Abstract
Protocirrineris mascaratus sp. nov., is described from intertidal reef flats in Oahu, Hawaii. It is unique among its congeners by the segmental origin of the feeding tentacles and branchiae in addition to the very distinct methyl green staining pattern on the anterior end. A redescription of Protocirrineris nuchalis based on syntypes and a comparative table of all valid species within Protocirrineris is presented.
Introduction
Multitentaculate cirratulids are organised into five genera based on the relative position of feeding tentacles and branchial filaments, number of branchiae per segment, and presence of modified chaetae. Of the four genera that possess modified chaetae, Cirriformia Hartman, 1936 and Timarete Kinberg, 1866 differ from Cirratulus Lamarck, 1801 and Fauvelicirratulus Çinar & Petersen, Citation2011 in having branchial filaments with an earlier segmental origin than feeding tentacles; however, the branchiae in Timarete shift to the mid-dorsum on posterior segments forming a lateral bulge over the notopodia while in Cirriformia they are inserted near the notopodial base throughout. Feeding tentacles and branchial filaments have the same segmental origin (chaetiger 1) in Cirratulus and Fauvelicirratulus but the latter differs in having multiple pairs of branchiae per segment and straight, tapering spines as opposed to a pair of branchiae per segment and stout, curved spines in the former (Çinar & Petersen Citation2011). Among the multitentaculate cirratulid genera, the genus Protocirrineris Czerniavsky, 1881 is unique in having only fimbriated capillaries throughout, lacking modified chaetae and feeding tentacles organised in longitudinal rows over several segments.
The genus Protocirrineris was originally proposed as a subgenus of Cirrhinereis Blainville, 1818, in Audouin & Milne Edwards (Citation1833). Hartman (Citation1959) erected the subgenus for Cirratulus tenuisetis Grube, 1860 and Petersen (Citation1991) reinstated and partly defined the subgenus as multitentaculate species which lack modified chaetae and included species of the Cirratulus chrysoderma complex within it. Blake (Citation1996) recognised Protocirrineris as a genus and described a new species, Protocirrineris socialis Blake, Citation1996, from California. Presently, the genus Protocirrineris has five described species: P. chrysoderma (Claparède, 1868) originally described from Gulf of Naples but reported to be widespread and belonging to a species complex (Petersen Citation1991), P. nuchalis (Ehlers, Citation1907) from New Zealand, P. antarctica (Monro, Citation1930) from Antarctica, P. socialis from California, USA, and P. angelicollatio Elías & Rivero, Citation2009 from Mar del Plata, Argentina. We describe Protocirrineris mascaratus sp. nov. from specimens collected in Oahu, Hawaii, and redescribe P. nuchalis from syntype material. A table with relevant morphological characteristics of all six species is presented and characters used for species-level distinction are discussed.
Materials and methods
The type series of the new species was collected on the invasive alga Avrainvillea amadelpha (Montagne) A. Gepp & E.S. Gepp 1908 present on nearshore reef flats in Maunalua Bay, south shore of Oahu, Hawaii. The area is predominantly composed of consolidated limestone reef flats covered by a shallow substratum of fine to coarse sand. All samples were fixed in buffered formalin and Rose Bengal mixture immediately after the sampling for a minimum of 48 h. Organisms were carefully removed from the crevices and branches of the algae and placed in 70% ethanol. Additional material has been found in samples taken during several monitoring programmes in Mamala Bay, south shore of Oahu in the vicinities of the sewage outfalls of Sand Island and Barbers Point. These samples were collected with a van Veen grab and fixed in a buffered solution of formalin and Rose Bengal. The fixed samples were elutriated over a 0.5-mm sieve and the organisms were sorted and preserved in 70% ethanol.
For scanning electron microscopy (SEM) examination, specimens were dehydrated through a series of increasing concentrations of ethanol ending with three changes of absolute ethanol followed by critical point drying (in a SAMDRI-795). They were mounted on stubs and coated with gold/palladium for about 2 min. SEM observations were carried out using the Hitachi S-4800 at the Biological Electron Microscopy Facility (BEMF), University of Hawaiıˆ at Mānoa.
Type specimens are deposited in the Bernice Pauahi Bishop Museum, Honolulu, Hawaii, USA (BPBM), and the United States National Museum of Natural History, Smithsonian Institution, Washington, DC, USA (USNM). Syntypes of Protocirrineris nuchalis were loaned from the Museum für Naturkunde, Berlin, Germany (MfN).
