Abstract
Background
Although pulmonary hypertension (PH) and Eisenmenger’s syndrome (ES) are common complications in adult congenital heart disease (ACHD), the frequency of diagnostic tests and the incidence of PH/ES in patients with ACHD in Japanese clinical practice are unclear. Therefore, we sought to clarify the frequency of diagnostic tests and incidence of PH/ES in patients with ACHD using the Medical Data Vision (MDV) database, the largest anonymized database of diagnosis procedure combination hospitals in Japan.
Methods
We conducted a retrospective cohort study using the MDV database (April 2008 to December 2021) of patients with ACHD (International Classificaiton of Diseases, 10th revision codes: Q203-204, Q210-213, Q250) aged ≥15 years. The frequency of laboratory/clinical tests and the incidence of PH/ES were calculated. Subgroup analyses were performed for the periods 2008–2015 and 2016–2021.
Results
Overall, 28219 ACHD patients were extracted from the MDV database (females 56.3%, males 43.7%; mean ± standard deviation age 44.7 ± 23.5 years). The mean ± standard deviation follow-up period was 2.5 ± 2.7 years. The frequencies of electrocardiography, ultrasonography, brain natriuretic peptide (BNP), N-terminal pro-BNP (NT-proBNP), right heart catheterization, and pulmonary function tests (DLCO) were 2149.8, 1054, 1233, 340, 40.0, and 6.0 per 1000 person-years, respectively. The incidence rate of PH/ES was 32.8 per 1000 person-years. The incidence rate of PH/ES increased from 24.6 to 46.7 per 1000 person-years from 2008–2015 to 2016–2021.
Conclusion
We have clarified the frequency of diagnostic tests related to PH/ES and the incidence of PH/ES in patients with ACHD in clinical practice in Japan, including non-specialist institutions for PH.
Introduction
Over the last 50 years, there have been considerable advances in the diagnosis, treatment, and ongoing care of patients with congenital heart disease (CHD), meaning patients with CHD are living longer than ever and the number of patients with adult CHD (ACHD) is increasingCitation1. Despite these advances, many treatments are not curative and patients with CHD frequently develop cardiac complications in adulthood. One leading complication is pulmonary hypertension (PH), which affects up to 10% of patients with ACHDCitation2–6. It has also been reported that the proportion of patients with CHD-related PH is higher in Asian countries than in Western countriesCitation7. Eisenmenger’s syndrome (ES), which occurs in patients with large unrepaired shunts or extracardiac shunts, is found in 11–19% of cases of PH and is one of the most severe types of PHCitation8.
The diagnosis of PH (including ES) in patients with ACHD is a significant clinical challenge due to the rarity of this disease, its complexity and heterogeneity in clinical features, the broad age-range at onset, and the vast array of anatomical defects and surgical repairs for CHD. The difficulty of diagnosing PH may also contribute to delayed or inappropriate treatment, increasing the risk of morbidity or mortality.
In attempts to improve the diagnosis of PH, clinical guidelines or recommendations have been developed in Europe and Japan that encourage diagnostic testing, including electrocardiography (ECG), echocardiography, right heart catheterization (RHC), and relevant blood testsCitation9–12. Regarding the diagnosis of PH/ES in Japanese patients with ACHD, important symptoms of ACHD, regardless of shunt repair, include shortness of breath, cyanosis, and clubbing. ECG, chest X-ray imaging, and transthoracic echocardiography (TTE) should be routinely performed when examining patients with ACHDCitation11.
Despite these guidelines, however, few studies have examined how frequently diagnostic tests for PH/ES are performed in patients with ACHD in real-world clinical practice. Therefore, our objective was to investigate the frequency of common diagnostic tests associated with PH/ES and the onset of PH/ES in a broad range of ACHD patients in real-world clinical practice in Japan. For this purpose, we used the Medical Data Vision (MDV) database, a nationwide administrative inpatient and outpatient health claims database, the largest involving diagnosis procedure combination (DPC) hospitals, in JapanCitation13.
Methods
Ethics
The study adhered to the ethical principles of the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Biological Research Involving Human Subjects. Owing to the use of retrospectively collected, de-identified data, ethical review and informed consent were not required.
Data source
For this descriptive study, we used the MDV database, which is described in more detail elsewhereCitation13. Between April 2008 and October 2022, the MDV database covered in excess of 40 million patients (approximately 9.3% of the total population) across more than 469 acute care hospitals (26.6% of such hospitals) in Japan, although patients are repeatedly counted if they visit different hospitals. The database records patient characteristics (age and sex), diagnoses, prescription receipts, and procedure receipt. Diagnoses are recorded using International Classification of Diseases, 10th Revision (ICD-10) codes, and text data in Japanese. The MDV database also collects DPC data from hospitalized patients that include multiple types of diagnoses, such as the main diagnosis, the most resource-consuming diagnosis, the second most resource-consuming diagnosis, complications, and the admission-precipitating diagnosisCitation14.
