ABSTRACT
Purpose/Aim: Substance P-NK-1R signaling has been implicated in fibrotic tendinopathies and myositis. Blocking this signaling with a neurokinin 1 receptor antagonist (NK1RA) has been proposed as a therapeutic target for their treatment.Materials and Methods: Using a rodent model of overuse injury, we pharmacologically blocked Substance P using a specific NK1RA with the hopes of reducing forelimb tendon, muscle and dermal fibrogenic changes and associated pain-related behaviors. Young adult rats learned to pull at high force levels across a 5-week period, before performing a high repetition high force (HRHF) task for 3 weeks (2 h/day, 3 days/week). HRHF rats were untreated or treated in task weeks 2 and 3 with the NK1RA, i.p. Control rats received vehicle or NK1RA treatments.Results: Grip strength declined in untreated HRHF rats, and mechanical sensitivity and temperature aversion increased compared to controls; these changes were improved by NK1RA treatment (L-732,138). NK1RA treatment also reduced HRHF-induced thickening in flexor digitorum epitendons, and HRHF-induced increases of TGFbeta1, CCN2/CTGF, and collagen type 1 in flexor digitorum muscles. In the forepaw upper dermis, task-induced increases in collagen deposition were reduced by NK1RA treatment.Conclusions: Our findings indicate that Substance P plays a role in the development of fibrogenic responses and subsequent discomfort in forelimb tissues involved in performing a high demand repetitive forceful task.
Introduction
Overuse-induced musculoskeletal disorders/injuries are also known as repetitive motion disorders and repetitive strain injuries, and include work-related musculoskeletal disorders. They are the leading cause of pain and physical disability worldwide,Citation1–Citation3 with diagnoses including tendinopathies, myalgia, and peripheral neuropathies. Risk factors include prolonged exposure to high repetition and high force,Citation4,Citation5 which is problematic as high repetition high force upper extremity tasks are performed by a multitude of people, including construction and health-care workers.Citation6,Citation7 These disorders are associated with motor declines, discomfort and pain,Citation8,Citation9 and fibrotic changes in biopsied tendons and muscles.Citation10,Citation11 Prevention is hampered by many problems,Citation12,Citation13 raising the need for effective treatments.Citation14,Citation15
We have a clinically relevant rodent model in which rats perform an operant grasping and isometric lever pulling task for a food reward that we use to examine the etiology and treatment of upper extremity overuse musculoskeletal disorders.Citation16 We and others have established an early inflammatory mechanism in rats and humans with these disorders,Citation17,Citation18 and have shown that ibuprofen and an anti-tumor necrosis factor alpha drug reduces this tissue inflammation.Citation19–Citation21 However, these drugs did not fully rescue task-induced functional declines, or return tissue fibrogenic proteins (e.g., TGFβ1 (transforming growth factor beta 1) and CCN2 (cell communication network protein 2, also known as connective tissue growth factor or CTGF) and intramuscular collagen deposition to control levels in rats performing a HRHF lever-pulling task for 4–12 weeks while undergoing these treatments. Flexor digitorum tendinopathy develops with performance of the HRHF task, as does flexor digitorum muscle fibrosis (both within and around the muscle) and forepaw upper dermis fibrosis.Citation19,Citation22–Citation24 An initial 5-week shaping period in which rats learn to pull at high force levels also induces elevated levels of extracellular and several fibrogenic proteins (periostin-like factor, CCN2, and TGFbeta1), relative to control rats.Citation19,Citation25,Citation26 We recently observed that performance of the HRHF task for 3 weeks increases ongoing nociceptor activity in the median nerve in parallel with increased extraneural fibrosis,Citation27 and that reducing this extraneural and musculoskeletal tissue fibrosis using an antibody directed against CCN2 or modeled manual therapy, improved sensorimotor behavioral declines.Citation24,Citation26,Citation28 This leads us to hypothesize that fibrosis within and around tissues may increase tissue strain and injury as a consequence of tethering to adjacent structures, changes that can also reduce dynamic tissue function and enhance discomfort.Citation29–Citation31
We sought to examine the effectiveness of blocking Substance P signaling, a tachykinin produced by tenocytes in association with tendinopathy in humans,Citation32,Citation33 in rabbit kicking models of tendinopathy,Citation34,Citation35 and in nerves, muscles, and tendons in our rat model of chronic overuse musculoskeletal disorders.Citation22,Citation36 Substance P has a high affinity for the neurokinin-1 receptor (NK1R). Substance P and NK1R increase in tendons and muscles with overuse,Citation32–Citation35 and the Substance P-NK1R pathway has been implicated as an inducer of collagen production of over-use fibrotic tendinopathies.Citation32,Citation37 The NK1R has been proposed as a therapeutic target for their treatment,Citation32,Citation37,Citation38 although this hypothesis has yet to be tested in vivo.
Therefore, our first goal was to determine if a specific NK-1R antagonist (NK1RA, L-732,138), delivered systemically, would reduce the early development of forearm tissue fibrosis and sensorimotor declines in rats performing a high repetition high force (HRHF) task for 3 weeks. We sought to determine its effectiveness not just in forelimb tendons but also in forelimb muscle and forepaw dermal tissues surrounding nerves involved in performing the task with the hopes of elucidating potential reasons underlying associated sensorimotor declines. We hypothesized that the development of fibrotic tissue changes and sensorimotor declines would be reduced in HRHF task animals by blocking Substance P signaling via the NK-1R pathway using this specific NK1RA.
Materials and methods
Overview of animals used in vivo studies
Experiments were approved by the Temple University Institutional Animal Care and Use Committee in compliance with NIH guidelines for the humane care and use of laboratory animals. Young adult female Sprague-Dawley rats (3 months of age at onset of experiments) from Charles Rivers (Wilmington, MA) were used. Female Sprague–Dawley rats were used since human females have a higher incidence of work-related musculoskeletal disorders than males.Citation3,Citation39,Citation40 Rats were housed individually in a central animal facility in a 12-h light:dark cycle with free access to water, gently handled at least twice per week and provided cage enrichment toys.
To motivate interest in food reward pellets, rats used were food restricted to 5% less than weights of age-matched control rats with free-access-to-food (used for weight comparison purposes only), yet allowed to gain weight over the course of the experiment. In addition to food reward pellets (a mix of 45 mg purified chocolate-flavored grain and banana-flavored pellets; Bio-Serv, NJ, USA), all rats received Purina rat chow (Woodstock, Ontario, Canada) in their cages daily. Task rats first underwent an initial shaping period for 5 weeks to learn the high force lever pulling task, before going on to perform a HRHF lever pulling task for 3 weeks. HRHF rats were left untreated or treated during the last two task weeks with a NK1RA. Two animals in the latter subgroup were lost due to unforeseen circumstances (a large forearm lipoma and a congenital gastric anomaly). Reach limbs were examined from untreated HRHF and HRHF+NK1RA rats (n = 21 and 11, respectively). Remaining food-restricted rats from which limbs were collected, bilaterally, were maintained as food-restricted controls (FRC) that were either treated with 95% ethanol in saline as a vehicle, saline as a vehicle, or NK1RA (n = 10 each FRC group, bilateral limb examination). Different numbers per group were needed to accommodate the development of new methods.
Shaping and task performance
Subcohorts of food-restricted rats underwent a five-week shaping period, as previously describedCitation16,Citation41 for 10 min/day, 5 days/week. Thereafter, a point equal to HRHF week 0, shaped rats began the HRHF task regimen for 2 h/day, 3 days/week for 3 weeks. The task was divided into 4, 0.5-h sessions separated by 1.5 h in order to avoid satiation. HRHF rats were cued to reach at a rate of 4 reaches/min and to extend their forearm forward into a portal, grasp a force lever bar, and then exert a fairly isometric pull for at least 90 milliseconds (ms) at a grasp force of their 48% maximum pulling force (mean of 1.37 N), for 2 h/day, in 30 min intervals with 1.5 h rest breaks between, 3 days/week, for 3 weeks. Rats were allowed to self-regulate their participation. The rats had a preferred reach limb that they used to pull on the lever bar, noted during each session. Additional details are as previously published.Citation16,Citation41 Food-restricted control rats (FRC) rested in their cages with daily handling and environmental toys until the end of the experiment, and received similar amounts of rat chow and food reward pellets as HRHF rats in their home cage.
