Abstract
Objectives
: Knowledge of the correspondence between clinical ICD diagnoses and classification criteria fulfilment is crucial to interpret studies identifying cases via ICD codes. We assessed the degree to which patients registered with ICD-10 diagnoses of psoriatic arthritis (PsA) in the Swedish National Patient Register (NPR) fulfil established PsA classification criteria.
Method
Four hundred patients with at least one outpatient visit to one of five rheumatology or internal medicine departments (three university/two county departments across Sweden) in 2013–2015, with a main ICD-10 diagnosis of PsA (L40.5, M07.0–M07.3), were randomly selected (80 cases/site). Through a structured medical record review, positive predictive values (PPVs) for fulfilment of the following classification criteria were assessed: CASPAR, Moll and Wright, Vasey and Espinoza, and modified ESSG criteria for PsA. A subset analysis regarding CASPAR fulfilment was also performed among cases with available rheumatoid factor and peripheral X-ray status (central CASPAR items; n = 227).
Results
Of the 400 patients with a main ICD-10 diagnosis of PsA, 343 (86%) fulfilled at least one of the four PsA classification criteria. PPVs for the different criteria were: CASPAR 69% (82% in the subset analysis), Moll and Wright 51%, Vasey and Espinoza 76%, and modified ESSG 64%. Overall, only 6.5% of the 400 PsA diagnoses were judged as clearly incorrect by the medical record reviewers.
Conclusion
The validity of rheumatologist-made, clinical ICD-10 diagnoses for PsA in the Swedish NPR is good, with PPVs of 69–82% for CASPAR fulfilment and 86% for meeting any established PsA classification criteria.
Psoriatic arthritis (PsA) is a chronic inflammatory disease associated with psoriasis and is part of the spondyloarthritis (SpA) group of disorders. The clinical presentation of PsA is heterogeneous, with musculoskeletal manifestations comprising arthritis, dactylitis, enthesitis, and spondylitis, and apart from psoriasis other extra-articular manifestations, such as inflammatory bowel disease and uveitis, may also occur (Citation1–3). In the absence of established diagnostic criteria or specific biomarkers, the clinical diagnosis of PsA and its differentiation from other arthritides, such as rheumatoid arthritis (RA), is sometimes a challenge, and the diagnostic work-up encompasses evaluation of personal and family history of psoriasis, clinical findings, laboratory tests, and imaging.
The heterogeneity of PsA has led to repeated attempts to construct classification criteria (Citation4, Citation5). The first to reach widespread use was proposed by Moll and Wright in the 1970s, defining PsA as a combination of psoriasis, inflammatory arthritis, or spondylitis, and absence of rheumatoid factor (RF) (Citation6). Later criteria are generally more complex (Citation7–11), some including synovial fluid analysis (Citation7) or human leucocyte antigen (HLA) profile (Citation11). As part of the SpA family, a modification of the European Spondyloarthropathy Study Group (ESSG) criteria for SpA has also been proposed to define PsA, by including a demand for psoriasis or a family history of this (Citation4, Citation12). Since their publication in 2006, however, the ClASsification criteria for Psoriatic ARthritis (CASPAR) have replaced all prior criteria sets as today’s standard PsA classification criteria (Citation13). Nonetheless, the differences in included items and their weights cause all the proposed PsA classification criteria to capture somewhat different patient populations, and no single, universally accepted gold standard exists.
In Sweden, clinical diagnoses in the form of International Classification of Diseases (ICD) diagnostic codes (Citation14) from visits and healthcare episodes in specialized (i.e. non-primary) care are systematically reported to the National Patient Register (NPR) at the National Board of Health & Welfare (Citation15). Based on ICD codes in the NPR, national cohorts of patients with specific diagnoses can thus be identified, and linking this to other data sources offers unique opportunities to study occurrence, risk factors, and outcomes of different diagnoses. For the interpretation of such register-based, epidemiological studies, however, knowledge of the correspondence between clinical ICD diagnoses and fulfilment of established classification criteria for the relevant condition is fundamental.
