ABSTRACT
Osteoarthritis (OA) is a degenerative joint condition characterized by painful cartilage lesions that impair joint mobility. Current treatments such as lavage, microfracture, and osteochondral implantation fail to integrate newly formed tissue with host tissues and establish a stable transition to subchondral bone. Similarly, tissue-engineered grafts that facilitate cartilage and bone regeneration are challenged by how to integrate the graft seamlessly with surrounding host cartilage and/or bone. This review centers on current approaches to promote cartilage graft integration. It begins with an overview of articular cartilage structure and function, as well as degenerative changes to this relationship attributed to aging, disease, and trauma. A discussion of the current progress in integrative cartilage repair follows, focusing on graft or scaffold design strategies targeting cartilage–cartilage and/or cartilage–bone integration. It is emphasized that integrative repair is required to ensure long-term success of the cartilage graft and preserve the integrity of the newly engineered articular cartilage. Studies involving the use of enzymes, choice of cell source, biomaterial selection, growth factor incorporation, and stratified versus gradient scaffolds are therefore highlighted. Moreover, models that accurately evaluate the ability of cartilage grafts to enhance tissue integrity and prevent ectopic calcification are also discussed. A summary and future directions section concludes the review.
Introduction
Articular cartilage is an avascular, aneural tissue that lines the surfaces of musculoskeletal joints, enabling load bearing and nearly frictionless joint articulation. Cartilage degeneration, which causes pain and loss of joint mobility, occurs in osteoarthritis (OA), a condition with tremendous social and economic burden (Citation1). Given its relative isolation from the blood supply, cartilage has a limited capacity for self-repair; thus, surgical intervention is often required to ease OA-associated pain and disability. Existing techniques for cartilage repair, such as microfracture and osteochondral transplantation, do not result in consistent cartilage regeneration or restoration of tissue continuity and integrity with the host cartilage or bone. For over two decades, tissue engineering strategies have been tested and optimized for cartilage repair (Citation2–Citation13), leading to the development of many promising cartilage grafts and sophisticated bioreactor systems for ex vivo graft culture (Citation14–Citation19). To facilitate clinical translation, the next challenge resides in the restoration of graft-to-host tissue continuity via functional integration of the graft or newly formed cartilage with both the host cartilage and subchondral bone. Therefore, the objective of this review is to highlight research efforts in cartilage–cartilage and cartilage–bone integration, focusing on the role of graft or scaffold design parameters including strategies for employing relevant growth factors for cartilage–host integration. This manuscript begins with a summary of the structure and function of healthy cartilage and disruptions of this relationship by degenerative changes that occur with age, disease, and trauma. Next, tissue engineering-based approaches for cartilage regeneration are discussed in the broader context of integrative cartilage repair with a focus on strategies that specifically aim to improve integration of cartilage grafts with native cartilage and bone. To this end, studies focused on exploring design parameters such as scaffold material, growth factor use, and cell source will be highlighted. This review concludes with promising future directions and a discussion of potential challenges in realizing the goal of integrative and functional cartilage repair.
Healthy articular cartilage structure and function
Healthy articular cartilage is composed of a mixture of liquid and solid phases that together enable load bearing and joint articulation (Citation20). The extracellular matrix, actively maintained by resident chondrocytes, is dense in collagen II and proteoglycans, including aggrecan as well as dermatan sulfate and keratin sulfate proteoglycans (Citation21,Citation22), with smaller amounts of collagen III, VI, IX, X, and XI also present (Citation23–Citation26). The charged proteoglycans attract water that swells the tissue, allowing it to support compressive loads, while the tensile properties of the collagen network contribute swelling resistance and shear strength (Citation20,Citation27–Citation31). In addition, interstitial fluid pressurization, combined with molecules released from the cartilage surface and synovium, lubricates the joint surfaces to facilitate painless motion (Citation32).
Articular cartilage is organized into non-mineralized and mineralized layers. The non- mineralized layer has three structurally contiguous zones, each differing in matrix composition, organization, and chondrocyte phenotype (Citation33–Citation35). These changes in matrix composition throughout the zones of articular cartilage are summarized in . Briefly, the surface zone, located at the articulating region of the cartilage, accounts for approximately 10% of the height of the total tissue and consists of thin, elliptical chondrocytes, and progenitor cells that are surrounded by a matrix with high water content (~78%), low proteoglycan content relative to the other zones, and collagen fibrils (4–12 nm) that are oriented parallel to the surface (Citation36–Citation44). The cells in this zone produce superficial zone protein which contributes to joint lubrication (Citation45,Citation46). Below the surface, the next 60% of the cartilage depth is the middle zone, within which spherical chondrocytes reside in a matrix rich in proteoglycans and unaligned collagen fibers (9–60 nm) (Citation39,Citation41–Citation43,Citation47). The deep zone, directly below the middle zone, comprises approximately 30% of the cartilage depth and is marked by spherical chondrocytes oriented in stacks that are perpendicular to the articular surface. These cells, while sparsely distributed, maintain a matrix of relatively high glycosaminoglycan content, low water content (~68%), and radially oriented collagen fibrils (60–140 nm) (Citation37,Citation40,Citation42–Citation44,Citation47). Although the collagen fibril diameter generally increases from the surface zone to the deep zone, fine fibrils (<100 Å) have been noted throughout the depth of the cartilage in human specimens (Citation40–Citation42).
Figure 1. Spatial change in matrix composition across the cartilage–bone junction. Progressing from surface zone cartilage (SZC), middle zone cartilage (MZC), to deep zone cartilage (DZC), then calcified cartilage (CC) and finally bone, Fourier transform infrared spectroscopy imaging analysis reveals regional differences in collagen (left), proteoglycan (middle), and mineral (right) distribution (neonatal bovine tibiofemoral joint). The white line in each image represents the average change in peak area across the specimen from the surface zone to bone. [Image modified from (61).]
![Figure 1. Spatial change in matrix composition across the cartilage–bone junction. Progressing from surface zone cartilage (SZC), middle zone cartilage (MZC), to deep zone cartilage (DZC), then calcified cartilage (CC) and finally bone, Fourier transform infrared spectroscopy imaging analysis reveals regional differences in collagen (left), proteoglycan (middle), and mineral (right) distribution (neonatal bovine tibiofemoral joint). The white line in each image represents the average change in peak area across the specimen from the surface zone to bone. [Image modified from (61).]](/cms/asset/89637189-0b1e-458f-9d40-0a58ed8d8ca2/icts_a_1231180_f0001_oc.jpg)
Situated between the deep zone and the subchondral bone is the calcified cartilage, comprised of hypertrophic chondrocytes embedded in a mineralized matrix that is rich in collagen II and proteoglycans. This osteochondral interface is reported to range from 20 to 243 µm in thickness in humans, with the wide range of values attributed to variability with age and total cartilage thickness (Citation35,Citation48,Citation49). Using scanning and transmission electron microscopy (SEM and TEM, respectively), Bullough et al. directly imaged the calcification front of adult canine cartilage harvested from the tibial plateau after removing the organic matrix either chemically or via exposure to high temperatures. Although the calcified cartilage is tightly interlocked with the underlying subchondral bone, a distinct border between the two tissues that is actively regulated by chondrocytes was observed (Citation50). While the subchondral bone is highly vascularized, the calcified cartilage is not. Electron micrographs of mature human, lapine, and canine cartilage-bone junctions reveal that most blood vessels terminate at the interface (Citation51).
The calcified cartilage matrix consists primarily of proteoglycans, mineral, and collagen II, IX, and X (Citation23,Citation52–Citation54). Highly aligned collagen II fibers run continuously from the deep zone into the calcified cartilage layer (Citation55–Citation57). The alignment of the collagen bundles at this transition and the interdigitated nature of the calcified cartilage–bone junction together serve to anchor the non-calcified cartilage to the bone (Citation56,Citation58). In a study by Redler et al., 30 human osteochondral specimens were examined using SEM, and the authors proposed that the undulating junction provides an ideal geometric configuration to resist the shearing action of articulation (Citation58). Furthermore, the network of branching collagen fibrils at the interface also contributes to the load bearing capabilities of the joint by diffusing and distributing load during force transmission from the cartilage to bone, reducing stress concentrations at the interface (Citation56,Citation58).