Pharmacological treatments of animals
Subcohorts of HRHF task rats were left untreated, or treated for 2 weeks with a NK1RA (L-732,138, Tocris Bioscience, R&D, Minneapolis, MN). This drug was delivered dehydrated and resuspended as recommended by company in 100% ethanol into a 100 mM stock solution (50 mg of powder in 1.058 ml of 100% ethanol). Stock solution was aliquoted (30 μl each) and frozen at −80 °C until use, at which time the aliquots were further diluted in saline for an end dose of 5 mg of NK1RA/kg body weight in 50 μl of 95% ethanol in saline, as recommended from past studies.Citation42,Citation43 Since: 1) a single dose of L-732,138 (5 mg/kg body weight, i.p.) reduces inflammatory events triggered by release of Substance P from nerve terminals;Citation44 2) most NK1R antagonists remain physiologically active for 5–40 h after a systemic dose;Citation45–Citation50 and 3) the window of opportunity for reducing adhesion formation using related NK1R antagonists when provided at 5 mg/kg body weight, i.p., is 5–24 h after a surgical procedure,Citation45,Citation46,Citation48,Citation50 we performed an initial pilot study in 3 task animals using L-732,138 at a dose of 5 mg/kg body weight, i.p., 3 days/week. The drug was administered after the last task session of Monday, Wednesday, and Fridays in task weeks 2 and 3 as we consider the task sessions as the inducing trauma.Citation28 This dose regimen proved effective at reducing inflammation and fibrogenic processes without also inducing negative side effects. Therefore, we repeated those studies with this dose and regimen with additional animals here, as well as in a longer term study.Citation51 Subcohorts of FRC rats were treated for 2 weeks with either the NK1RA drug or vehicles (50 μl of 95% ethanol in saline, or 50 μl of saline).
Behavioral assays
All HRHF and FRC rats underwent behavioral assays. Grip strength was assayed after onset of food restriction, 5 weeks later after shaping (HRHF week 0), and at the end of HRHF week 3 using a rat grip strength meter (1027SR-D58, Columbus Instruments, Columbus, Ohio). The test was repeated 5 times/limb, and maximum grip strength per trial is reported. Forepaw sensitivity to mechanical probing was assayed at end of HRHF task week 3, bilaterally, using nylon monofilaments (Semmes-Weinstein monofilaments, Stoelting, Woodale, IL) and described methods.Citation52 This test was repeated 10 times/limb/trial, and the mean number of limb withdrawal responses out of 10 reported for each monofilament. A two-choice temperature place preference test was used to determine cold aversion at the end of HRHF task week 3 using a temperature testing apparatus (T2CT, BioSeb, France) and described methods.Citation23 The reference plate was 22 °C while the test plate was adjusted from 22 to 12 °C across 35 min. Rats were free to choose their preferred position. Temperature place preference was tested only the day before euthanasia to avoid any confounds of learning preference.
Histology and immunohistochemistry
Reach limbs were collected from subcohorts of HRHF rats (10 HRHF and 7 HRHF+NK1RA), and one limb each of age-matched FRC rats (5 FRC+95% ethanol/saline, 6 FRC+saline, and 5 FRC+NK1RA). Animals were anesthetized with 5% isoflurane in oxygen and euthanized by cardiac exsanguination, serum collected and limbs to be used for ELISAs removed, before undergoing transcardial perfusion with first saline and then with 4% paraformaldehyde in phosphate buffer (pH 7.4). Perfused limbs were postfixed 24 h. Soft tissues were collected and cryoprotected in 10% and then 30% sucrose in phosphate buffer (48 h each). A 2.5 mm piece of mid-to-proximal flexor digitorum muscle was removed using a scalpel and placed in cryomolds for crossectional cryosectioning; remaining forearm muscle-tendon-nerve mass underwent longitudinal cryosectioning (15 μm thick sections), before placing onto charged slides. Slides were dried overnight at rm temp and stored at −80 °C until use. Muscle-tendon-nerve mass cryosections were stained in batched subsets with hematoxylin, or for collagen type 1, CCN2, transforming growth factor beta 1 (TGFβ1) and Substance P using previously described antibodies and methods,Citation19,Citation23,Citation25 before coverslipping with 80% glycerol in PBS, using DAPI as a nuclear counterstain. Specificity of the antibodies used has been previously published.Citation19,Citation23 Subsets also underwent Masson’s Trichrome staining and coverslipping with DPX (a resin mountant media, #44,581, Sigma-Aldrich, Inc., St. Louis, MO).
Fixed forepaws were processed for paraffin embedding and sectioned longitudinally using a microtome (5 μm); sections were placed onto charged slides. Sections were deparaffinized before staining with antibodies as described above, hematoxylin and eosin, or Masson’s Trichrome.
Histomorphometry
Epitendon thickness and cellularity (number of cells per βm2) and endotendon cellularity were assayed in wrist level flexor digitorum tendons in longitudinal cryosections after hematoxylin staining. The cell shape factor analysis feature of an imaging program was used to assay if endotendon cells were more spindle-shaped (<0.5) versus rounded (>0.5 to 1 indicates a more rounded shape) (Life Science, Bioquant Image Analysis Corporation, Nashville, TN). Shortest diameters of myofibers were assayed in collagen/DAPI immunostained crossectional cryosections of flexor digitorum muscles, in 3 fields/muscle, using a 20x objective, with data reported as mean circular mil (mcm), using Bioquant software.
Quantification was performed in batched sets by individuals blinded to group assignment, using upright microscopes with bright field and epifluorescence (E800, Nikon, Melville, NY) interfaced to digital cameras (Retiga 4000R QImaging Firewire, Surry, BC Canada), computer and image analysis systems (Bioquant Image Analysis Corporation, Nashville, TN). In flexor digitorum muscle cross-sectional cryosections, TGFβ1 positive cells were counted with results reported as a number of cells per mm2. A thresholded pixel count, 20x objective, in 3 fields/tissue was used to quantify: collagen type 1 and CCN2 immunostaining in crossectional cryosections, and blue staining (collagen) in the upper dermis of mid forepaw pads after Masson’s Trichrome staining of paraffin-processed sections. Thresholded pixel counts are reported as a percent of total number of pixels in the region of interest chosen using the irregular region of interest tool (Bioquant software).Citation53
ELISAs
Animals were deeply anesthetized with 5% isoflurane in oxygen and euthanized by cardiac exsanguination. Forelimb tissues were collected for ELISA from subcohorts of rats. This included reach limbs from subcohorts of HRHF rats (11 untreated HRHF and 4 HRHF+NK1RA), and one limb each of FRC rats (5 FRC+95% ethanol/saline, 6 FRC+saline, and 5 FRC+NK1RA), the latter removed prior to perfusion of rats with fixative for histological assays described above. Samples were flash frozen and stored at −80 °C until thawed and homogenized in sterile, ice-cold, phosphate-buffered saline (PBS) containing proteinase inhibitors (#5,056,489,001, Sigma-Aldrich), before being centrifuged (12,000 rpm, 15 min, 4 °C). Supernatants were stored at −80 °C until assayed in duplicate for TGFβ1 (#ADI-900–155, Enzo, Farmingdale, NY), CCN2 (#024398, USBiological, San Diego, CA), and collagen type 1 (#LS-F5638, LifeSpan BioSciences, Seattle, WA), collagen type 3 (LS-F23886, LifeSpan). Data (pg of protein) were normalized to μg of total protein, determined using bicinchoninic acid assays (#3225, BCA Protein assay, PierceTM, Thermo Fisher Scientific, Waltham, MA, USA).
Western blots
Aliquots of rat muscle lysates were also analyzed using Western blot methods for phosphorylated ERKA1/2 (pERK1/2) and total ERK protein expression. Laemmli buffer (4×) and 5% beta-mercaptoethanol (BME) were added and samples boiled. Equal amounts of protein (20 µg/20 µL) were resolved using 4–12% Tris-Glycine gels. Gels were blotted onto nitrocellulose membranes, blocked 1 h in 5% BSA/Tris-buffered saline (TBS)/0.05% Tween-20, and incubated overnight with primary antibodies diluted 1:1000 (anti-p44/42 MAPK (total Erk1/2), #137F5, Cell Signaling Technologies, Danvers, MA, USA; anti-Phospho-p44/42 MAPK (pERK 1/2), #D.133.14.4E, Cell Signaling Technologies). Membranes were washed in TBS/Tween and incubated 1 h at room temp with secondary antibodies (IRDye® Infrared Dyes, LI-COR, Lincoln, NE, USA) diluted 1:5000. Images were obtained using a LI-COR System and densitometrically analyzed using Image J. Band densities were normalized to the same internal control sample loaded into one well of each gel. Normalized bands of pERK and total ERK were compared as a ratio (pERK/total ERK), and to total protein determined per lane using Ponceau-S staining (Sigma-Aldrich, St. Louis, MO, USA). The densitometry data for Ponceau S total protein-stained images were obtained from all proteins visible in each entire lane using Image J (i.e., the entire lane was chosen rather than a single band). These ratios were compared statistically and graphed. Gels and blots were repeated until four different samples per group were assayed.
Statistical analyses
GraphPad PRISM v.7 was used for all statistical analyses. All data are expressed as the mean ± standard error of the mean (SEM). A p-value of <0.05 was considered significant. No differences between FRC+Saline and FRC+95% ethanol/saline were observed, so data from these two groups were combined for statistical comparisons and are termed FRC+Vehicle rats hereafter. Two way ANOVAs were used to assay 1) Grip strength differences between groups with treatment and week as factors; 2&3) forepaw mechanical sensitivity and cold temperature aversion (tested in week 3 only) with treatment and filament, and treatment and temperature, respectively, as factors; and 4) remaining rat data with group and treatment as factors. Tukey multiple comparison tests were used for post hoc assays, and adjusted p values are reported.
