Abstract
Objectives
This study aims to assess the feasibility of the Coventry ultidisciplinary fast-track cranial giant cell arteritis (FTGCA) pathway, which was set up in 2013 in collaboration with vascular physiology and ophthalmology to enable prompt multidisciplinary assessment, including ultrasound (US). This study also looks at the impact of prior corticosteroid (CS) use on the performance of US in real life.
Method
Data were collected retrospectively for patients who attended the Coventry FTGCA pathway between 1 January 2014 and 31 December 2017. Patients were identified from US lists and clinical details were obtained from electronic medical records.
Results
In total, 620 eligible patients were included in this study. US had a sensitivity of 50%, which improved to nearly 56% in CS-naïve patients. The median duration of CS use prior to US was 2 days, and sensitivity was around 46% in this group. The specificity of US was > 96%, and CS use was avoided completely in 345 patients (56%). CSs natively impacted on the utility of US, with US more likely to be false negative.
Conclusions
This novel multidisciplinary pathway demonstrates excellent feasibility and minimizes the use of CSs in patients without giant cell arteritis. US was performed promptly, was cost effective- and had reassuring real-life sensitivity and specificity in this cohort, with excellent patient feedback. CS-naïve patients showed higher sensitivity for US despite the short duration of CS use.
Giant cell arteritis (GCA) commonly presents with headache in the temporal region, scalp tenderness, jaw claudication, and visual disturbance (Citation1). It is the most common type of vasculitis and is characterized by systemic inflammation, arteritis, and critical ischaemia (Citation2). Permanent loss of vision, resulting from ischaemic optic neuropathy, is the most feared complication of GCA and can affect up to one-fifth of patients (Citation3). Strokes and aortitis leading to aortic aneurysms are recognized later stage complications, but visual loss tends to occur early, making cranial presentations of GCA a medical emergency (Citation4). Permanent visual loss leads to long-term economic, health, and social consequences (Citation5). Consequently, guidelines emphasize the importance of prompt recognition and management of GCA (Citation2, Citation6).
The diagnosis of GCA is challenging owing to the lack of specific symptoms and urgency of recognition. Temporal artery biopsy (TAB) has traditionally been the gold standard for diagnosis, despite limitations of cost, skillset, and delay. Patients are typically commenced on high-dose corticosteroids (CSs) while awaiting the biopsy results. TAB has high specificity but lacks sensitivity as skip lesions may result in false negatives (Citation7). The emerging use of Doppler ultrasound (US) in the diagnosis of GCA has helped to overcome some of the challenges of TAB (Citation8). It can be performed quickly, inexpensively, and non-invasively, with typical findings of hypoechoic haloes, stenoses, or occlusions on US having diagnostic sensitivity and specificity of 78% and 79%, respectively, compared to TAB and 87% and 96% compared to American College of Rheumatology (ACR) classification criteria for GCA, albeit in small studies (Citation8–10). Drawbacks of US include the need for skilled sonographers, and that the halo sign, the most diagnostic feature on US, can disappear within 48–72 h of starting CSs (Citation11, Citation12). Nonetheless, TAB remains the gold standard for diagnosis of cranial GCA, and in difficult or uncertain cases, biopsies can be extremely useful.
University Hospital Coventry and Warwickshire NHS Trust (UHCW) established a multidisciplinary fast track pathway (FTP) for GCA in 2013. It sought to address previously unmet need by offering patients same-day clinical review, Doppler US, diagnosis, and treatment for patients with cranial GCA. Doppler US included scanning of the main stem and parietal and frontal branches of the temporal arteries bilaterally. We did not include a scan of the axillary arteries at that time; this was subsequently introduced in 2019. This service uniquely ran across three departments: rheumatology, ophthalmology, and vascular ultrasound. Treatment was initiated after review (immediately after US results) for most patients, in contrast to previous models. Prior to the introduction of this service, patients experienced a delay of at least 4 weeks from initial presentation to final diagnosis by TAB, although CSs were started in primary care by the general practitioner (GP). In these cases, often the biopsy had been carried out late and the clinicians were unsure whether or not the patient had GCA. The core aims of this pathway were: (i) to avoid unnecessarily exposing unaffected patients to CSs; (ii) to minimize delays and anxiety for patients; and (iii) to reduce complications through early diagnosis and treatment.