For RA and ankylosing spondylitis (AS), prior validation studies of the respective ICD codes in the NPR found positive predictive values (PPVs) for the fulfilment of relevant classification criteria of 91% for RA and 70–89% (depending on the criteria used) for AS (Citation16, Citation17). Regarding PsA, only a smaller, regional study is available, reporting the proportion of PsA ICD diagnoses from specialized care that could be confirmed by medical record review to range from 69% to 93%, according to the assessors’ judgement (Citation18).
Thus, the main objective of the present study was to validate the ICD codes for PsA in the Swedish NPR in relation to fulfilment of the CASPAR and other established PsA classification criteria by a nationwide approach.
Method
Study setting
In Sweden, administrative data, including dates and ICD codes, from visits and healthcare episodes in specialized (i.e. non-primary) care are reported to the NPR (Citation15). The NPR consists of the inpatient register (IPR), containing information regarding inpatient care episodes since 1964, and with 100% coverage from 1987 onwards, and the outpatient care register (OPR). The latter was started in 2001, and since then it has been mandatory for healthcare providers in specialized care to report data on outpatient visits to physicians to the OPR, although the coverage remains lower than in the IPR, mainly lacking information from some private caregivers. In 2011, the OPR coverage was estimated at 87% (Citation19). For each reported healthcare visit/episode in the NPR, one main and, optionally, one or more secondary ICD diagnoses are recorded. Since 1997, clinical diagnoses in Sweden have been registered according to the Swedish version of the ICD-10. Before this, previous versions were used, as follows: ICD-7, 1964–1968; ICD-8, 1968–1986; and ICD-9, 1987–1996.
Prescription drugs dispensed by Swedish pharmacies have been registered in the Prescribed Drug Register (PDR) since 2005. Linkage of information from different administrative/healthcare registers within Sweden can be done via the personal identification number, unique to every permanent resident.
Study population
All individuals, alive and residing in Sweden on 31 December 2015, and having received at least one ICD code for PsA (ICD-8: 696.00; ICD-9: 696A; ICD-10: L40.5, M07.0, M07.1, M07.2, or M07.3) as main or secondary diagnosis in 1968–2015, were identified from the NPR. For the current validation, a subpopulation of all such prevalent PsA patients in 2015 was selected, by defining inclusion criteria requiring at least one ICD-10 code for PsA as the main diagnosis from an outpatient visit (i.e. in the OPR) to a rheumatology or internal medicine department in 2013–2015. The rationale for the 2013–2015 time period was to ensure that the medical records would be accessible, sometimes spanning several decades, but at the same time to allow some years to have passed before the medical record review was performed in 2019 (see ‘Validation process and outcomes’, below), to enable some accumulation of clinical information also for incident cases. Furthermore, PsA patients in Sweden are typically diagnosed and treated in specialized outpatient care (Citation18), normally at departments of rheumatology or internal medicine (in non-university hospitals, rheumatology is often part of internal medicine), although milder cases, not requiring disease-modifying anti-rheumatic drug therapy, may be referred back to primary care after the diagnosis has been made.
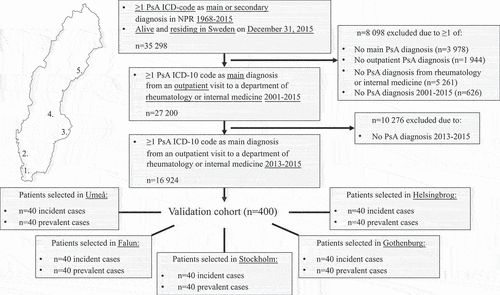
For the validation process, 400 patients (the validation cohort) were selected from five hospitals across Sweden (80 patients per site), all fulfilling the inclusion criteria described above (see ‘Study population’). The five validation sites – Helsingborg Hospital (Helsingborg), Sahlgrenska University Hospital (Gothenburg), Karolinska University Hospital (Stockholm), Falun Hospital (Falun), and Norrland University Hospital (Umeå) – were chosen to ensure a geographical spread across Sweden, as well as including both university (n = 3) and county (n = 2) clinics (). At each site, 40 patients who had received their first ever PsA ICD code in 2013–2015 (i.e. incident-appearing from a register identification perspective) and 40 with at least one PsA ICD code prior to 2013 (prevalent-appearing) were selected. Apart from this stratification, the case selection at each site was carried out randomly.