Results
HRHF-induced declines in grip strength and sensory declines improved with NK1RA treatment
Since declines in forelimb muscle strength correlated with increasing muscle fibrosis in past studies using this model,Citation19,Citation30 we assayed reflexive grip strength to determine the effectiveness of NK1RA treatment in reducing onset of this motor decline. We observed reduced grip strength in reach limbs of HRHF rats immediately after shaping (HRHF week 0), compared to baseline and FRC+Vehicle rats ()). These declines persisted in 3-week untreated HRHF rats, yet improved toward control levels in HRHF+NK1RA rats ()).
Figure 1. Functional assays in untreated HRHF, HRHF+NK1RA, and food-restricted control (FRC) rats groups treated with vehicle or NK1RA. (a) Reflexive grip strength reported in centiNewton (cN), assayed using a rat grip meter. HRHF rats were tested immediately prior to the 5-week shaping period, immediately post shaping (which is task week 0) and after performing the HRHF task for 3 weeks. FRC rats were tested at similar time points. (b) Mechanical sensitivity tested at the end of task week 3 with nylon monofilaments that bend at the forces indicated in milliNewtons (mN). Number of limb withdrawals out of 10 total probings per filament is reported. (c) Place preference testing for cold temperature sensitivity, performed at the end of task week 3. Time spent on a variable plate cooling from 22 °C to 12 °C, versus total time (300 sec) is presented for FRC and FRC+Vehicle groups combined, HRHF and HRHF+NK1RA rats. Symbols: *: p <0.05, compared to age-matched FRC group (color coded); †: p <0.05, compared to HRHF+NK1RA rats.
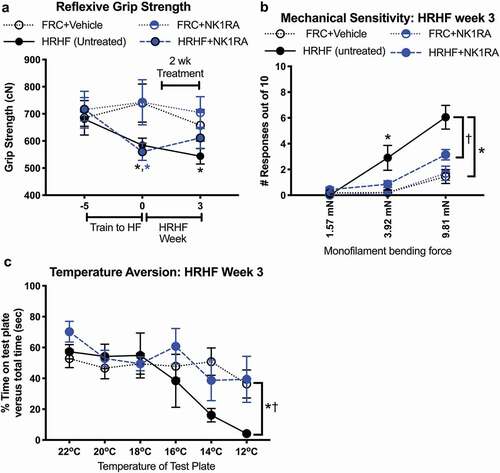
Forepaw sensitivity to mechanical probing was assayed in week 3 using nylon monofilaments and showed increased numbers of responses to 3.92 sized filaments (out of 10 total probings/filament) in reach limbs of untreated HRHF rats, compared to FRC+Vehicle rats, and to 9.81 mN sized filaments, compared to FRC+Vehicle and HRHF+NK1RA rats ()). Avoidance of noxious cold temperatures in a temperature place preference assay was assayed in task week 3 as an indicator of discomfort. Untreated HRHF rats spent less time on the variable test plate when it reached noxious cold temperatures (12 °C), compared to FRC+Vehicle rats (). The NK1RA treatment ameliorated this cold temperature aversion in HRHF+NK1RA, compared to untreated HRHF rats.
HRHF-induced tendinopathies partially reduced by NK1RA treatment
Tendinopathies increase with repetitive overuse and can contribute to grip strength declines.Citation22,Citation54 Therefore, flexor digitorum tendons were examined at the level of the wrist for indices of tendinopathies. HRHF rats showed increased epitendon cellularity and thickness, as well as endotendon cell shape changes (increased rounded cells within the endotendon), compared to FRC+Vehicle and FRC+NK1RA rat tendons (–; a representative tendon image for the FRC+Vehicle only is shown as no differences were observed between the FRC+Vehicle and FRC+NK1RA groups in –). HRHF+NK1RA rats showed reduced epitendon cellularity and thickness, compared to untreated HRHF rats (,,,). Supplemental Figure 1 shows additional examples of tendon changes in response to task and treatment.
Figure 2. Responses of flexor digitorum tendon to task and treatments. (a–d) Quantification of epitendon cellularity, epitendon thickness, endotendon cellularity, and endotendon cell shape, respectively. (e–g) Representative images of hematoxylin-stained tendons, coverslipped with glycerol to reduce tissue shrinking. (h) Substance P immunoreactivity (red fluorescence) in a HRHF rat’s tendon (T), showing immunoreactivity in tenocytes in the endotendon and by many cellular profiles in the epitendon region (arrows). (i) Pgp9.5 immunoreactivity (green), a pan-neuronal marker, in axons in a HRHF rat’s median nerve (N) and in an outer tendon region (T). Substance P immunoreactivity (red fluorescence) is associated with pgp9.5+ axons entering the outer tendon region (red arrows). Symbols: *: p <0.05 and **: p <0.01, compared between groups as shown; n.s. = not significant. Scale bars = 50 µm.
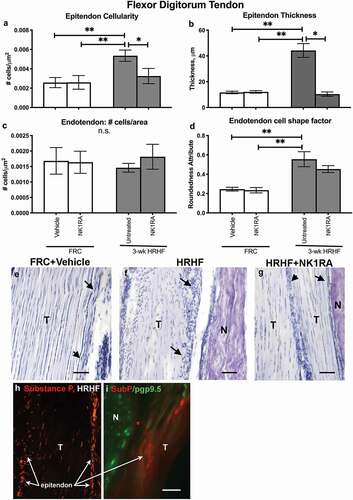
We next examined tendons for potential sources of Substance P, and observed small cells with substance P immunoreactivity in untreated HRHF rat epi- and endotendons (), and in axons (axons showed co-localization with pgp9.5, a pan-neuronal marker) in untreated HRHF rat endotendons ().
In contrast to changes in epitendons of HRHF+NK1RA rats, whole flexor digitorum tendon homogenates showed increased collagen type 1 levels in both HRHF groups, compared to FRC+Vehicle and FRC+NK1RA rats’ tendons (Supplemental Figure 2(a)). Tendon levels of collagen type 3 showed no task or treatment-induced differences (Supplemental Figure 2(b)).
HRHF-induced muscle increases of several muscle fibrogenic proteins reduced by NK1RA treatment
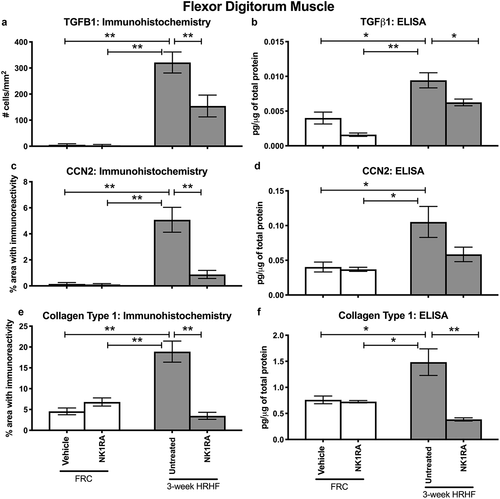
Increased production of TGFβ1, CCN2, and collagen are key features of muscle fibrosisCitation55. Therefore, we examined flexor digitorum muscles for these proteins as this muscle is heavily utilized in performing the operant task. TGFβ1 immunoreactivity was higher in flexor digitorum muscles of untreated HRHF rats, compared to FRC groups and HRHF+NK1RA rats, in small cells around individual myofibers (). CCN2 immunoreactive deposition was higher around myofibers in flexor digitorum muscles of untreated HRHF rats, compared to the FRC and HRHF+NK1RA groups (). Collagen deposition, examined first after Trichrome staining, showed perimyseal increases in untreated HRHF rat muscles, compared to the FRC and HRHF+NK1RA groups (). Lastly, collagen type 1 immunoreactive deposition was higher around myofibers and in the perimyseum of untreated HRHF rat muscles, compared to FRC and HRHF+NK1RA groups (; only the FRC+Vehicle is shown as no differences were observed between the FRC+Vehicle and FRC+NK1RA groups with regard to collagen and other fibrogenic protein expression in muscles, as shown in and ). Quantification of these results confirmed these findings (–), as did ELISA of muscles from reach limbs from additional animals (–).
Figure 3. Representative images of TGFβ1 and CCN2 immunoreactivity, and Masson’s Trichrome staining, in cross-sectionally cut flexor digitorum muscles of FRC+Vehicle, FRC+NK1RA, untreated 3-week HRHF, and 3-week HRHF+NK1RA rats. (a) TGFβ1 immunoreactivity in flexor digitorum muscles. (b) CCN2 immunoreactivity in flexor digitorum muscles. (c) Masson’s Trichrome staining. Inset shows Masson’s Trichrome staining at higher power. Scale bars = 100 µm.
