Abstract
Objective
To describe long-term (24-month) treatment patterns of patients initiating galcanezumab versus standard of care (SOC) preventive migraine treatments including anticonvulsants, beta-blockers, antidepressants, and onabotulinumtoxinA using administrative claims data.
Methods
This retrospective cohort study, which used Optum de-identified Market Clarity data, included adults with migraine with ≥1 claim for galcanezumab or SOC preventive migraine therapy (September 1, 2018 − March 31, 2020) and continuous database enrollment for 12 months before (baseline) and 24 months after (follow-up) the index date (date of first claim). Baseline patient demographics, clinical characteristics, and treatment patterns were analyzed after 24-month follow-up, including adherence (measured as the proportion of days covered [PDC]), persistence, discontinuation (≥60-day gap), restart, and treatment switch. Propensity score matching (1:1) was used to balance the galcanezumab and SOC cohorts.
Results
The study included 2307 matched patient pairs with 24-month follow-up. The mean age across cohorts was 44.5 years (females: ∼87%). Patients in the galcanezumab versus SOC cohort demonstrated greater treatment adherence (PDC: 48% vs. 38%), with more patients considered adherent (PDC ≥80%: 26.6% vs. 20.7%) and persistent (322.1 vs. 236.4 d) (all p < .001). After 24-month follow-up, fewer galcanezumab-treated patients had discontinued compared with SOC-treated patients (80.1% vs. 84.7%; p < .001), of which 41.3% and 39.6% switched to a non-index medication, respectively. The most prevalent medication patients switched to in both cohorts was erenumab. Significantly greater proportions of patients who initiated galcanezumab versus SOC medications switched to fremanezumab (p < .001) and onabotulinumtoxinA (p = .016).
Conclusion
Patients who initiated galcanezumab for migraine prevention had higher treatment adherence and persistence compared with those who initiated SOC medications after 24-month follow-up.
PLAIN LANGUAGE SUMMARY
Only few patients (3 − 13%) with migraine, who qualify for preventive treatment, are using them. Conventional preventive treatments have not been developed specifically for migraine treatment, and more than half of the patients stop using them prematurely. Calcitonin gene-related peptide monoclonal antibodies such as galcanezumab, fremanezumab, and erenumab are newer treatments that provide migraine-specific preventive treatment. Prior studies have compared 6- to 12-month migraine medication use by patients starting galcanezumab versus those starting traditional standard of care (SOC) migraine preventive medications. We compared long-term (24-month) migraine medication use in patients starting galcanezumab versus those starting SOC migraine preventive medications to confirm if the results are sustained over a longer period. Over 24 months, patients who used galcanezumab followed the prescribed treatment regimen to a greater extent compared with those who used SOC medications (48% vs. 38%, respectively). Additionally, patients using galcanezumab continued treatment for a longer time compared with those using SOC. Over 24 months, about 85% of patients stopped taking SOC medications, while around 80% of patients stopped taking galcanezumab. Our findings indicate that patients with migraine are more likely to continue using galcanezumab as a preventive treatment for a longer period compared with SOC medications. This study helps identify gaps in the preventive treatment of migraine and provides insights on how they are not being used enough.
Introduction
Migraine is a common, recurrent, disabling nervous system disorder that affects one in seven people in the United States (US)Citation1,Citation2. According to the National Health Interview Survey, the overall age-adjusted prevalence of migraine or severe headache in the US was 15.9% in 2018 (range from 2005–2018: 12.3–16.6%)Citation3. Headaches, including migraine, were reported as the fifth leading cause of emergency department visits in the US in 2016 and the second leading cause of disability worldwide in 2019Citation3,Citation4. Migraine interferes with education, employment, and patients’ quality of life.
Typical migraine treatments manage attacks using acute medications and prevent attacks using preventive medicationsCitation5. The American Headache Society (AHS) consensus statement (2021) and the American Academy of Neurology (AAN) guidelines (2012) recommend the initiation of appropriate preventive therapies to effectively manage migraine based on the frequency of attacks, degree of disability experienced, response to existing acute treatments, and patient preferenceCitation6,Citation7. Of note, about 40% of the overall population of patients with migraine are eligible for preventive treatments; however, only 3–13% receive conventional oral migraine preventives, and high discontinuation rates are reported among these patientsCitation6,Citation8,Citation9. This could be due partly to the limitations associated with some conventional oral migraine preventive treatments. None of the currently available standard of care (SOC) preventive medications, including anticonvulsants, beta-blockers, and antidepressants were specifically developed for migraine treatmentCitation6, but considered efficacious or possibly effective therapies for episodic migraine in the 2012 AAN guidelinesCitation7. However, many of the SOC treatments have demonstrated burdensome side effectsCitation10,Citation11. OnabotulinumtoxinA was regulatory approved for the prevention of chronic migraine by the FDA in 2010 and was not included in the 2012 AAN guidelines on episodic migraine.
The approval of calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) for migraine prevention have changed the preventive treatment landscape, owing to reliable evidence supporting efficacy and safetyCitation6. Guidelines and consensus statements recommend the use of CGRP mAbs after at least two oral SOC preventive medications or at least two quarterly injections of onabotulinumtoxinA that have failed due to intolerance or inadequate response, or for those with moderate disabilityCitation6.