Results
Family Cirratulidae Ryckholt, 1851
Genus Protocirrineris Czerniavsky, 1881
Type Species: Cirratulus tenuisetis Grube, 1860, designated by Hartman (1959)
Diagnosis (from Blake, 1996)
Prostomium bluntly conical to wedge-shaped, with or without eyes. Body nearly round in cross section, with distinct segments. Grooved tentacular filaments few, arising singly or in paired groups on several anterior chaetigers following chaetiger 1; individual tentacular filaments often arranged in longitudinal rows. Branchiae occurring singly, usually first present from segments with tentacular filaments, sometime on more anterior chaetigers. Chaetae all capillaries.
Protocirrineris mascaratus sp. nov.
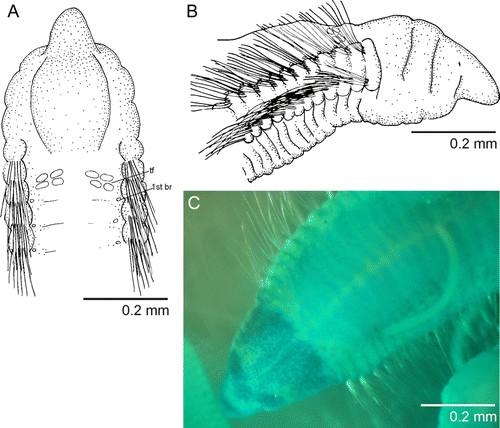
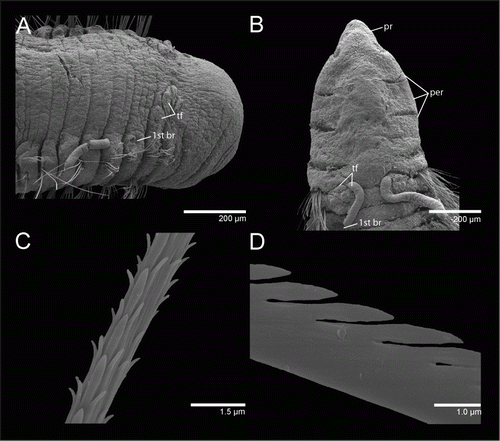
(A–C) and (A–D)
Material examined
Holotype: sandy reef flat near Paiko Lagoon Sanctuary, south shore of Oahu Island, Hawaii, 21° 16' 48.13” N, 157° 43' 52.38” W, shallow subtidal, 0.5 m depth, associated with Avrainvillea amadelpha, 03/06/2010, colls. W. Magalhães and B. Dugan (USNM 1191168). Paratypes: from the same locality, date, and collector as holotype (5, BPBM R3638; 4 mounted on stubs, USNM 1191169). Additional non-type material: Mamala Bay, Sand Island outfall, south shore of Oahu Island, Hawaii, station E5R1, 101.5 m, Aug/08 (1), station C1R3, 18.9 m, Aug/09 (1); Mamala Bay, Barbers Point outfall, south shore of Oahu Island, Hawaii, station HB2R3, 59.4 m, Feb/06 (1); Hanauma Bay, southwest shore of Oahu Island, Hawaii, 4/2000, coll. R. Brock (1). Maui Island, Alii Kahekili Nui Ahumonu Beach Park, collected on Halimeda kanaloana Vroom, 2006 meadows, 30 m, 2005, coll. A. Fukunaga (1).
Description
Holotype 8.0 mm long, 0.4 mm wide for about 126 chaetigers. Additional specimens, including paratypes, were 4–24 mm long, 0.5–1.0 mm wide for 46–212 chaetigers. Body rounded dorsally and ventrally flattened; wider on thoracic chaetigers and tapering posteriorly; last chaetigers indistinct from posterior end chaetigers. Pygidium a simple lobe, anal aperture placed terminally. Body colour pale yellow; inter-segmental areas of peristomium and first and second chaetigers dusky ventrally; some preserved specimens with thoracic chaetigers light to dark brown.
Prostomium short, conical, with a pair of postero-lateral nuchal organs (A, B; A, B); peristomium with three sub-equal annulations, dorsal crest extending over anterior of chaetiger 1 (A, B; A, B). Tentacle filaments two pairs on each side; first pair on posterior of chaetigers 1 or 2, second pair on anterior end of chaetigers 2 or 3 (A; A, B). Branchial filaments arising from posterior-most tentacle-bearer chaetiger, usually above chaetigers 3 or 4 (A; A), one pair per segment, more abundant on first third and absent on last third of the body.