Figure 1. Flowchart describing the patient selection to the validation cohort. Validation sites shown in the map of Sweden: 1. Helsingborg Hospital (Helsingborg); 2. Sahlgrenska University Hospital (Gothenburg); 3. Karolinska University Hospital (Stockholm); 4. Falun Hospital (Falun); 5. Norrland University Hospital (Umeå). Inclusion criteria for the validation cohort were having received at least one ICD-10 code for PsA as the main diagnosis from an outpatient visit to a department of rheumatology or internal medicine in 2013–2015. From each of the five different study sites, 40 incident (with first ever ICD code for PsA 2013–2015) and 40 prevalent (at least one PsA ICD code prior to 2013) cases were randomly selected. ICD-10, International Classification of Diseases, 10th Revision; NPR, Swedish National Patient Register; PsA, psoriatic arthritis.
Ethics
Ethical approval was granted by the Regional Ethics Committee in Stockholm, Sweden (Dnr. 2015/1844-31/2 with amendment Dnr. 2018/182-32). Consent from individual patients was not required by the approval.
Validation process and outcomes
For the validation of ICD-10 codes for PsA against established classification criteria, the medical records from specialized care (i.e. non-primary care, but also encompassing specialities beyond rheumatology and internal medicine) of the 400 patients in the validation cohort were reviewed according to a structured protocol (see online supplement). The reviews were performed during 2019 by five physicians (coauthors JKW, GMA, EK, VS, and SW), all specialists in rheumatology, and each responsible for all 80 reviews at their study site. The data extraction protocol had been jointly developed and tested by the five assessors prior to the start of the reviews. Records were reviewed from as far back in time as deemed relevant and possible for logistical/access reasons and up until the time of the review in 2019.
The primary outcome was the PPV of having received at least one main ICD-10 code for PsA from a department of rheumatology or internal medicine in the NPR in 2013–2015 for the fulfilment of the CASPAR criteria for PsA (Citation13). As secondary outcomes, the corresponding PPVs were also assessed for the fulfilment of the Moll and Wright (Citation6), Vasey and Espinoza (Citation8), and modified ESSG criteria for PsA (Citation4, Citation12), as well as for fulfilment of at least one of the four included PsA criteria. Classification criteria sets requiring synovial fluid or HLA profiling were not included owing to the low likelihood of finding such information in the medical records (Citation7, Citation11). Moreover, fulfilment of the Assessment of SpondyloArthritis international Society (ASAS) classification criteria for peripheral or axial SpA (Citation20) and of the modified 1987 American College of Rheumatology (ACR) classification criteria for RA (Citation21) was also evaluated. PPVs were calculated for the full validation cohort, as well as for the prevalent and incident groups separately. In keeping with how medical records are structured, missing information regarding a specific criteria item was treated as negative. For items on imaging, the information available in the clinical radiologist reports was used.
Apart from assessing PPVs in the full validation cohort (n = 400), stricter PsA case definitions were also evaluated: (i) requiring at least two PsA ICD codes ever, of which at least one as main diagnosis from a department of rheumatology or internal medicine in 2013–2015 (n = 353); (ii) excluding cases with at least one main RA diagnosis from a department of rheumatology or internal medicine ever (n = 345); and (iii) applying a combination of (i) and (ii) (n = 301). Because of the retrospective design, a fair amount of missing data was expected regarding imaging, RF, and HLA-B27 status, thus limiting the ability to assess fulfilment of classification criteria including such items. Therefore, subset analyses were also performed, restricted to cases for whom such information was present.
As further secondary outcomes, the degrees of overlap between fulfilment of different criteria were evaluated: (i) between the four PsA criteria sets; and (ii) between fulfilment of any PsA criteria and the ASAS peripheral or axial SpA and ACR RA criteria. Moreover, we aimed to assess the clinical certainty of the PsA diagnoses in three ways: (i) as judged by the rheumatologists performing the medical record reviews (scored as: Certain PsA/Uncertain PsA/Not PsA); (ii) by the proportion for whom PsA remained the clinical diagnosis according to the treating physician at the latest available follow-up visit prior to the medical record review; and (iii) by assessing the proportion of cases who had also received ICD codes for other arthritic diseases.
