Abstract
Objective: The efficacy of anti-tumour necrosis factor-α (anti-TNF-α) treatment with infliximab (IFX) may be reduced by the development of anti-drug antibodies (ADAs). This study evaluated drug concentration and the presence of ADAs, relative to response, in rheumatoid arthritis (RA) patients treated with IFX.
Method: Ninety-four RA patients were consecutively included and assessed for disease activity at baseline, and after 14, and 30 or 52 weeks. Serum IFX concentration and ADAs were analysed using in-house enzyme-linked immunosorbent assays. ADA analysis was based on binding to TNF-α-coated plates, with the lower detection limit set at mean + 2 sd of controls.
Results: At 14 and 52 weeks, 74.5% of the patients had moderate to good response. Good responders had significantly higher IFX concentrations than moderate and poor responders at 52 weeks (6.6 ± 1.4 µg/mL vs 3.6 ± 1.3 µg/mL and 2.6 ± 1.6 µg/mL, respectively). An IFX concentration ≥4.66 µg/mL at 14 weeks yielded a moderate to good response at 30/52 weeks, with 91.3% specificity and 39.3% sensitivity. Eleven patients dropped out owing to lack of efficacy and eight owing to side effects; three with IFX concentration ≤ 0.5 µg/mL were ADA positive. At an IFX concentration ≤ 0.5 µg/mL, 43.8% and 30.1% at 14 and 52 weeks, respectively, were ADA positive. None of the good responders had ADAs.
Conclusion: One-quarter of patients had an IFX concentration ≤ 0.5 µg/mL but only 11.7% had ADAs. High IFX concentration was related to a good response, suggesting that the lack of response could be due to a lack of IFX, rather than to the presence of ADAs.
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease characterized by joint inflammation, with a prevalence of 0.5–1% (Citation1, Citation2). Tumour necrosis factor-α (TNF-α) has been identified as having a key role in the inflammatory process and, consequently, the introduction of TNF-α blockade by drugs such as infliximab (IFX) has led to successful treatment of the disease (Citation3). However, sometimes IFX fails to produce a satisfactory clinical response in patients with RA, and this is frequently reported as being due to the generation of anti-drug antibodies (ADAs) or to other side effects (Citation4, Citation5). Previous studies have shown that up to 44% of patients treated with IFX develop ADAs (Citation4, Citation6–Citation8). Too low a treatment dosage in relation to disease activity or an increased metabolism of IFX could also affect the response to the drug (Citation9).
In this study, we evaluated the response to IFX treatment in relation to the concentration of IFX and the presence of ADAs during treatment in consecutively included patients with RA in clinical practice.
Method
Ninety-four (78 female and 16 male) patients fulfilling the 1987 American Rheumatism Association criteria for RA (Citation10) were consecutively included in the study between 1999 and 2006. The patients were treated with IFX at baseline, at 2 and 6 weeks, and thereafter every 8 weeks. The patients were followed for 52 weeks, although in 19 patients the IFX treatment was terminated before the follow-up reached 52 weeks owing to side effects (n = 8) or lack of efficacy (n = 11).
Patients were assessed before the infusion at baseline, and after 14 and 52 weeks, for number of swollen and tender joints, erythrocyte sedimentation rate (ESR; mm/h), patients’ global assessment, Disease Activity Score based on 28-joint count (DAS28), and response to treatment (Citation11, Citation12). C-reactive protein (CRP; mg/mL) was measured. Data on treatment with disease-modifying anti-rheumatic drugs (DMARDs: methotrexate, sulfasalazine, leflunomide, azathioprine, and cyclosporine), and with oral glucocorticoids (prednisolone-equivalent dosage < 10 mg/day) were also registered. All data were collected into the Swedish National Quality Register, a register for patients treated with biologics. Supplementary data were also retrieved from the patients’ medical records. Data on the patients are presented in . Plasma was sampled before every infusion, at baseline, and at 14, and 30 or 52 weeks, and stored at −80°C until analysed. Three patients were lost from sampling at 52 weeks.
Table 1. Patient characteristics at baseline.
