conventionally not collected in clinical trials such as the population size and representativeness of patient groups, the duration of follow-up, assessments of outcomes between multiple sites globally over time, and an assessment of the effect of medicines in lowering riskCitation8.
Accumulation of these data may reveal previously undetected trends and provide a research platform capable of providing meaningful answers to research questions, and potentially commercial benefits, as shown by recently published evidence of the utility of regulatory real-world evidence (RWE) for the pharmaceutical industryCitation9. This study, therefore, sought to define the gaps in scientific knowledge using specialist clinicians treating TG-IAP by generating a list of potential research questions (priorities) that could be answered by a Hypertriglyceridemia Patient Registry. The potential research priorities set here will help with drafting policies that determine levels of investment and how research is practiced.
Materials and methods
Participants
Ten TG-IAP treatment experts (composed of eight pancreatologists and two lipidologists) from the United States, the United Kingdom, France, Germany, and Spain were recruited into the study after providing written informed consent. The research was performed by applicable guidelines/regulations, in agreement with the WMA Declaration of Helsinki (2013), and approved by the King’s College London Research Ethics Committee (MRA-21/22-26960).
Identifying research priorities using the Jandhyala method for observing a consensus of expert opinion
Participants were invited to complete an unidentifiable qualitative survey, online, regarding what research questions a patient registry should be able to answer, using a consensus method known as the Jandhyala methodCitation10. The Jandhyala method is a validated novel approach that is different from other consensus methods, such as Delphi and modified-Delphi, as it is limited to just two online surveys, provides novel measurements at the awareness and consensus stages to provide values for participants’ awareness of, and agreement with, items produced within a list. The method profile also preserves the integrity of the items suggested by all participants and ensures any consensus on items to be retained from this list is observed in contrast to other methodologies whether items are added or removed in repeated roundsCitation10. Previously, the method was also used to develop and validate quality-of-life scales, disease-severity scales, core datasets, and definitionsCitation11–13.
For Awareness Round 1, participants were invited to provide a minimum of three-50 free-text responses on an online survey, each referring to one item in response to the question: “As a clinician, what are the research questions that the Hypertriglyceridemia Patient Registry should be able to answer?” Two research analysts coded responses, with disagreements settled by the author. Participants’ survey responses were assigned a score of “one” for each code they referred to. This included the awareness score, which displayed how much information each participant contributed. Coded and aggregated responses were categorized into six broad categories relating to different areas of disease management and evidence generation. The categorized research questions were then presented to the participants in an anonymized survey for Consensus Round 2 online.
The research questions generated (participants’ aggregated codes) were listed and participants were asked to measure their agreement with the inclusion of each research question on a 5-point Likert scale from strongly agree to strongly disagree. Items that had a consensus index of > 50% (CI > = 0.51) were retained.
Results
In the Awareness Round (1) of the Jandhyala method (), 38 unique items were produced. Item awareness indices ranged from 0.03 (Item 25) to 1.00 (Item 11), which all participants mentioned in Awareness Round 1 (A). Four items had an awareness index of 0.5 or above, including Items 8, 9, 11, and 14, and in the Consensus Round 2 (C), 58% of the items achieved unanimous agreement for inclusion. Other items received consensus scores of 0.51 or above, apart from Item 30 which scored 0.31, meaning that all items met the threshold for inclusion as research priorities. Six participants (participants 1, 4, 6, 7, 9, and 10) provided unique items in Awareness Round 1 (). Consequently, data saturation was reached by six participants because no other unique items were generated. Furthermore, the content analysis showed that the consensus-generated list of research questions could be grouped into broader categories related to diagnosis, management, and evidence generation ().
Figure 1. Data saturation. The percentage of unique items from participants is represented by the bars, and in the order that they joined the study, with the x-axis representing both the participant number and the number of participants required for data saturation as represented by the cumulative percentage of unique items. The y-axis presents a continuous scale for percentages from which the % uniqueness and the cumulative percentage can be derived.
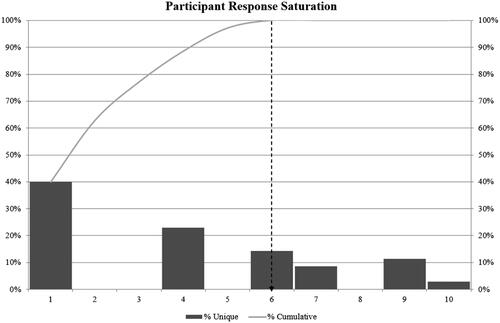
Table 1. Awareness and consensus scores for research questions.
Table 2. Categorical research questions attaining consensus in survey 2 (CR2).