Study population
The definitions of PH and CHD in this study were based on those used in prior studiesCitation2,Citation15–17. Patients who were aged ≥15 years at the index date were eligible. The index date was defined as the date of the first confirmed diagnosis of CHD after 15 years of age. Supplementary Table 1 lists the ICD-10 codes for PH, ES, and CHD subtypes. At least two recorded diagnoses of CHD were required to avoid false-positive diagnoses. Previous validation studies of CHD diagnoses in Japan showed that the definition of CHD using ICD-10 codes showed high positive predictive valuesCitation18. Patients without claims data after but excluding the index date were excluded from the study.
Patients were followed up from the index date to any of the following events (follow-up period): the date of death, end of the study period, no consecutive claims within 12 months, or the first confirmed diagnoses of PH/ES, whichever came first. Follow-up was discontinued in patients without consecutive claims within 12 months because the MDV database comprises hospital claims data and cannot link data if patients visit multiple hospitals or are transferred to another hospital.
Outcomes
Data were collected for 6 months before the index date (pre-index/baseline period) and the follow-up period (from the index date to the date of death, end of the study period, no consecutive claims within 12 months, or the first confirmed diagnoses of PH/ES, whichever came first). As patient characteristics, we retrieved the age and sex.
We investigated the following outcomes to explore the diagnostic practice and prognosis of ACHD. The first outcome was diagnostic screening for PH/ES in the follow-up period in terms of ECG (conventional ECG [D208], stress ECG [D209], Holter/other ECG [D210], and real-time/portable ECG [D212]), ultrasound (cardiac ultrasound or TTE), brain natriuretic hormone (BNP), N-terminal-pro-BNP (NT-proBNP), RHC, and pulmonary diffusing capacity test (DLCO). The procedure codes and corresponding names are given in Supplementary Table 2.
The second outcome was the incidence of PH/ES during the follow-up period. We defined PH/ES by combining disease and procedure codes to avoid false positives. The diagnosis of PH/ES required a recorded disease code for PH or ES (ICD-10 codes: I270, I272, or I278) at least twice (≥1 month apart) as well as a procedure code for RHC (procedure code: D2061 or E0028) recorded 1 month before or after the first confirmed diagnoses of PH/ES.
Statistical analyses
We analyzed patient characteristics using the frequency and percentage for categorical variables or the mean, standard deviation (SD), median, and quartiles for continuous variables. For the outcomes of interest, we calculated the following: number of patients observed, observed patient-year, number of events, and incidence rate (per 1000 person-years with 95% confidence intervals [CI]). We also determined the number and incidence (per 1000 person-years with 95% CI) of diagnostic tests for PH/ES for each type of test. We conducted time-to-event analyses of PH/ES using Kaplan–Meier (KM) curves, and determined the number of events per month together with the median survival time (with 25th and 75th percentiles and 95% CI), and the proportion surviving per month with 95% CI. The analyses were conducted for the entire patient population, by CHD type, and by index year (2008–2015 and 2016–2021). These study periods were chosen to coincide with the approval of Macitentan in Japan (2015). Patients included in the first period could be included in the second period if their follow-up was continued into the second period. All analyses were done using SAS software version 9.4 or higher (SAS Institute, Cary, NC, USA).
Results
Patients
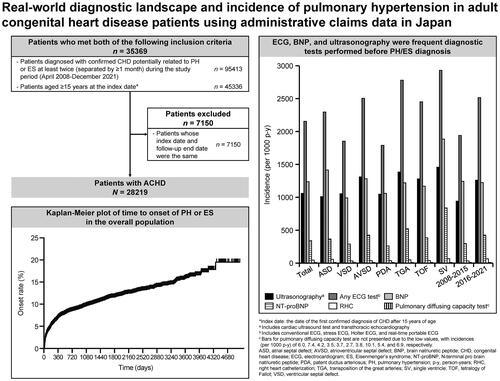
Overall, 28219 patients with ACHD were included in this study (). The mean ± SD age was 44.7 ± 23.5 years, and 56.3% of the patients were females and 43.7% were males (). There were 13248 (46.9%) patients with atrial septal defect (ASD), 10171 (36.0%) with ventricular septal defect (VSD), 1078 (3.8%) with atrioventricular septal defect (AVSD), 1871 (6.6%) with patent ductus arteriosus (PDA), 705 (2.5%) with transposition of the great arteries (TGA), 2757 (9.8%) with tetralogy of Fallot (TOF), and 678 (2.4%) with a single ventricle (SV). There were 12555 (44.5%) patients for the period 2008–2015 and 15664 (55.5%) the period 2016–2021. The mean ± SD follow-up period was 923.1 ± 980.1 days.