To assess the generalizability of results from the validation cohort to all PsA cases in Sweden, age, sex, and register-based frequencies of psoriasis, extra-articular manifestations, and use of relevant drugs [according to ICD codes in the NPR and Anatomical Therapeutic Chemical classification (ATC) codes in the PDR] were compared between the validation cohort and all PsA cases in the NPR, according to different case definitions.
Finally, for the incident-appearing PsA cases (n = 200), the time from symptom onset to the first recorded PsA ICD code was assessed to determine the feasibility of identifying incident PsA based on ICD codes in the NPR.
Statistics
The proportions of cases fulfilling the different classification criteria were compared between the five study sites, males and females, and younger and older patients by the chi-squared test. (Results are presented in Supplementary Tables S5 and S6.) Apart from this, only descriptive statistics were used.
Results
Study population and disease characteristics
In total, 35 298 individuals, alive and residing in Sweden on 31 December 2015, with at least one ICD code for PsA in the NPR 1968–2015, were identified. Of these, 16 924 fulfilled the study inclusion criteria, from whom the validation cohort of 400 patients was selected (). From the generalizability assessments performed, the included validation cohort appeared to be representative of the overall PsA population in Sweden (Supplementary Table S2).
Characteristics of the validation cohort are presented in , while a more detailed description of all classification criteria items assessed is displayed in Supplementary Table S3. For some items, a high degree of missing data hampers interpretation (Supplementary Table S3), and also affects the possibility for patients to fulfil the corresponding classification criteria. Of the 400 subjects, 353 (88%) had clinically verified skin/nail psoriasis, while the corresponding figure for a medical history of psoriasis was 375 (94%; four prevalent- and 21 incident-appearing cases lacked psoriasis history). Regarding PsA manifestations, 368 patients (92%) displayed clinically verified arthritis (seen in 88%), dactylitis (in 24%), and/or enthesitis (in 39%) at least once, whereas inflammatory back pain was reported by 95 cases (24%). The combination of a medical history of psoriasis and any PsA manifestation (arthritis, dactylitis, enthesitis, or inflammatory back pain) was present in 355 (89%), and arthritic changes in the hands/feet on plain X-rays in 124 patients (31%; a figure that could potentially be higher, since 20% of cases lacked X-ray data).
Table 1. Demographics and disease characteristics.
The numerically lower frequencies of several criteria items observed in the incident group (; Supplementary Table S3) may partly reflect their shorter disease duration and thus follow-up time (with fewer visits/less available information) in the medical records.
Classification criteria fulfilment
Of the 400 patients in the validation cohort, 275 (69%) fulfilled the CASPAR criteria for PsA based on the information available in their medical records (i.e. the PPV for CASPAR fulfilment was 69%) (). The use of stricter PsA case definitions resulted in similar estimates (PPV 68–73%) (). Since RF status and X-ray changes in hands/feet are both central items for CASPAR, missing information regarding these items (33% and 20%, respectively) will, however, have negatively affected the estimation. When limiting the assessment to patients for whom RF and X-ray information was available (n = 227), the PPV rose to 82% ().
Figure 2. Classification criteria fulfilment. Proportions of patients in the full validation cohort (n = 400) fulfilling different classification criteria (i.e. percentages corresponding to positive predictive values of having received at least one main ICD-10 code for PsA from an outpatient visit to a department of rheumatology or internal medicine in NPR 2013–2015 for the fulfilment of the different criteria). *CASPAR, Moll and Wright, Vasey and Espinoza, or modified ESSG criteria for PsA. ACR, American College of Rheumatology; ASAS, Assessment of SpondyloArthritis international Society; CASPAR, ClASsification criteria for Psoriatic ARthritis; ESSG, European Spondyloarthropathy Study Group; periph., peripheral; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SpA, spondyloarthritis.
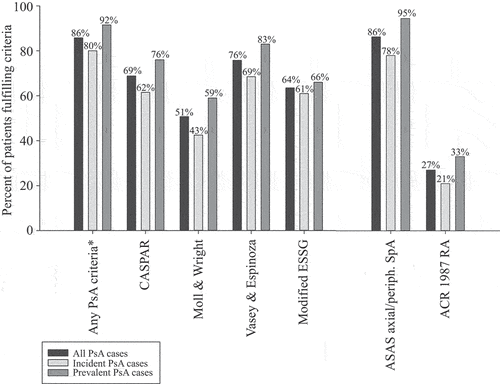
Table 2. Subset analyses regarding classification criteria fulfilment.