Parapodia well developed in thoracic region forming lateral shoulders (B), becoming more ventral than lateral in mid-body and posterior chaetigers, not forming shoulders or a ventral groove. Chaetae all fimbriated capillaries (C, D); 8–10 on each anterior noto- and neuropodia, reducing to 4–6 posteriorly.
Parapodia well developed in thoracic region forming lateral shoulders (B), becoming more ventral than lateral in mid-body and posterior chaetigers, not forming shoulders or a ventral groove. Chaetae all fimbriated capillaries (C, D); 8–10 on each anterior noto- and neuropodia, reducing to 4–6 posteriorly.
Methyl green staining pattern (MGSP)
Distinctive staining pattern apparent on prostomium, peristomium and anterior end of the first chaetiger; prostomium intensely speckled, with an unstained ocular region; peristomium also intensely speckled, leaving an unstained band between the dorsum of the first and second annulation; very fine line over dorsum of anterior end unstained (C). Remainder of body staining uniformly with a light green; segmental areas more intensely stained than inter-segmental regions (C).
Remarks
summarises the relevant morphological characteristics of all described species of Protocirrineris. Protocirrineris mascaratus sp. nov., is distinct from the recently described species P. angelicollatio and P. socialis by the very distinctive MGSP of the anterior segments. The segmental origin of tentacles and branchiae is very important for differentiating species in this genus. For instance, the segmental origin of tentacles of P. mascaratus sp. nov. occurs much more anteriorly than all other known species. Protocirrineris chrysoderma needs to be revised and redescribed due to the lack of relevant taxonomical information on the original description. Type material of P. nuchalis is darkly coloured (light brown to dark greenish brown) with no distinct MGSP and tentacles are arranged in two longitudinal rows at each side above chaetigers 7–16 (see redescription below and ).
Table 1 Synoptic table of morphological characteristics of species currently assigned to Protocirrineris.
Etymology
Named for the distinct MGSP leaving a ‘mask’ of unstained bands on the peristomium and prostomium.
Biology/ecology
This species was found inhabiting the branches and crevices among blades of the invasive alga Avrainvillea amadelpha and on the calcareous alga Halimeda kanaloana from intertidal to 100 m in depth.
Distribution
Known only from the south coasts of Oahu and Maui islands, Hawaii, USA.
Protocirrineris nuchalis (Ehlers, 1907)
(A and B) and (A–F)
Cirratulus (Cirrineris) nuchalis Ehlers, 1907: 22–23.
Material examined
Syntypes: Waiheke, Auckland harbour, New Zealand, no date (2, MfN #6757).
Description
Syntype 1 complete, 42 mm long, 3 mm wide in thoracic region, reducing to 2 mm wide on abdominal region for about 388 chaetigers (375 in original description). Syntype 2 lacking far posterior chaetigers and in two pieces; first fragment 40 mm long, 1.5 mm wide on thoracic region and 1.0 mm wide on abdominal region for about 240 chaetigers; second fragment a posterior abdominal region 13 mm long, 1 mm wide for 52 chaetigers but lacking pygidium. Body elongate, dorsally inflated, ventrally flattened; thoracic region of 15–20 chaetigers followed by crowded abdominal chaetigers one third as long as thoracic chaetigers. Posterior end not tapering anterior to pygidium; pygidium with terminal anus and two vertical bean-shaped slits (E). Colour in ethanol light brown to dark greenish brown (A–E); tentacles and branchial filaments indistinctly colour, same colour as body for darker specimen and darker than body for lighter specimen, sometimes bicoloured with dorsum darker than ventrum.
Figure 3 Protocirrineris nuchalis. A, anterior end, dorsal view (syntype 1); B, anterior end, lateral view (syntype 1). (tf, tentacular filaments; br, branchia).
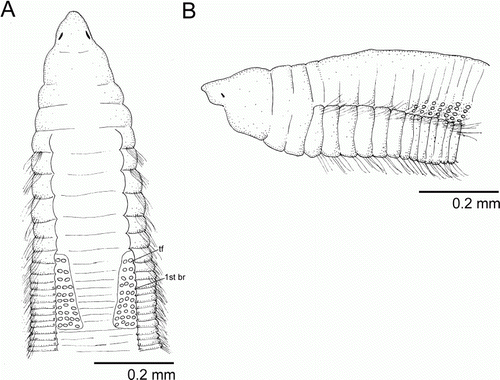
Figure 4 Protocirrineris nuchalis. A, anterior end, dorsal view; B, anterior end, dorso-lateral view showing prostomium and peristomium; C, insertion of tentacular filaments from chaetigers 7–16; D, mid-body in ventral view; E, posterior end with pygidium; F, MGSP of anterior end of syntype 2. (pr, prostomium; per, peristomium; nuO, nuchal organ; ch, chaetiger; br, branchia).