The initial treatment dose of IFX was 3.3 ± 0.1 mg/kg (mean ± sem). If no satisfactory response was achieved, the dose was increased, in eight patients between weeks 6 and 14, and in 13 patients before their last infusion. Furthermore, the treatment intervals between infusions were decreased for 19 patients between 6 and 14 weeks and for 31 patients before 30/52 weeks. The adjustments to achieve low or no disease activity were decided by the patient’s treating physician.
All patients gave their informed consent to participate and the regional ethics committee approved the study.
Analyses of levels of IFX and ADAs
The levels of IFX and ADAs were analysed for all samples on one occasion after 52 weeks using in-house enzyme-linked immunosorbent assays (ELISAs), as previously developed and described by Marits et al (Citation9). The method for analysing IFX concentration using TNF-α-coated plates was performed as previously described (Citation9, Citation13, Citation14). ADAs were analysed when the trough concentration of IFX was ≤ 0.5 µg/mL before the next infusion. The ELISA for the ADA analysis was based on inhibition of binding of IFX to the ELISA plates coated with TNF-α. The cut-off level for ADAs was set at the mean value + 2 sd of the percentage of inhibition of binding by the plasma from healthy individuals. We tested 39 healthy individuals and the mean value + 2 sd resulted in a cut-off value of 28.88% of inhibition, which is in agreement with the original presentations for the method (Citation9, Citation13).
Statistical analysis
Non-parametric tests were used for comparative analyses between continuous data. The Mann–Whitney U-test was used to analyse two groups, the Kruskal–Wallis test for more than two groups, and Friedman’s test for related samples. Univariate analyses of variance were used to investigate the relative strengths of the relationships between the variables and outcome. The receiver operating characteristics curve was used to determine the optimal value of IFX for moderate to good response at 52 weeks. All statistical analyses were performed using SPSS 21.0 software (IBM Corp., Armonk, NY, USA).
Results
IFX levels and clinical response
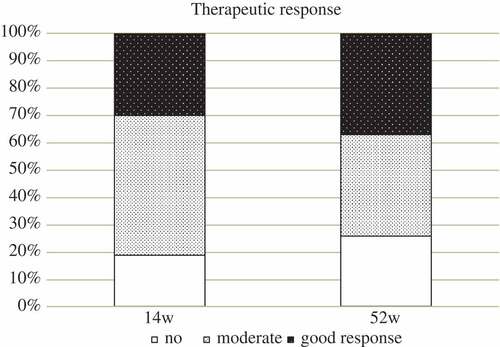
This study included 94 patients with RA, 72 of whom were followed and sampled for 52 weeks and another five for 30 weeks, while 17 patients were, in addition to baseline measures, sampled only at 14 weeks. After 14 weeks, 70 of 94 patients (74.5%) had moderate to good response (as defined by European League Against Rheumatism criteria), with similar results at week 52, when 54 of 72 patients (75.0%) had a moderate to good response ().
Good responders had significantly higher concentrations of IFX, e.g. at 52 weeks good responders had an IFX concentration of 6.6 ± 1.4 µg/mL, compared with 3.6 ± 1.3 µg/mL in patients with a moderate response and 2.6 ± 1.6 µg/mL in those with a poor response (p < 0.001) (). An IFX concentration ≥ 4.66 µg/mL at 14 weeks predicted a moderate to good response at 52 weeks, with a specificity of 91.3% and a sensitivity of 39.3% (area under the curve 0.689). Patients with a good response at 14 weeks and an IFX concentration > 0.5 µg/mL were still good to moderate responders after 52 weeks (16/18; 88.9%), while those with an IFX concentration ≤ 0.5 µg/mL did not improve their response from week 14 to week 52, despite the dose of IFX being increased from (mean ± sd) 220.6 ± 49.1 mg to 235.7 ± 49.7 mg (p = 0.066) or 3.24 ± 0.62 mg/kg to 3.54 ± 0.77 mg/kg, respectively ().
Figure 2. Infliximab concentrations (IFX conc) at 14 and 52 weeks, respectively, stratified by response to treatment. ns, not significant.