Real-world studies with 6- and 12-month follow-ups have described the treatment patterns of CGRP mAbs versus SOC medications, specifically galcanezumab versus SOC medications, and found that patients who initiate treatment with galcanezumab generally have greater adherence and persistence to therapy compared with those initiating SOC treatmentCitation12,Citation13. Generation of long-term real-world evidence is needed to understand whether earlier initiation of CGRP mAbs and their continued use can be of benefit for patients with migraine. Hence, we aimed to confirm if the results from the previous 6- and 12- month studies are sustained over a longer period of time. The current study describes the demographics, clinical characteristics, medication use, and long-term treatment patterns (including treatment adherence, persistence, discontinuation, restart, and switch) after 24 months of follow-up of US patients with migraine who newly initiated treatment with galcanezumab versus SOC medications.
Material and methods
Study design and data source
This real-world analysis was a retrospective, observational cohort study in the US using integrated clinical and claims data from the de-identified Optum Market Clarity database (September 1, 2018 − March 31, 2020) (Supplementary Figure 1). The study assessed treatment patterns over a 24-month follow-up period in adults with migraine newly initiating preventive treatment with galcanezumab or SOC treatments. The SOC treatments included medications described in the 2021 AHS consensus statement as well as additional medications for migraine prevention (Supplementary Table 1)Citation7,Citation13. The National Drug Code list was obtained from First Databank.
De-identified data from the Optum Market Clarity database were used in this study. The Optum de-identified Market Clarity data set deterministically links medical and pharmacy claims with electronic health record data from providers across the continuum of careCitation14. The clinical data combined with adjudicated medical and pharmacy claims from both Optum-affiliated and non-affiliated payers provided a complete assessment of patients’ clinical profiles and treatment patterns. Institutional review board approval or patient informed consent were not required to conduct this study because only de-identified patient records were used.
Patient selection
Data were selected for adult patients aged ≥18 years (as of the index date) with ≥1 claim for galcanezumab or SOC preventive migraine treatment between September 1, 2018, and March 31, 2020 (identification period) (). The index date was defined as the first date of a claim for galcanezumab or SOC preventive migraine treatment during the identification period, and the medication claim on the index date was identified as the index medication. For patients identified with an index date for a SOC treatment that had overlapping days’ supply with a claim for galcanezumab, the index date was set to the first date of the galcanezumab claim. Patients were required to have continuous enrollment in medical and pharmacy benefits for 12 months before the index date (baseline period) and 24 months after the index date (24-month follow-up period). Only patients with complete claims, enrollment, and demographics data were included. Included patients were naïve to CGRP inhibitor use for migraine prevention. Patients were newly initiated on the index SOC medication but may have used non-index drug classes previously for migraine prevention during baseline.
Figure 1. Patient selection and data attrition for 24-month follow-up. Conventional preventive treatments for migraine in the SOC cohort included level A drugs (antiepileptic drugs [divalproex sodium, sodium valproate, or topiramate], beta-blockers [metoprolol, propranolol, or timolol]), level B drugs (tricyclic antidepressants [amitriptyline or venlafaxine], beta-blockers [atenolol or nadolol]), and other nonspecific drug (onabotulinumtoxinA). Abbreviations: CGRP, calcitonin gene-related peptide; HCPCS, Healthcare Common Procedure Coding System; mAbs, monoclonal antibodies; N, number of patients identified at each selection step; NDC, National Drug Code; SOC, standard of care preventive migraine medications.
![Figure 1. Patient selection and data attrition for 24-month follow-up. Conventional preventive treatments for migraine in the SOC cohort included level A drugs (antiepileptic drugs [divalproex sodium, sodium valproate, or topiramate], beta-blockers [metoprolol, propranolol, or timolol]), level B drugs (tricyclic antidepressants [amitriptyline or venlafaxine], beta-blockers [atenolol or nadolol]), and other nonspecific drug (onabotulinumtoxinA). Abbreviations: CGRP, calcitonin gene-related peptide; HCPCS, Healthcare Common Procedure Coding System; mAbs, monoclonal antibodies; N, number of patients identified at each selection step; NDC, National Drug Code; SOC, standard of care preventive migraine medications.](/cms/asset/261afb33-5e45-47aa-9ba0-863fce79e4c1/icmo_a_2316864_f0001_c.jpg)
Migraine diagnosis was confirmed based on the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code from baseline through the index date. Patients with evidence of using the index drug class during baseline or evidence of multiple index drug use within the same class on the index date were excluded. Patients were excluded if they had evidence of pregnancy, epilepsy, cancer, or cluster headache or had undergone preventive treatment with dosing indicated for cluster headaches (i.e. galcanezumab 300 mg) at any time during the baseline or follow-up periods.
Study measures
Baseline demographics and clinical features
Baseline demographics measured on the index date included age, gender, race, ethnicity, geographic region, insurance plan type, and provider specialty. Provider specialty was based on the prescriber of the index preventive migraine therapy claim(s). Baseline clinical features assessed during the 12-month period before the index date included migraine type, preventive and acute medications for migraine, and comorbidity as measured by the Quan-Charlson comorbidity score and Agency for Healthcare Research and Quality (AHRQ) comorbid conditionsCitation7,Citation15,Citation16. Comorbid medical conditions were identified based on ICD-10-CM diagnosis codes. The top 20 comorbid conditions present among patients in both cohorts were reported. Medications were summarized by drug class, individual drugs for preventive medications, and migraine-specific and nonspecific drugs for acute medications.