In addition to anchoring cartilage to bone, collagen fibers also serve as a template for mineralization (Citation50,Citation59). In a study, that characterized the mineral in human bone and calcified cartilage, Zizak et al. noted a striking change in the mineral particle orientation from perpendicular to the surface in the calcified cartilage to parallel to the surface in the bone, reflecting the underlying collagen alignment (Citation60). While the collagen fibers run continuously from the calcified cartilage to the deep zone, the mineral is localized to the calcified cartilage and bone regions (see ). An exponential increase in mineral content within the calcified cartilage region has been reported, and this trend persists with age (Citation61). This mineralized collagen and proteoglycan network collectively impart strength to the calcified cartilage matrix. Using a three-point bending test, bovine calcified cartilage modulus was measured to be approximately 0.32 ± 0.25 GPa, which is intermediate between that of uncalcified cartilage above and bone below (Citation62). A modulus ranging from 10 to 30 GPa (Citation63,Citation64) has been reported for nanoindentation measurements of human femoral calcified cartilage. Moreover, a direct positive correlation between mineralization and indentation modulus was demonstrated in adult human femoral calcified cartilage (Citation64).
The mineral found in the calcified cartilage has been identified to be poorly crystalline carbonated hydroxyapatite that is similar to bone mineral in terms of both chemistry and size (2–4.2 nm) (Citation53,Citation55,Citation60,Citation65). It is reported that calcified cartilage measures a higher calcium content than bone, although values reported for the calcium content of the interface range from 1 to 28 wt% (Citation60,Citation63). The dense mineralized matrix acts as a barrier that limits diffusion and prevents osseous invasion from the subchondral bone (Citation66). In the calcified cartilage of mature murine (Citation67) and equine (Citation68) specimens, the diffusion coefficient of small molecules (~400 Da) was reported to be 0.26 and 0.9 µm2/s, respectively. In the equine model, the diffusion coefficient in the calcified cartilage was fivefold lower than in the uncalcified region, highlighting the barrier effect of the calcified cartilage layer. However, Fluorescence Loss Induced Photobleaching (FLIP) analysis revealed that patches of non-mineralized regions, which account for 22% of the volume of calcified cartilage in the murine model, may serve as transport pathways for small molecules. This finding suggests that, in addition to its integrative and mechanical functions, the calcified cartilage plays a complex multifaceted role in modulating molecular transfer between the bone and articular cartilage (Citation67).
Age- and disease-related changes
Structural and functional changes in cartilage with age are well established and have recently been reviewed in detail (Citation69); a brief summary is provided here. Hallmarks of the normal aging process in human cartilage include decreased thickness and cellularity (Citation70) as well as accumulation of advanced glycation end-products that alter collagen crosslinking, disrupting matrix integrity (Citation71). The earliest changes are observed in the superficial zone, with a 50% decrease in chondrocyte density reported between 20 and 90 years of age. As cell density declines, it is likely that any intrinsic repair potential is diminished, leading to an increased risk of fibrillation and osteoarthritis (Citation70).
Changes are also observed in the calcified cartilage with age. The thickness of human calcified cartilage decreases with maturity (Citation48), and the interface becomes less permeable as it transitions to serve as a barrier to solute diffusion from the bone in adult years (Citation72). A steady reduction in the number of blood vessels is also observed with age in human femoral and humoral heads. This decline continues into the seventh decade of life, at which point the trend reverses to increased vascularization (Citation73). Parallel trends in remodeling have been reported, suggesting that the presence of blood vessels may be correlated to an active remodeling state (Citation73).
Accumulation of these age-related changes can lead to OA, which results from the disruption between the inherent homeostasis between anabolic and catabolic processes and leads to fibrillation of the articulating surface, osteophyte formation, and subchondral bone thickening (Citation47,Citation74–Citation76). This process may be initiated gradually by wear that occurs during aging or rapidly due to trauma, such as a ligamental or meniscal injury (Citation77). While the specific factors that initiate joint degeneration are unknown, Bullough noted that age-related degeneration first occurs in areas of unloaded cartilage (Citation78). From this observation, he proposed that, as the joint remodels with age, areas of cartilage which were previously unloaded and had thus degenerated are exposed to new levels of load, leading to overtaxing. This process results in further degeneration that may lead to OA (Citation78,Citation79). Conversely, OA may begin with changes in the subchondral bone, such as cracking, that result from overloading and occur underneath intact hyaline cartilage, leading to subchondral bone collapse and eventual lesion formation in the articular cartilage (Citation80,Citation81).
Although catabolic processes overtake anabolic activities, the disease state is accompanied by an increase in overall chondrocyte activity (Citation82). The net loss of proteoglycans and the disruption of the collagen network have been attributed to elevated matrix metalloproteinase (MMP) and aggrecanase activity as well as changes in chondrocyte phenotype, such as collagen III production (Citation83,Citation84). Shifts in the underlying matrix architecture consequently result in lower compressive and tensile mechanical properties (Citation28,Citation85–Citation87), ultimately compromising joint function.
Current progress in integrative cartilage repair
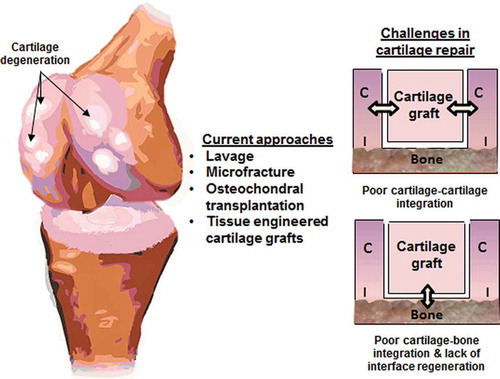
Surgical intervention is often employed to encourage a healing response in partial thickness defects and restore a congruent articular surface in symptomatic, focal full thickness lesions. Clinical treatments that provide access to the osseous vascular supply include subchondral drilling and microfracture (Citation88–Citation90). For larger defects, allografts or autografts are transplanted into the damaged area, a technique termed osteochondral transplantation (Citation91,Citation92). While these traditional treatments relieve symptoms such as pain, stable integration of the graft with surrounding bone and cartilage post repair has yet to be realized.
More recently, cell-based approaches (Citation93) and tissue-engineered constructs have emerged as a promising alternative to existing treatment options. Several scaffold-based grafts have been approved in Europe, and many are pending FDA approval in the United States (Citation94). These early products improve upon traditional osteochondral transplantation, because they are not associated with donor site morbidity or the risk of graft-related disease transmission. Despite their promise, current grafts have focused primarily on cartilage and bone regeneration, and as a result, two challenges are integration of cartilage grafts with (Citation1) surrounding articular cartilage and (Citation2) subchondral bone (see ). Cartilage–cartilage integration is critical for stable repair because, in the absence of continuity between host and neo cartilage, micromotion between the graft and adjacent tissue can fuel further cartilage degeneration (Citation95). Integration of the cartilage to bone via the regeneration of the osteochondral interface is also necessary for stable repair because this calcified cartilage layer contributes to cartilage–bone homeostasis, mechanical functionality, and long-term stability. Moreover, during cartilage healing, the importance of a structural barrier separating bone and cartilage was demonstrated by Hunziker et al. using a full-thickness cartilage defect porcine model. It was observed that a “structural barrier,” in this case a Gore-Tex® membrane (0.2 µm pore diameter), placed between the cartilage and bone compartments, was necessary to maintain the integrity of the newly formed cartilage. The barrier limited vascular ingrowth from the subchondral bed into the cartilage compartment effectively preventing ectopic mineralization in the newly formed repair tissue (Citation66). Therefore, in order to achieve functional and integrative cartilage repair, both cartilage–cartilage and cartilage–bone integration must be considered in current cartilage graft designs. These integration strategies are highlighted in the following sections.