Figure 1. Patient flow. *Index date: the date of the first confirmed diagnosis of CHD after 15 years of age. Abbreviations. ACHD, adult congenital heart disease; CHD, congenital heart disease; ES, Eisenmenger’s syndrome; PH, pulmonary hypertension.
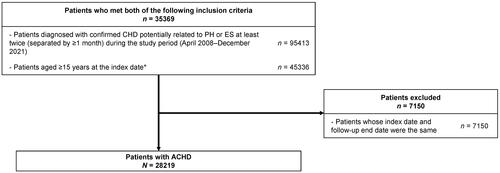
Table 1. Patient characteristics.
Incidence of diagnostic testing
and Supplementary Table 3 show the frequency of diagnostic testings for PH/ES among patients with ACHD. The incidence per 1000 person-years was 2149.8 for any ECG test, 1054.2 for ultrasonography, 1232.8 for BNP, 339.7 for NT-proBNP, 40 for RHC, 6.0 for DLCO in the total population. The table also shows the incidence of diagnostic tests for each CHD subtype and by index period. The incidence (per 1000 person-years) of any ECG test (from 1938.1 to 2511.9), ultrasonography (from 937.0 to 1254.7), and NT-proBNP (from 291.3 to 422.5), and RHC (from 28.6 to 59.7) increased between 2008–2015 and 2016–2021, whereas no marked changes were seen for BNP (from 1239.1 to 1221.9) and DLCO (from 5.4 to 6.9).
Figure 2. Incidence rates of diagnostic tests in the total population, by type of CHD, and by index year. (a) Includes cardiac ultrasound test and transthoracic echocardiography. (b) Includes conventional ECG, stress ECG, Holter ECG, and real-time portable ECG. (c) Bars for pulmonary diffusing capacity test are not presented due to the low values, with incidences (per 1000 p-y) of 6.0, 7.4, 4.2, 3.5, 3.7, 2.7, 3.8, 10.1, 5.4, and 6.9, respectively. Abbreviations. ASD, atrial septal defect; AVSD, atrioventricular septal defect; BNP, brain natriuretic peptide; CHD, congenital heart disease; ECG, electrocardiogram; NT-proBNP, N-terminal pro brain natriuretic peptide; PDA, patent ductus arteriosus; p-y, person-years; RHC, right heart catheterization; TGA, transposition of the great arteries; SV, single ventricle; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
Incidence of PH/ES
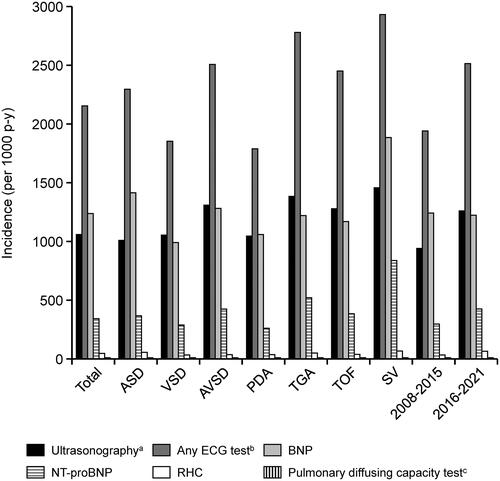
shows the incidence rate of PH/ES during the follow-up period. In the overall population, the incidence rate of PH/ES was 33 per 1000 person-years. The incidence of PH/ES by CHD subtype (per 1000 person-years) was 34 for ASD, 30 for VSD, 65 for AVSD, 53 for PDA, 37 for TGA, 26 for TOF, and 103 for SV. shows the KM curve from the index date to the incidence of PH/ES during the follow-up period, shows the KM curves by index year, and shows the KM curves by type of CHD. The characteristics of patients with first confirmed diagnosis of PH/ES after the index date are shown in Supplementary Table 5. There were no marked differences between ACHD patients and the patients with PH/ES in terms of sex and age.
Figure 3. Kaplan–Meier curves of the time to onset of pulmonary hypertension or Eisenmenger’s syndrome. (a) Overall population. (b) By index year. (c) By type of congenital heart disease. Abbreviations. ASD, atrial septal defect; AVSD, atrioventricular septal defect; CI, confidence interval; NR, not reached; PDA, patent ductus arteriosus; q1, quartile 1; q3, quartile 3; SV, single ventricle; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
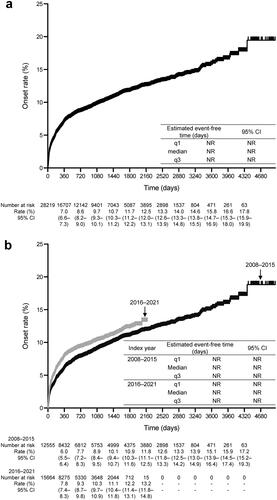
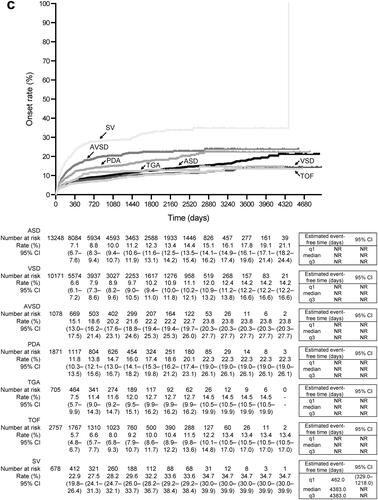
Table 2. Incidence rate of first confirmed diagnosis of pulmonary hypertension or eisenmenger’s syndrome during the follow-up period.