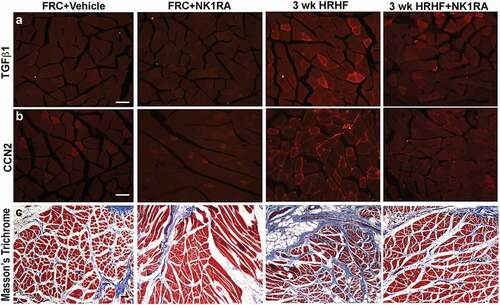
Figure 4. Representative images of Collagen type 1 (green) and Substance P (red) immunoreactivity in cross-sectionally cut flexor digitorum muscles of FRC+Vehicle, untreated 3-week HRHF, and 3-week HRHF+NK1RA rats. DAPI used as a nuclear counterstain. Representative images are shown for: (a) FRC+Vehicle rat; (b) 3 week HRHF rat; and (c) 3 week HRHF + NK1RA rat. A representative image for the FRC+Vehicle group only is shown as no differences were observed between the FRC+Vehicle and FRC+NK1RA groups. Scale bars = 50 µm.
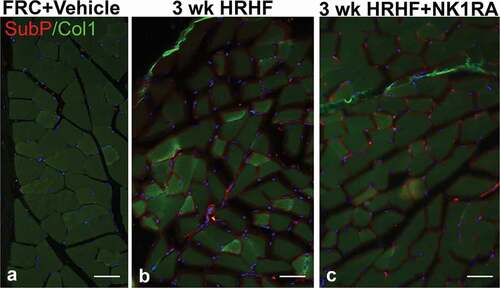
Figure 5. TGFβ1 and CCN2 and Collagen type 1 in flexor digitorum muscles of FRC+Vehicle, FRC+NK1RA, untreated 3-week HRHF, and 3-week HRHF+NK1RA rats, assayed using immunohistochemistry and ELISA. Immunohistochemical quantification was performed in cross-sections through the mid-muscle mass. Whole muscle homogenates were used for the ELISAs. (a) Number of TGFβ1 immunoreactive cells per mm2. (b) TGFβ1 levels assayed using ELISA. (c) CCN2 immunoreactivity, quantified using a thresholded pixel count method. (d) CCN2 levels assayed using ELISA. (e) Collagen type 1 immunostaining, quantified using a thresholded pixel count method. (f) Collagen type 1 levels assayed using ELISA. Symbols: *: p <0.05 and **: p <0.01, compared between groups as shown.
We next examined flexor digitorum muscles for potential sources of Substance P, and found increases in neuronal fascicle-like structures around myofibers and in the perimysium in both untreated HRHF and HRHF+NK1RA rats (,), relative to FRC+Vehicle rat muscles (). A representative image for the FRC+Vehicle group only is shown as no differences were observed between the FRC+Vehicle and FRC+NK1RA groups.
HRHF-induced fibrosis of forepaw’s upper dermis reduced by NK1RA treatment
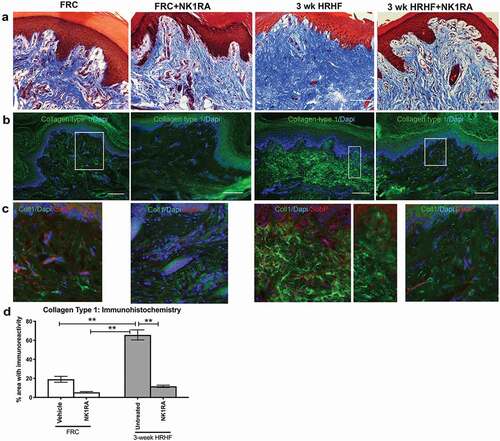
In an effort to examine possible reasons for task-induced increases in forepaw mechanical sensitivity and cold temperature aversion, dermal regions of forepaw tissues were examined using Masson’s Trichrome staining and collagen type 1 immunoreactivity. We observed increased collagen deposition in the upper dermis of pads in untreated 3 week HRHF rats, compared to the FRC and HRHF+NK1RA groups (–). Increased collagen type 1 immunoreactivity was particularly evident in dermal papillae regions of untreated 3 week HRHF rat forepaws that also contained nerves immunoreactivity for Substance P (see red fluorescent spots in dermal papillae regions of middle panels of ,). Quantification of collagen type 1 immunostaining after Masson’s Trichrome staining of forepaw sections showed confirmed these observations ().
Figure 6. Masson’s Trichrome staining and collagen type 1 immunoreactivity in the upper dermis of forepaw pads of groups as shown. (a) Masson’s Trichrome staining, which stains collagen blue. (b) Collagen type 1 immunoexpression (green fluorescence), with DAPI used as a nuclear counterstain. (c) Substance P immunoexpression (red fluorescence), Collagen type 1 immunoexpression (green) and DAPI staining. Boxes in B indicate areas shown enlarged in panels C. Scale bars = 100 µm. (d) Quantification of collagen type 1 immunoexpression in upper dermis. **: p <0.01, compared between groups as shown.
Neither task nor treatment affected myofiber diameters, phospho-ERK or total ERK levels
Mean myofiber diameters did not differ between groups (Supplemental Figure 3). There were no effects of task or treatment on pERK or total ERK levels (Supplemental Figure 4), likely as rats were euthanized and tissues collected at 36 h after the last task session to avoid activity-induced inflammatory cytokine/chemokine changes, and pERK responses occur rapidly (meaning, any potential pERK response was missed due to experimental design).
Discussion
Substance P-NK-1R signaling has been implicated as inducing hypercellularity and collagen production in fibrotic tendinopathies and myositis, and has been proposed as a therapeutic target for their treatment,Citation32,Citation37,Citation38 although to our knowledge, these are first studies of this kind in an animal model of overuse-induced musculoskeletal disorders. We sought here to determine if Substance P—NK-1R signaling contributes to the early progression of sensorimotor declines and fibrogenic responses in multiple tissue types involved in performing a HRHF task using a specific NK1R antagonist (L-732,138) delivered intraperitoneally in the final 2 weeks of a 3-week task. We found that the NK1RA treatment reduced fibrogenic responses in not only tendon tissues but also in involved muscle and dermal tissues, and improved motor declines, mechanical hypersensitivity, and cold temperature aversion induced by the high demand task.
Substance P is a tachykinin involved in nociception, inflammatory processes (such as in myositis and plasma extravasation), tendinosis and postoperative peritoneal adhesion formation.Citation32,Citation34,Citation44,Citation56–Citation58 We have previously shown that Substance P immunoexpression increased transiently in epitendons in vivo (in axons, fibroblasts, and macrophages) in week 3 of a HRHF task, and then again in task week 12, compared to control rats.Citation22 This was a task-dependent finding as no increases were observed in rats performing a low repetition negligible force task for up to 12 weeks.Citation22 NK1R antagonists have been used to block cardiac fibrosis, fibrogenic factors in colitis-induced fibrosis, intraperitoneal adhesions post surgery, and TGFβ1 production by lung epithelial cells in lung fibrosis.Citation43,Citation45,Citation46,Citation48,Citation50,Citation59–Citation61 We show here that systemic treatment of rats with the NK1RA, L-732,138, also reduces fibrogenic-related proteins induced in multiple tissue types (muscles, tendons, and nerves) impacted by the performance of a high demand repetitive task.