Cartilage–cartilage integration
Cartilage–cartilage integration is necessary for the success of graft systems in partial- or full-thickness defects over time. Clinically, surgical preparation for cartilage graft placement involves the removal of damaged cartilage directly surrounding a lesion to ensure that the graft is well shouldered by healthy tissue. This process can result in chondrocyte apoptosis and necrosis (Citation96), leaving a hypocellular region surrounding the defect (Citation97). As a result, the integration of the cartilage graft with host tissue poses a significant hurdle (Citation98,Citation99). Approaches to overcome this challenge have largely focused on the use of chemotactic agents to draw viable cells into the gap between the graft and native tissue, as well as digestion rinses that break down the dense matrix at the border of autographs/allografts, facilitating cell migration to the host-graft junction.
The use of digestion reagents such as hyaluronidase (Citation100–Citation102), collagenase (Citation100–Citation102), and chondroitinase ABC (Citation100,Citation103,Citation104) have been investigated. Lee et al. demonstrated the utility of digestive rinses for improving cartilage integration by showing that treatment of cartilage explants with chondroitinase ABC (1 U/ml for 5 minutes or 0.5 U/ml or 1 U/ml for 15 minutes) leads to enhanced chondrocyte adhesion, as measured by a micropipette micromanipulation system (Citation105). Similarly, treatment with collagenase at 10 U/ml (Citation102) and 30 U/ml (Citation101) increased cellularity in the edges of immature (6-month old) bovine full-thickness explants in vitro. Combined in vitro treatment with hyaluronidase (0.1%, 24 hours) followed by collagenase (10 U/ml or 30 U/ml, 24 hours) eventually resulted in cellular concentrations in the wound edge that exceeded that of healthy tissue (Citation102). Adjusting the doses and treating with the two enzymes simultaneously (300 U/ml collagenase with 3% hyaluronidase for 1 hour) improved cartilage–cartilage integration, which was demonstrated histologically, between bovine explants that were cultured subcutaneously in athymic rats for 35 days (Citation102). The biosafety of these treatments has been investigated by Quinn and Hunziker (Citation103) with regard to changes in cell density, structure, and cell-mediated matrix deposition that result from choindroitinase ABC treatment (1 U/ml for 5 minutes). While chondroitinase ABC exposure did appear to further decrease cell densities within 100 µm of the defect surfaces, the decrease was small relative to the effect of the defect itself and was contained to the area immediately adjacent to the defect (within 100 µm), demonstrating that digestive treatments can be spatially controlled to break down matrix within the zone of chondrocyte death.
Given the low cell density naturally surrounding defects and the further reduction in cell number immediately following digestive rinses, methods which actively recruit cells into the edge of the graft are an attractive option to counteract these trends and draw viable cells to the wound edge. Chemotactic agents that have been explored for this purpose include platelet derived growth factor (PDGF) (Citation106,Citation107), insulin-like growth factor I (IGF-1) (Citation106–Citation108), basic fibroblast growth factor (bFGF) (Citation106,Citation107), vascular endothelial growth factor (VEGF) (Citation107), and a variety of bone morphogenic proteins (BMPs) (Citation107). Cell migration analysis via boyden chamber assays indicate that PDGF, IGF-1, and BFGF effectively stimulate the migration of bovine articular chondrocytes at 25, 50, and 100 ng/ml, but are ineffective at lower doses, such as 5 ng/ml (Citation106). A combination of matrix digestion and chemotactic stimulation was evaluated by McGregor et al. using bovine metacarpal-phalangeal joint cartilage explants, in which the zone of death resulting from OATS™ harvesting tools was approximately 173 μm. Several growth factors alone and in combination with a collagenase rinse led to an improvement in the repopulation of the zone of death. Combined treatment with collagenase (0.6%, 10 mins) and 25 ng/ml IGF-1 resulted in the greatest reduction in the depth of the death zone (~70% of the untreated control) and increased cellularity within this region (~4.5 times higher than the untreated control) at four weeks (Citation106).
Biomaterial-based approaches to promote cartilage–cartilage integration have also been explored. Wang et al. designed a tissue “glue” by functionalizing chondroitin sulfate with methacrylate and aldehyde groups that form chemical bonds with both the native tissue and an acrylate-based scaffold (Citation109). This glue was tested in vitro via application to a cartilage explant and in vivo using a subcutaneous mouse model. The glue results in stable integration between the cartilage and a poly (ethylene glycol) diacrylate hydrogel construct, exhibiting an integration strength that exceeds the bulk strength of the hydrogel when measured using a custom shear testing device. Maher et al. (Citation110) tested the hypothesis that the introduction of a biodegradable, chondrocyte-laden nanofibrous hydrogel (PuraMatrix™) in the gap between the native and grafted cartilage, would improve the mechanical stability of the interface. When the scaffold system was seeded with bovine chondrocytes, supplemented with transforming growth factor-β3, and tested in an in vitro bovine cartilage gap model, matrix elaboration was enhanced, resulting in a significant increase in maximum push-out strength after six weeks.
Overall, these studies highlight the promise of strategies that improve cell migration and graft integration at the cartilage–cartilage interface. Initial studies with digestion rinses, chemotactic agents, and material-based approaches have shown potential to support stable, integrative cartilage repair. Given the complexity of the events immediately following postsurgical intervention, it is likely that an optimized combination of these approaches will be needed to maximize cartilage–cartilage integration over time.
Cartilage–bone integration
In addition to cartilage–cartilage integration, regeneration of the calcified cartilage region is critical for stable and functional integration with the subchondral bone. Published approaches to regenerate the osteochondral interface include both cell-based and scaffold-based strategies. In a series of pioneering studies, Kandel et al. seeded interface-relevant deep zone chondrocytes (DZCs) on Millicell CM® filter inserts pre-coated with collagen II, and subsequently cultured them in mineralization media containing 10 mM beta-glycerophosphate (Citation111). Mineralized cartilage formation was observed in the region directly adjacent to the insert. Scaling up to an in vivo sheep model, biphasic constructs were formed by seeding ovine full-thickness chondrocytes atop calcium polyphosphate substrates. The full-thickness chondrocytes formed an unmineralized cartilage layer on top of the bone scaffold, while calcified cartilage was not observed. The lack of a calcified cartilage layer likely contributed to the decrease in interfacial shear strength between the bone and cartilage that was measured. The cartilage tissue was sheared off in some of the defects underscoring the importance of the calcified cartilage layer in functional cartilage–bone integration (Citation112). This finding was later confirmed by Allan et al. in a study in which DZCs were seeded directly above a calcium phosphate-coated calcium polyphosphate scaffold in mineralization media, establishing a calcified cartilage-like zone above the ceramic (Citation113). The mineral formed in vitro was shown to be biomimetic, with enhanced interfacial mechanical properties compared to constructs that lacked a mineralized cartilage zone (Citation114). These studies demonstrated the feasibility and importance of calcified cartilage formation, and the next step is to address the functional requirements of the cartilage–bone interface through scaffold design.
Stratified scaffolds for cartilage–bone integration
An alternative approach to cell layers seeded atop a bone scaffold is a multi-phased graft that guides the simultaneous regeneration of bone, cartilage, and a calcified cartilage intermediate. A scaffold-based approach is advantageous because fewer chondrocytes are required than the cell-based approach, and functional mechanical properties of each tissue type can be readily achieved through scaffold design. First generation stratified scaffolds contained distinct cartilage and bone regions joined together using either sutures or sealants. For example, Schaefer et al. seeded bovine full-thickness chondrocytes on polyglycolic acid (PGA) meshes and periosteal cells on polylactide-co-glycolide (PLGA)/polyethylene glycol foams. The two constructs were subsequently sutured together at either one or four weeks post-seeding. Integration was enhanced when the constructs were joined together after one week compared to four weeks, illustrating the importance of early cell-cell interactions during tissue integration (Citation9). This general approach has since been tested using a variety of biomaterials and scaffolds (Citation3,Citation115–Citation127) and growth factors (Citation128–Citation131). Collectively, these studies demonstrate the feasibility of engineering both cartilage- and bone-like tissues and underscore the need for a consistent biological barrier between neo-cartilage and the bone region. It is likely that the biphasic design alone is not sufficient to achieve consistent and functional cartilage and bone formation and integration.