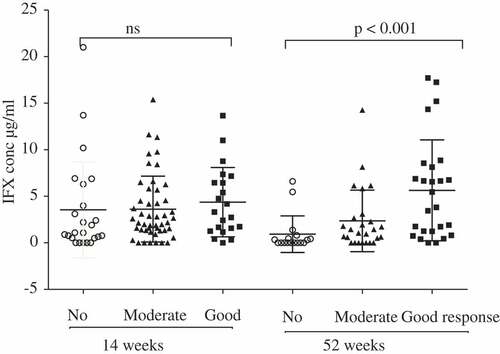
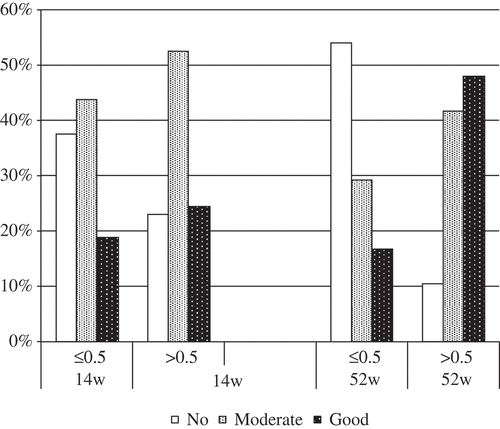
Figure 3. Response to treatment stratified by infliximab concentration > 0.5 µg/mL or ≤ 0.5 µg/mL at 14 and 52 weeks.
The concentration of IFX was inversely correlated with DAS28, and with levels of ESR and CRP at 14 weeks (p < 0.01, p < 0.05, and p < 0.001, respectively) and 52 weeks (p < 0.001 for all three analyses). At week 14, 16 of 94 patients (17%) had an IFX concentration ≤ 0.5 µg/mL in the analysed samples. After 30 or 52 weeks, 26 of 77 patients (33.8%) had an IFX concentration ≤ 0.5 µg/mL. The treatment dose of IFX was increased significantly during the treatment period, from 3.3 ± 0.1 mg/kg at baseline to 3.4 ± 0.1 mg/kg at 14 weeks and 3.6 ± 0.1 mg/kg at 52 weeks, and was unrelated to the therapeutic response and presence of ADAs (Friedman p < 0.001). There was no correlation between the dose in mg/kg and the measured concentration of IFX at either 14 or 52 weeks. Twelve of the 16 patients with an IFX concentration ≤ 0.5 µg/mL were analysed on one later occasion (30 or 52 weeks), with 10 exhibiting an IFX concentration ≤ 0.5 µg/mL, and seven of the 12 patients (58%) were ADA positive. In most of these cases (11/12), the dose of IFX given was constant, but in one patient who was positive for ADAs after 14 weeks the dose was increased from 180 mg to 300 mg; the ADA positivity was lost despite a low IFX concentration (≤ 0.5 µg/mL) being detected.
Clinical response and ADAs
Non-responding patients after 52 weeks were significantly more often ADA positive (23.8% vs moderate to good responders 7.3%, p = 0.046). None of the patients who were good responders at 14 or at 52 weeks or in remission (26.4%; DAS28 < 2.6) was ADA positive. Seven patients out of 16 (44%) with low IFX concentration at week 14 were ADA positive; this represents 7.4% of all patients who started with IFX therapy. At 30 weeks, in two of the five patients who terminated early, the infusions were ADA positive, and at 52 weeks six other patients (10.4%) with an IFX concentration of ≤ 0.5 µg/mL were ADA positive. In total, 11 (11.7%) of the patients were ADA positive at 14 or 52 weeks and four patients were positive on both occasions. These individuals were significantly more often non-responders (56.0% vs those with higher concentrations 12.0%, chi-square 17.14, p < 0.001).
ADA-positive patients had significantly higher DAS28 scores at both 14 and 52 weeks and significantly higher CRP levels at 52 weeks. The presence of ADAs was unrelated to positivity for antibodies [anti-citrullinated protein antibody (ACPA), rheumatoid factor (RF), antinuclear antibodies (ANAs), or double-stranded DNA antibodies], age, gender, body mass index (BMI), or smoking. The treatment dose of IFX, in total or per kilogram, did not affect the presence of ADAs. Treatment with DMARDs (in 95% and 97% of cases at 14 and 52 weeks, respectively) and/or prednisolone (in 35% and 46% at 14 and 52 weeks, respectively) did not affect the presence of ADAs. In 72% and 77% of patients at 14 and 52 weeks, respectively, the treatment was methotrexate (mean dose 11.3 ± 4.7 mg/week). The corresponding figures for corticosteroid treatment were 14.3% in ADA-positive and 42.6% in ADA-negative patients, respectively (non-significant).