The aims of this study were: (i) to assess the feasibility of running an FTP without prior introduction of CSs; and (ii) to assess the impact of the use of CSs on performance of US in a real-life cohort.
Method
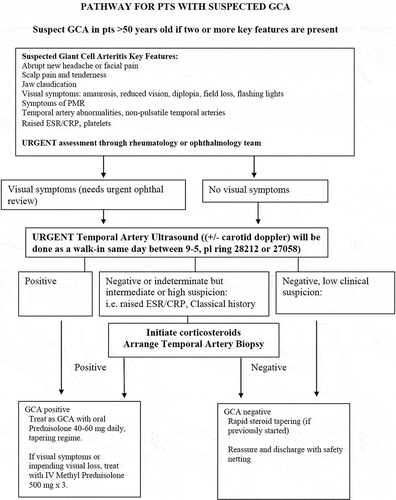
A detailed description of the UHCW GCA pathway is provided in another article (Citation13). Telephone referrals to the FTP are accepted if the patient is aged over 50 years and has appropriate clinical features (). Patients are seen on the same day as the referral, or the next working day if they are referred out of hours. Patients who are referred out of hours are usually commenced on CSs immediately to avoid treatment delays. Comprehensive clinical assessments (including ophthalmic assessment), relevant blood investigations, and vascular Doppler US studies are performed urgently and a management plan is instituted – all on the same day. In patients with negative or inconclusive US and clinical suspicion, TAB is performed by the ophthalmic team, usually within 2 weeks. Patients with low clinical suspicion of GCA and a negative US are reassured and discharged following the initial consultation. They undergo rapid CS tapering if CSs have been started. Patients with high suspicion or positive US are immediately commenced on oral CSs or intravenous methylprednisolone if there is a risk of vision loss.
Figure 1. Coventry multidisciplinary fast-track pathway for giant cell arteritis (GCA). CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ophthal, ophthalmology; pts, patients; PMR, polymyalgia rheumatica; US: ultrasound.
Our pathway started in middle of 2013 and initially we piloted this for a few months, obtaining TABs alongside US, with excellent results (Citation13). In this article, we present data from 1 January 2014 to 31 December 2017, a period of 4 years. The collected data were collated to examine the impact on outcomes, reliability of US in the clinical setting, and the practical experience of running an FTP.
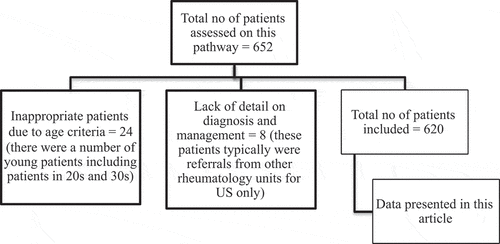
Patients were identified from ultrasonography lists, as all patients attending the FTP are required to undergo a Doppler US of their temporal arteries. The clinical records of all patients with suspected GCA seen on the UHCW GCA FTP between, and including, January 2014 and December 2017 were retrospectively reviewed so that we could obtain data relating to final diagnosis and outcomes. A predetermined piloted proforma was used to collect data regarding patient demographics, clinical features, investigative results, CS use, and final diagnosis. Since this is a real-life study, we felt that it would be appropriate to use the final clinical diagnosis. There are also reservations around the performance of ACR criteria for GCA in real-life settings (Citation14). All patients who had been referred with suspected GCA were included, and clinical data were sought from electronic and paper records. Patients who were less than 50 years old were excluded from this analysis since they did not fulfil age criteria for this referral.