Recently, tri-layered scaffolds with cartilage, interface, and bone regions have been designed for osteochondral regeneration. Heymer et al. tested a tri-layered scaffold produced by Kensey Nash Corporation. The scaffold consists of a hydrophobic interface that separates the cartilage and bone portions that are comprised of collagen I fibers with incorporated hyaluronan or polylactic acid, respectively (Citation132). When stem cells were seeded on the polylactic acid portion above the interface and cultured for three weeks in vitro, cartilage-like tissue was formed. While calcified cartilage formation was not observed, the three layers remained distinguishable and structurally stable after culture. Jiang et al. reported on a scaffold composed of an agarose hydrogel and PLGA/45S5 bioactive glass composite microspheres, which supported the region-specific coculture of chondrocytes and osteoblasts. This design led to the production of three compositionally distinct yet structurally continuous regions containing cartilage, calcified cartilage, and bone-like matrices in vitro (Citation133). Similarly, Kon et al. developed an acellular tri-layered osteochondral scaffold that controls the spatial distribution of calcium phosphate and collagen to recapitulate the cartilage-to-bone transition. The upper, intermediate, and lower layers consisted of 100% collagen I, 60% collagen I and 40% HA, and 30% collagen I and 70% HA, respectively (Citation134). The layers were joined via freeze drying and tested in an adult equine osteochondral defect model. Distinct unmineralized and mineralized regions were present after six months of implantation, and the new tissue was well integrated with surrounding cartilage and bone. Recently, this scaffold was tested in a pilot clinical trial of 30 patients in which safety and potential clinical benefit, measured by patient reports on the Cartilage Standard Evaluation Form, were demonstrated after a two-year follow-up (Citation135). Marquass et al. introduced a tri-phasic scaffold consisting of a collagen I hydrogel to mimic cartilage and a layer of bioactive ceramic TCP to simulate bone that were joined together by an intermediate activated plasma phase. When seeded with autologous MSCs, the tri-layered scaffold performed comparably to osteochondral autografts both histologically and mechanically after one year in an ovine model (Citation136). In a study by Cheng et al., MSCs encapsulated in collagen microspheres were pre-differentiated into chondrocytes or osteoblasts using a novel collagen microencapsulation technology (Citation137). The microspheres were first aggregated in a collagen I gel to form chondrogenic and osteogenic layers that were then combined to produce an osteochondral scaffold. Additionally, a middle layer of undifferentiated MSCs in collagen I gel was sandwiched between the osteogenic and chondrogenic regions. Formation of a calcified cartilage interface layer was present only in the group with all three layers, while no interface was observed when undifferentiated MSCs were cultured on bi-layered scaffolds with either chondrogenic or osteogenic layers. Collectively, these studies reveal that tri-layered scaffolds offer direct regional control; however, in order to be translated, methods are needed to achieve functional integration of all three layers and support the robust formation of a physiologically relevant-sized calcified cartilage interface.
Scaffold designs utilizing a compositional gradient eliminate the need to integrate distinct layers and circumvent the challenge of fabricating a physiologically relevant or very thin calcified cartilage scaffold that must be pre-integrated with the bone and cartilage sections. To this end, Sherwood et al. formed an osteochondral graft with graded porosity and composition between the cartilage and bone regions using the TheriForm™ three-dimensional printing process (Citation138). Ovine chondrocytes attached to the cartilage phase of the scaffold and produced cartilage-like tissue after six weeks of in vitro culture. Harley et al. reported on a gradient scaffold using liquid-phase co-synthesis with an unmineralized collagen II-chondroitin-6-sulfate cartilage region and a mineralized collagen I-chondroitin-6-sulfate bone region (Citation8). At the interface, the solutions were interdiffused, leading to a gradual transition in composition between the cartilage and bone regions. Targeting mechanical properties, Singh et al. developed a multi-phased PLGA microsphere scaffold with continuous macroscopic gradients of stiffness using an ethanol-based solvent evaporation technique. Structural gradients were produced by varying the spatial distribution of microspheres that were reinforced with a stiff nano-phase material (CaCO3 or TiO2) and softer microspheres made of 100% PLGA (Citation139). More recently, Salerno et al. reported the fabrication of a PCL-HA osteochondral scaffold using a combination of CO2 foaming and salt leaching. Concentration gradients of salt particles and foaming temperatures were manipulated to create gradients in pore size, porosity, and scaffold morphology (Citation140). Utilizing a novel extrusion method, Erisken et al. fabricated PCL nanofiber scaffolds with graded calcium phosphate content. In vitro studies showed that culturing MC3T3-E1 cells on these scaffolds led to a gradient of calcified matrix within four weeks (Citation141). In a separate study by the same group, a novel twin-screw extrusion and electrospinning method was used to fabricate gradients of insulin and β-glycerophosphate (β-GP) in a PCL nanofiber mesh (Citation142). These scaffolds were then seeded with human adipose-derived stromal cells, and chondrogenic differentiation of the stem cells increased at insulin-rich locations while mineralization increased at β-GP-rich locations.
Gradients of growth factors have also been incorporated into osteochondral scaffolds. In a study by Dormer et al., chondrogenic and osteogenic growth factors were added to PLGA microsphere-based scaffolds in opposing gradients. This scaffold was seeded with human MSCs, resulting in a corresponding gradient from cartilage-like to bone-like matrices (Citation143). While scaffolds with dual growth factor gradients did not outperform sham repair in the mandibular model (Citation144), osteochondral regeneration was enhanced in a rabbit knee model when both growth factor and HA gradients were simultaneously incorporated into the scaffold design (Citation145). Although these studies demonstrate the feasibility of forming unmineralized and mineralized tissues on a continuous scaffold, the consistent and robust regeneration of a distinct calcified cartilage layer at the soft-to-hard tissue junction remains elusive. Furthermore, physiologically relevant gradient scaffolds are relatively challenging to fabricate and require methods which enable precise spatial and temporal control, posing a barrier to future scale-up efforts. More studies are also needed to determine if a gradient of properties is necessary to recapitulate the native interface in a scaffold system or if stratified layers are sufficient.
Calcified cartilage regeneration
To address the challenge of consistently regenerating calcified cartilage, recent studies have focused on identifying design parameters for osteochondral interface formation. Khanarian et al. evaluated and optimized a biomimetic hydrogel-ceramic composite scaffold for calcified cartilage formation by seeding the scaffold with bovine deep zone chondrocytes that were made hypertrophic via thyroid hormone (T3) stimulation (Citation146). Higher matrix deposition and mineralization potential were exhibited by the hypertrophic cells in the presence of HA particles (Citation147). Furthermore, while cell hypertrophy was independent of ceramic size, matrix deposition was significantly greater with the addition of micron-sized ceramic particles but not with the addition of nano-sized particles. The highest matrix content, mechanical properties, and mineralization potential were found in composite gels with 3 wt/vol% micro-HA, which approximates the mineral content of the native osteochondral interface. Evaluation of a hybrid alginate hydrogel-hydroxyapatite (HA) scaffold showed that the HA phase of the composite scaffold promoted the formation of a proteoglycan- and collagen II-rich matrix by DZCs. These cells became hypertrophic with the addition of T3 in these gels (Citation146). Importantly, the enhanced biosynthesis translated into significant increases in both compressive and shear moduli relative to the ceramic-free control. Presence of HA, and likely the associated high local calcium concentration of the alginate matrix, also promoted chondrocyte hypertrophy and collagen X deposition. These results demonstrate that a hydrogel with biomimetic ceramic content is optimal for calcified cartilage formation. Clinically, it is possible that the composite hydrogel scaffold could be used to augment existing cartilage grafts, providing anchorage to the bone and a diffusion barrier during the healing process. For example, in a full-thickness defect, the ceramic phase would promote osteointegration with bone while the hydrogel phase would facilitate integration with other hydrogel-based cartilage grafts.