In total, 343 (86%) of the 400 patients fulfilled at least one of the four assessed PsA classification criteria (i.e. the PPV for fulfilment of at least one criteria set was 86%) (). The corresponding estimates when using stricter case definitions ranged from 88% to 91% (). The Vasey and Espinoza criteria were met by the numerically highest proportion of patients (76%) and the Moll and Wright definition by the lowest (55%) (), but a substantial overlap was observed, with 36% fulfilling all four PsA criteria and 61% three or more of these ().
Figure 3. Overlap of classification criteria fulfilment. (A) Venn diagram displaying the numbers of cases in the full validation cohort (n = 400) meeting each of the four different PsA classification criteria, and the overlap between fulfilment of these. Patients not fulfilling any of the assessed PsA classification criteria (n = 57) are not included in the figure. (B) Venn diagram displaying the numbers of cases in the full validation cohort (n = 400) meeting any of the four assessed PsA classification criteria, the modified 1987 ACR criteria for RA, and the ASAS criteria for axial or peripheral SpA, and the overlap between fulfilment of these. Patients not fulfilling any of the included classification criteria (n = 30) are not included in the figure. *CASPAR, Moll and Wright, Vasey and Espinoza, or modified ESSG criteria for PsA. ACR, American College of Rheumatology; ASAS, Assessment of SpondyloArthritis international Society; CASPAR, ClASsification criteria for Psoriatic ARthritis; ESSG, European Spondyloarthropathy Study Group; Mod., modified; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SpA, spondyloarthritis.
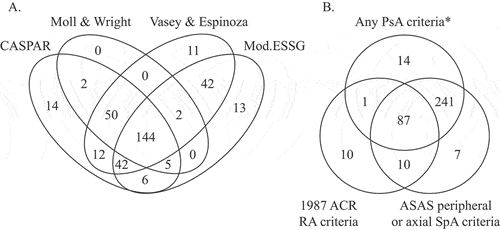
Most patients not fulfilling any PsA criteria had either no verified arthritis (23 of 57 cases) or polyarticular disease (25 of 57 cases) (Supplementary Table S4). Of the 343 subjects meeting any of the PsA criteria, 12 (3.5%) did so despite lacking a medical history of skin/nail psoriasis. Owing to the lower frequencies of several criteria items among the incident patients, fulfilment of all PsA criteria was somewhat lower in this group ( and ).
The ASAS criteria for axial or peripheral SpA were fulfilled by 345 (86%) of the 400 patients (). Of these, only 24 met the axial criteria, although this should be viewed with great caution owing to the high numbers of missing data regarding sacroiliac joint imaging and HLA-B27 status (74–86%) (Supplementary Table S3), which are central to this criteria set. Of the 345 patients fulfilling either set of ASAS criteria, only 17 did not also fulfil any of the four PsA criteria (). Of the 400 patients, 108 (27%) met the ACR RA criteria () [RF positive/negative/missing: n = 18/66/24; anti-citrullinated protein antibody (ACPA) positive/negative/missing: 9/61/38; RF and ACPA positive: n = 5], again with a substantial overlap with PsA criteria fulfilment (only 20 patients fulfilled the RA but none of the four PsA criteria) ().
Clinical certainty of PsA diagnoses
According to the clinical judgement of the rheumatologists performing the medical record reviews, only 6.5% (n = 26) of the 400 PsA diagnoses were judged as clearly incorrect, with 76% (n = 305) considered certain (). Moreover, for 87% of the cases, PsA remained the clinical diagnosis according to the treating physician at the most recent follow-up visit available in the medical record at the time of review (). Results regarding the proportions of cases also having received ICD codes for other arthritic diseases are presented in the online supplement.
Table 3. Assessors’ judgement and clinical diagnoses at the latest available follow-up visit.