Discussion
The correlation between investment in research and medical needs is weakCitation14–16. Examples of factors that can cause biased perceptions of medical needs can include drug sales, roles of advocacy groups in orphan diseases, and also key opinion leaders who support their researchCitation17.
Generating research priorities with the Jandhyala method
The present study aimed to generate a list of research questions that may be answered by clinicians or healthcare providers observing patients within a Hypertriglyceridemia Patient Registry. This study presents the first use of the Jandhyala Method for developing a set of research priorities, considering the perspectives and experience of 10 specialist clinicians in the management of TG-IAP, and the identified gaps in real-world evidence for the disease area.
The majority of the items generated were related to hypertriglyceridemia-induced pancreatitis (as per grouping identified by Freedman et al.), covering parameters such as the impact of hepatic complications, the risk of development, and the demographic and diagnostic criteria for the condition. Whilst items related to hypertriglyceridemia-induced pancreatitis are prominent in this study, current literature shows that this form of pancreatitis is known, but uncommonCitation18,Citation19, and not understood fully in pediatric casesCitation20 which correlates with the prominence of these generated items, meaning there may be a need for further research to account for limited data available. Furthermore, a significant portion of items was also related to RWE, covering therapeutic intervention, efficacy, and outcomes for hypertriglyceridemia-induced pancreatitis which corresponds with the fact that there is currently no global standard for treating the conditionCitation21, further highlighting the need for research in hypertriglyceridemia-induced pancreatitis.
Of the 38 items generated, the participants were most aware of Item 11 “What are the diagnostic conditions of hypertriglyceridemia-induced pancreatitis?”. The same participants were also involved in the development of the core dataset for TG-IAP5 which in part, aimed to improve diagnostic processes and overall patient management. This could indicate that there is a considerable amount of awareness of unmet needs related to the diagnostic aspects of the disease. Additionally, and as mentioned previously, pediatric hypertriglyceridemia-induced pancreatitis is not fully understood in pediatric cases and highlights a need to elucidate the pathophysiological characteristics of the conditionCitation20. This too corresponds with the significant awareness of Item 11.
Overall, the items were agreed upon by all participants during the consensus round and could be further grouped into 6 broader categories providing confidence in the reliability and validity of our findings. Furthermore, the Jandhyala method had previously been used to develop and observe consensus-of-opinion-guided definitions for medical affairs and real-world evidenceCitation22,Citation23. In this study, bearing in mind that the TG-IAP core dataset requires validation in a real-world clinical setting (patient registry), the novel application of the Jandhyala method for the development of research questions (priorities) to be answered specifically by a patient registry is the most pragmatic course available for validation of a core dataset.
Hypertriglyceridemia-induced diseases and the requirement for a multidisciplinary approach to diagnosis and treatment guided by a core dataset remain outstanding challenges. The tools described in this study are intended to be a ‘starting point’ for the further development and refinement of a globally unified understanding of patients with TG-IAP. This will aid with diagnosing TG-IAP and monitoring its severity and progression. The rapid and conclusive power held by the core dataset will also provide a platform for valuable answers to research questions using a patient population sample more representative of that found in the real world.
Insights observed on inclusivity and developing scientific knowledge
Priority-setting needs to be determined by researchers and members of the public with lived experience, in this instance, specialist clinicians working in the field. By utilizing the perspectives of researchers, ethicists, and community organization staff, observed that building trust, personal qualities and skills of researchers and community members, funding, as well as cultural standards, were the critical influences to enabling shared decision-making in health research priority-settingCitation24. Broader research on the topic has also found that forming connections, trust, and personal qualities of researchers are key driving factors to achieving inclusionCitation25–30.
Engaging people with lived experience as collaborators is, also crucial as a matter of justiceCitation24,Citation31,Citation32 . It allows their voices to be heard and their concerns highlighted in agenda-setting and during the generation of scientific knowledgeCitation33. Additionally, this approach can help address the injustices faced by many with rare diseases by generating research project topics and questions focused on improving access and affordability of health care and servicesCitation31,Citation32,Citation34. Consequently, the involvement of rare disease patients in priority-setting is fundamentalCitation34. On this occasion, the scope of the work was limited to TG-IAP clinicians. However, the future work planned involves engaging patients.
Limitations
The Jandhyala method presented 38 items to the same experts who developed the core dataset for TG-IAP. As a consequence, bias may have been introduced by the experts. However, it may be deemed necessary in this case as meeting the unmet needs of these specialist clinicians was the focus of setting these research priorities within the framework of a patient registry. Ten experts from the U.S. and E.U (5 from each territory) were recruited for the study which invited questions regarding the applicability of the work to territories not represented by an expert.