Summary and future directions
This review highlighted promising cartilage regeneration strategies with a focus on approaches that target functional integration between the graft and native cartilage and subchondral bone. Specifically, digestive rinses, chemotactic factors, and strategically designed biomaterials have been employed to promote integration of the graft with native cartilage. Custom scaffolds with graded or continuous changes in composition have demonstrated promise for the regeneration of composite tissues of cartilage, calcified cartilage and bone in vitro and in vivo. The tremendous potential of these approaches is evident and has been established both in vitro and in vivo to promote integrative cartilage repair. An optimized combination of these strategies that promotes simultaneous integration with both host cartilage and subchondral bone will ensure integrative cartilage repair.
In general, published cartilage–cartilage integration approaches have been confined largely to the investigation of digestive and chemotactic factors to encourage repopulation of the hypocellular cartilage–cartilage junction, although promising results have also been reported for biomaterial-based approaches. More research into these strategies is needed to establish the role of scaffolding systems in expediting the integration between both native and newly formed cartilage. Relative to the cartilage–cartilage interface, substantially more effort has been directed toward scaffold development for the cartilage–bone interface. However, it is still unclear which scaffold design is best suited to regenerate the osteochondral interface and anchor the neo-cartilage to underlying bone. Tri-layered scaffolds and gradient scaffolds offer tunable platforms that can be used to direct interface development and prevent delamination; however, the scalability of these highly controlled and regionally distinct systems requires further testing to determine their relative advantage in vivo.
The scarcity of in vitro and in vivo models to test integrative scaffolds has limited the study of these systems and contributed to the lack of conclusive data pertaining to each system. Integration performance cannot be assessed using traditional in vitro studies because the scaffolds are cultured in isolation from native tissues. Meanwhile, in vivo studies provide integration data but are extremely expensive to conduct. Moreover, it is unclear if small animal studies, while more cost-effective than large animal models, offer meaningful, translatable data because the cartilage in these animals is far thinner than that found in humans and healing potential varies markedly across species. The emergence of organ culture models offers a promising modality to asses integration in large animal (Citation110,Citation148–Citation150) or human (Citation151) tissues without the prohibitive cost of a live animal study. These models are advantageous as they present an opportunity to study integration and the impact of scaffolds on the surrounding native tissue with a higher level of control experimentally. Characterization, optimization, and standardization of these models will be critical in the next phase of integrative cartilage repair research.
In addition to the development of better culture models, further investigation is also needed to fully understand interface maintenance and homeostasis to ensure the long-term success of integrative regeneration strategies. Work by Jiang et al. highlighted the importance of chondrocyte–osteoblast interactions using a three-dimensional model in which a micromass of chondrocytes was cultured with a monolayer of osteoblasts. Coculture of these cell types regulated cell-mediated mineralization and matrix production (Citation152). Later, the importance of the zonal organization of cartilage as a regulator in chondrocyte biosynthesis and mineralization was demonstrated through studies performed with monolayer cocultures (Citation75). Collectively, these results emphasize the importance of cellular interaction in the long-term regulation and stability of osteochondral systems.
In summary, custom-designed scaffold systems combined with chemotactic factors, and digestive washes represent a promising platform for integrative cartilage repair. However, further exploration into appropriate culture models with specific emphasis on tissue integrity and the prevention of ectopic calcification is required to develop a long-term solution to the treatment of full-thickness cartilage defects. It is anticipated that these efforts will lead to the development of a new generation of clinical management strategies for cartilage degeneration that result in functional and stable cartilage repair with improved long-term outcomes.
Declaration of interest
The authors report no conflicts of interest. No third party was involved in the drafting of this article.
Acknowledgments
The authors thank Christopher Mosher for his assistance with .
Funding
This work was funded by the NIH Ruth L. Kirschstein National Research Service Award T32 AR059038 (MKB), the New York Stem Cell Institute (NYSTEM C029551), the Presidential Early Career Award for Scientists and Engineers (HHL), and NIH-NIAMS 5R01AR055280 (HHL).
Additional information
Funding
References
- Centers for Disease Control and Prevention Public Health Service, U.S. Department of Health and Human Services. Osteoarthritis and you: Patient information from the CDC. J Pain Palliat Care Pharmacother 2010;24(4):430–438.
- Vacanti CA, Langer R, Schloo B, Vacanti JP. Synthetic polymers seeded with chondrocytes provide a template for new cartilage formation. Plastic and Reconstructive Surgery 1991;88(5):753–759.
- Gao J, Dennis JE, Solchaga LA, Awadallah AS, Goldberg VM, Caplan AI. Tissue- engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng 2001;7(4):363–371.
- Jiang J, Tang A, Ateshian GA, Guo XE, Hung CT, Lu HH. Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann Biomed Eng 2010;38(6):2183–2196.
- Holland TA, Bodde EW, Cuijpers VM, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthr Cartilage 2007;15(2):187–197.
- Chao PH, Yodmuang S, Wang X, Sun L, Kaplan DL, Vunjak-Novakovic G. Silk hydrogel for cartilage tissue engineering. J Biomed Mater Res B 2010;95(1):84–90.
- Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 2000;122:252–260.
- Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a multiphase osteochondral scaffold III: Fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res A 2010;92(3):1078–1093.
- Schaefer D, Martin I, Shastri P, Padera RF, Langer R, Freed LE, Vunjak-Novakovic G. In vitro generation of osteochondral composites. Biomaterials 2000;21(24):2599–2606.
- Yu H, Grynpas M, Kandel RA. Composition of cartilagenous tissue with mineralized and non-mineralized zones formed in vitro. Biomaterials 1997;18(21):1425–1431.
- Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials 2011;32(34):8771–8782.
- Sampat SR, Dermksian MV, Oungoulian SR, Winchester RJ, Bulinski JC, Ateshian GA, Hung CT. Applied osmotic loading for promoting development of engineered cartilage. Journal of Biomechanics 2013;46(15):2674–2681.
- Bhumiratana S, Eton RE, Oungoulian SR, Wan LQ, Ateshian GA, Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proceedings of the National Academy of Sciences of the United States of America 2014;111(19):6940–6945.
- Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res 1999;17(1):130–138.
- Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng 2000;122(3):252–260.
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci 1995;108(Pt 4):1497–1508.
- Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res 1989;7(5):619–636.
- Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res 2001;19(1):11–17.
- Frank EH, Jin M, Loening AM, Levenston ME, Grodzinsky AJ. A versatile shear and compression apparatus for mechanical stimulation of tissue culture explants. J Biomech 2000;33(11):1523–1527.
- Zhu W, Mow VC, Koob TJ, Eyre DR. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J Orthop Res 1993;11(6):771–781.
- Bayliss MT, Venn M, Maroudas A, Ali SY. Structure of proteoglycans from different layers of human articular cartilage. Biochem J 1983;209(2):387–400.
- Gurr E, Mohr W, Pallasch G. Proteoglycans from human articular cartilage: The effect of joint location on the structure. J Clin Chem Clin Biochem 1985;23(12):811–819.
- Wachsmuth L, Soder S, Fan Z, Finger F, Aigner T. Immunolocalization of matrix proteins in different human cartilage subtypes. Histol Histopathol 2006;21(5):477–485.
- Young RD, Lawrence PA, Duance VC, Aigner T, Monaghan P. Immunolocalization of collagen types II and III in single fibrils of human articular cartilage. J Histochem Cytochem 2000;48(3):423–432.
- Wotton SF, Duance VC. Type III collagen in normal human articular cartilage. Histochem J 1994;26(5):412–416.
- Soder S, Hambach L, Lissner R, Kirchner T, Aigner T. Ultrastructural localization of type VI collagen in normal adult and osteoarthritic human articular cartilage. Osteoarthritis Cartilage 2002;10(6):464–470.
- Ogston AG. The Biological Functions of the Glycosaminoglycans. In: Balazs EA, editor. Chemistry and Molecular Biology of the Intercellular Matrix. London: Academic Press; 1970. p. 1231–1240.