Ethical approval was granted by the UHCW Research and Development Department (approval number GF0264). The Coventry branch of the national GCA patient group (PMRGCA UK) was involved in the design of this model of care. Patient feedback was obtained through a piloted questionnaire for a small proportion of the patients.
Statistical analyses
Descriptive analyses were performed using Microsoft Excel, and freely available online calculators were used for Fisher’s exact test to prove statistical differences. Sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs) were estimated, together with 95% confidence intervals (CIs), using Wilson’s method (Citation15).
Results
Overall group
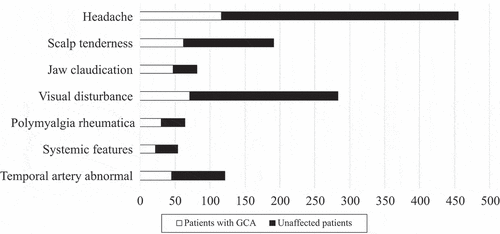
Over the 4 year period of the UHCW GCA FTP, we found 620 eligible patients (see for details of excluded patients). Patients who were less than 50 years old (24 patients, none of whom was diagnosed with or treated as GCA), or where we did not have a final diagnosis and management data (Citation8), were excluded. Patients were predominantly female (411/620; 66%) and white (84%), although 75 patients (12%) were of South Asian descent. The median age was 70 years (range 50–100, mean 70.2 years); details are shown in . In the whole cohort, 169 patients (27%) received CSs for suspected GCA while awaiting assessment, whereas 451 patients did not. The main referral sources for US were rheumatology (248), ophthalmology (219), and medicine (145), and patients who had presented out of hours were more likely to have been started on CS. In some of these cases, US was delayed by a number of days owing to issues with requesting an US. The usual starting dose of prednisolone in these patients was 60 mg daily in patients with a high risk of visual complications (jaw claudication, eye symptoms but no signs), or 40 mg where there was low visual risk. Some of these were subsequently diagnosed as GCA (37 patients), and CSs were continued. The common symptoms in this cohort were headaches (n = 451, 73%), scalp tenderness (n = 198, 32%), and visual symptoms, including blurring of vision or some loss of vision (n = 282, 45%) (). Polymyalgia-type symptoms were present in 72 patients (12%) and jaw claudication in 86 patients (14%), with systemic features such as fever and sweats being present in 47 patients (8%). The mean C-reactive protein reading in this cohort was 20.2 mg/L (normal level < 3 mg/L). The main difference in presentations between the various referring teams was that patients with visual problems were much more likely to come through ophthalmology than through rheumatology or medicine.
Table 1. Demographic and ethnicity details for patients on the giant cell arteritis (GCA) pathway.
Patients with GCA
A final diagnosis of GCA was made in 143 patients (23%) within the whole cohort. Of these, 92 patients (64%) were female and 129 (90%) were white. The median age at presentation was 75 years (range 52–95 years). The patients’ demographic details are shown in . Very few patients from black, Asian, and minority ethnic groups were given a diagnosis of GCA (14 patients overall). US was positive in five of these patients, and biopsies were negative in the three patients where they were performed.
Of the 143 patients with CGA, 37 patients had been on CSs prior to the diagnosis and 106 had not been exposed to CSs prior to the diagnosis of GCA. Overall, the diagnosis of GCA was given to 70 (31 US-positive) patients who had presented via rheumatology, 42 (22 US-positive) who had presented via ophthalmology, and 31 (17 US-positive) who had presented through other routes, predominantly medicine.