Conclusion
This work intends to develop a global harmonized framework, where multiple populations of TG-IAP patients can be effectively combined and observed using the same set of indicators. The research questions presented may be answered by larger and uniform real-world populations. This may address the longstanding issues associated with incomplete data sets in observational studies by facilitating higher-quality research and the thorough validation of new tools, geared toward improving the overall management of patients with TG-IAP. This will be achieved through improved diagnosis, monitoring, and the detection of changes in disease severity and subsequent disease progression which will inform personalized patient management plans and improve patient outcomes along with their quality of life.
Transparency
Declaration of funding
Declaration of financial/other relationships
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. R. Jandhyala is a visiting senior lecturer at the Centre for Pharmaceutical Medicine Research at King’s College London and is responsible for research into real-world evidence approaches. He is also the founder and CEO of Medialis Ltd, a medical affairs consultancy and contract research organization involved in the design and delivery of real-world evidence in the pharmaceutical industry. The Jandhyala method was developed by R Jandhyala but is free of commercial licensing restrictions and while used as part of proprietary methodology, is not a direct means of commercial gain for the author.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
RJ conducted the study and prepared, authored, and approved the manuscript. SF, RS, VR, EM, VS, ML, and EB prepared, authored, and approved the manuscript. The authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Acknowledgements
The authors would like to acknowledge the contributions to the development of the core dataset made by the non-author advisors Robert Sutton, Vikesh K. Singh, Michael Davidson, Andres Gelrud, and Chris Forsmark. They would also like to acknowledge the contribution of Medialis personnel (Omolade Femi-Ajao, Radek Wojcik, Brendon Pearce, Mohammed Kabiri, Ziyaad Rahman and Obuchinezia Anyanwu) to the development of the core dataset and this manuscript. All persons mentioned here have consented to be named.
Data availability statement
The data that support the findings of this study are available from the corresponding author, R Jandhyala, upon reasonable request.
Additional information
Funding
References
- Nasser M, Ueffing E, Welch V, et al. An equity lens can ensure an equity-oriented approach to agenda setting and priority setting of cochrane reviews. J Clin Epidemiol. 2013;66(5):511–521. doi:10.1016/j.jclinepi.2012.11.013.
- GFHR. The 3D Combined Approach matrix: an improved tool for setting priorities in research for health. Geneva, Switzerland. 2009. https://www.files.ethz.ch/isn/111447/2009_The-3D-Combined-Approach-Matrix.pdf
- Fleurence RL, Torgerson DJ. Setting priorities for research. Health Policy. 2004;69(1):1–10. doi:10.1016/j.healthpol.2003.11.002.
- Jandhyala R. A medicine adoption model for assessing the expected effects of additional real-world evidence (RWE) at product launch. Curr Med Res Opin. 2021;37(9):1645–1655. doi:10.1080/03007995.2021.1947218.
- Freedman S, de-Madaria E, Singh VK, et al. A simple core dataset for triglyceride-induced acute pancreatitis. Curr Med Res Opin. 2022;23:1–10.
- Yang AL, McNabb-Baltar J. Hypertriglyceridemia and acute pancreatitis. Pancreatology. 2020;20(5):795–800. doi:10.1016/j.pan.2020.06.005.
- Carr RA, Rejowski BJ, Cote GA, et al. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent aetiology? Pancreatology. 2016;16(4):469–476. doi:10.1016/j.pan.2016.02.011.
- Ferreira-González I, Marsal JR, Mitjavila F, et al. Patient registries of acute coronary syndrome: assessing or biasing the clinical real world data? Circ Cardiovasc Qual Outcomes. 2009;2(6):540–547. doi:10.1161/CIRCOUTCOMES.108.844399.
- Jandhyala R. Commercial impact of adding real-world evidence to clinical trials at regulatory approval: a markovian-like transition model. Curr Med Res. Opin. 2023;:1–8. doi:10.1080/03007995.2023.2174330.
- Jandhyala R. Delphi, non-RAND modified delphi, RAND/UCLA appropriateness method and a novel group awareness and consensus methodology for consensus measurement: a systematic literature review. Curr Med Res Opin. 2020;36(11):1873–1887. doi:10.1080/03007995.2020.1816946.
- Jandhyala R. Development and validation of the medical affairs pharmaceutical physician value (MAPPval) instrument. Pharmaceut Med. 2022;36(1):47–57. doi:10.1007/s40290-021-00413-9.