- Kempson GE, Muir H, Pollard C, Tuke M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim Biophys Acta 1973;297(2):456–472.
- Schmidt MB, Mow VC, Chun LE, Eyre DR. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res 1990;8(3):353–363.
- Basser PJ, Schneiderman R, Bank RA, Wachtel E, Maroudas A. Mechanical properties of the collagen network in human articular cartilage as measured by osmotic stress technique. Arch Biochem Biophys 1998;351(2):207–219.
- Maroudas AI. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature 1976;260(5554):808–809.
- Ateshian GA. The role of interstitial fluid pressurization in articular cartilage lubrication. J Biomech 2009;42(9):1163–1176.
- Aydelotte MB, Greenhill RR, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Connect Tissue Res 1988;18(3):223–234.
- Aydelotte MB, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. I. Morphology and cartilage matrix production. Connect Tissue Res 1988;18(3):205–222.
- Hunziker EB, Quinn TM, Hauselmann HJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthr Cartilage 2002;10(7):564–572.
- Grogan SP, Miyaki S, Asahara H, D’Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: Normal distribution and changes in osteoarthritis. Arthritis Res Ther 2009;11(3):R85.
- Brocklehurst R, Bayliss MT, Maroudas A, Coysh HL, Freeman MA, Revell PA, Ali SY. The composition of normal and osteoarthritic articular cartilage from human knee joints. With special reference to unicompartmental replacement and osteotomy of the knee. J Bone Joint Surg Am 1984;66(1):95–106.
- Bullough P, Goodfellow J. The significance of the fine structure of articular cartilage. J Bone Joint Surg Br 1968;50(4):852–857.
- Clarke IC. Articular cartilage: A review and scanning electron microscope study. 1. The interterritorial fibrillar architecture. J Bone Joint Surg Br 1971;53(4):732–750.
- Hunziker EB, Michel M, Studer D. Ultrastructure of adult human articular cartilage matrix after cryotechnical processing. Microsc Res Tech 1997;37(4):271–284.
- Weiss C, Rosenberg L, Helfet AJ. An ultrastructural study of normal young adult human articular cartilage. J Bone Joint Surg Am 1968;50(4):663–674.
- Muir H, Bullough P, Maroudas A. The distribution of collagen in human articular cartilage with some of its physiological implications. J Bone Joint Surg Br 1970;52(3):554–563.
- Minns RJ, Steven FS. The collagen fibril organization in human articular cartilage. J Anat 1977;123(Pt 2):437–457.
- Venn MF. Chemical composition of human femoral and head cartilage: Influence of topographical position and fibrillation. Ann Rheum Dis 1979;38(1):57–62.
- Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys 1994;311(1):144–152.
- Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, Caterson B. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun 1999;254(3):535–541.
- Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol 2008;22(2):351–384.
- Lane LB, Bullough PG. Age-related changes in the thickness of the calcified zone and the number of tidemarks in adult human articular cartilage. J Bone Joint Surg Br 1980;62(3):372–375.
- Muller-Gerbl M, Schulte E, Putz R. The thickness of the calcified layer of articular cartilage: A function of the load supported? J Anat 1987;154:103–111.
- Bullough PG, Jagannath A. The morphology of the calcification front in articular cartilage. Its significance in joint function. J Bone Joint Surg Br 1983;65(1):72–78.
- Clark JM. The structure of vascular channels in the subchondral plate. J Anat 1990;171:105–115.
- Gannon JM, Walker G, Fischer M, Carpenter R, Thompson RC, Jr., Oegema TR, Jr. Localization of type X collagen in canine growth plate and adult canine articular cartilage. J Orthop Res 1991;9(4):485–494.
- Boskey AL. Mineral-matrix interactions in bone and cartilage. Clin Orthop Relat Res 1992;281:244–274.
- Muller-Glauser W, Humbel B, Glatt M, Strauli P, Winterhalter KH, Bruckner P. On the role of type IX collagen in the extracellular matrix of cartilage: Type IX collagen is localized to intersections of collagen fibrils. J Cell Biol 1986;102(5):1931–1939.
- Hough AJ, Banfield WG, Mottram FC, Sokoloff L. The osteochondral junction of mammalian joints. An ultrastructural and microanalytic study. Lab Invest 1974;31(6):685–695.
- Broom ND, Poole CA. A functional-morphological study of the tidemark region of articular cartilage maintained in a non-viable physiological condition. J Anat 1982;135(Pt 1):65–682.
- Clark JM, Huber JD. The structure of the human subchondral plate. J Bone Joint Surg Br 1990;72(5):866–873.
- Redler I, Mow VC, Zimny ML, Mansell J. The ultrastructure and biomechanical significance of the tidemark of articular cartilage. Clin Orthop Relat Res 1975;(112):357–362.
- Fawns HT, Landells JW. Histochemical studies of rheumatic conditions. I. Observations on the fine structures of the matrix of normal bone and cartilage. Ann Rheum Dis 1953;12(2):105–113.
- Zizak I, Roschger P, Paris O, Misof BM, Berzlanovich A, Bernstorff S, Amenitsch H, Klaushofer K, Fratzl P. Characteristics of mineral particles in the human bone/cartilage interface. J Struct Biol 2003;141(3):208–217.
- Khanarian NT, Boushell MK, Spalazzi JP, Pleshko N, Boskey AL, Lu HH. FTIR-I compositional mapping of the cartilage-to-bone interface as a function of tissue region and age. J Bone Miner Res 2014;29(12):2643-2652.
- Mente PL, Lewis JL. Elastic modulus of calcified cartilage is an order of magnitude less than that of subchondral bone. J Orthop Res 1994;12(5):637–647.
- Gupta HS, Schratter S, Tesch W, Roschger P, Berzlanovich A, Schoeberl T, Klaushofer K, Fratzl P. Two different correlations between nanoindentation modulus and mineral content in the bone-cartilage interface. J Struct Biol 2005;149(2):138–148.
- Ferguson VL, Bushby AJ, Boyde A. Nanomechanical properties and mineral concentration in articular calcified cartilage and subchondral bone. J Anat 2003;203(2):191–202.
- Duer MJ, Friscic T, Murray RC, Reid DG, Wise ER. The mineral phase of calcified cartilage: Its molecular structure and interface with the organic matrix. Biophys J 2009;96(8):3372–3378.
- Hunziker EB, Driesang IM, Saager C. Structural barrier principle for growth factor-based articular cartilage repair. Clin Orthop Relat Res 2001;(391 Suppl):S182–S189.
- Pan J, Zhou X, Li W, Novotny JE, Doty SB, Wang L. In situ measurement of transport between subchondral bone and articular cartilage. J Orthop Res 2009;27(10):1347–1352.
- Arkill KP, Winlove CP. Solute transport in the deep and calcified zones of articular cartilage. Osteoarthr Cartilage 2008;16(6):708–714.
- Lotz M, Loeser RF. Effects of aging on articular cartilage homeostasis. Bone 2012;51(2):241–248.
- Vignon E, Arlot M, Patricot LM, Vignon G. The cell density of human femoral head cartilage. Clin Orthop Relat Res 1976;(121):303–308.
- Verzijl N, DeGroot J, Oldehinkel E, Bank RA, Thorpe SR, Baynes JW, Bayliss MT, Bijlsma JW, Lafeber FP, TeKoppele JM. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J 2000;350(Pt 2):381–387.
- Ogata K, Whiteside LA. Barrier to material transfer at the bone-cartilage interface: Measurement with hydrogen gas in vivo. Clin Orthop Relat Res 1979;(145):273–276.
- Lane LB, Villacin A, Bullough PG. The vascularity and remodelling of subchondrial bone and calcified cartilage in adult human femoral and humeral heads. An age- and stress-related phenomenon. J Bone Joint Surg Br 1977;59(3):272–278.
- Hargrave-Thomas EJ, Thambyah A, McGlashan SR, Broom ND. The bovine patella as a model of early osteoarthritis. J Anat 2013;223(6):651–664.