Time from symptom onset to PsA diagnosis among incident cases
The median (interquartile range) time from reported symptom onset to the first recorded PsA ICD code in the NPR for the incident-appearing group was 3.0 (1.1–10) years (). This estimate may be artificially long, however, since for 37 incident-appearing patients the reported symptom onset actually occurred before 2001, when the recording of outpatient care ICD diagnoses in the NPR was initiated. If the true diagnosis dates for these patients occurred prior to 2001 (and in outpatient care), they would thus have been missed. Even if excluding these 37 cases, however, the median (interquartile range) time from symptom onset to first PsA ICD code remained quite long, at 2.1 (0.9–5.0) years.
Discussion
Main findings
In this nationwide validation study of ICD codes for PsA in the Swedish NPR (n = 400 cases, found to be representative of the overall PsA population), the PPV of having received at least one ICD-10 code for PsA as the main diagnosis from an outpatient visit to a rheumatology or internal medicine department was 86% for the fulfilment of at least one of four established PsA classification criteria (CASPAR, Moll and Wright, Vasey and Espinoza, and modified ESSG). For the CASPAR alone, the corresponding PPV was 69%, but rising to 82% when limiting the assessment to cases for whom relevant RF and X-ray information was available. Moreover, according to the rheumatologists performing the medical record reviews, only 6.5% of the assessed diagnoses were judged as clearly wrong. Although 27% of the patients fulfilled ACR criteria for RA, only 20 patients (5% of all the 400 cases) did so without simultaneously meeting any of the PsA criteria.
On the other hand, in light of the rather long median time from symptom onset to the first recorded PsA ICD code in the NPR for the incident-appearing group, it does not appear feasible to use the date of the first ICD code in NPR as a proxy for the time of PsA onset. However, such relationships may potentially change over time.
Previous research
To our knowledge, the validity of ICD codes for PsA in Sweden has previously only been assessed in a smaller, regional study from Skåne county (Citation18), encompassing both cases identified based on ICD codes from any specialized care department (and thus in the NPR; n = 60), and from primary care only (not reported to the NPR; n = 40). According to the assessors’ judgement, 69% of the PsA diagnoses from specialized care could be verified, while 24% were judged as unverified PsA owing to insufficient information, and 6.8% as not PsA. These figures are quite comparable to the present results (76%, 17%, and 6.5%, respectively) (). However, only 49% of patients in the regional assessment were found to fulfil CASPAR criteria, versus 69% in the current study. Reasons for this discrepancy may include the requirement for a main diagnosis from rheumatology or internal medicine in the present study, and a disproportionally high share (one-third) of patients in the regional study having received only one PsA ICD code from specialized care ever. In fact, of the 20 cases with two or more such ICD codes, 74% fulfilled CASPAR criteria, a finding very similar to our corresponding 73% estimate ().
In the few international PsA validation studies published to date, the gold standards used were not classification criteria fulfilment, making comparisons to the current results difficult. Instead, most use diagnosis according to the responsible physician/rheumatologist as standard, reporting PPVs of various ICD-9 or Read (Citation22) code-based PsA definitions for such a diagnosis in the range of 57–100% (Citation23–26). One study, however, reported 67% of 73 cases with at least one ICD-9 code for PsA from rheumatology to have a correct diagnosis according to the medical record reviewer (Citation27), a figure fairly consistent with our estimate of 76% with certain PsA according to the assessors. Moreover, case definitions requiring diagnostic codes specifically from rheumatology have been shown to render higher PPVs (Citation26, Citation27), as has the requirement for two or more diagnostic events (as also seen in the present study) () (Citation27).
Strengths and limitations
The nationwide approach and structured medical record review (from as far back in time as deemed relevant, and not limited to rheumatology or internal medicine records) by specialists in rheumatology, and using a jointly predefined and tested protocol, are important strengths of this study. The inclusion of four different established PsA classification criteria, as well as various means to evaluate the clinical certainty of the PsA diagnoses, provides a comprehensive assessment of the validity of the PsA ICD codes in the NPR.