Unaffected patients
We were able to discharge 477 patients (77%) referred to the FTP as they did not have GCA. Of these, 345 were not given any CS treatment. In view of the retrospective nature of data collection, the notes were also reviewed to see whether they were subsequently diagnosed to have GCA, and none of these patients was subsequently given a diagnosis of GCA or large-vessel vasculitis. Complete data regarding CS use were only available for 112 of the 132 unaffected patients who were exposed to CSs. The median duration of CS exposure before US was 2 days. These patients had either presented out of hours, or come through other departments, or been admitted to hospital under a medical team and then referred to rheumatology, which created delays. Overall, CSs were given for a median of 30 days (range 1–412 days, sd 78 days); some patients had a diagnosis of polymyalgia rheumatica or were on CSs for another reason, hence the prolonged course in some patients.
Doppler ultrasound
All patients who attended the UHCW GCA FTP underwent US, and those with typical GCA features were labelled ‘positive’. The majority of patients (66%) received their US on the day of their referral. US was performed in 90% of patients within 3 days and 96% within 1 week; the main reasons for delay were that the request was not marked as urgent and not followed up with a telephone call to the vascular laboratory or that the US was requested under the wrong department. The lead vascular scientist audited the images from all vascular scientists and found no significant differences or discrepancies.
US was positive overall in 84 patients, 70 of whom were diagnosed with GCA. With regard to the source of referral, there did not seem to be any differences, with 35 of the 84 patients having been referred from rheumatology, 28 from ophthalmology, and 21 from other sources (primarily medicine). The sensitivity of US overall was 50.0% (95% CI 42.9–59.1%) () compared to clinical diagnosis, and improved further in patients with no CS exposure, to 55.8% (95% CI 44.7–66.4%). In patients who had been started on CSs prior to US, sensitivity was 45.5% (95% CI 34.0–57.4%). There was excellent correlation between positive US and clinical diagnosis of GCA. Patients started on CSs still showed halos within the first 6 days, and no definite halos were found from day 7 onwards, although only 4% of patients were in this category. Specificity was less affected by CS exposure and the figures were well above 90% in all groups.
Table 2. Performance of ultrasound (US) using the final clinical diagnosis with or without corticosteroid (CS) use.
The PPV was lower for the year 2015 (74.1%), but otherwise the numbers were consistently above 85%. The overall PPV for US over the 4 years was 84.9%. The NPV also seems reasonable, at 86.7%, which improves further to 91.2% in patients who are CS naïve. As one would expect, the NPV was lower for patients already on CSs prior to US. False-negative scans were seen in 34/443 in the CS-naïve group and in 36/169 of the patients who had already started on CSs prior to US; these differences were highly statistically significant (p < 0.0001, Fisher’s exact test, two-tailed). Some US results were indeterminate, and these patients were investigated further, usually with TAB, if there was ongoing clinical suspicion of GCA.
Visual loss data
Visual symptoms had been present in 281 patients (45.3%), including blurring of vision, visual loss, flashing lights, etc. Typically, these patients presented to eye casualty, which covers not only Coventry but all of Warwickshire, and hence a greater proportion of patients with visual features end up within this pathway. Visual loss (including temporary or permanent reduction in visual acuity, or field defect) had been present in 68 (47.5%) of the GCA patients at presentation (36 of these had presented through eye casualty, 11 through medicine, two through neurology, and 19 through rheumatology), with complete recovery in 42 patients (62%), and 26 patients (38%) had some permanent visual loss. This represents 18.1% of the total number of patients with GCA. Of the 68 patients in this group, 19 patients had not been on CSs prior to US, reflecting a higher number of out-of-area referrals. A number of these patients reported improvement within a few days of initiation of CSs, usually within the first couple of days, although one patient reported gradual improvement in vision until about 3 months. Some of these patients did not present with headaches and had very few other features suggesting GCA. No patients developed visual problems after their initial presentation in this pathway that could be attributed to GCA; one patient was subsequently diagnosed with retinal melanoma, which was the cause of progressive visual loss (this patient also had a positive US, and no biopsy was performed).