The sensorimotor behavioral declines in untreated HRHF rats suggest that the rats have discomfort and/or reduced tissue function. The improved reflexive grip strength with NK1RA treatment could be due to reduced collagen deposition in epitendons and around myofibers, since increased muscle fibrosis can decrease skeletal muscle strength.Citation30,Citation62 The improved forepaw mechanical sensitivity may be the result of both HRHF-induced musculotendinous fibrogenic changes and dermal nerve irritation, since this is a limb withdrawal assay that requires full function of muscles, tendons, and nerves. NK1RA treatment also reduced HRHF task rats’ aversion to noxious cold temperatures in temperature place preference testing. Cold hypersensitivity is thought to be a clear sign of peripheral neuropathy.Citation63,Citation64 We hypothesize that the increased collagen deposition in upper dermal regions of forepaw pads of HRHF rats is compressing nerves in that region, enhancing injury-induced release of Substance P from dermal nerve terminals. This involvement of fibrosis is supported by recent studies from our lab also examining 3 week HRHF rats showed improved sensorimotor function after modeled manual therapy that reduced collagenous constraints on neural tissuesCitation24,Citation27 and use of an anti-CCN2 agent that reduced collagen and other fibrogenic protein expression,Citation26and that miniscalpel-needle treatment effectively reduces discomfort (and inflammation) in subjects with overuse musculoskeletal disorders.Citation65 The authors of the latter study suggest that the miniscalpel-needle treatment suggest it may detach taut bands, thereby relaxing compressed nerves and vessels and improving microcirculation similar to microinvasive surgery methods.Citation65,Citation66 Blocking NK1R signaling may also promote anti-nociception in rodents, as shown in studies in which NK1R antagonists reduce visceral hyperalgesia and acid-induced muscle pain.Citation67,Citation68 Analgesic properties of NK1RA may not be relevant in humans as several clinical trials have shown they are ineffective at reducing pain [reviewed in,Citation69] although three trials showed mild to moderate relief of postoperative pain using CP-99,994 (weaker than ibuprofen),Citation70 gynecological abdominal surgery using aprepitant,Citation71 or lower limb surgery using aprepitant.Citation72 Although still attractive as potential tools for pain management in combination with other drugs,Citation69 NK1R antagonists have shown success as antiemetics in humans.Citation49
The 3 week HRHF task increased epitendon cellularity, thickness, and hypercellularity in untreated HRHF rats, enhanced the rounding of endotendon cells, changes normalized by the 2-week NK1RA treatment toward or to control levels. A clear role of Substance P in the development of other overuse-induced tendinosis-like responses was established in past studies using exogenous injections of either Substance P or NaCl into Achilles tendons, specifically showing enhanced vascular density and inflammation.Citation32 Substance P levels and immunoexpression increase in Achilles tendons of rats subjected to overuse exercise, as does NK1R immunoreactivity in blood vessel walls.Citation34 Their findings encouraged us to test a systemic NK1RA in our model of overuse activity (that is, a high repetitive high force work task), the results of which are promising. We did not observe a full reduction of epitendon cellularity to control levels. Such a decline may require rest as well so as to reduce mechanical loading induced increases in TGFβ1, since others have shown that TGFβ1 is a co-mediator of Substance P signaling induced proliferation in cultured tenocytes undergoing mechanical loading.Citation73
TGFβ1 levels increase in flexor digitorum muscles of rats performing this HRHF task immediately post shaping (task week 0) and remain increased with continued performance of the HRHF task for up to 18 weeks.Citation19,Citation23 In this current study, numbers of cells immunopositive for TGFβ1 increased in muscles of untreated 3-week HRHF rats, compared to controls, as did ELISA detected levels of TGFβ1. TGFβ1 immunoreactivity and levels did not reduce fully to control levels in HRHF+NK1RA rats, suggesting that as in tendons, that the repeated mechanical movement increases TGFβ1 production as shown previously.Citation74 The reductions in CCN2 immunoreactivity and collagen type 1 immunoreactivity and levels in response to the 2-week NK1RA treatment also support a role for substance P signaling in their production in muscles. Increased Substance P immunoreactivity in nerve-like fascicles did not decrease in the flexor digitorum muscles suggesting that sprouts did not decline in density. Perhaps Substance P-NK1R signaling was impaired by the antagonist, reducing collagen production and perhaps even nociception, but not intramuscular axonal sprouting. The increased Substance P labeled axonal processes in the muscle are further supported by studies showing increased sprouting of Substance P labeled axons in skeletal muscles following injury.Citation75,Citation76
The mean diameter of the myofibers did not decline in the 3-week HRHF rats relative to controls, indicating that myofiber atrophy did not contribute to the grip strength declines. In a longer study examining flexor digitorum muscles of rats performing this same task for 12 weeks, an increase in the overall cross-sectional area of the muscle was observed,Citation20 suggestive of muscle growth and some adaptation to the task (although inflammatory responses increased and grip strength was lower than in controls, and by task week 18, muscle tears were evident.Citation23) These findings are in contrast to those in a recent study by Fujiwara et al. showing myofiber atrophy in rats performing a negligible force, food pellet retrieval task.Citation77 Their study showed strong evidence of autophagy-related responses to that task. We have also examined the effects of performing a similar negligible force food pellet retrieval for 6 weeks, and observed greater macrophage influx, pro-inflammatory cytokine, and matrix metalloproteinase increases than in studies examining HRHF rats for up to 6 weeks,Citation17,Citation19,Citation78 yet fewer indices of musculotendinous fibrosis. When considering Fujiwara and our past and current results in combination, we suggest that precise hand pinching activities needed for the food pellet retrieval task affects the tissues differently than this isometric lever bar pulling task.
We have previously shown that in primary tenocyte cultures that Substance P stimulates collagen type 1 production independently from TGFβ1 in a time-dependent manner. Specifically, the independent signaling was observed only in the first 24 h of stimulation; thereafter, by 48 h, Substance P stimulation of collagen type 1 production was blocked by a TGFβ1 receptor inhibitor, indicating signaling though the TGFβ1 pathway at the later time point.Citation79 The downregulation of TGFβ1 in flexor digitorum muscles after in vivo NK1RAtreatment may be suggestive of a similar independent phase, although co-mediation of Substance P signaling with TGFβ1 signaling should not be ruled out.Citation80 Our upper dermal findings also suggest an early role for Substance P-NK1R signaling in the development of dermal fibrosis.
There are several limitations in this study. The numbers of animals in the NK1RA group assessed using ELISA were low (n = 4). Two animals were lost in this subgroup due to unforeseen circumstances. However, we believe that the higher numbers (n = 7 HRHF+NK1RA reach limbs) used for the histomorphological and immunohistochemical results fill this gap. Therefore, the ELISA data for HRHF+NK1RA rats should be considered as confirmatory only. This study only assessed the early effects of the NK1RA. Long-term reversal studies for persistent fibrosis have also been performed in 12-week HRHF rats and also show reductions in collagen expression following NK1RA treatment.Citation51 Lastly, this is both a rat study and only females were studied; results should be considered in that light.
These data support a role of Substance P in the development of overuse-induced fibrosis, and that blocking its signaling with a NK1RA successfully reduces several musculotendinous and dermal fibrogenic responses occurring as a consequence of repetitive high force lever pulling tasks. As we also have evidence that reduction of musculoskeletal and neural fibrosis relieves strain and compression on tissues that is contributing to sensorimotor declines, it is our hope that similar treatment will be useful in reducing fibrotic changes in humans with painful overuse-induced injuries since novel treatments are needed for clinical management of persistent pain and motor dysfunction occurring with chronic musculoskeletal disorders. That said, it remains to be determined if NK1RA can reduce fibrogenic changes in humans.
Supplemental_Fig_4_ERK_in_NK1RA.tif
Download TIFF Image (1.8 MB)Supplemental_Fig_3_Myocyte_diameter.tif
Download TIFF Image (62.7 KB)Supplemental_Fig_2_tendon_ELISA_June_2019.tif
Download TIFF Image (520.3 KB)Supplemental_Fig_1_tendon_H_E_June_2019.tif
Download TIFF Image (2.4 MB)Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR056019, and by American Society of Bone and Mineral Research Gap Award 1025. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Hauret KG, Jones BH, Bullock SH, Canham-Chervak M, Canada S. Musculoskeletal injuries description of an under-recognized injury problem among military personnel. Am J Prev Med. 2010;38(1 Suppl):S61–70. Epub 2010/02/13. PubMed PMID: 20117601. doi:https://doi.org/10.1016/j.amepre.2009.10.021.
- Occhionero V, Korpinen L, Gobba F. Upper limb musculoskeletal disorders in healthcare personnel. Ergonomics. 2014;57(8):1166–1191. PubMed PMID: 24840049. doi:https://doi.org/10.1080/00140139.2014.917205.
- World Health Organization. Gender, equity and human rights: women on the move: migration, care work and health [pdf]. Geneva: World Health Organization; 2018 [cited 2018 November 21]. Available from: http://www.who.int/gender-equity-rights/en/.
- Harris-Adamson C, Eisen EA, Kapellusch J, Garg A, Hegmann KT, Thiese MS, Dale AM, Evanoff B, Burt S, Bao S, Silverstein B. Biomechanical risk factors for carpal tunnel syndrome: a pooled study of 2474 workers. Occup Environ Med;2014. Epub 2014/ 10/18. PubMed PMID: 25324489. doi:https://doi.org/10.1136/oemed-2014-102378.
- Gallagher S, Heberger JR. Examining the interaction of force and repetition on musculoskeletal disorder risk: a systematic literature review. Hum Factors. 2013;55(1):108–124. Epub 2013/ 03/23. PubMed PMID: 23516797. doi:https://doi.org/10.1177/0018720812449648.
- Gupta AA, Mhaske SA, Ahmad MA, Yuwanati MB, Prabhu S, Pardhe N. Ergonomic microscope: need of the hour. J Clin Diagn Res. 2015;9(5):ZC62–5. Epub 2015/ 07/15. PubMed PMID: 26155565; PubMed Central PMCID: PMCPMC4484157. doi:https://doi.org/10.7860/JCDR/2015/11742.5952.
- Bureau of Labor Statistics. Number, median days away from work, and incidence rate for nonfatal occupational injuries and illnesses involving days away from work by ownership, industry, musculoskeletal disorders, and event or exposure, 2015. 2016 [cited 2018 October 15]. Available from: www.bls.gov/news.release/osh2.t01.htm.