Preventive and acute medications for migraine
The baseline and follow-up preventive and acute migraine medications analyzed were based on the AAN 2012 guidelines and the 2021 AHS consensus statementCitation6,Citation7. Preventive migraine medications included CGRP mAbs (erenumab, fremanezumab, galcanezumab, and eptinezumab); oral CGRP antagonists (gepants) (rimegepant and atogepant); and certain medications from other preventive drug categories such as beta-blockers, anticonvulsants, tricyclic antidepressants, calcium channel blockers, angiotensin II receptor antagonists, and onabotulinumtoxinA. Acute medications included migraine-specific (triptans, gepants [rimegepant and ubrogepant], ergotamine derivatives, and serotonin 5-HT1F receptor agonists) and migraine nonspecific medications (nonsteroidal anti-inflammatory drugs [NSAIDs], opioids [short-acting], antiemetics, acetaminophen and combinations, butalbital-containing combinations, and isometheptene-containing compounds). Rimegepant was considered as a preventive medication if the quantity of tablets taken was >8 per month. The number of patients with claims for specific drug categories and the number of unique preventive and acute migraine treatments per patient in each cohort during baseline and follow-up were reported. Medication overuse was determined by calculating the total days’ supply for each acute medication class over the baseline and follow-up periodsCitation17,Citation18.
Treatment patterns during the 24-month follow-up period
Treatment adherence
Treatment adherence to the index drug was reported using the proportion of days covered (PDC) and the medication possession ratio (MPR). The PDC was calculated by dividing the number of days on which the index therapy was available (based on pharmacy and medical claims) by the number of days between the index date through the follow-up end date (PDC observation period). For patients with multiple SOC index drug classes, the numerator was the sum of days when either drug class was present.
The MPR was calculated by adding the number of days the index medication was supplied for all but the last refill in the observation period, divided by the number of days between the first and the last refill. Patients with ≥2 prescription fills of the index drug were eligible for the MPR evaluation. The MPR was capped at 100%. Patients with a PDC or MPR of ≥80% were considered treatment adherent.
Persistence
Persistence to the index drug was defined as the number of days of continuous therapy from the index date until the end of the follow-up period, allowing for a maximum gap of <60 d (<60-day gap) between prescription fills. Persistence to the index drug until the end of follow-up and the number of days of persistent index drug use among all patients in the cohort were calculated. Persistence was measured in those with complete medication claims data for the full 24-month follow-up period. Patients were excluded from the persistence analysis in cases where the gap between the end of the patient’s last prescription fill and the end of the 24-month follow-up was <60 d and if a patient with health plan enrollment beyond the end of the 24-month follow-up did not have medication refills.
Discontinuation
Discontinuation of the index drug was defined as a minimum gap in therapy of 60 d. The date of discontinuation was defined by the date the patient had run out of the days’ supply of the last prescription filled before the first observed gap in therapy. For patients with multiple SOC index drug classes, discontinuation of either component class equated to discontinuation of the index therapy. Similar to the persistence calculation, discontinuation was not measured for patients who had a < 60-day gap between the end of their last prescription fill and the end of the 24-month follow-up in the main analysis; however, these patients were included as censored in the Kaplan − Meier analysis.
Restart of index drug after discontinuation
Restart of the index drug was evaluated among patients who discontinued the index drug (≥60-day gap). Restart was defined as the presence of a prescription filled for the index drug after the discontinuation date and no later than the end of the study period. The time to first restart was calculated as the days between the discontinuation date and the restart date of the index drug.
Switch to non-index drug after discontinuation
A treatment switch was defined as the first switch to a non-index migraine preventive treatment after discontinuation that was not part of the index treatment regimen. The index treatment regimen comprised all migraine preventive medications with overlapping days’ supply within a ± 30-day period of the index date. Treatment switch could occur between individual CGRP mAbs/gepants or between different SOC drug classes of migraine preventive treatment. A treatment switch between SOC drugs within the same drug class was not considered a switch. The time to first switch was calculated as the number of days between the index date and the first switch date to a drug that was not part of the index treatment regimen.
Statistical analyses
Propensity score matching
Propensity score matching was used to control for selection bias and confounders due to the study’s observational nature. The propensity score was defined as the probability of receiving a treatment conditional on the patient’s observed baseline and clinical characteristics. Propensity scores were estimated using unconditional logistic regression with “receiving galcanezumab” as the dependent variable and the following baseline characteristics as independent variables: demographics (index year, age, age group, geographic region, insurance type, and provider specialty); clinical characteristics (Quan-Charlson comorbidity score, AHRQ comorbidities, baseline migraine medication use, and number of monthly migraine daysCitation19,Citation20) and economic characteristics (baseline all-cause and migraine-related utilization and costs). All-cause and migraine-specific utilization (defined using ICD-10-CM diagnosis codes for migraine in any position on a claim) included ambulatory visits (i.e. physician office and hospital outpatient), emergency department visits, inpatient admissions, and other medical visits. Costs (all-cause and migraine-specific) included pharmacy costs and medical costs (ambulatory costs [physician office and hospital outpatient], emergency services costs, inpatient costs, and other medical costs).