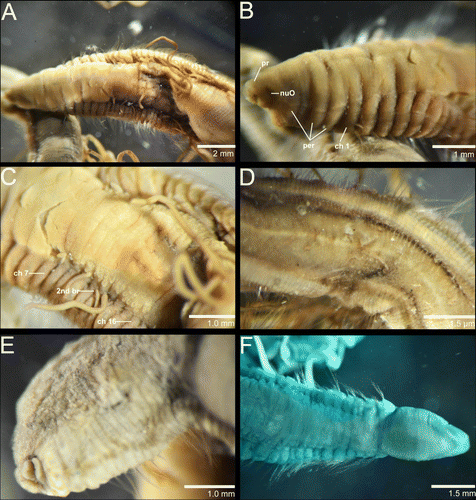
Prostomium conical with large postero-lateral nuchal organs (A, B; A, B). Peristomium with three annuli in syntype 1 (A; B); first annulus longer with 2–3 sub-annulations, second and third ones sub-equals; syntype 2 without distinct dorsal peristomial annuli and with a dorsal crest along peristomium (F). Tentacular filaments paired groups of 2–4 longitudinal rows on each side above chaetigers 7–16 on syntype 1 (A; A, C) and paired double rows above chaetigers 6–16 on syntype 2. Branchial filaments arising from chaetiger 10 in a groove formed by parapodial shoulders, one pair per segment, absent on posterior third of the body (A; C).
Parapodia well-developed forming lateral shoulders from chaetigers 7–10 throughout; noto- and neuropodia project laterally in anterior chaetigers, becoming more ventral in abdominal chaetigers with neuropodia projecting ventrally giving the appearance of a ventral groove (D). Chaetae all fimbriated capillaries arranged in two longitudinal rows of 10–12 capillaries in anterior noto- and neuropodia, reducing in length and number to two rows of 6–8 capillaries posteriorly.
MGSP
Entire body stained light green without a distinct pattern (F).
Remarks
A redescription of this species was needed to meet the recent taxonomic standards for this family following the work of Blake (Citation1991, Citation1996) and because the original description by Ehlers (Citation1907) lacks illustrations. Ehlers (Citation1907) discusses the similarities of P. nuchalis to Cirratulus tenuisetis based on the arrangement of tentacles and dark colouration of the body, differing from the latter by the absence of modified chaetae. Protocirrineris nuchalis differs from its congeners mainly in that its feeding tentacles extend for up to 10 chaetigers while it is restricted to 2–4 chaetigers in other species ().
Distribution
This species is believed to be endemic to New Zealand (Read Citation2004); type locality is Auckland harbour but it has also been recorded at Port of Tauranga (Inglis et al. Citation2006).
Discussion
The genus Protocirrineris is the only multitentaculate cirratulid genus lacking modified chaetae. This could be a plesiomorphic condition as juveniles of the related multitentaculate genus Cirriformia have also been described as having only capillaries throughout (Blake Citation1996). However, the phylogenetic relationships within the Cirratulidae have never been investigated in detail. The lack of reliable characters is problematic in the understanding of the phylogeny of this group, as it has been shown that most of the characters used in species-level descriptions of multitentaculate cirratulids, i.e. segmental origin of feeding tentacles and acicular spines vary ontogenetically (e.g. Magalhães & Bailey-Brock Citation2010).
The six described species of Protocirrineris may be distinguished by the segmental origin, extent and number of longitudinal rows of feeding tentacles (). The origin of branchiae is usually from the posterior-most tentacle-bearer segments but these are sometimes difficult to see without the aid of SEM images. Methyl green staining patterns have been described for four out of the six species but only Protocirrineris mascaratus sp. nov., has a distinctive staining pattern. The ultrastructure of capillary fibrils might be a relevant character but it has only been shown for P. mascaratus sp. nov. and P. angellicolatio and they appear to be distinct in width, shape, and number of fibrils along the capillary chaetal blade (compare C, D herein to Elías & Rivero Citation2009: E, F). The usefulness of capillary fibrils to cirratulid taxonomy has been discussed for the bitentaculate genus Aphelochaeta (Doner & Blake Citation2009) but a unifying terminology is lacking.