By including prevalent as well as incident cases from both university and county clinics across Sweden, we believe the generalizability of our results to be good in relation to the entire PsA population, fulfilling our case definition with at least one PsA ICD code as main diagnosis from an outpatient visit to a rheumatology or internal medicine department (corresponding to 77% of all individuals with at least one PsA ICD code in the NPR) (). This was also strengthened by the generalizability assessments performed (Supplementary Table S2). However, patients diagnosed with PsA in primary care, only in inpatient care, or within specialized care departments other than rheumatology or internal medicine were not part of the present validation. As for primary care, the prior regional validation study found that only 8.4% of all PsA cases had never been diagnosed in specialized care (Citation18). Moreover, diagnoses from private rheumatologists, some of whom may not report ICD codes to the NPR, were also not validated. The extent of private care is, however, limited in Sweden, and most patients followed at such units will occasionally also consult public rheumatology departments.
The fact that certain rheumatologists at some of the study sites (but not all) had an academic interest in PsA may potentially have affected the validation results positively. Included cases were, however, randomly selected (rather than among those clinically followed by rheumatologists with such academic interest), and the good agreement of criteria fulfilment across all study sites speaks against such bias (Supplementary Table S5). Moreover, with the continuously evolving knowledge of PsA and its typical characteristics, we cannot rule out secular trends in ICD-code validity. Such problems, however, are likely to increase the further back in time one looks, and the outpatient part of the NPR, which the current validation concerns, was not started until 2001.
Regarding differentiation from RA, only 22 of the 108 patients meeting the ACR RA criteria were known to be RF and/or ACPA positive, and it is possible that use of the 2010 ACR/European League Against Rheumatism (EULAR) RA criteria instead, with their greater weighting of serology, would have resulted in less overlap between PsA and RA criteria fulfilment (Citation28).
The relatively high degree of missing data for some criteria items (Supplementary Table S3) is partly a consequence of the retrospective design. In medical records, the lack of information regarding some types of items (e.g. ‘dactylitis diagnosed by physician’) will often correspond to a true absence, since negative findings are often not noted down. This may, however, not always be the case, and by treating missing data as negative, the current results regarding classification criteria fulfilment should be viewed as conservative estimates, since more cases would be likely to meet the criteria if all relevant information were available.
Conclusion
The validity of clinical PsA diagnoses from outpatient rheumatology or internal medicine units in the Swedish NPR is good, with a positive predictive value of 69–82% of having received at least one main ICD-10 diagnosis of PsA for CASPAR criteria fulfilment, and of 86% for meeting at least one of four established PsA classification criteria. Thus, the use of this definition to identify PsA cases for future epidemiological studies on the basis of ICD codes in the NPR appears feasible.
Authors contributions
JKW contributed to study conception and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. GMA, EK, VS, SW, and DDG contributed to study conception and design, acquisition and interpretation of data, and critically revised the manuscript, making intellectual contribution to its content. SE, UL, JA, and LTHJ contributed to study concept and design, interpretation of data and critically revised the manuscript, making intellectual contribution to its content. All authors read and approved the final manuscript.
Supplemental Material
Download MS Word (62.9 KB)Acknowledgement
Special thanks to our patient representative Britt Nilsson for her valuable input.
Disclosure statement
JKW: consultant for AbbVie, Amgen, Celgene, Eli Lilly, and Novartis. EK: speakers’ bureaux for Eli Lilly; consultant for Novartis; grant/research support from Roche. VS: consultant for Astra Zeneca, Novartis, and Sanofi. SE: consultant for AbbVie, Janssen and Novartis. JA: grant/research support for the Swedish biologics register ARTIS from AbbVie, BMS, Eli Lilly, Merck, Pfizer, Roche, Samsung Bioepis, Sanofi, and UCB. LTHJ: consultant for AbbVie, Eli Lilly, Janssen, Novartis, and Pfizer. No potential conflicts of interest were reported by the remaining authors (GMA, SW, UL, and DDG).
Supplemental material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/03009742.2022.2066807.
References
- Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016;68:1060–71.
- Kasiem FR, Luime JJ, Vis M, Kok MR, Wervers K, Gerards AH, et al. Lessons learned from clinical phenotypes in early psoriatic arthritis: the real-world Dutch south west early Psoriatic ARthritis study. Scand J Rheumatol 2021;50:124–31.
- Pittam B, Gupta S, Harrison NL, Robertson S, Hughes DM, Zhao SS. Prevalence of extra-articular manifestations in psoriatic arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2020;59:2199–206.