Running the FTP
As the FTP has become more established it has become busier. The number of referrals to the FTP has risen each year, although outcomes have remained consistent (). Although referral rates tend to fluctuate, 2017 was far busier than any of the previous years. The number of patients coming through from ophthalmology has changed significantly over the 4 years of this service, from six in 2014 to 123 in 2017. Referral patterns for sudden visual loss have changed from 2014 (when the patients were being referred from within Coventry) to regional referrals (within Warwickshire). The other local hospitals, such as George Eliot Hospital and Warwick Hospital, have rheumatology services, so patients without visual symptoms are managed by their local rheumatology service. There has also been an increase in the referrals coming from medicine, from 23 in 2014 to 69 in 2017. The direct referrals to rheumatology have remained consistent across the 4 years (57, 65, 58, and 68). This has led to service constraints, but we have increased middle-grade cover (core medical trainees, specialist trainees, and fellows) to support this. An internal quality assurance led by the lead vascular scientist did not find any discrepancies and there have been regular multidisciplinary team meetings to discuss complex cases.
Patient satisfaction
Patient feedback was obtained from all patients attending this pathway for 2 months (October and November 2017) and completed responses were obtained from 40 patients, some of whom had undergone TAB as well. Overall, the feedback confirmed high patient satisfaction with the FTP, and 100% of patients either ‘agreed’ or ‘strongly agreed’ with the following statements: ‘I was happy with the standard of care for this service’, ‘I was reviewed promptly at the hospital following my GP referral’, ‘I had a comprehensive assessment on the day’, ‘I had vascular Doppler (ultrasound scan) performed promptly in the X-ray department’, ‘The doctors and sonographer treated me with courtesy and respect’, and ‘I would recommend this service to my friends and family’.
For patients diagnosed as GCA positive through the FTP, 100% (14/14) strongly agreed with the statement that ‘Treatment for GCA was initiated promptly’. This demonstrates how the FTP has not compromised the rapid initiation of CSs for patients in need, while also successfully avoiding potentially harmful CS exposure for GCA-negative patients. Many patients also left positive comments about staff, reflecting their happiness with the service.
Discussion
CSs are associated with significant adverse effects, even with short-term use. An increase in the risk of infections, arrhythmias, hyperglycaemia, and diabetes mellitus, heart failure, inability to sleep, and psychological side-effects can be seen within a few days of initiation (Citation16–18). Other common side-effects that have led to controlled trials for new agents such as tocilizumab (Citation19) include osteoporosis, cataracts, skin thinning, avascular necrosis, hypertension, sudden death, and gastrointestinal disturbances (Citation20). CS-induced side-effects are quite common, with about 80% developing at least one side-effect. A cost evaluation of the seven most common side-effects with CSs (fracture, cataract, diabetes, peptic ulcer, stroke, myocardial infarction, and non-Hodgkin’s lymphoma) resulted in extra costs of £165 per person-year using CSs (Citation21). Other studies have reported similar findings (Citation22, Citation23). One study showed that even a short course of CSs is associated with significant adverse effects (Citation24). Hence, there are significant benefits to reducing CS burden both for patients with GCA and for those who do not have GCA. The Coventry fast-track cranial giant cell arteritis (FTGCA) pathway successfully demonstrates the feasibility of a multidepartmental model of care whereby patients can be assessed and treated urgently, with the additional benefit of minimizing CS use in patients not thought to have GCA. In the current series, > 75% of patients who are being referred do not have GCA, so avoiding or reducing CS for these patients is an important achievement.
This model of delivery of care for suspected GCA patients is novel and has not been reported previously. Other models have relied on individuals with an interest in and limited (in terms of diagnoses) US training. All of our vascular scientists have undergone the Society for Vascular Ultrasound Accreditation scheme, and additional specific training in scanning temporal arteries was performed in house. They provide urgent scans for the transient ischaemic attack (TIA) service. Some patients presenting with visual loss have had strokes rather than GCA, and these reports, with acceptability to other departments, are an important element of this service. Despite the limitations associated with multiple team members performing US, we have seen high sensitivity, specificity, and predictive values for US within this pathway. These results are comparable to the findings of other research studies on GCA. Although often there is a drop-off in daily practice compared to results from clinical trials (Citation25, Citation26), we have not seen this within our FTP. A number of units in the UK have now adopted this model.