The genus Protocirrineris is present in the Antarctic (P. antarctica), southern Atlantic (P. angellicolatio), northeastern Pacific (P. socialis), northwestern Pacific (P. mascaratus), southwestern Pacific (P. nuchalis) and Mediterranean (P. chrysoderma). The geographical distribution of most species is restricted to the type locality with the exception of P. chrysoderma, which has been reported to be widespread in the Atlantic, Mediterranean and Pacific. Petersen (Citation1991), however, argues that P. chrysoderma may be a complex of species and a closer look at these morphologically similar taxa using SEM imaging could reveal that Protocirrineris may be a much more species-rich taxon.
Acknowledgements
This is contributed paper WRRC-CP-2013-03 of the Water Resources Research Center, University of Hawai'i at Mānoa, Honolulu. Tina M Carvalho (BEMF) assisted with SEM procedure. Some specimens were collected with the help of the City and County of Honolulu Oceanographic Team and collection permits. Dr B Neuhaus (MfN) kindly loaned the syntypes of P. nuchalis. B Dugan helped elutriating and sorting the algae samples. This research was partly funded by the Edmondson Research Grants, Department of Zoology, University of Hawai'i at Mānoa to W Magalhães and The National Institutes for Water Resources to JH Bailey-Brock. Two anonymous reviewers greatly improved the manuscript.
References
- Audouin , JV and Milne Edwards , H . 1833 . Classification des Annélides, et description de celles qui habitent les côtes de la France . Annales des Sciences Naturelles , 27 : 337 – 447 . Paris
- Blake , JA . 1991 . Revision of some genera and species of Cirratulidae (Polychaeta) from the western North Atlantic . Ophelia Supplement , 5 : 17 – 30 .
- Blake JA 1996 . 8. Family Cirratulidae Ryckholdt, 1851. Including a revision of the genera and species from the eastern North Pacific . In : Blake JA , Hilbig B , Scott PH Taxonomic atlas of the benthic fauna of the Santa Maria Basin and the Western Santa Barbara Channel . Volume 6 – The Annelida Part 3, Polychaeta: Orbiniidae to Cossuridae . Santa Barbara , CA Santa Barbara Museum of Natural History . Pp. 263 – 384 .
- Çinar , ME and Petersen , ME . 2011 . Re-description of Cirratulus dollfusi (Polychaeta: Cirratulidae), and Fauvelicirratulus as a new genus . Journal of the Marine Biological Association of the United Kingdom , 91 : 415 – 418 . doi: 10.1017/S0025315410000895
- Doner , SA and Blake , JA . 2009 . Two new species of Aphelochaeta (Polychaeta: Cirratulidae) from deep water off northern California . Zoosymposia , 2 : 127 – 137 .
- Ehlers , E . 1907 . Neuseelandische Anneliden II . Abhandlungen der Koniglichen Gesellschaft der Wissenschaften zu Gottingen Mathematisch-Physikalische Klasse neue folge , 5 : 3 – 31 .
- Elías , R and Rivero , MS . 2009 . Two new species of Cirratulidae (Annelida: Polychaeta) from Mar del Plata, Argentina (SW Atlantic) . Zoosymposia , 2 : 139 – 148 .
- Fauvel P 1953 . Annelida Polychaeta . The fauna of India, including Pakistan, Ceylon, Burma, and Malaya . Allahabad . Pp. 1 – 507 .
- Hartman , O . 1959 . Catalogue of the polychaetous annelids of the world . Allan Hancock Foundation Publications, Occasional Paper , 23 : 1 – 628 .
- Inglis G , Gust N , Fitridge I , Floerl O , Woods C , Hayden B , Fenwick G 2006 . Port of Tauranga: baseline survey for non-indigenous marine species (Research Project ZB2000/04) . Biosecurity New Zealand Technical Paper No. 2005/05 .
- Magalhães , WF and Bailey-Brock , JH . 2010 . Redescription of Cirriformia crassicollis (Kinberg, 1866) and Timarete hawaiensis (Hartman, 1956) new combination, (Polychaeta: Cirratulidae), endemic polychaetes to the Hawaiian Islands . Zootaxa , 2625 : 53 – 62 .
- Monro , CCA . 1930 . Polychaete worms . Discovery Reports , 2 : 1 – 222 .
- Petersen ME 1991 . A review of asexual reproduction in the Cirratulidae (Annelida: Polychaeta), with redescription of Cirratulus gayheadius (Hartman, 1965), new combination, and emendation or reinstatement of some cirratulid genera . Bulletin of Marine Science 48 : 592 ( Abstract ).
- Read GB 2004 . Guide to New Zealand Shore Polychaetes . http://www.annelida.net/nz/Polychaeta/References/NZPolySpeciesListV2.htm (accessed 4 June 2012)