- Helliwell PS, Taylor WJ. Classification and diagnostic criteria for psoriatic arthritis. Ann Rheum Dis 2005;64:ii3–8.
- Rudwaleit M, Taylor WJ. Classification criteria for psoriatic arthritis and ankylosing spondylitis/axial spondyloarthritis. Best Pract Res Clin Rheumatol 2010;24:589–604.
- Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973;3:55–78.
- Bennett RM. Psoriatic arthritis. In: McCarty DJ, editor. Arthritis and related conditions. Philadelphia, PA: Lea & Febiger, 1979:645.
- Vasey F, Espinoza LR. Psoriatic arthropathy. In: Calin A, editor. Spondyloarthropathies. Orlando, FL: Grune & Stratton, 1984:151–85.
- Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA) – an analysis of 220 patients. Q J Med 1987;62:127–41.
- McGonagle D, Conaghan PG, Emery P. Psoriatic arthritis: a unified concept twenty years on. Arthritis Rheum 1999;42:1080–6.
- Fournie B, Crognier L, Arnaud C, Zabraniecki L, Lascaux-Lefebvre V, Marc V, et al. Proposed classification criteria of psoriatic arthritis. A preliminary study in 260 patients. Rev Rhum Engl Ed 1999;66:446–56.
- Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum 1991;34:1218–27.
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006;54:2665–73.
- World Health Organization. International statistical classification of diseases and related health problems (ICD). [ cited 2022 January 14]. Available from: https://www.who.int/classifications/classification-of-diseases.
- Socialstyrelsen. The National Patient Register. [ cited 2022 January 14]. Available from: https://www.socialstyrelsen.se/en/statistics-and-data/registers/register-information/the-national-patient-register.
- Waldenlind K, Eriksson JK, Grewin B, Askling J. Validation of the rheumatoid arthritis diagnosis in the Swedish National Patient Register: a cohort study from Stockholm County. BMC Musculoskelet Disord 2014;15:432.
- Lindstrom U, Exarchou S, Sigurdardottir V, Sundstrom B, Askling J, Eriksson JK, et al. Validity of ankylosing spondylitis and undifferentiated spondyloarthritis diagnoses in the Swedish National Patient Register. Scand J Rheumatol 2015;44:369–76.
- Lofvendahl S, Theander E, Svensson A, Carlsson KS, Englund M, Petersson IF. Validity of diagnostic codes and prevalence of physician-diagnosed psoriasis and psoriatic arthritis in southern Sweden–a population-based register study. PLoS One 2014;9:e98024.
- Socialstyrelsen. Kodningskvalitet i patientregistret. Ett nytt verktyg för att mäta kvalitet. 2013 [ cited 2022 January 14]. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2013-3-10.pdf.
- Rudwaleit M, van der Heijde D, Landewe R, Akkoc N, Brandt J, Chou CT, et al. The assessment of SpondyloArthritis international society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011;70:25–31.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24.
- Chisholm J. The Read clinical classification. BMJ 1990;300:1092.
- Singh JA, Holmgren AR, Krug H, Noorbaloochi S. Accuracy of the diagnoses of spondylarthritides in Veterans Affairs medical center databases. Arthritis Rheum 2007;57:648–55.
- Love TJ, Cai T, Karlson EW. Validation of psoriatic arthritis diagnoses in electronic medical records using natural language processing. Semin Arthritis Rheum 2011;40:413–20.
- Ogdie A, Alehashemi S, Love TJ, Jiang Y, Haynes K, Hennessy S, et al. Validity of psoriatic arthritis and capture of disease modifying antirheumatic drugs in the health improvement network. Pharmacoepidemiol Drug Saf 2014;23:918–22.
- Lee H, Ford JA, Jin Y, Cho SK, Santiago Ortiz AJ, Tong AY, et al. Validation of claims-based algorithms for psoriatic arthritis. Pharmacoepidemiol Drug Saf 2020;29:404–8.
- Asgari MM, Wu JJ, Gelfand JM, Salman C, Curtis JR, Harrold LR, et al. Validity of diagnostic codes and prevalence of psoriasis and psoriatic arthritis in a managed care population, 1996–2009. Pharmacoepidemiol Drug Saf 2013;22:842–9.
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8.