This is the largest reported cohort undergoing US as the first line of management for assessment of cranial GCA in the world. Although the median duration of CS exposure before US was only 2 days, there was a significant drop in sensitivity. US was statistically significantly more likely to be negative for these patients, and this has important implications for FTPs. We found sensitivities of 55.8% in CS-naïve patients and 50.0% in the whole cohort, including patients who had been on CSs, with a specificity of > 95% in all groups. The TABUL study, which included 430 patients (Citation27), had a sensitivity of 54% and specificity of 81% for US. In the TABUL trial, there were 162 patients with US consistent with GCA; only nine of these had changes in the axillary arteries, with seven patients having changes in only the axillary arteries, and inclusion of the axillary arteries increased absolute sensitivity by < 5% (Citation27). Some smaller studies have shown higher sensitivities for US in GCA, but with serious methodological limitations (Citation28). Two separate meta-analyses have been completed to investigate the role of US in the diagnosis of GCA (Citation9, Citation29). For the first meta-analysis, the weighted sensitivity and specificity of the halo sign were 69% (95% CI 57–79%) and 82% (CI 75–87%), respectively, compared with biopsy, and 55% (CI 36–73%) and 94% (CI 82–98%), respectively, compared with ACR criteria. In the second meta-analysis, the pooled sensitivity was 0.68 and pooled specificity was 0.91. Our results are comparable to these, despite the caveats of using real-life data over a substantial period of time and involving multiple clinicians.
This pathway is reliant on three teams working together to deliver the service: vascular ultrasound, ophthalmology, and rheumatology. We have not come across other examples of such team working for the delivery of GCA pathways, but there are multiple advantages with this approach. The traditional models suffer from an inability to scan other blood vessels, including carotids, particularly for patients presenting with amaurosis fugax or features suggesting TIA. Occipital artery involvement is another area where the vascular team may have an advantage (Citation30). Furthermore, it becomes problematic when individuals are on leave, and this puts serious constraints on the service. The recent publication of a Getting It Right First Time (GIRFT) document, which is part of an aligned set of programmes within National Health Service (NHS) England and NHS Improvement, also emphasizes that the GCA service should not be run single-handedly (Citation31). Our pathway received a commendation from British Society for Rheumatology (BSR) in 2018 as part of best practice awards. We also perform carotid artery scanning for patients with unilateral visual loss, which is not part of other FTPs.
In this cohort, the majority of patients with visual symptoms have recovered completely. This is reassuring and supports observations from other FTPs that early treatment can result in benefits in patients with visual problems (Citation32). Only about 18% of patients with GCA had permanent visual loss in this cohort, despite > 45% presenting with visual symptoms. Our cohort has a disproportionate of patients with visual symptoms owing to the success of the regional eye casualty, which has led to increasing numbers of referrals from outside Coventry but within the county of Warwickshire (around an additional 600 000 patients) with visual problems. Hence, the overall proportion of patients with visual features is higher than one would expect. Previous studies have suggested that up to 20% of patients have visual problems (Citation3). We did not routinely collect specific data relating to the duration of symptoms and delays in referral, although this is likely to be particularly important with regard to visual loss. Delays on the part of the patient and the GP are both likely to contribute to a delay in presentation, resulting in increased risk of complications such as visual loss (Citation33). Previous studies have highlighted that patients at highest risk of neuro-ophthalmic complications do not always mount high inflammatory responses (Citation34). In our cohort, patients presenting atypically to ophthalmologists with visual symptoms were included, although this is not universal for GCA FTPs. A number of these patients would not have been included in the results for other FTPs owing to their uncertain diagnosis and not meeting ACR criteria.