Patients were matched 1:1 with a caliper of 0.01. Patients were hard matched on gender and migraine type (chronic, episodic, or unknown). A standardized difference of greater than 10% in absolute value was considered for potential imbalances that warranted further investigation or adjustment.
Significance level, hypotheses testing, and multiplicity adjustment
Statistical analyses were performed using SAS software, version 9.4. All analyses were planned, and the significance level was set at a two-sided α level of 0.05 a priori. Descriptive analyses were performed before and after matching, and the mean, standard deviation, number of observations, and percentages were reported. For post-matching, the z-test using robust standard errors in an ordinary least-squares regression was used for continuous measures and the Rao-Scott test was used for binary measures. No multiplicity adjustments were performed. Discontinuation of the index drug (median time to discontinuation) over the 24-month follow-up period was described using Kaplan − Meier curves. Patients who did not discontinue the index drug were censored at the end of follow-up.
Results
Patient sample and baseline characteristics
A total of 2363 and 61,576 patients were identified as initiating galcanezumab and SOC medications, respectively, as the index drug (). Before matching, patients in the galcanezumab cohort were similar in age to those in the SOC cohort (mean: 44.5 vs. 43.3 years; standardized difference: 9.1%), and 87.1% and 81.9% of the patients were female in the respective cohorts (). The galcanezumab cohort included more patients with a neurologist (44.0% vs. 20.9%; standardized difference: 50.8%) and fewer patients with a primary care physician (19.7% vs. 33.7%; standardized difference: −32.1%) as their provider specialty on the index claim as compared to the SOC cohort. Chronic migraine rates were higher in the galcanezumab cohort than in the SOC cohort (44.8% vs. 20.1%; standardized difference: 54.6%).
Table 1. Baseline patient demographics before and after matching in the galcanezumab and SOC cohorts.
After 1:1 propensity score matching, the galcanezumab and SOC cohort matched population comprised 2307 patients each (). The matched cohorts were well-balanced across all variables (Supplementary Table 2; ). Patients in the matched cohorts had a mean age of approximately 44.5 years and comprised mostly females (87.3%). Most patients resided in the South (∼35%) or Midwest (∼34%) region of the US. The proportion of patients with chronic migraine was 44.4% in both cohorts. The top three chronic comorbid conditions were “spondylosis, intervertebral disc disorders, other back problems,” “other connective tissue disease,” and “respiratory infections.”
Index medication classes used by patients in the SOC cohort were anticonvulsants (33.3%), tricyclic antidepressants (25.8%), beta-blockers (23.8%), onabotulinumtoxinA (15.9%), calcium channel blockers (1.3%), and angiotensin II receptor antagonists (0.7%).
Baseline and follow-up preventive and acute medications used for migraine in the matched population
During the baseline period, there were no significant differences between the galcanezumab and SOC cohorts in the use of any preventive or acute migraine medications (87.1% vs. 86.4%; ). About 40% of patients in each cohort received three or more unique preventive or acute drug classes. Around 17% of patients received preventive therapy, of which most received a single preventive drug class. Anticonvulsants, beta-blockers, and tricyclic antidepressants were the most prescribed baseline preventive drug classes in both cohorts (). The majority of patients (∼85%) received acute medications, of which about 34% of patients received three or more unique acute drug classes (). Most common acute medications included triptans (58.2% and 57.7%), NSAIDs (38.7% and 38.8%), opioids (36.4% and 38.8%), and antiemetics (33.9% and 33.7%) in the galcanezumab and SOC cohorts, respectively (). At baseline, 33.2% and 30.6% of patients were presumed to have acute medication overuse in the galcanezumab and SOC cohorts, respectively (standardized difference: 5.6%). Presumed overuse of triptans was found to be most prevalent among the cohorts, followed by opioids and butalbital ().
Figure 2. Medications used during the 12-month (baseline) and 24-month (follow-up) periods in the matched galcanezumab and SOC cohorts. Temporal comparisons were not conducted. Any result that included less than 12 patients was not reported due to re-identification risk. Proportions of patients were compared using the Rao-Scott test for categorical variables. Abbreviations: CGRP, calcitonin gene-related peptide; DHE, dihydroergotamine; mAb, monoclonal antibody; SOC, standard of care preventive migraine medications. *p<.05 between galcanezumab and SOC.
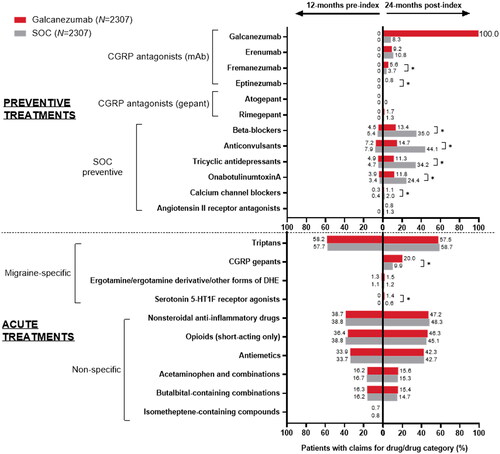
Table 2. Baseline and follow-up preventive and acute medications used for migraine in the galcanezumab and SOC matched population.