Early-terminating patients
Nineteen patients terminated IFX treatment early, 11 of them owing to a lack of efficacy between 14 and 32 weeks (). Their DAS28 score at the time of the latest infusion was 5.2 ± 0.6 (mean ± sd), compared with 5.2 ± 1.6 at baseline. Eight patients ended treatment because of side effects of IFX treatment, two of whom were positive for ADAs ().
Table 2. Characteristics of patients who terminated treatment before week 52.
Discussion
The loss of clinical response or increased frequency of side effects in patients with RA treated with IFX has been suggested to be due to immunogenicity of the drug (Citation6, Citation15, Citation16). In this study, in which 94 patients with RA were consecutively included, the concentrations of IFX and ADAs were evaluated in relation to their response to treatment after 14 and 52 weeks. The frequency of ADAs was fairly low, at 11.7% (11/94), compared with previous studies reporting frequencies of 26–43% (Citation4, Citation7, Citation17–Citation19), and even up to 54% (Citation5). However, a few studies report similar frequencies (Citation8, Citation20, Citation21), including a 2017 publication with a frequency of 17% (Citation20). Analysing samples for the presence of anti-IFX antibodies using radioimmunoassays yielded generally higher frequencies, with a few exceptions, compared with ELISAs with TNF-α-coated plates or functional cell-based reporter gene assays (RGAs) (Citation4, Citation7, Citation21). In a comparative study on different assays for analysing IFX concentration and ADAs, the ELISA and RGA methods showed fairly similar frequencies, while the sensitivity for ADAs using a radioimmunoassay or homogeneous mobility shift assay was much higher. This suggests that these last two methods detect not only drug-neutralizing antibodies but also those that form immune complexes with circulating IFX (Citation21). Furthermore, it has been suggested that the presence of ADAs may be a transient phenomenon, which makes it more difficult to evaluate the results (Citation14). The method used in our study of analysing ADAs on plates coated with TNF-α has been evaluated in other studies (Citation9, Citation13, Citation14), although the frequencies of ADAs were higher, 55–61% in patients with Crohn’s disease (Citation9, Citation14). This method of analysing ADAs is used today for routine clinical analyses in Sweden. The cut-off value for measuring ADAs in this study was set at an IFX concentration ≤ 0.5 µg/mL. Measuring ADAs at a higher concentration of IFX could yield false-positive results since IFX interferes in the test (Citation17). When analysing patients with RA, there is also a possibility of interference due to RF in RA patients leading to falsely elevated results in immunoassays, and also by using bridging ELISAs with IFX-coated wells, which was not observed using the method in this study (Citation9, Citation22). However, in this study there were no differences in frequencies of ADAs irrespective of RF status in patients with low concentrations of ADA. A limitation of the method used in this study is that ADAs can only be detected in samples with undetectable IFX levels owing to interference with the method. Of those individuals with an IFX concentration ≤ 0.5 µg/mL, only seven of 16 were ADA positive at 14 weeks and eight of 26 at 30/52 weeks, with a similar distribution of RF positivity in each group.
In this study, 23.4% of patients (18/77) had an IFX concentration ≤ 0.5 µg/mL at 30 or 52 weeks without being ADA positive, which is comparable to the frequency reported by van den Bemt et al (Citation19) of almost 30% of patients with low IFX concentrations without ADAs. We found that these patients were significantly more often non-responders, implying that they would not benefit from treatment with IFX. The presence of ADAs was clearly related to a lack of response and remission, and therefore ADA positivity was significantly related to higher DAS28 and also CRP. In general, a lower serum trough concentration of IFX correlated significantly with higher DAS28, ESR, and CRP, as observed by others (Citation7, Citation23), and values potentially resulting from the presence of ADAs.