Discussion
To our knowledge, this is the first study to unveil the diagnostic landscape for PH/ES in ACHD patients in real-world clinical practice in Japan following the implementation of Japanese guidelinesCitation11,Citation12. Of note, the frequency of relevant diagnostic tests (including ECG, ultrasonography, RHC and laboratory tests [NT-proBNP]) increased between the two study periods (2008–2015 and 2016–2021), which broadly correspond to the periods before and after the introduction of clinical recommendations for performing such tests in ACHD patients. This increase in testing was also accompanied by an increase in the incidence of the first confirmed diagnosis of PH or ES between 2008–2015 and 2016–2021 (24.6 vs 46.7 cases per 1000 person-years, respectively). Furthermore, the incidence of PH or ES was greatest in patients with a SV compared with other types of CHD.
Diagnostic testing
To understand these findings, we should first consider the relevant clinical guidelines and recommendations that encourage diagnostic testingCitation9–12. The European guidelines (2015 ESC/ERS)Citation9, and the Japanese guidelines (JCS 2017/JPCPHS 2017),Citation12 recommend echocardiography as the first-line, non-invasive test in patients with suspected PH, and that patients with high or intermediate probability of PH should undergo diagnostic tests such as ECG, pulmonary function, pulmonary diffusing capacity, chest radiography, high-resolution computed tomography, and arterial blood gases before proceeding to RHC. Similar recommendations were incorporated in the guidelines for the management of congenital heart diseases in adultsCitation11 and guidelines for treatment of pulmonary hypertensionCitation12 published in 2018. Here, we observed a marked increase in incidence of diagnostics tests over time, especially ECG, ultrasound, and RHC, suggesting that clinicians in Japan are adhering to recommendations regarding the use of diagnostic tests. The high number of ECG, ultrasound, and BNP tests suggests that these are the mainstay tests performed in Japan. In fact, ultrasound is becoming increasingly more accessible to patients without long wait times, and in many places without a need for an appointment. By comparison, NT-proBNP and DLCO may be reserved for higher-risk patients. In fact, the incidence of these tests varied considerably across the different types of CHD.
Echocardiography, in particular, is a well-established method for the diagnosis of PH and the European guidelines include threshold values of the peak tricuspid regurgitation velocity for predicting high probability of PH, especially when combined with other echocardiographic signs of PHCitation9. These recommendations have also been incorporated into the Japanese guidelinesCitation12. Echocardiography is also useful for diagnosis of chronic heart failure (CHF), another significant complication in CHD patients, and can help visualize the underlying structural/functional abnormalities contributing to CHFCitation19.
Supporting our finding that the use of ultrasonography increased between the two periods, a recent study has revealed that the number of transthoracic echocardiograms performed in hospitals in Kumamoto, Japan, increased steadily between 2013 and 2017Citation20. The authors attributed this finding to the increased importance of using this procedure to evaluate valvular heart disease.
The cardiac biomarkers BNP and NT-proBNP were conventionally associated with the risk of HFCitation21,Citation22. In clinical settings, these tests are generally used for the purpose of assessing the risk of morbidity or mortality of chronic HF patientsCitation23,Citation24. In the context of CHD, however, their role is less clear. For example, the American Heart Association considered that there was not enough information regarding the benefit or harm of measuring BNP or other markers of neurohormonal activation in CHD patientsCitation19. Whereas the European guidelines suggested that NT-proBNP may be elevated in patients with PH and is an independent predictor of riskCitation9. Therefore, we suspect that the increased use of NT-pro-BNP tests may reflect more comprehensive evaluation of ACHD patients, particularly those deemed at highest riskCitation12.
The incidence of BNP tests remained high in both periods (2008–2015 and 2016–2021), which might reflect common Japanese clinical practice for suspicious cardiac disorders, including HF and PH, in accordance with clinical guidelinesCitation11,Citation12,Citation21,Citation22.