During the 24-month follow-up, a similar proportion of patients in the galcanezumab and SOC cohorts received three or more unique preventive and acute medication classes (77.4% vs. 76.1%) (). Significantly more patients in the galcanezumab versus SOC cohort received a single preventive drug class (60.3% vs. 56.1%; p = .003), whereas significantly fewer patients received three or more preventive drug classes (11.8% vs. 14.0%; p = .027). Additionally, a similar proportion of patients in the galcanezumab versus SOC cohort received three or more unique acute medication classes (45.8% vs. 43.5%). Similar proportions of patients in the galcanezumab and SOC cohorts used acute treatments such as triptans, NSAIDs, opioids, and antiemetics (). Additionally, a significantly greater proportion of patients received gepants for acute treatment in the galcanezumab versus SOC cohort (20.0% vs. 9.9%; p < .001). At 24-month follow-up, about 33% of patients in either cohort were found to have overused acute medications such as triptans, opioids, and butalbital, in the observed order of prevalence ().
Treatment patterns in the matched population
Index drug refill and treatment adherence
During the 24-month follow-up, patients in the galcanezumab cohort had twice as many prescription fills for their index drug as the SOC cohort (mean number of fills: 11.9 vs. 6.6; p < .001) (). At 24 months, patients who initiated galcanezumab showed significantly greater treatment adherence than those who initiated SOC medications as measured by PDC (mean: 48% vs. 38%) and MPR (mean: 82% vs. 76%) (both p < .001). Significantly more patients in the galcanezumab cohort were considered treatment adherent than those in the SOC cohort as measured by PDC ≥80% (26.6% vs. 20.7%; p < .001) and MPR ≥80% (67.6% vs. 59.5%; p < .001).
Table 3. Treatment patterns in patients with migraine prescribed an index treatment of galcanezumab or SOC during 24-month follow-up.
Persistence and discontinuation of index treatment
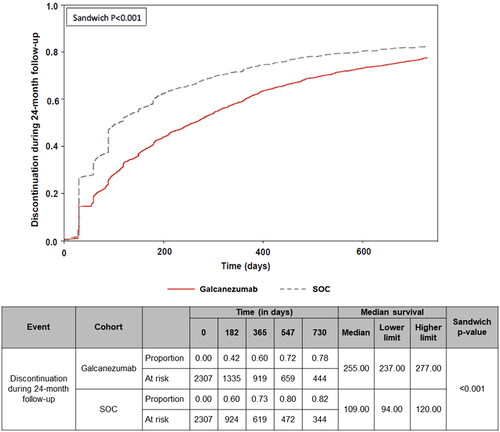
Persistence and discontinuation results excluded 74 patients from galcanezumab and 63 from SOC cohorts, at the end of the 24-month follow-up period. The galcanezumab cohort showed significantly greater persistence to their index treatment (allowing for <60-day gaps) than did the SOC cohort (mean: 322.1 vs. 236.4 days; p < .001) (). Significantly fewer patients in the galcanezumab cohort discontinued treatment during follow-up compared with the SOC cohort (80.1% vs. 84.7%; p < .001) (). Likewise, patients who discontinued in the galcanezumab cohort had a longer mean treatment duration before discontinuation than patients in the SOC cohort (220.8 vs. 147.1 days; p < .001) (). The Kaplan − Meier analysis included the censored 74 and 63 patients, which were previously excluded from the respective cohorts. Based on the Kaplan − Meier analysis, a significantly lower percentage (censoring-adjusted percentage) of patients who initiated galcanezumab discontinued their index treatment at 24 months versus those who initiated SOC medications (77.7% vs. 82.4%), with a median time to discontinuation of 255.0 versus 109.0 d, respectively (p < .001) ().
Figure 3. Proportion of patients that discontinued the index drug during the 24-month follow-up period. The Wald chi-squared test using robust standard errors in a proportional hazard model was used for assessing equality of hazard rates. Abbreviation. SOC, standard of care preventive migraine medications.