- Piligian G, Herbert R, Hearns M, Dropkin J, Landsbergis P, Cherniack M. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75–93. Epub 1999/ 11/26. PubMed PMID: 10573598.
- Gold JE, Hallman DM, Hellstrom F, Bjorklund M, Crenshaw AG, Djupsjobacka M, Heiden M, Mathiassen SE, Piligian G, Barbe MF. Systematic review of biochemical biomarkers for neck and upper-extremity musculoskeletal disorders. Scand J Work Environ Health. 2016;42(2):103–124. PubMed PMID: 26599377. doi:https://doi.org/10.5271/sjweh.3533.
- Dennett X, Fry HJ. Overuse syndrome: a muscle biopsy study. Lancet. 1988;1(8591):905–908. PubMed PMID: 2895831. doi:https://doi.org/10.1016/s0140-6736(88)91714-x.
- Lluch AL. Thickening of the synovium of the digital flexor tendons: cause or consequence of the carpal tunnel syndrome? J Hand Surg Br. 1992;17(2):209–212. Epub 1992/ 04/01. PubMed PMID: 1588206.
- Bureau of Labor Statistics. Nonfatal occupational injuries and illnesses requiring daya away from work. 2014 [cited 2018October 15]. Available from: https://www.bls.gov/news.release/archives/osh2_11192015.pdf.
- Stephens AS, Stephens SR, Morrison NA. Internal control genes for quantitative RT-PCR expression analysis in mouse osteoblasts, osteoclasts and macrophages. BMC Res Notes. 2011;4:410. Epub 2011/10/15. PubMed PMID: 21996334; PubMed Central PMCID: PMC3204251. doi:https://doi.org/10.1186/1756-0500-4-410.
- van der Beek AJ, Dennerlein JT, Huysmans MA, Mathiassen SE, Burdorf A, van Mechelen W, van Dieën JJ, Frings-Dresen MH, Holtermann AA, Janwantanakul PP, Van Der Molen HF. A research framework for the development and implementation of interventions preventing work-related musculoskeletal disorders. Scand J Work Environ Health. 2017;43(6):526–539. PubMed PMID: 28945263. doi:https://doi.org/10.5271/sjweh.3671.
- Sayegh ET, Sandy JD, Virk MS, Romeo AA, Wysocki RW, Galante JO, Trella KJ, Plaas A, Wang VM. Recent scientific advances towards the development of tendon healing strategies. Curr Tissue Eng. 2015;4(2):128–143. Epub 2016/ 01/12. PubMed PMID: 26753125; PubMed Central PMCID: PMCPMC4706369. doi:https://doi.org/10.2174/2211542004666150713190231.
- Barbe MF, Gallagher S, Massicotte VS, Tytell M, Popoff SN, Barr-Gillespie AE. The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet Disord. 2013;14(1):303. Epub 2013/10/26. PubMed PMID: 24156755. doi:https://doi.org/10.1186/1471-2474-14-303.
- Barbe MF, Elliott MB, Abdelmagid SM, Amin M, Popoff SN, Safadi FF, Barr AE. Serum and tissue cytokines and chemokines increase with repetitive upper extremity tasks. J Orthop Res. 2008;26(10):1320–1326. Epub 2008/05/09. PubMed PMID: 18464247. doi:https://doi.org/10.1002/jor.20674.
- Matute Wilander A, Karedal M, Axmon A, Nordander C. Inflammatory biomarkers in serum in subjects with and without work related neck/shoulder complaints. BMC Musculoskelet Disord. 2014;15:103. Epub 2014/03/29. PubMed PMID: 24669872; PubMed Central PMCID: PMC3973377. doi:https://doi.org/10.1186/1471-2474-15-103.
- Abdelmagid SM, Barr AE, Rico M, Amin M, Litvin J, Popoff SN, Safadi FF, Barbe MF. Performance of repetitive tasks induces decreased grip strength and increased fibrogenic proteins in skeletal muscle: role of force and inflammation. PLoS One. 2012;7(5):e38359. Epub 2012/ 06/08. PubMed PMID: 22675458; PubMed Central PMCID: PMC3364991. doi:https://doi.org/10.1371/journal.pone.0038359.
- Jain NX, Barr-Gillespie AE, Clark BD, Kietrys DM, Wade CK, Litvin J, Popoff SN, Barbe MF. Bone loss from high repetitive high force loading is prevented by ibuprofen treatment. J Musculoskelet Neuronal Interact. 2014;14(1):78–94. PubMed PMID: 24583543; PubMed Central PMCID: PMCPMC4067254.
- Rani S, Barbe MF, Barr AE, Litivn J. Role of TNF alpha and PLF in bone remodeling in a rat model of repetitive reaching and grasping. J Cell Physiol. 2010;225(1):152–167. Epub 2010/05/12. PubMed PMID: 20458732. doi:https://doi.org/10.1002/jcp.22208.
- Fedorczyk JM, Barr AE, Rani S, Gao HG, Amin M, Amin S, Litvin J, Barbe MF. Exposure-dependent increases in IL-1beta, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J Orthop Res. 2010;28(3):298–307. Epub 2009/ 09/11. PubMed PMID: 19743505; PubMed Central PMCID: PMC2807907. doi:https://doi.org/10.1002/jor.20984.
- Fisher PW, Zhao Y, Rico MC, Massicotte VS, Wade CK, Litvin J, Bove GM, Popoff SN, Barbe MF. Increased CCN2, substance P and tissue fibrosis are associated with sensorimotor declines in a rat model of repetitive overuse injury. J Cell Commun Signal. 2015:1–18. doi:https://doi.org/10.1007/s12079-015-0263-0.
- Bove GM, Harris MY, Zhao H, Barbe MF. Manual therapy as an effective treatment for fibrosis in a rat model of upper extremity overuse injury. J Neurol Sci. 2016;361:168–180. PubMed PMID: 26810536; PubMed Central PMCID: PMCPMC4729290. doi:https://doi.org/10.1016/j.jns.2015.12.029.
- Rani S, Barbe MF, Barr AE, Litvin J. Induction of periostin-like factor and periostin in forearm muscle, tendon, and nerve in an animal model of work-related musculoskeletal disorder. J Histochem Cytochem. 2009;57(11):1061–1073. Epub 2009/07/22. PubMed PMID: 19620321; PubMed Central PMCID: PMC2762884. doi:https://doi.org/10.1369/jhc.2009.954081.
- Barbe MF, Hilliard BA, Delany SP, Iannarone VJ, Harris MY, Amin M, Cruz GE, Barreto‐Cruz Y, Tran N, Day E, Hobson L. Blocking CCN2 reduces progression of sensorimotor declines and fibrosis in a rat model of chronic repetitive overuse. J Orthop Res;2019. Epub 2019/05/02. PubMed PMID: 31041999. doi:https://doi.org/10.1002/jor.24337.
- Bove GM, Delany SP, Hobson L, Cruz GE, Harris MY, Amin M, Chapelle SL, Barbe MF. Manual therapy prevents onset of nociceptor activity, sensorimotor dysfunction, and neural fibrosis induced by a volitional repetitive task. Pain;2018. Epub 2018/ 11/22. PubMed PMID: 30461558. doi:https://doi.org/10.1097/j.pain.0000000000001443.
- Bove GM, Delany SP, Hobson L, Cruz GE, Harris MY, Amin M, Chapelle SL, Barbe MF. Manual therapy prevents onset of nociceptor activity, sensorimotor dysfunction, and neural fibrosis induced by a volitional repetitive task. Pain. 2019;160(3):632–644. Epub 2018/ 11/22. PubMed PMID: 30461558; PubMed Central PMCID: PMCPMC6377318. doi:https://doi.org/10.1097/j.pain.0000000000001443.
- Driscoll M, Blyum L. The presence of physiological stress shielding in the degenerative cycle of musculoskeletal disorders. J Bodyw Mov Ther. 2011;15(3):335–342. PubMed PMID: 21665110. doi:https://doi.org/10.1016/j.jbmt.2010.05.002.
- Stauber WT, Knack KK, Miller GR, Grimmett JG. Fibrosis and intercellular collagen connections from four weeks of muscle strains. Muscle Nerve. 1996;19(4):423–430. Epub 1996/04/01. PubMed PMID: 8622719. doi:https://doi.org/10.1002/mus.880190402.
- Zugel M, Maganaris CN, Wilke J, Jurkat-Rott K, Klingler W, Wearing SC, Findley T, Barbe MF, Steinacker JM, Vleeming A, Bloch W. Fascial tissue research in sports medicine: from molecules to tissue adaptation, injury and diagnostics. Br J Sports Med. 2018. PubMed PMID: 30072398. doi:https://doi.org/10.1136/bjsports-2018-099308.
- Andersson G, Backman LJ, Scott A, Lorentzon R, Forsgren S, Danielson P. Substance P accelerates hypercellularity and angiogenesis in tendon tissue and enhances paratendinitis in response to Achilles tendon overuse in a tendinopathy model. Br J Sports Med. 2011;45(13):1017–1022. Epub 2011/05/05. PubMed PMID: 21540192. doi:https://doi.org/10.1136/bjsm.2010.082750.