- Jiang J, Leong NL, Mung JC, Hidaka C, Lu HH. Interaction between zonal populations of articular chondrocytes suppresses chondrocyte mineralization and this process is mediated by PTHrP. Osteoarthr Cartilage 2008;16(1):70–82.
- Oettmeier R, Abendroth K, Oettmeier S. Analyses of the tidemark on human femoral heads. I. Histochemical, ultrastructural and microanalytic characterization of the normal structure of the intercartilaginous junction. Acta Morphol Hung 1989;37(3–4): 155–168.
- Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, Buckwalter JA. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J Orthop Res 2011;29(6):802–809.
- Bullough PG. The geometry of diarthrodial joints, its physiologic maintenance, and the possible significance of age-related changes in geometry-to-load distribution and the development of osteoarthritis. Clin Orthop Relat Res 1981;(156):61–66.
- Bullough PG. The role of joint architecture in the etiology of arthritis. Osteoarthr Cartilage 2004;12(Suppl A):S2–S9.
- Turley SM, Thambyah A, Riggs CM, Firth EC, Broom ND. Microstructural changes in cartilage and bone related to repetitive overloading in an equine athlete model. J Anat 2014;224(6):647–658.
- Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, Carrino JA, Cosgarea A, Artemov D, Chen Q, Zhao Z, Zhou X, Riley L, Sponseller P, Wan M, Lu WW, Cao X. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med 2013;19(6):704–712.
- Collins DH, McElligott TF. Sulphate (35SO4) uptake by chondrocytes in relation to histological changes in osteoarthritic human articular cartilage. Ann Rheum Dis 1960;19:318–330.
- Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van WH, Poole AR. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest 1997;99(7):1534–1545.
- Aigner T, McKenna L. Molecular pathology and pathobiology of osteoarthritic cartilage. Cell Mol Life Sci 2002;59(1):5–18.
- Roberts S, Weightman B, Urban J, Chappell D. Mechanical and biochemical properties of human articular cartilage in osteoarthritic femoral heads and in autopsy specimens. J Bone Joint Surg Br 1986;68(2):278–288.
- Kempson GE, Spivey CJ, Swanson SA, Freeman MA. Patterns of cartilage stiffness on normal and degenerate human femoral heads. J Biomech 1971;4(6):597–609.
- Akizuki S, Mow VC, Muller F, Pita JC, Howell DS, Manicourt DH. Tensile properties of human knee joint cartilage: I. Influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J Orthop Res 1986;4(4):379–392.
- Beiser IH, Kanat IO. Subchondral bone drilling: A treatment for cartilage defects. J Foot Surg 1990;29(6):595–601.
- Insall J. The Pridie debridement operation for osteoarthritis of the knee. Clin Orthop Relat Res 1974;(101):61–67.
- Sledge SL. Microfracture techniques in the treatment of osteochondral injuries. Clin Sports Med 2001;20(2):365–377.
- Czitrom AA, Langer F, McKee N, Gross AE. Bone and cartilage allotransplantation. A review of 14 years of research and clinical studies. Clin Orthop Relat Res 1986;(208):141–145.
- Hangody L, Kish G, Karpati Z, Szerb I, Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc 1997;5(4):262–267.
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331(14):889–895.
- Kon E, Filardo G, Di MA, Marcacci M. ACI and MACI. J Knee Surg 2012;25(1):17–22.
- Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full- thickness defects of articular cartilage. J Bone Joint Surg Am 1993;75(4):532–553.
- Tew SR, Kwan AP, Hann A, Thomson BM, Archer CW. The reactions of articular cartilage to experimental wounding: Role of apoptosis. Arthritis Rheum 2000;43(1):215–225.
- Hunziker EB, Quinn TM. Surgical removal of articular cartilage leads to loss of chondrocytes from cartilage bordering the wound edge. J Bone Joint Surg Am 2003;85A:85–92.
- Redman SN, Dowthwaite GP, Thomson BM, Archer CW. The cellular responses of articular cartilage to sharp and blunt trauma. Osteoarthr Cartilage 2004;12(2):106–116.
- Huntley JS, Bush PG, McBirnie JM, Simpson AH, Hall AC. Chondrocyte death associated with human femoral osteochondral harvest as performed for mosaicplasty. J Bone Joint Surg Am 2005;87(2):351–360.
- Qiu W, Murray MM, Shortkroff S, Lee CR, Martin SD, Spector M. Outgrowth of chondrocytes from human articular cartilage explants and expression of alpha-smooth muscle actin. Wound Repair Regen 2000;8(5):383–391.
- Bos PK, DeGroot J, Budde M, Verhaar JA, van Osch GJ. Specific enzymatic treatment of bovine and human articular cartilage: Implications for integrative cartilage repair. Arthritis Rheum 2002;46(4):976–985.
- Janssen LM, In der Maur CD, Bos PK, Hardillo JA, van Osch GJ. Short-duration enzymatic treatment promotes integration of a cartilage graft in a defect. Ann Otol Rhinol Laryngol 2006;115(6):461–468.
- Quinn TM, Hunziker EB. Controlled enzymatic matrix degradation for integrative cartilage repair: Effects on viable cell density and proteoglycan deposition. Tissue Eng 2002;8(5):799–806.
- Hunziker EB, Kapfinger E. Removal of proteoglycans from the surface of defects in articular cartilage transiently enhances coverage by repair cells. J Bone Joint Surg Br 1998;80(1):144–150.
- Lee MC, Sung KL, Kurtis MS, Akeson WH, Sah RL. Adhesive force of chondrocytes to cartilage. Effects of chondroitinase ABC. Clin Orthop Relat Res 2000;(370):286–294.
- McGregor AJ, Amsden BG, Waldman SD. Chondrocyte repopulation of the zone of death induced by osteochondral harvest. Osteoarthr Cartilage 2011;19(2):242–248.
- Mishima Y, Lotz M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J Orthop Res 2008;26(10):1407–1412.
- Chang C, Lauffenburger DA, Morales TI. Motile chondrocytes from newborn calf: Migration properties and synthesis of collagen II. Osteoarthr Cartilage 2003;11(8):603–612.
- Wang DA, Varghese S, Sharma B, Strehin I, Fermanian S, Gorham J, Fairbrother DH, Cascio B, Elisseeff JH. Multifunctional chondroitin sulphate for cartilage tissue- biomaterial integration. Nat Mater 2007;6(5):385–392.
- Maher SA, Mauck RL, Rackwitz L, Tuan RS. A nanofibrous cell-seeded hydrogel promotes integration in a cartilage gap model. J Tissue Eng and Regen Med 2010;4(1):25–29.
- Kandel RA, Hurtig M, Grynpas M. Characterization of the mineral in calcified articular cartilagenous tissue formed in vitro. Tissue Eng 1999;5(1):25–34.
- Kandel RA, Grynpas M, Pilliar R, Lee J, Wang J, Waldman S, Zalzal P, Hurtig M. Repair of osteochondral defects with biphasic cartilage-calcium polyphosphate constructs in a sheep model. Biomaterials 2006;27(22):4120–4131.
- Allan KS, Pilliar RM, Wang J, Grynpas MD, Kandel RA. Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue Eng 2007;13(1):167–177.
- St-Pierre JP, Gan L, Wang J, Pilliar RM, Grynpas MD, Kandel RA. The incorporation of a zone of calcified cartilage improves the interfacial shear strength between in vitro- formed cartilage and the underlying substrate. Acta Biomater 2012;8(4):1603–1615.
- Zhang W, Chen JL, Tao JD, Hu CC, Chen LK, Zhao HS, Xu GW, Heng BC, Ouyang HW. The promotion of osteochondral repair by combined intra-articular injection of parathyroid hormone-related protein and implantation of a bi-layer collagen-silk scaffold. Biomaterials 2013;34(25):6046–6057.
- Galperin A, Oldinski RA, Florczyk SJ, Bryers JD, Zhang MQ, Ratner BD. Integrated bi- layered scaffold for osteochondral tissue engineering. Adv Healthcare Mater 2013;2(6):872–883.