RHC is the gold standard test for diagnosis and classification of PH, and its role was highlighted in the 2015 ESC/ERS and JCS 2017/JPCPHS 2017 guidelinesCitation9,Citation12. The increased frequency between 2008–2015 to 2016–2021 may reflect the positive impact of these guidelines in Japanese clinical practice. Among the CHD types investigated, RHC was more frequent in patients with ASD and SV. For patients with ASD, one possible explanation might be that physicians recognized the importance of evaluating pulmonary vascular resistance and the reversibility of pulmonary vascular lesions due to oxygen or drug loading, as stated in the JCS 2017 guidelines for the management of congenital heart diseases in adultsCitation11. In patients with SV, the physicians may carefully evaluate their pulmonary circulation through RHC because these patients develop abnormalities of the pulmonary vascular tree even at a low pulmonary artery pressureCitation17.
DLCO was the least frequently used test in this study, and its use increased only slightly between 2008–2015 and 2015–2021 (5.4 vs 6.9 per 1000 person-years). Clinical guidelines recommend DLCO tests if echocardiography indicated intermediate or high probability of PH patients after echocardiography as recommended in PH guidelinesCitation9,Citation10,Citation12. In clinical practice, the 6-min walking test, liver ultrasound, pulmonary vascular CT, and pulmonary function tests may be performed to improve the diagnostic accuracy of PH complicationsCitation11. The results of ECG, echocardiography, and BNP usually raise suspicion of PH, and a definitive diagnosis is made by right heart catheterization. Therefore, many physicians may not believe DLCO to be necessary for diagnosis of PH. Considering the clinical guidelines, DLCO may be used to as part of the diagnostic workup in patients with or at high risk of severe lung disease, particularly chronic obstructive pulmonary disease or idiopathic pulmonary fibrosisCitation25.
The incidence of cardiac tests and the severity of CHD is well correlated,Citation26 and physicians might manage patients with severe CHD more carefully by performing relevant cardiac clinical examinations more frequently or by using a variety of different types of test. Although we did not include any index of the severity of CHD, the incidence of tests varied among the seven types of CHD, which may partly reflect the relative severities of these disease or the perception that some tests may be more useful for certain types of CHD.
Incidence of PH/ES
Earlier studies reported prevalence rates of PH in ACHD patients of up to 10%,Citation2–6 similar to our result (8.29%). To our knowledge, however, few studies have determined the incidence of PH/ES (32.8 per 1000 person-years). This incidence seems reasonable when we consider the results of the Global Burden of Disease in which the incidence of PH varied from 0.008 to 1.4 cases per 100,000 person-years in general populationsCitation27 and 18.2 cases per 1000 person-years in patients with systemic sclerosisCitation28.
We also show that the incidence of PH/ES among ACHD patients has increased over time in Japan, from 24.6 to 46.7 per 1000 person-years from 2008–2015 to 2016–2021, although the prevalence decreased slightly (from 8.84% to 7.85%). This increased incidence is most likely due to increased diagnostic testing, including RHC, for PH in ACHD patients following the introduction of the clinical guidelines in JapanCitation11,Citation12. However, further research will be required to evaluate the true impact of relevant guidelines because other national or regional projects in Japan that have shown other factors might be involved in the increased number of diagnostic tests, such as echocardiography in KumamotoCitation20.
Nitta et al. found that among patients referred to a specialist center for ACHD, 16.5% of patients referred by non-specialized doctors had a history of loss of follow-up compared with 3.7% of patients referred by specialized doctorsCitation16. Thus, it is possible that a large number of patients with possible PH were not under active management, and improved follow-up may lead to the detection of PH in more patients with CHD.
We found that the incidence and prevalence of PH were highest (102.8 per 1000 person-years and 26.99%) in patients with SV than in patients with other types of ACHD, despite SV being the least-frequent type of CHD (678 patients). Fontan surgery is a treatment of choice for patients with a SVCitation29. The diagnosis of Fontan-derived PH may have been accelerated following the incorporation of Fontan circulation into the updated clinical classification of PH published in 2013Citation30 and the Japanese PH guideline published in 2017Citation12. Options for managing patients with Fontan circulation are emerging, and include bosentan and sildenafilCitation31–33 (unpublished data). Such drugs may help to improve hemodynamics and prevent PH in patients with SV. We should also note that patients with a SV also had the highest incidences of diagnostic tests, in consideration of life-long care and the risk of clinically significant comorbidities in patients with Fontan circulationCitation5. The higher frequency of tests in a small population may therefore contribute to the high frequency of diagnosis of PH/ES.
Patients with AVSD had the second-highest prevalence and incidence of PH/ES in this study. One explanation for this is that left heart disease is common in patients with PH; for example, in an Italian study of 69 adults (>65 years) with PH or CTEPH, 23 (33%) exhibited a left heart disease phenotypeCitation34. Furthermore, a study using two-dimensional (2D) speckle tracking strain showed that patients with PH were more likely to exhibit LV diastolic dysfunction, as well as impaired RV function, than controlsCitation35. In our study, the incidence of PH/ES was also relatively high in patients with PDA (53.4 per 1000 person-years) compared with patients with the other types of CHD (26.5–36.6 per 1000 person-years). The association between less-severe types of CHD and PH/ES seems uncertain.