- Andersson G, Danielson P, Alfredson H, Forsgren S. Presence of substance P and the neurokinin-1 receptor in tenocytes of the human Achilles tendon. Regul Pept. 2008;150(1–3):81–87. Epub 2008/04/09. PubMed PMID: 18394729. doi:https://doi.org/10.1016/j.regpep.2008.02.005.
- Backman LJ, Andersson G, Wennstig G, Forsgren S, Danielson P. Endogenous substance P production in the Achilles tendon increases with loading in an in vivo model of tendinopathy-peptidergic elevation preceding tendinosis-like tissue changes. J Musculoskelet Neuronal Interact. 2011;11(2):133–140. Epub 2011/06/01. PubMed PMID: 21625050.
- Song Y, Stal PS, Yu J, Forsgren S. Marked effects of tachykinin in myositis both in the experimental side and contralaterally: studies on NK-1 receptor expressions in an animal model. ISRN Inflamm. 2013;2013:907821. Epub 2013/ 09/21. PubMed PMID: 24049666; PubMed Central PMCID: PMC3765760. doi:https://doi.org/10.1155/2013/907821.
- Elliott MB, Barr AE, Clark BD, Wade CK, Barbe MF. Performance of a repetitive task by aged rats leads to median neuropathy and spinal cord inflammation with associated sensorimotor declines. Neuroscience. 2010;170(3):929–941. Epub 2010/08/03. PubMed PMID: 20673790. doi:https://doi.org/10.1016/j.neuroscience.2010.07.041.
- Song Y, Stal PS, Yu JG, Lorentzon R, Backman C, Forsgren S. Inhibitors of endopeptidase and angiotensin-converting enzyme lead to an amplification of the morphological changes and an upregulation of the substance P system in a muscle overuse model. BMC Musculoskelet Disord. 2014;15:126. Epub 2014/04/15. PubMed PMID: 24725470; PubMed Central PMCID: PMC3992129. doi:https://doi.org/10.1186/1471-2474-15-126.
- Han SH, Choi W, Song J, Kim J, Lee S, Choi Y, Byun SE, Ahn T, Ahn H, Ding C, Baik L. The implication of substance P in the development of tendinopathy: a case control study. Int J Mol Sci. 2017;18(6). Epub 2017/ 06/10. PubMed PMID: 28598390; PubMed Central PMCID: PMCPMC5486064. doi:https://doi.org/10.3390/ijms18061241.
- Cote JN. A critical review on physical factors and functional characteristics that may explain a sex/gender difference in work-related neck/shoulder disorders. Ergonomics. 2012;55(2):173–182. Epub 2011/ 08/19. PubMed PMID: 21846285. doi:https://doi.org/10.1080/00140139.2011.586061.
- World Health Organization. Gender, health and work [pdf]. Geneva: World Health Organization; 2018 [cited 2018 November 21]. Available from: http://www.who.int/occupational_health/topics/gender/en/.
- Barbe MF, Massicotte VS, Assari S, Monroy MA, Frara N, Harris MY, Amin M, King T, Cruz GE, Popoff SN. Prolonged high force high repetition pulling induces osteocyte apoptosis and trabecular bone loss in distal radius, while low force high repetition pulling induces bone anabolism. Bone. 2018;110:267–283. PubMed PMID: 29476978. doi:https://doi.org/10.1016/j.bone.2018.02.014.
- Melendez GC, Li J, Law BA, Janicki JS, Supowit SC, Levick SP. Substance P induces adverse myocardial remodelling via a mechanism involving cardiac mast cells. Cardiovasc Res. 2011;92(3):420–429. PubMed PMID: 21908647; PubMed Central PMCID: PMC3211974. doi:https://doi.org/10.1093/cvr/cvr244.
- Dehlin HM, Manteufel EJ, Monroe AL, Reimer MH Jr., Levick SP. Substance P acting via the neurokinin-1 receptor regulates adverse myocardial remodeling in a rat model of hypertension. Int J Cardiol. 2013;168(5):4643–4651. Epub 2013/08/22. PubMed PMID: 23962787; PubMed Central PMCID: PMCPMC4043399. doi:https://doi.org/10.1016/j.ijcard.2013.07.190.
- Saunders CJ, Christensen M, Finger TE, Tizzano M. Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc Natl Acad Sci U S A. 2014;111(16):6075–6080. Epub 2014/04/09. PubMed PMID: 24711432; PubMed Central PMCID: PMCPMC4000837. doi:https://doi.org/10.1073/pnas.1402251111.
- Cohen PA, Aarons CB, Gower AC, Stucchi AF, Leeman SE, Becker JM, Reed KL. The effectiveness of a single intraperitoneal infusion of a neurokinin-1 receptor antagonist in reducing postoperative adhesion formation is time dependent. Surgery. 2007;141(3):368–375. Epub 2007/03/14. PubMed PMID: 17349849. doi:https://doi.org/10.1016/j.surg.2006.09.007.
- Cohen PA, Gower AC, Stucchi AF, Leeman SE, Becker JM, Reed KL. A neurokinin-1 receptor antagonist that reduces intraabdominal adhesion formation increases peritoneal matrix metalloproteinase activity. Wound Repair Regener. 2007;15(6):800–808. Epub 2007/ 11/22. PubMed PMID: 18028127. doi:https://doi.org/10.1111/j.1524-475X.2007.00291.x.
- Guo TZ, Wei T, Li WW, Li XQ, Clark JD, Kingery WS. Immobilization contributes to exaggerated neuropeptide signaling, inflammatory changes, and nociceptive sensitization after fracture in rats. J Pain. 2014;15(10):1033–1045. Epub 2014/ 07/27. PubMed PMID: 25063543; PubMed Central PMCID: PMCPMC4182120. doi:https://doi.org/10.1016/j.jpain.2014.07.004.
- Reed KL, Stucchi AF, Leeman SE, Becker JM. Inhibitory effects of a neurokinin-1 receptor antagonist on postoperative peritoneal adhesion formation. Ann N Y Acad Sci. 2008;1144:116–126. Epub 2008/ 12/17. PubMed PMID: 19076371. doi:https://doi.org/10.1196/annals.1418.010.
- Ibrahim MA, Preuss CV. Antiemetic neurokinin-1 receptor blockers. Treasure Island (FL): StatPearls; 2019.
- Esposito AJ, Heydrick SJ, Cassidy MR, Gallant J, Stucchi AF, Becker JM. Substance P is an early mediator of peritoneal fibrinolytic pathway genes and promotes intra-abdominal adhesion formation. J Surg Res. 2013;181(1):25–31. Epub 2012/ 07/07. PubMed PMID: 22765994. doi:https://doi.org/10.1016/j.jss.2012.05.056.
- Barbe MF, White AR, Hilliard BA, Salvadeo DM, Amin M, Harris MY, GE Cruz, L Hobson, SN Popoff. Comparing effects of rest with or without a NK1RA on fibrosis and sensorimotor declines induced by a voluntary moderate demand task. J Musculoskeletal Neuronal Interact. 2019. In Press. Available from: http://www.ismni.org/jmni/accepted/JMNI_19M-01-008.pdf
- Xin DL, Hadrevi J, Elliott ME, Amin M, Harris MY, Barr-Gillespie AE, Barbe MF. Effectiveness of conservative interventions for sickness and pain behaviors induced by a high repetition high force upper extremity task. BMC Neurosci. 2017;18(1):36. PubMed PMID: 28356066; PubMed Central PMCID: PMCPMC5371184. doi:https://doi.org/10.1186/s12868-017-0354-3.
- Al-Shatti T, Barr AE, Safadi FF, Amin M, Barbe MF. Increase in inflammatory cytokines in median nerves in a rat model of repetitive motion injury. J Neuroimmunol. 2005;167(1–2):13–22. Epub 2005/07/20. PubMed PMID: 16026858; PubMed Central PMCID: PMC1552098. doi:https://doi.org/10.1016/j.jneuroim.2005.06.013.
- Ettema AM, Amadio PC, Zhao C, Wold LE, An KN. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86–A(7):1458–1466. Epub 2004/07/15. PubMed PMID: 15252093. doi:https://doi.org/10.2106/00004623-200407000-00014.
- Uchio K, Graham M, Dean NM, Rosenbaum J, Desmouliere A. Down-regulation of connective tissue growth factor and type I collagen mRNA expression by connective tissue growth factor antisense oligonucleotide during experimental liver fibrosis. Wound Repair Regener. 2004;12(1):60–66. PubMed PMID: 14974966. doi:https://doi.org/10.1111/j.1067-1927.2004.012112.x.