Restart of index drug and switch to non-index drug after discontinuation
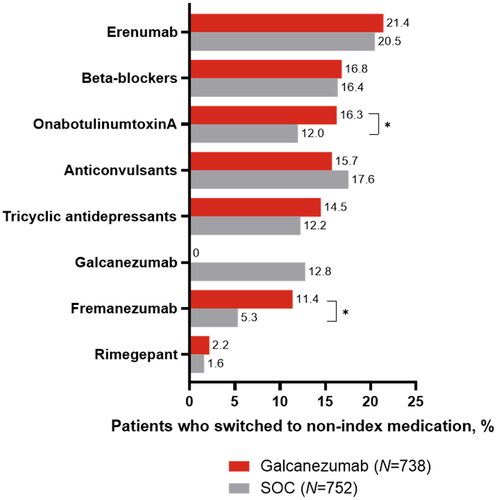
Among patients who discontinued their index treatment during the 24-month follow-up period (galcanezumab: n = 1789; SOC: n = 1900), significantly fewer patients in the galcanezumab cohort restarted the index drug compared with those in the SOC cohort (28.0% vs. 32.1%; p = .007), while a similar proportion of patients switched to a non-index preventive drug (41.3% vs. 39.6%; p = .301) (). Among patients who restarted or switched treatments, those in the galcanezumab cohort had a significantly shorter mean time from discontinuation to the first restart of their index drug (161.6 vs. 201.0 d; p < .001) and a longer mean time from the index date to their first switch to a non-index drug (355.9 vs. 317.9 days; p < .001) versus the SOC cohort, respectively. Among patients who switched treatment, erenumab was the most prevalent subsequent treatment in both cohorts, but the difference was not significant (). A significantly greater proportion of patients who initiated galcanezumab versus SOC medications switched to fremanezumab (11.4% vs. 5.3%; p < .001) and onabotulinumtoxinA (16.3% vs. 12.0%; p = .016).
Figure 4. Switch to non-index preventive migraine drug class among patients who switched treatment in the galcanezumab versus SOC cohorts during 24-month follow-up. Treatment switch could have occurred within the calcitonin gene-related peptide class or between different SOC drug classes of preventive migraine treatments. Proportions of patients were compared using the Rao-Scott test for categorical variables. Any result that included less than 12 patients was not reported due to re-identification risk. Abbreviations. N, number of patients who switched to a non-index drug; SOC, standard of care preventive migraine medications. *p < .05 between galcanezumab and SOC.
Discussion
This real-world study compared the long-term treatment patterns of patients with migraine who initiated galcanezumab versus SOC migraine preventive treatments over a 24-month follow-up period. Our findings demonstrated that patients who initiated galcanezumab had significantly greater treatment adherence and persistence to the index drug than those who initiated SOC medications. The majority of patients in both cohorts (>80%) discontinued their index medication during the 24-month follow-up period. Fewer patients discontinued in the galcanezumab cohort, and the mean time to discontinuation was longer than that of patients in the SOC cohort among those who discontinued. Of those who discontinued, similar proportions of patients in the galcanezumab and SOC cohorts restarted their index therapy (28% vs. 32%) and switched therapies (41% vs. 40%) during follow-up.
After propensity score matching, both galcanezumab and SOC cohorts were well-balanced, and the matched characteristics reflected those of the galcanezumab cohort. About half of the patients in this study had comorbidities of spondylosis, intervertebral disc disorders, other back problems, other connective tissue diseases, and respiratory infections. Previous studies have explored the association of migraine and/or other headaches with these conditionsCitation21–24. While some studies report that migraine could be a clinical manifestation of an underlying disease, other studies have described a potential pathophysiological link between migraine and these comorbid conditions. In the current study, the most commonly initiated SOC medication was anticonvulsants followed by tricyclic antidepressants, beta-blockers, and onabotulinumtoxinA. This finding is consistent with results from previous studies describing the use of different SOC therapiesCitation11,Citation13,Citation25,Citation26. Future studies or surveys may help in understanding the reasons behind the choice of preventive treatment prescribed, as some prescribed SOC therapies may have been selected in consideration of comorbid conditions present in patients with migraine while others may lack quality evidence in support of migraine preventionCitation7.
Previous studies have established that galcanezumab initiation reduced monthly headache days with acute medication useCitation27. Similar findings were observed in the current real-world study. In the baseline period, the majority of patients (>85%) used acute medications, while only around 17% used preventive medications. This is attributed to the design of this study where patients were selected based on the first medication they initiated. Hence, only those patients in the galcanezumab cohort with overlapping SOC medication use at the index date or patients identified early in the identification period would have used baseline preventive medication.
In general, the treatment patterns observed in this study were consistent with the previous 12-month follow-up study, which concluded that the galcanezumab cohort had greater treatment adherence and persistence with fewer discontinuations and switches than the SOC cohortCitation13.
It is worthwhile to note that the galcanezumab discontinuation rate in the current 24-month study is almost twice as that in the previous 12-month follow-up study (80% vs. 49%), while the SOC treatment discontinuation rate remained around 80% in both studiesCitation13. Higher discontinuation rates with SOC medications may possibly be due to their suboptimal efficacy or tolerability issues. Moreover, differences in discontinuation rates between galcanezumab and SOC medications could be attributed to the different reimbursement policies associated with CGRP inhibitors and SOC medications as well as the lack of evidence outlining long-term effects of CGRP inhibitors, specifically the criteria for prior authorization renewal that requires confirmation of treatment effectiveness/tolerability for continued coverageCitation28,Citation29. A recently published study emphasized the need for robust guidelines on CGRP mAb discontinuation in the US developed based on other pathophysiological mechanisms and not only effectivenessCitation29. Additionally, previous observational studies have demonstrated that patients who discontinued CGRP mAbs including erenumab and galcanezumab experienced increased migraine frequency and disability a year after initiationCitation30,Citation31.