Clinical implications
Several recent studies have provided insight into ACHD in Japan, including the prevalence of PHCitation5,Citation26. We are still lacking adequate epidemiological and diagnostic information from Japanese clinical practice, including general hospitals. Our present findings indicate that the incidence of PH/ES among ACHD has increased between the two study periods, and is associated with the type of CHD. On the one hand, this increased incidence may reflect the increased frequency of relevant diagnostic tests. Thus, our findings may encourage physicians to conduct appropriate medical examinations, particularly diagnostic tests, including echocardiography and RHC, in patients with ACHD suspected of having PH/ES, or to consider incorporating such tests into routine clinical practice. On the other hand, we should also consider that the number of adults with CHD is steadily increasing due to advances in surgical procedures and improved management, including the use of appropriate drug therapies. Therefore, physicians should be increasingly vigilant for the risk of PH/ES, or other complications, in an increasing number of patients with ACHD. This study was the first to clarify the actual situation regarding the diagnosis of PH/ES in general practice in Japanese patients with ACHD. We believe our findings may be useful for researchers in other countries performing research into the diagnostic situation regarding PH/ES.
Limitations
There are some limitations of this study that deserve mention. Typical clinical examinations, such as chest X-ray, for detecting suspicious PH, were not included in this study. Although the diagnostic tests assessed in this study are commonly utilized for patients with suspected PH/ES, the specific reason for performing these tests was not recorded in the database. Therefore, we cannot exclude the possibility that some tests were performed for other reasons. CHF, a major comorbidity in patients with ACHD, was not extracted using ICD-10 codes in this study due to its low validity. Unfortunately, without this information, we cannot ascertain the reason why the BNP and NT-proBNP tests were performed. The timing and combination of clinical examinations leading to PH/ES diagnosis was not planned for the analyses. We did not include a “washout period” to ensure the CHD diagnosis was followed sequentially by a diagnosis of PH/ES, although the 6-month pre-index period could partially overcome this limitation. The ICD-10 codes do not specify the underlying cause of PH. Therefore, PH in this study may include PH caused by underlying diseases other than CHD. The study only included patients with ACHD (≥15 years) who visited the same hospital several consecutive times. Patients who transferred to other hospitals, or were lost to clinical follow-up, cannot be tracked using the MDV database. Therefore, we should take care when generalizing the results to the wider Japanese population who receive a diagnosis of PH at a health facility other than the health facility that provides the diagnosis of CHD. Additionally, the validity of the definition of PH/ES used in the study has not been validated. Although we tried to reduce the likelihood of misidentification by combining ICD-10 codes and diagnostic codes, it is possible that we incorrectly classified or missed some patients with PH/ES. Another limitation is that we did not compare the proportions of patients who underwent each diagnostic test according to the type of CHD or between the two time-periods due to marked differences in follow-up duration and considering the conditions for censoring patients. Moreover, some patients were included in both study periods if their follow-up was not completed in the first period. Finally, several clinically relevant prognostic variables, including the PH WHO functional class and hemodynamics, were not recorded in the MDV database.
Conclusions
To our knowledge, this is the first study to provide real-world evidence regarding the diagnostic landscape and incidence of PH/ES in a diverse population of patients with ACHD at acute care hospitals in Japan. ECG, ultrasonography, and BNP tests were commonly used in accordance with relevant Japanese guidelines. The incidence of PH/ES in this cohort of ACHD patients also seemed appropriate considering published data. Among the CHD types examined, the incidence of PH/ES was greatest in patients with a SV. We also observed a trend towards increased utilization of clinical examinations and PH/ES diagnosis in 2016–2021 than in 2008–2015, suggesting the use of these tests and resultant diagnosis of PH/ES has increased over time, although further studies are needed required to evaluate true impact of relevant guidelines.
In summary, this real-world study in Japan demonstrated a high risk of PH/ES in patients with ACHD. Diagnostic tests, including ECG, ultrasound, and BNP are becoming more frequently performed over time. The frequency of PH/ES diagnosis is also increasing, indicating a need for greater vigilance of this complication in patients with ACHD.
Transparency
Author contributions
Tomoko Ishizu: conceptualization, methodology, writing – review and editing. Yasuhiro Hayashi: project administration, supervision, visualization, writing – review and editing. Natsuko Tokushige: conceptualization, methodology, project administration, supervision, writing – review and editing. Junichi Omura: conceptualization, methodology, writing – review and editing. The sponsor funded the study, writing support and publication; was involved in study design, the collection, analysis and interpretation of data; and reviewed the manuscript.
Prior presentation
Results of this study were presented at the 71st Scientific Sessions of the Japanese College of Cardiology (September 8–10, 2023) and to the 270th Annual Scientific Meeting of Kantokoushinetsu Regional Office of the Japanese Circulation Society (December 16, 2023).