This study has some limitations. The study includes real-life data, which are not as strictly controlled as clinical trials. Studies of this nature are subject to biases, which would be applicable to this research. In this cohort, we have counted all the patients in whom CSs were continued in GCA treatment doses as having a positive diagnosis, although, in reality, a lot of these cases do not have a clear diagnosis and different clinicians have different thresholds for continuing CSs. In addition, patients with aortitis would have been treated as GCA, although these patients are not expected to have any US findings, and hence the utility of US may seem lower in this study. Vascular scientists were not required to go through a formal assessment to demonstrate their expertise in temporal artery US. We did not have details on CS dosing for a number of patients. This may have influenced the utility of US. There may be bias in the way in which patients were given CSs, as patients with visual symptoms may have been given CSs preferentially. We only scanned axillary arteries for the very few patients who had arm claudication in this cohort (although we now routinely scan these), and this may have increased US sensitivity by around 5%.
Conclusion
The Coventry FTGCA pathway for cranial GCA represents a novel model for healthcare delivery through collaborative multidisciplinary teamwork between ophthalmology, rheumatology, and vascular physiology. This innovative pathway demonstrates excellent feasibility and minimizes the use of CSs in patients without GCA. US was performed promptly, was cost effective, and had reassuring real-life sensitivity and specificity in this cohort with excellent patient feedback. CS-naïve patients showed higher sensitivity for US despite the short duration of CS use.
Authors’ contributions
SD led the development of the FTGCA pathway in Coventry; all authors contributed to the study design, data collection, analysis, and write-up of the article.
Acknowledgements
We wish to thank Professor N Krishnan, UHCW NHS Trust, and Ines Rombach, Institute for Statistics, University of Oxford, for help with statistics and for reviewing the manuscript; Dr K Chaudhuri and all the rheumatology and ophthalmology consultants at UHCW NHS Trust for help with running the pathway, and the vascular surgeons for their help with biopsies and access to the vascular laboratory; the Coventry branch of the GCA patient group (PMRGCA UK) for their help and support in creating this model; and the British Society for Rheumatology for their support for this model of care.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dejaco C, Brouwer E, Mason JC, Buttgereit F, Matteson EL, Dasgupta B. Giant cell arteritis and polymyalgia rheumatica: current challenges and opportunities. Nat Rev Rheumatol 2017;13:578.
- Dasgupta B, Borg FA, Hassan N, Alexander L, Barraclough K, Bourke B, et al. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology 2010;49:1594–7.
- Soriano A, Muratore F, Pipitone N, Boiardi L, Cimino L, Salvarani C. Visual loss and other cranial ischaemic complications in giant cell arteritis. Nat Rev Rheumatol 2017;13:476.
- Ninan J, Lester S, Hill C. Giant cell arteritis. Best Pract Res Clin Rheumatol 2017;30:169–88.
- Frick KD. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol 2007;125:544–50.
- Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, et al., European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009;68:318–23.
- Kaltsonoudis E, Pelechas E, Papoudou-Bai A, Markatseli TE, Elisaf M, Voulgari PV, et al. The impact of temporal artery biopsy for the diagnosis of giant cell arteritis in clinical practice in a tertiary university hospital. Plos One 2019;14:e0210845.
- Schmidt WA, Kraft HE, Volker L, Vorpahl K, Gromnica-Ihle EJ. Colour Doppler sonography to diagnose temporal arteritis. Lancet 1995;345:866.
- Bley TA, Reinhard M, Hauenstein C, Markl M, Warnatz K, Hetzel A, et al. Comparison of duplex sonography and high-resolution magnetic resonance imaging in the diagnosis of giant cell (temporal) arteritis. Arthritis Rheum 2008;58:2574–8.
- Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med 2005;142:359–69.
- Santoro L, D’Onofrio F, Bernardi S, Gremese E, Ferraccioli G, Santoliquido A. Temporal ultrasonography findings in temporal arteritis: early disappearance of halo sign after only 2 days of steroid treatment. Rheumatology 2013;52:622.