Future studies may help characterize factors associated with improved adherence and persistence to galcanezumab for migraine. Additionally, understanding reasons for treatment discontinuation through real-world studies and patient/provider surveys is important for patients, providers, policymakers, payers, and other stakeholders to appropriately address the non-adherence to preventive treatment observed among patients with migraine. This along with patient education play a crucial role in improving medication adherenceCitation5,Citation32. Increasing awareness about the importance of treatment, their potential benefits and side effects can empower patients to actively participate in their migraine management journey.
Strengths and limitations
This is one of the first real-world long-term follow-up studies that has assessed treatment patterns with galcanezumab. The long-term 24-month follow-up adds reliability to the existing data on 6- and 12-month follow-up studies. Secondly, this study addressed selection or confounder bias that is inherent to observational studies by using propensity score matching to balance the cohorts. For the SOC cohort, the study included index drugs with favorable evidence-based efficacy; this ensured appropriate comparisons as drugs with inconsistent or poor efficacy may skew results.
The study has the following limitations. The study sample predominantly represents those commercially enrolled in medical and pharmacy benefits, so findings may not be generalizable to other patients. The data source had limited clinical data that indicated severity. Demographic and clinical data could have been potentially misclassified due to data coding and/or entry errors. As the study used claims databases, filled prescriptions may not have translated to patients taking these medications; prescriptions that are written but not filled were not captured. Over-the-counter medications were not captured. This might have confounded the treatment adherence calculations or estimate of patients with medication overuse. A substantial proportion of the study population had several comorbidities including hypertension, heart diseases, and anxiety disorders for which SOC treatment might have been used. This may have confounded the results and treatment choice, and hence, index drug treatment patterns across all cohorts. However, considering the rate of comorbidities was largely balanced between cohorts, it might not have affected the differences observed between matched cohorts. Some medications have on- and off-label indications, and it is hard to determine the specific indication for which they were prescribed; this may have affected follow-up medication use and switching outcomes. Another limitation of the current study is that it compared a single drug (galcanezumab) with a drug class (e.g. beta-blockers).
Conclusion
This study demonstrated greater treatment adherence and persistence to galcanezumab over SOC migraine preventive treatments. At the end of the 24-month follow-up, discontinuation rates were lower for galcanezumab than for the SOC cohort. Despite the difference in discontinuation rates between the cohorts, discontinuation was high in both cohorts indicating that overall persistence to migraine preventive medications is still low. To improve treatment adherence, future studies and surveys exploring patient characteristics and reasons for discontinuing migraine preventive treatments are needed. This can help patients, providers, policymakers, payers, and other stakeholders to appropriately address non-adherence to preventive treatments observed in patients with migraine. With emerging real-world evidence supporting galcanezumab as a preventive treatment for migraine, the current migraine therapeutic landscape may need revisiting to ensure faster and continuous access to appropriate treatments.
Transparency
Author contributions
OJV and HT contributed to conception of the work. OJV, MV, GK, MH, and HT contributed to design of the work. MV, EB, and FC contributed to acquisition of data. EB, GA, FC, and LV contributed to analysis of data. OJV, MV, EB, GK, GA, MH, and LV contributed to interpretation of data. OJV, GK, and LV contributed to drafting of work. OJV, MV, EB, GA, MH, HT, and FC contributed to critical reviewing of the work for important intellectual content.
Supplemental Material
Download MS Word (27 KB)Supplemental Material
Download TIFF Image (341.2 KB)Acknowledgements
The authors thank Sarah Hague of Optum Life Sciences HEOR for project management support; Randall Gerdes of Optum Life Sciences HEOR for programming verification support; and Keerthana Muthiah of Eli Lilly Services India Private Limited for medical writing support.
Declaration of funding
This work was supported by Eli Lilly and Company.
Declaration of financial/other relationships
OJV, LV, MH, and GK are employees and minor stockholders of Eli Lilly and Company. MV, HT, EB, and FC are employees of Optum Life Sciences. GA and HT were employees of Optum Life Sciences when the study was conducted.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Data availability statement
The data that support the study findings were provided by Optum. Restrictions apply to the availability of these data, which were used under license for this study and therefore are not publicly available. Requests may be sent to Optum for more information on data availability and licensing.
References
- National Institute of Neurological Disorders and Stroke (NINDS). Migraine. https://www.ninds.nih.gov/Disorders/All-Disorders/Migraine-Information-Page.
- Law HZ, Chung MH, Nissan G, et al. Hospital burden of migraine in United States adults: a 15-year national inpatient sample analysis. Plast Reconstr Surg Glob Open. 2020;8(4):e2790. doi: 10.1097/GOX.0000000000002790.
- Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: updated age, sex, and socioeconomic-specific estimates from government health surveys. Headache. 2021;61(1):60–68. doi: 10.1111/head.14024.
- Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631–649. doi: 10.1016/j.ncl.2019.06.001.
- Eigenbrodt AK, Ashina H, Khan S, et al. Diagnosis and management of migraine in ten steps. Nat Rev Neurol. 2021;17(8):501–514. doi: 10.1038/s41582-021-00509-5.
- Ailani J, Burch RC, Robbins MS. The American headache society consensus statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021–1039. doi: 10.1111/head.14153.
- Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the quality standards subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337–1345. doi: 10.1212/WNL.0b013e3182535d20.
- Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia. 2017;37(5):470–485. doi: 10.1177/0333102416678382.