Supplementary file.pdf
Download PDF (271.4 KB)Acknowledgements
The authors express their gratitude to Naoki Hirose MPH, PhD (Janssen Pharmaceutical K.K.), for his constructive comments, expertise, and statistical advice regarding this study, as well as the assistance with developing the protocol and interpreting the results. His willingness and generosity are greatly appreciated. The authors thank Sigel Byron MSc and Ryosuke Yamada MD for assistance with developing the study concept and protocol. The authors also thank EPS Corporation for support with developing the protocol, data curation, study conduct, and performing data analyses. The authors acknowledge Nicholas D. Smith PhD (EMC K.K.) for medical writing support, which was funded by Janssen Pharmaceutical K.K.
Declaration of funding
This study was funded by Janssen Pharmaceutical K.K., Japan.
Declaration of financial/other relationships
Tomoko Ishizu has received consulting fee/honorarium from Janssen Pharmaceutical K.K., lecture fee/honorarium from Janssen Pharmaceutical K.K., travel fee for invited lectures from Janssen Pharmaceutical K.K. and is a deputy representative director in the Japanese Society for Adult Congenital Heart Disease (unpaid). Yasuhiro Hayashi is an employee of Janssen Pharmaceutical K.K. and holds stock in Johnson & Johnson. Natsuko Tokushige is an employee of Janssen Pharmaceutical K.K. and holds stock in Johnson & Johnson. Junichi Omura is an employee of Janssen Pharmaceutical K.K. and holds stock in Johnson & Johnson. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no other relevant financial relationships to disclose.
Data availability statement
The data analyzed in this study were used under contract with Medical Data Vision and cannot be shared with external researchers. Researchers interested in using these data should contact Medical Data Vision directly (https://en.mdv.co.jp/).
References
- Liu A, Diller GP, Moons P, et al. Changing epidemiology of congenital heart disease: effect on outcomes and quality of care in adults. Nat Rev Cardiol. 2023;20(2):126–137. doi: 10.1038/s41569-022-00749-y.
- Chiu SN, Lu CW, Lin MT, et al. Pulmonary hypertension in adult congenital heart disease in asia: a distinctive feature of complex congenital heart disease. J Am Heart Assoc. 2022;11(7):e022596. doi: 10.1161/jaha.121.022596.
- Lowe BS, Therrien J, Ionescu-Ittu R, et al. Diagnosis of pulmonary hypertension in the congenital heart disease adult population impact on outcomes. J Am Coll Cardiol. 2011;58(5):538–546. doi: 10.1016/j.jacc.2011.03.033.
- van Riel AC, Schuuring MJ, van Hessen ID, et al. Contemporary prevalence of pulmonary arterial hypertension in adult congenital heart disease following the updated clinical classification. Int J Cardiol. 2014;174(2):299–305. doi: 10.1016/j.ijcard.2014.04.072.
- Yao A, Inuzuka R, Mizuno A, et al. Status of adult outpatients with congenital heart disease in Japan: the Japanese network of cardiovascular departments for adult congenital heart disease registry. J Cardiol. 2022;80(6):525–531. doi: 10.1016/j.jjcc.2022.07.019.
- Schwartz SS, Madsen N, Laursen HB, et al. Incidence and mortality of adults with pulmonary hypertension and congenital heart disease. Am J Cardiol. 2018;121(12):1610–1616. doi: 10.1016/j.amjcard.2018.02.051.
- Anderson JJ, Lau EM. Pulmonary hypertension definition, classification, and epidemiology in asia. JACC Asia. 2022;2(5):538–546. doi: 10.1016/j.jacasi.2022.04.008.
- Arvanitaki A, Giannakoulas G, Baumgartner H, et al. Eisenmenger syndrome: diagnosis, prognosis and clinical management. Heart. 2020;106(21):1638–1645. doi: 10.1136/heartjnl-2020-316665.
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317.
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–3731. doi: 10.1093/eurheartj/ehac237.
- Joint Research Group Societies. Guidelines for management of congential heart diseases in adults (JCS 2017), March 23, 2018. In Japanese.
- Joint Research Group Societies. Guidelines for treatment of pulmonary hypertension (JCS 2017/JPCPHS 2017), Published March 23, 2018. Corrigendum published December 23, 2021. In Japanese.
- Laurent T, Simeone J, Kuwatsuru R, et al. Context and considerations for use of two Japanese real-world databases in Japan: medical data vision and Japanese medical data center. Drugs Real World Outcomes. 2022;9(2):175–187. doi: 10.1007/s40801-022-00296-5.
- Yasunaga H. Real world data in Japan: chapter II the diagnosis procedure combination database. ACE. 2019;1(3):76–79. doi: 10.37737/ace.1.3_76.