- Reed KL, Stucchi AF, Becker JM. Pharmacologic inhibition of adhesion formation and peritoneal tissue-type plasminogen activator activity. Semin Reprod Med. 2008;26(4):331–340. Epub 2008/08/30. PubMed PMID: 18756410. doi:https://doi.org/10.1055/s-0028-1082391.
- Fong G, Backman LJ, Hart DA, Danielson P, McCormack B, Scott A. Substance P enhances collagen remodeling and MMP-3 expression by human tenocytes. J Orthop Res. 2013; 31(1):91–98. doi:https://doi.org/10.1002/jor.22191.
- Butler DSC, Ambite I, Nagy K, Cafaro C, Ahmed A, Nadeem A, Filenko N, Tran TH, Andersson K-E, Wullt B, Puthia M, Svanborg C. Neuroepithelial control of mucosal inflammation in acute cystitis. Sci Rep. 2018;8(1):11015. Epub 2018/07/22. PubMed PMID: 30030504; PubMed Central PMCID: PMCPMC6054610. doi:https://doi.org/10.1038/s41598-018-28634-0.
- Koon HW, Shih D, Karagiannides I, Zhao D, Fazelbhoy Z, Hing T, Xu H, Lu B, Gerard N, Pothoulakis C. Substance P modulates colitis-associated fibrosis. Am J Pathol. 2010;177(5):2300–2309. PubMed PMID: 20889569; PubMed Central PMCID: PMC2966789. doi:https://doi.org/10.2353/ajpath.2010.100314.
- Yaraee R, Ghazanfari T. Substance P potentiates TGFbeta-1 production in lung epithelial cell lines. Iran J Allergy Asthma Immunol. 2009;8(1):19–24. PubMed PMID: 19279355.
- Lim R, Morrill JM, Prushik SG, Reed KL, Gower AC, Leeman SE, Stucchi AF, Becker JM. An FDA approved neurokinin-1 receptor antagonist is effective in reducing intraabdominal adhesions when administered intraperitoneally, but not orally. J Gastrointest Surg. 2008;12(10):1754–1761. Epub 2008/08/19. PubMed PMID: 18709513. doi:https://doi.org/10.1007/s11605-008-0634-4.
- Cutlip RG, Baker BA, Hollander M, Ensey J. Injury and adaptive mechanisms in skeletal muscle. J Electromyogr Kinesiol. 2009;19(3):358–372. Epub 2008/ 09/05. PubMed PMID: 18768331. doi:https://doi.org/10.1016/j.jelekin.2008.06.007.
- Mishra SK, Hoon MA. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci. 2010;43(1):157–163. PubMed PMID: 19853036; PubMed Central PMCID: PMC2818468. doi:https://doi.org/10.1016/j.mcn.2009.10.006.
- Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. Embo J. 2009;28(9):1308–1318. PubMed PMID: 19279663; PubMed Central PMCID: PMC2683043. doi:https://doi.org/10.1038/emboj.2009.57.
- Li S, Shen T, Liang Y, Bai B, Zhang Y. Miniscalpel-needle treatment is effective for work-related neck and shoulder musculoskeletal disorders. Evid Based Complement Alternat Med. 2016;2016:5760240. Epub 2016/07/07. PubMed PMID: 27382406; PubMed Central PMCID: PMCPMC4921642. doi:https://doi.org/10.1155/2016/5760240.
- Zhang Y, Quan WC, Yin P, Li H, Jin F, Zeng GG, Guo CQ. Analysis on indications and dominant diseases of acupotomology. Zhongguo Zhen Jiu. 2010;30(6):525–528. Epub 2010/06/29. PubMed PMID: 20578397.
- Lin CC, Chen WN, Chen CJ, Lin YW, Zimmer A, Chen CC. An antinociceptive role for substance P in acid-induced chronic muscle pain. Proc Natl Acad Sci U S A. 2012;109(2):E76–83. Epub 2011/ 11/16. PubMed PMID: 22084095; PubMed Central PMCID: PMCPMC3258641. doi:https://doi.org/10.1073/pnas.1108903108.
- Kamp EH, Beck DR, Gebhart GF. Combinations of neurokinin receptor antagonists reduce visceral hyperalgesia. J Pharmacol Exp Ther. 2001;299(1):105–113. Epub 2001/09/19. PubMed PMID: 11561069.
- Kleczkowska P, Nowicka K, Bujalska-Zadrozny M, Hermans E. Neurokinin-1 receptor-based bivalent drugs in pain management: the journey to nowhere? Pharmacol Ther. 2018. Epub 2018/11/24. PubMed PMID: 30468743. doi:https://doi.org/10.1016/j.pharmthera.2018.11.007.
- Dionne RA, Max MB, Gordon SM, Parada S, Sang C, Gracely RH, Sethna NF, MacLean DB. The substance P receptor antagonist CP-99,994 reduces acute postoperative pain. Clin Pharmacol Ther. 1998;64(5):562–568. Epub 1998/12/02. PubMed PMID: 9834049. doi:https://doi.org/10.1016/S0009-9236(98)90140-0.
- Soga T, Kume K, Kakuta N, Hamaguchi E, Tsutsumi R, Kawanishi R, Fukuta K, Tanaka K, Tsutsumi YM. Fosaprepitant versus ondansetron for the prevention of postoperative nausea and vomiting in patients who undergo gynecologic abdominal surgery with patient-controlled epidural analgesia: a prospective, randomized, double-blind study. J Anesth. 2015;29(5):696–701. Epub 2015/03/25. PubMed PMID: 25801542. doi:https://doi.org/10.1007/s00540-015-2006-z.
- Kakuta N, Kume K, Hamaguchi E, Tsutsumi R, Mita N, Tanaka K, Tsutsumi YM. The effects of intravenous fosaprepitant and ondansetron in the prevention of postoperative nausea and vomiting in patients who underwent lower limb surgery: a prospective, randomized, double-blind study. J Anesth. 2015;29(6):836–841. Epub 2015/07/27. PubMed PMID: 26210166. doi:https://doi.org/10.1007/s00540-015-2054-4.
- Backman LJ, Fong G, Andersson G, Scott A, Danielson P. Substance P is a mechanoresponsive, autocrine regulator of human tenocyte proliferation. PLoS One. 2011;6(11):e27209. PubMed PMID: 22069500; PubMed Central PMCID: PMC3206074. doi:https://doi.org/10.1371/journal.pone.0027209.
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84(2):649–698. Epub 2004/03/27. PubMed PMID: 15044685. doi:https://doi.org/10.1152/physrev.00031.2003.
- Saxler G, Brankamp J, von Knoch M, Loer F, Hilken G, Hanesch U. The density of nociceptive SP- and CGRP-immunopositive nerve fibers in the dura mater lumbalis of rats is enhanced after laminectomy, even after application of autologous fat grafts. Eur Spine J. 2008;17(10):1362–1372. Epub 2008/08/16. PubMed PMID: 18704516; PubMed Central PMCID: PMCPMC2556465. doi:https://doi.org/10.1007/s00586-008-0741-7.
- Mense S. Functional anatomy of muscle: muscle, nociceptors and afferent fibers. In: SMaRDG, editor. Muscle pain: understanding the mechanisms. Berlin Heidelberg: Springer-Verlag; 2010. p. 17–48.
- Fujiwara M, Iwata M, Inoue T, Aizawa Y, Yoshito N, Hayashi K, Suzuki S. Decreased grip strength, muscle pain, and atrophy occur in rats following long-term exposure to excessive repetitive motion. FEBS Open Bio. 2017;11(7):1737–1749. Epub 2017/11/11. PubMed PMID: 29123982; PubMed Central PMCID: PMCPMC5666401. doi:https://doi.org/10.1002/2211-5463.12315.
- Frara N, Abdelmagid SM, Tytell M, Amin M, Popoff SN, Safadi FF, Barbe MF. Growth and repair factors, osteoactivin, matrix metalloproteinase and heat shock protein 72, increase with resolution of inflammation in musculotendinous tissues in a rat model of repetitive grasping. BMC Musculoskelet Disord. 2016;17:34. PubMed PMID: 26781840; PubMed Central PMCID: PMCPMC4717665. DOI: https://doi.org/10.1186/s12891-016-0892-3.
- Frara N, Fisher PW, Zhao Y, Tarr JT, Amin M, Popoff SN, Barbe MF. Substance P increases CCN2 dependent on TGF-beta yet collagen type I via TGF-beta1 dependent and independent pathways in tenocytes. Connect Tissue Res. 2018;59(1):30–44. PubMed PMID: 28399671; PubMed Central PMCID: PMCPMC5581284. doi:https://doi.org/10.1080/03008207.2017.1297809.
- Fong G, Backman LJ, Alfredson H, Scott A, Danielson P. The effects of substance P and acetylcholine on human tenocyte proliferation converge mechanistically via TGF-beta1. PLoS One. 2017;12(3):e0174101. Epub 2017/03/17. PubMed PMID: 28301610; PubMed Central PMCID: PMCPMC5354451. doi:https://doi.org/10.1371/journal.pone.0174101.