- Hauenstein C, Reinhard M, Geiger J, Markl M, Hetzel A, Treszl A, et al. Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology 2012;51:1999–2003.
- Dubey S, Pinnell J, Tiivas C, Mehta P. Coventry fast track pathway for managing giant cell arteritis. Int J Clin Rheumatol 2020;15:21–5.
- Seeliger B, Sznajd J, Robson JC, Judge A, Craven A, Grayson PC, et al. Are the 1990 American College of Rheumatology vasculitis classification criteria still valid? Rheumatology 2017;56:1154–61.
- Piegorsch WW. Sample sizes for improved binomial confidence intervals. Comput Stat Data Anal 2004;46:309–16.
- Chan CCK. Steroid management in giant cell arteritis. Br J Ophthalmol 2001;85:1061–4.
- Andersson R, Malmvall BE, Bengtsson BA.Long-term corticosteroid treatment in giant cell arteritis. Acta Med Scand 1986:220;465–9.
- Ling MHM, Tsuang PPJ. Side effects of corticosteroid therapy: psychiatric aspects. Arch Gen Psychiatry 1981;38:471–7.
- Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017;377:317–28.
- Martínez-Taboada VM, Rodríguez-Valverde V, Carreño L, López-Longo J, Figueroa M, Belzunegui J, et al. A double-blind placebo controlled trial of etanercept in patients with giant cell arteritis and corticosteroid side effects. Ann Rheum Dis 2008;67:625–30.
- Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med 2009;103:975–94.
- Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther 2011;33:1413–32.
- Gale S, Wilson JC, Chia J, Trinh H, Tuckwell K, Collinson N, et al. Risk associated with cumulative oral glucocorticoid use in patients with giant cell arteritis in real-world databases from the USA and UK. Rheumatol Ther 2018;5:327–40.
- Yao T-C, Huang Y-W, Chang S-M, Tsai S-Y, Wu AC, Tsai H-J. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med 2020;173:325–30.
- McDonald L, Lambrelli D, Wasiak R, Ramagopalan SV. Real-world data in the United Kingdom: opportunities and challenges. BMC Med 2016;14:97.
- Bartlett VL, Dhruva SS, Shah ND, Ryan P, Ross JS. Feasibility of using real-world data to replicate clinical trial evidence. JAMA Netw Open 2019;2:e1912869.
- Luqmani R, Lee E, Singh S, Gillett M, Schmidt WA, Bradburn M, et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess 2016;20:1–238.
- Mackie SL, Dejaco C, Appenzeller S, Camellino D, Duftner C, Gonzalez-Chiappe S, et al. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis. Rheumatology 2020;59:e1–e23.
- Arida A, Kyprianou M, Kanakis M, Sfikakis PP. The diagnostic value of ultrasonography-derived edema of the temporal artery wall in giant cell arteritis: a second meta-analysis. BMC Musculoskelet Disord 2010;11:44.
- Pinnell J, Tiivas C, Perkins P, Blake T, Saravana S, Dubey S. Ultrasonography of occipital arteries to diagnose giant cell arteritis: a case series and literature review. Clin Rheumatol 2018;37:569–73.
- Getting It Right First Time. Rheumatology (https://www.gettingitrightfirsttime.co.uk/medical-specialties/rheumatology/). Accessed 21 February 2022.
- Diamantopoulos AP, Haugeberg G, Lindland A, Myklebust G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology 2016;55:66–70.
- Prior JA, Ranjbar H, Belcher J, Mackie SL, Helliwell T, Liddle J, et al. Diagnostic delay for giant cell arteritis – a systematic review and meta-analysis. BMC Med 2017;15:120.
- Royal College of Physicians. Diagnosis and management of giant cell arteritis. 2010 (https://www.rcplondon.ac.uk/guidelines-policy/diagnosis-and-management-giant-cell-arteritis). Accessed 7 October 2020.