There was a clear relationship between the concentration of IFX and the response to treatment, as has been shown in several other studies (Citation6, Citation18). This relationship was unaffected by BMI or smoking. The results of this study suggest that patients who fail to respond to IFX treatment possibly could have benefited from a higher dosage treatment, independently of formation of ADAs, as suggested by others (Citation9, Citation24). A higher metabolism of IFX could be one explanation, although this was not proven in this study, or higher levels of TNF-α in the circulation in the non-responding group, which has been reported in non-responding patients with Crohn’s disease (Citation25). Thus, in clinical practice all patients, and especially non-responding ones, could benefit from measurement of IFX in serum before the following infusion to personalize and optimize dosage, and therefore the clinical response (Citation9, Citation16, Citation24, Citation26). The treating physician should monitor IFX levels and aim for a concentration of at least 4.66 µg/mL, shown in this study to obtain a specificity of 91.3%, to achieve a moderate to good response.
This study was rather small and also retrospective. Furthermore, some samples had been stored for longer than others, which could affect the results of the analyses. However, the patients were consecutively recruited from the clinical practice, with the treating physician being unaware of the analysed results.
Several groups have suggested that treatment with non-biological DMARDs, such as methotrexate, would yield less ADA production or lower ADA concentrations compared with single IFX treatment (Citation7, Citation14, Citation17, Citation27). In our study, as well as other studies, ADA formation was not affected by concomitant DMARD treatment (Citation5, Citation18, Citation19, Citation26). A 2017 study measuring TNF-α bioactivity found that methotrexate was more effective than other DMARDs in preventing ADA formation (Citation28).
Conclusion
From this study, we can conclude that despite a low concentration of IFX in 17–33% of the RA patients, the presence of ADAs was rather low. A high IFX concentration was related to a good response, which suggests that a lack of response could be due to a lack of medication rather than to the presence of ADAs.
Acknowledgements
This work was supported by the Swedish Rheumatism Association, the Swedish Foundation for Strategic Research, King Gustaf V’s 80-Year Fund, the Swedish Research Council [K2013-52X-20307-07-3], and Västerbotten County Council.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Neovius M, Simard JF, Askling J. Nationwide prevalence of rheumatoid arthritis and penetration of disease-modifying drugs in Sweden. Ann Rheum Dis 2011;70:624–9.
- Silman AJ. Epidemiology of rheumatoid arthritis. APMIS 1994;102:721–8.
- Feldman M, Taylor P, Paleolog E, Brennan FM, Maini RN. Anti-TNFα therapy is useful in rheumatoid arthritis and Crohn’s disease: analysis of the mechanism of action predicts utility in other diseases. Transplantation Proc 1998;30:4126–7.
- Wolbink GJ, Vis M, Lems W, Voskuyl AE, De Groot E, Nurmohamed MT, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:711–15.
- Krintel SB, Grunert VP, Hetland ML, Johansen JS, Rothfuss M, Palermo G, et al. The frequency of anti-infliximab antibodies in patients with rheumatoid arthritis treated in routine care and the associations with adverse drug reactions and treatment failure. Rheumatology (Oxford) 2013;52:1245–53.
- Radstake TRDJ, Svenson M, Eijsbouts AM, van den Hoogen FHJ, Enevold C, van Riel PLCM, et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis 2009;68:1739–45.
- Bendtzen K, Geborek P, Svenson M, Larsson L, Kapetanovic MC, Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor α inhibitor infliximab. Arthritis Rheum 2006;54:3782–9.
- Finckh A, Dudler J, Wermelinger F, Ciurea A, Kyburz D, Gabay C, et al. Influence of anti-infliximab antibodies and residual infliximab concentrations on the occurrence of acquired drug resistance to infliximab in rheumatoid arthritis patients. Joint Bone Spine 2010;77:313–18.
- Marits P, Landucci L, Sundin U, Davidsdottir L, Nilsson J, Befrits R, et al. Trough s-infliximab and antibodies towards infliximab in a cohort of 79 IBD patients with maintenance infliximab treatment. J Crohn’s Colitis 2014;8:881–9.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24.