- Jandhyala R. Design, validation and implementation of the post-acute (long) COVID-19 quality of life (PAC-19QoL) instrument. Health Qual Life Outcomes. 2021;19(1):229. doi:10.1186/s12955-021-01862-1.
- Damy T, Conceição I, García-Pavía P, et al. A simple core dataset and disease severity score for hereditary transthyretin (ATTRv) amyloidosis. Amyloid. 2021;28(3):189–198. doi:10.1080/13506129.2021.1931099.
- Yao L, Li Y, Ghosh S, et al. Health ROI as a measure of misalignment of biomedical needs and resources. Nat Biotechnol. 2015;33(8):807–811. doi:10.1038/nbt.3276.
- Gross CP, Anderson GF, Powe NR. The relation between funding by the national institutes of health and the burden of disease. N Engl J Med. 1999;340(24):1881–1887. doi:10.1056/NEJM199906173402406.
- Gillum LA, Gouveia C, Dorsey ER, et al. NIH disease funding levels and burden of disease. PLoS One. 2011;6(2):e16837. doi:10.1371/journal.pone.0016837.
- Dogan S, Pistilli B, Andre F. Defining research priorities without biases: what is the optimal process? Ann Oncol. 2017;28(2):195–196. doi:10.1093/annonc/mdw629.
- de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6(5):649–655. doi:10.1177/2050640618755002.
- Garg R, Rustagi T. Management of hypertriglyceridemia induced acute pancreatitis. Biomed Res Int. 2018;2018:e4721357. doi:10.1155/2018/4721357.
- Grisham JM, Tran AH, Ellery K. Hypertriglyceridemia-induced acute pancreatitis in children: a mini-review. Front Pediatr. 2022;10:931336. doi:10.3389/fped.2022.931336.
- Marić N, Mačković M, Bakula M, et al. Hypertriglyceridemia-induced pancreatitis treated with continuous insulin infusion—case series. Clin Endocrinol (Oxf). 2022;96(2):139–143. doi:10.1111/cen.14554.
- Jandhyala R. Development of a definition for medical affairs using the jandhyala method for observing consensus opinion among medical affairs pharmaceutical physicians. Front Pharmacol. 2022;13:842431. doi:10.3389/fphar.2022.842431.
- Jandhyala R. Development of a definition for real-world evidence using the jandhyala method for observing consensus opinion among medical affairs pharmaceutical physicians. Curr Med Res Opin. 2023. doi:10.1080/03007995.2023.2172261.
- Pratt B. Towards inclusive priority-setting for global health research projects: recommendations for sharing power with communities. Health Policy Plan. 2019;34(5):346–357. doi:10.1093/heapol/czz041.
- Cargo M, Mercer SL. The value and challenges of participatory research: strengthening its practice. Annu Rev Public Health. 2008;29:325–350. doi:10.1146/annurev.publhealth.29.091307.083824.
- Cornwall A. Whose voices? Whose choices? reflections on gender and participatory development. In: Cornwall A, editor. The participation reader. New York (NY): Zed Books; 2011. p. 203–223.
- Gaventa J. Towards participatory governance: assessing transformative possibilities. In: Hickey S, Mohan G, editors. Participation from tyranny to transformation. London: Zed Books; 2004. p. 25–58.
- Muhammad M, Wallerstein N, Sussman AL, et al. Reflections on the researcher identity and power: the impact of positionality on community based participatory research (CBPR) processes and outcomes. Crit Sociol. 2015;41(7-8):1045–1063. doi:10.1177/0896920513516025.
- Ponic P, Reid C, Frisby W. Cultivating the power of partnerships in feminist participatory action research on women’s health. Nurs Inq. 2010;17(4):324–335. doi:10.1111/j.1440-1800.2010.00506.x.
- Wallerstein N, Duran B. The theoretical, historical, and practical roots of CBPR. In: Wallerstein N, Minkler M, editors. Community-based participatory research for health: from process to outcomes. San Francisco (CA): John Wiley & Sons; 2010. p. 25–46.
- Oswald K, Gaventa J, Leach M. Introduction: interrogating engaged excellence in research. IDS Bull. 2016;47(6):1–18.
- Pratt B. Engagement as “co-constructing knowledge”: a moral necessity in public health research. Bioeth. 2019;33(7):805–813. doi:10.1111/bioe.12591.
- Reynolds L, Sariola S. The ethics and politics of community engagement in global health research. Crit Public Health. 2018;28(3):257–268. doi:10.1080/09581596.2018.1449598.
- Ahmed SM, Palermo AS. Community engagement in research: frameworks for education and peer review. Am J Public Health. 2010;100(8):1380–1387. doi:10.2105/AJPH.2009.178137.