- Ibrahim NS, Krishnamurithy G, Raghavendran HRB, Puvaneswary S, Min NW, Kamarul T. Novel HA-PVA/NOCC bilayered scaffold for osteochondral tissue- engineering applications: Fabrication, characterization, in vitro and in vivo biocompatibility study. Mater Lett 2013;113:25–29.
- Ding CM, Qiao ZG, Jiang WB, Li HW, Wei JH, Zhou GD, Dai KR. Regeneration of a goat femoral head using a tissue-specific, biphasic scaffold fabricated with CAD/CAM technology. Biomaterials 2013;34(28):6706–6716.
- Grayson WL, Bhumiratana S, Grace Chao PH, Hung CT, Vunjak-Novakovic G. Spatial regulation of human mesenchymal stem cell differentiation in engineered osteochondral constructs: effects of pre-differentiation, soluble factors and medium perfusion. Osteoarthr Cartilage 2010;18(5):714–723.
- Zhang S, Chen L, Jiang Y, Cai Y, Xu G, Tong T, Zhang W, Wang L, Ji J, Shi P, Ouyang HW. Bi-layer collagen/microporous electrospun nanofiber scaffold improves the osteochondral regeneration. Acta Biomater 2013;9(7):7236–7247.
- Chiang H, Liao CJ, Hsieh CH, Shen CY, Huang YY, Jiang CC. Clinical feasibility of a novel biphasic osteochondral composite for matrix-associated autologous chondrocyte implantation. Osteoarthritis Cartilage 2013;21(4):589–598.
- Swieszkowski W, Tuan BHS, Kurzydlowski KJ, Hutmacher DW. Repair and regeneration of osteochondral defects in the articular joints. Biomol Eng 2007;24(5):489–495.
- Shao X, Goh JC, Hutmacher DW, Lee EH, Zigang G. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng 2006;12(6):1539–1551.
- Chen G, Sato T, Tanaka J, Tateishi T. Preparation of a biphasic scaffold for osteochondral tissue engineering. Mater Sci Eng C 2006;26(1):118–123.
- Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am 2005;87(5):936–944.
- Yunos D, Ahmad Z, Salih V, Boccaccini A. Stratified scaffolds for osteochondral tissue engineering applications: Electrospun PDLLA nanofibre coated Bioglass(R)-derived foams. J Biomater Appl 2013;27(5):537–551.
- Scotti C, Wirz D, Wolf F, Schaefer DJ, Burgin V, Daniels AU, Valderrabano V, Candrian C, Jakob M, Martin I, Barbero A. Engineering human cell-based, functionally integrated osteochondral grafts by biological bonding of engineered cartilage tissues to bony scaffolds. Biomaterials 2010;31(8):2252–2259.
- Castro NJ, O’Brien CM, Zhang LG. Biomimetic Biphasic 3-D Nanocomposite scaffold for osteochondral regeneration. AIChE J 2014;60(2):432–442.
- Seo JP, Tanabe T, Tsuzuki N, Haneda S, Yamada K, Furuoka H, Tabata Y, Sasaki N. Effects of bilayer gelatin/beta-tricalcium phosphate sponges loaded with mesenchymal stem cells, chondrocytes, bone morphogenetic protein-2, and platelet rich plasma on osteochondral defects of the talus in horses. Res Vet Sci 2013;95(3):1210–1216.
- Chen J, Chen H, Li P, Diao H, Zhu S, Dong L, Wang R, Guo T, Zhao J, Zhang J. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials 2011;32(21):4793–4805.
- Re’em T, Witte F, Willbold E, Ruvinov E, Cohen S. Simultaneous regeneration of articular cartilage and subchondral bone induced by spatially presented TGF-beta and BMP-4 in a bilayer affinity binding system. Acta Biomater 2012;8(9):3283–3293.
- Heymer A, Bradica G, Eulert J, Noth U. Multiphasic collagen fibre-PLA composites seeded with human mesenchymal stem cells for osteochondral defect repair: an in vitro study. J Tissue Eng and Regen Med 2009;3(5):389–3897.
- Jiang J, Tang A, Ateshian GA, Guo XE, Hung CT, Lu HH. Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann Biomed Eng 2010;38(6):2183–2196.
- Kon E, Mutini A, Arcangeli E, Delcogliano M, Filardo G, Nicoli AN, Pressato D, Quarto R, Zaffagnini S, Marcacci M. Novel nanostructured scaffold for osteochondral regeneration: Pilot study in horses. J Tissue Eng and Regen Med 2010;4(4):300–308.
- Kon E, Delcogliano M, Filardo G, Busacca M, Di MA, Marcacci M. Novel nano- composite multilayered biomaterial for osteochondral regeneration: A pilot clinical trial. Am J Sports Med 2011;39(6):1180–1190.
- Marquass B, Somerson JS, Hepp P, Aigner T, Schwan S, Bader A, Josten C, Zscharnack M, Schulz RM. A novel MSC-seeded triphasic construct for the repair of osteochondral defects. J Orthop Res 2010;28(12):1586–1599.
- Cheng HW, Luk KD, Cheung KM, Chan BP. In vitro generation of an osteochondral interface from mesenchymal stem cell-collagen microspheres. Biomaterials 2011;32(6):1526–1535.
- Sherwood JK, Riley SL, Palazzolo R, Brown SC, Monkhouse DC, Coates M, Griffith LG, Landeen LK, Ratcliffe A. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials 2002;23(24):4739–4751.
- Singh M, Dormer N, Salash J, Christian J, Moore D, Berkland C, Detamore M. Three- dimensional macroscopic scaffolds with a gradient in stiffness for functional regeneration of interfacial tissues. J Biomed Mater Res A 2010. [Epub ahead of print]
- Salerno A, Iannace S, Netti PA. Graded biomimetic osteochondral scaffold prepared via CO2 foaming and micronized NaCl leaching. Mater Lett 2012;82:137–140.
- Erisken C, Kalyon DM, Wang H. Functionally graded electrospun polycaprolactone and beta-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials 2008;29(30):4065–4073.
- Erisken C, Kalyon DM, Wang HJ, Ornek-Ballanco C, Xu JH. Osteochondral tissue formation through adipose-derived stromal cell differentiation on biomimetic polycaprolactone nanofibrous scaffolds with graded insulin and beta- glycerophosphate concentrations. Tissue Eng Pt A 2011;17(9–10):1239–1252.
- Dormer NH, Singh M, Wang L, Berkland CJ, Detamore MS. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Ann Biomed Eng 2010;38(6):2167–2182.
- Dormer NH, Singh M, Zhao L, Mohan N, Berkland CJ, Detamore MS. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J Biomed Mater Res A 2012;100(1):162–170.
- Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue Eng Pt A 2011;17(21–22):2845–2855.
- Khanarian NT, Jiang J, Wan LQ, Mow VC, Lu HH. A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Eng Pt A 2012;18 (5–6):533–545.
- Khanarian NT, Haney NM, Burga RA, Lu HH. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration. Biomaterials 2012;33(21):5427–5258.
- Obradovic B, Martin I, Padera RF, Treppo S, Freed LE, Vunjak-Novakovic G. Integration of engineered cartilage. J Orthop Res 2001;19(6):1089–1097.
- de Vries-van Melle ML, Mandl EW, Kops N, Koevoet WJ, Verhaar JA, van Osch GJ. An osteochondral culture model to study mechanisms involved in articular cartilage repair. Tissue Eng Pt C Meth 2012;18(1):45–53.
- Theodoropoulos JS, De Croos JN, Park SS, Pilliar R, Kandel RA. Integration of tissue- engineered cartilage with host cartilage: An in vitro model. Clin Orthop Relat Res 2011;469(10):2785–2795.
- Secretan C, Bagnall KM, Jomha NM. Effects of introducing cultured human chondrocytes into a human articular cartilage explant model. Cell Tissue Res 2010;339(2):421–427.
- Jiang J, Nicoll SB, Lu HH. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem Biophys Res Commun 2005;338(2):762–770.