- Prevoo MLL, Van’T Hof MA, Kuper HH, Van Leeuwen MA, Van De Putte LBA, Van Riel PLCM. Modified disease activity scores that include twenty-eight-joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis: modified disease activity scores. Arthritis Rheum 1995;38:44–8.
- van Gestel AM, Prevoo MLL, van’t Hof MA, van Rijswijk MH, van de Putte LBA, van Riel PLCM. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis: comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism criteria. Arthritis Rheum 1996;39:34–40.
- Candon S, Mosca A, Ruemmele F, Goulet O, Chatenoud L, Cézard J-P. Clinical and biological consequences of immunization to infliximab in pediatric Crohn’s disease. Clin Immun 2006;118:11–19.
- Baert F, Noman M, Vermeire S, Van Assche G, D’ Haens G, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med 2003;348:601–8.
- Haraoui B, Cameron L, Ouellet M, White B. Anti-infliximab antibodies in patients with rheumatoid arthritis who require higher doses of infliximab to achieve or maintain a clinical response. J Rheumatol 2006;33:31.
- Aarden L, Ruuls SR, Wolbink G. Immunogenicity of anti-tumor necrosis factor antibodies—toward improved methods of anti-antibody measurement. Curr Opin Immunol 2008;20:431–5.
- Abe T, Takeuchi T, Miyasaka N, Hashimoto H, Kondo H, Ichikawa Y, et al. A multicenter, double-blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. J Rheumatol 2006;33:37.
- Pascual-Salcedo D, Plasencia C, Ramiro S, Nuño L, Bonilla G, Nagore D, et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology (Oxford) 2011;50:1445–52.
- van den Bemt BJ, den Broeder AA, Wolbink G, Hekster YA, van Riel PL, Benraad B, et al. Anti-infliximab antibodies are already detectable in most patients with rheumatoid arthritis halfway through an infusion cycle: an open-label pharmacokinetic cohort study. BMC Musculoskelet Disord 2011;12:12.
- Moots RJ, Xavier RM, Mok CC, Rahman MU, Tsai W-C, Al-Maini MH, et al. The impact of anti-drug antibodies on drug concentrations and clinical outcomes in rheumatoid arthritis patients treated with adalimumab, etanercept, or infliximab: results from a multinational, real-world clinical practice, non-interventional study. PLoS One 2017;12:e0175207.
- Steenholdt C, Bendtzen K, Brynskov J, Thomsen O, Ainsworth MA. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn’s disease: post hoc analysis of a randomized controlled trial. Am J Gastroenterol 2014; 109:1055–64.
- Marks V. False-positive immunoassay results: a multicenter survey of erroneous immunoassay results from assays of 74 analytes in 10 donors from 66 laboratories in seven countries. Clin Chem 2002;48:2008–16.
- Wolbink GJ. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 2005;64:704–7.
- Mulleman D, Méric J-C, Paintaud G, Ducourau E, Magdelaine-Beuzelin C, Valat J-P, et al. Infliximab concentration monitoring improves the control of disease activity in rheumatoid arthritis. Arthritis Res Ther 2009;11:R178.
- Martínez-Borra J, López-Larrea C, González S, Fuentes D, Dieguez A, Deschamps EM, et al. High serum tumor necrosis factor-alpha levels are associated with lack of response to infliximab in fistulizing Crohn’s disease. Am J Gastroenterol 2002;97:2350–6.
- Ducourau E, Mulleman D, Paintaud G, Miow Lin D, Lauféron F, Ternant D, et al. Antibodies toward infliximab are associated with low infliximab concentration at treatment initiation and poor infliximab maintenance in rheumatic diseases. Arthritis Res Ther 2011;13:R105.
- Maneiro JR, Salgado E, Gomez-Reino JJ. Immunogenicity of monoclonal antibodies against tumor necrosis factor used in chronic immune-mediated inflammatory conditions: systematic review and meta-analysis. JAMA Intern Med 2013;173:1416.
- Dénarié D, Rinaudo-Gaujous M, Thomas T, Paul S, Marotte H. Methotrexate reduced TNF bioactivity in rheumatoid arthritis patients treated with infliximab. Mediators Inflamm 2017;2017:3708250.