- Kaemmerer H, Gorenflo M, Huscher D, et al. Pulmonary hypertension in adults with congenital heart disease: real-world data from the international COMPERA-CHD registry. J Clin Med. 2020;9(5):1456. doi: 10.3390/jcm9051456.
- Nitta M, Ochiai R, Nakano S, et al. Characteristics of patients with adult congenital heart disease treated by non-specialized doctors: the potential loss of follow-up. J Cardiol. 2021;77(1):17–22. doi: 10.1016/j.jjcc.2020.06.018.
- Pascall E, Tulloh RM. Pulmonary hypertension in congenital heart disease. Future Cardiol. 2018;14(4):343–353. doi: 10.2217/fca-2017-0065.
- Ishikawa T, Oyanagi G, Obara T, et al. Validity of congenital malformation diagnoses in healthcare claims from a university hospital in Japan. Pharmacoepidemiol Drug Saf. 2021;30(7):975–978. doi: 10.1002/pds.5244.
- Stout KK, Broberg CS, Book WM, et al. Chronic heart failure in congenital heart disease: a scientific statement from the American heart association. Circulation. 2016;133(8):770–801. doi: 10.1161/cir.0000000000000352.
- Usuku H, Yamamoto E, Oike F, et al. Current awareness and status of transthoracic echocardiography in Kumamoto prefecture – a report of the Kumamoto cardiovascular echocardiography standardization project. Circ Rep. 2020;2(6):297–305. doi: 10.1253/circrep.CR-20-0028.
- Joint Research Group Societies. Guidelines for treatment of acute heart failure (JCS 2011), September 20, 2013. In Japanese.
- Joint Research Group Societies. Guidelines for treatment of chronic heart failure (JCS 2010), September 13, 2013. In Japanese.
- Masson S, Latini R, Anand IS, et al. Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the valsartan heart failure (Val-HeFT) data. Clin Chem. 2006;52(8):1528–1538. doi: 10.1373/clinchem.2006.069575.
- Tsutamoto T, Sakai H, Nishiyama K, et al. Direct comparison of transcardiac increase in brain natriuretic peptide (BNP) and N-terminal proBNP and prognosis in patients with chronic heart failure. Circ J. 2007;71(12):1873–1878. doi: 10.1253/circj.71.1873.
- Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D109–D116. doi: 10.1016/j.jacc.2013.10.036.
- Nitta M, Shimizu S, Kaneko M, et al. Outcomes of women with congenital heart disease admitted to acute-care hospitals for delivery in Japan: a retrospective cohort study using nationwide Japanese diagnosis procedure combination database. BMC Cardiovasc Disord. 2021;21(1):409. doi: 10.1186/s12872-021-02222-z.
- Emmons-Bell S, Johnson C, Boon-Dooley A, et al. Prevalence, incidence, and survival of pulmonary arterial hypertension: a systematic review for the global burden of disease 2020 study. Pulm Circ. 2022;12(1):e12020. doi: 10.1002/pul2.12020.
- Rubio-Rivas M, Homs NA, Cuartero D, et al. The prevalence and incidence rate of pulmonary arterial hypertension in systemic sclerosis: systematic review and meta-analysis. Autoimmun Rev. 2021;20(1):102713. doi: 10.1016/j.autrev.2020.102713.
- Jolley M, Colan SD, Rhodes J, et al. Fontan physiology revisited. Anesth Analg. 2015;121(1):172–182. doi: 10.1213/ane.0000000000000717.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–41. doi: 10.1016/j.jacc.2013.10.029.
- Hebert A, Mikkelsen UR, Thilen U, et al. Bosentan improves exercise capacity in adolescents and adults after Fontan operation: the TEMPO (treatment with endothelin receptor antagonist in Fontan patients, a randomized, placebo-controlled, double-blind study measuring peak oxygen consumption) study. Circulation. 2014;130(23):2021–2030. doi: 10.1161/circulationaha.113.008441.
- Mori H, Park IS, Yamagishi H, et al. Sildenafil reduces pulmonary vascular resistance in single ventricular physiology. Int J Cardiol. 2016;221:122–127. doi: 10.1016/j.ijcard.2016.06.322.
- Van De Bruaene A, La Gerche A, Claessen G, et al. Sildenafil improves exercise hemodynamics in Fontan patients. Circ Cardiovasc Imaging. 2014;7(2):265–273. doi: 10.1161/circimaging.113.001243.
- Toma M, Miceli R, Bonsante E, et al. Left heart disease phenotype in elderly patients with pulmonary arterial hypertension: insights from the italian PATRIARCA registry. J Clin Med. 2022;11(23):7136. doi: 10.3390/jcm11237136.
- de Amorim Corrêa R, de Oliveira FB, Barbosa MM, et al. Left ventricular function in patients with pulmonary arterial hypertension: the role of two-dimensional speckle tracking strain. Echocardiography. 2016;33(9):1326–1334. doi: 10.1111/echo.13267.