- Kawata AK, Shah N, Poon JL, et al. Understanding the migraine treatment landscape prior to the introduction of calcitonin gene-related peptide inhibitors: results from the assessment of TolerabiliTy and effectiveness in MigrAINe patients using preventive treatment (ATTAIN) study. Headache. 2021;61(3):438–454. doi: 10.1111/head.14053.
- Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48(8):1157–1168. doi: 10.1111/j.1526-4610.2008.01217.x.
- Ferreira A, Marques SR, Lopes S, et al. Preventive oral treatment in migraine: efficacy and dropout rates observed at a tertiary headache center. SN Compr Clin Med. 2022;5(1):38. doi: 10.1007/s42399-022-01369-w.
- Mavridis T, Deligianni CI, Karagiorgis G, et al. Monoclonal antibodies targeting CGRP: from clinical studies to real-world evidence–what do we know so far? Pharmaceuticals. 2021;14(7):700. doi: 10.3390/ph14070700.
- Varnado OJ, Manjelievskaia J, Ye W, et al. Treatment patterns for calcitonin gene-related peptide monoclonal antibodies including galcanezumab versus conventional preventive treatments for migraine: a retrospective US claims study. Patient Prefer Adherence. 2022;16:821–839. doi: 10.2147/PPA.S346660.
- Market Clarity Data. 2007–2022. [Internet]. https://www.optum.com/business/life-sciences/real-world-data/market-clarity-data.html.
- Healthcare Cost and Utilization Project (HCUP). Clinical Classifications Software (CCS) for ICD-9-CM [Internet]. 2017. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp.
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433.
- Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71(22):1821–1828. doi: 10.1212/01.wnl.0000335946.53860.1d.
- Silberstein SD, Olesen J, Bousser MG, et al. The international classification of headache disorders, 2nd edition (ICHD-II)–revision of criteria for 8.2 medication-overuse headache. Cephalalgia. 2005;25(6):460–465. doi: 10.1111/j.1468-2982.2005.00878.x.
- Pharmacy Quality Alliance (PQA). One new health plan performance measure and two new pharmacy measures recommended for endorsement. https://www.pqaalliance.org/assets/docs/PQA_Summary-KeyPoints_Endorse_MPT_SP-TAT_PDC-CMP-PH.pdf.
- Pharmacy Quality Alliance (PQA). Measure overview and rationale: migraine preventive therapy (MPT) 2021. https://www.pqaalliance.org/assets/docs/PQA_MPT_Rationale.pdf.
- Biscetti L, De Vanna G, Cresta E, et al. Headache and immunological/autoimmune disorders: a comprehensive review of available epidemiological evidence with insights on potential underlying mechanisms. J Neuroinflammation. 2021;18(1):259. doi: 10.1186/s12974-021-02229-5.
- Lin WS, Huang TF, Chuang TY, et al. Association between cervical spondylosis and migraine: a nationwide retrospective cohort study. Int J Environ Res Public Health. 2018;15(4):587. doi: 10.3390/ijerph15040587.
- Vivekanantham A, Edwin C, Pincus T, et al. The association between headache and low back pain: a systematic review. J Headache Pain. 2019;20(1):82. doi: 10.1186/s10194-019-1031-y.
- Yoon MS, Manack A, Schramm S, et al. Chronic migraine and chronic tension-type headache are associated with concomitant low back pain: results of the German headache consortium study. Pain. 2013;154(3):484–492. doi: 10.1016/j.pain.2012.12.010.
- Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53(4):644–655. doi: 10.1111/head.12055.
- Parikh SK, Silberstein SD. Current status of antiepileptic drugs as preventive migraine therapy. Curr Treat Options Neurol. 2019;21(4):16. doi: 10.1007/s11940-019-0558-1.
- Abu-Zaid A, AlBatati SK, AlHossan AM, et al. Galcanezumab for the management of migraine: a systematic review and Meta-Analysis of randomized Placebo-Controlled trials. Cureus. 2020;12(11):e11621. doi: 10.7759/cureus.11621.
- DiGrande S. The current landscape of CGRP inhibitor coverage. 2019. https://www.ajmc.com/view/the-current-landscape-of-cgrp-inhibitor-coverage.
- Al-Hassany L, Lyons HS, Boucherie DM, et al. The sense of stopping migraine prophylaxis. J Headache Pain. 2023;24(1):9. doi: 10.1186/s10194-023-01539-8.
- Raffaelli B, Terhart M, Overeem LH, et al. Migraine evolution after the cessation of CGRP(-receptor) antibody prophylaxis: a prospective, longitudinal cohort study. Cephalalgia. 2022;42(4–5):326–334. doi: 10.1177/03331024211046617.
- Vernieri F, Brunelli N, Messina R, et al. Discontinuing monoclonal antibodies targeting CGRP pathway after one-year treatment: an observational longitudinal cohort study. J Headache Pain. 2021;22(1):154. doi: 10.1186/s10194-021-01363-y.
- Seng EK, Rains JA, Nicholson RA, et al. Improving medication adherence in migraine treatment. Curr Pain Headache Rep. 2015;19(6):24. doi: 10.1007/s11916-015-0498-8.
