Abstract
The New Zealand giraffe weevil (Lasiorhynchus barbicornis) is an endemic, wood-boring insect (Coleoptera: Brentidae). Despite being a large and charismatic species, very little is known about its life history. We conducted observations of L. barbicornis behaviour and ecology in a wild population west of Auckland and developed a marking system in preparation for a future large-scale observational experiment. We found that giraffe weevils were easily located and observable in the field, and therefore provide a useful model species for behavioural ecology. Adults were found to aggregate, copulate and oviposit on 17 host tree species from 16 families, extending the current host plant association records for this species. A breeding experiment determined that the length of the larval stage is a minimum of 2 years and that the population at emergence has an unbiased sex ratio.
Introduction
The giraffe weevil, Lasiorhynchus barbicornis (Fabricius, 1775), (Coleoptera: Brentidae) is endemic, and is the only member of the Brentidae present in New Zealand. It is distributed across the North Island and in the northwestern regions of the South Island (Kuschel Citation2003). Giraffe weevils are generally thought to be rare and cryptic and it is likely that for this reason they are virtually unstudied to date and consequently very little is known about their life history. It is recognized, however, that there is remarkable sexual dimorphism in adults. This is mostly due to the extreme elongation of the male rostrum, which makes up approximately half of its total body length (Kuschel Citation2003). Males use their rostrum during fights with other males for access to females (Meads Citation1976), whereas females use their rostrum to drill holes in wood before oviposition (Hudson Citation1934). In addition, both sexes are highly size variable, with males ranging in body length from 15 to 90 mm and females from 12 to 50 mm (Kuschel Citation2003; CJ Painting unpublished data). The large size variation in males can lead to disparity in reproductive success, which in many species has led to the evolution of alternative reproductive tactics (Gadgil Citation1972; Shuster & Wade Citation2003; Brockmann et al. Citation2008). This, in combination with the presence of an exaggerated trait (the elongated rostrum) in males (Painting & Holwell Citation2013) suggests that L. barbicornis would make an interesting new model species for research on sexual selection and behavioural ecology. For this reason, in the current study we determined whether large-scale observations of behaviour, such as mating and fighting, would be possible in the field by developing methods for observation and insect marking. Furthermore, we aimed to build on previous work (Broun Citation1880; Hudson Citation1934; Pritchard Citation1952; Meads Citation1976; May Citation1993; Kuschel Citation2003) to extend our understanding of the natural history and host plant associations of L. barbicornis.
Materials and methods
Behavioural observations were conducted at Matuku Reserve (36°51.92′S, 174°28.32′E), west of Auckland city over a 3-year period (2010–2013) between late October and April in each year. Adult L. barbicornis aggregate on host trees in the warmer months, during which females drill holes before oviposition while males locate the females and copulate with them. To prepare for a large-scale study on their behavioural ecology, we first located trees around the reserve that hosted aggregations of adult weevils, and then established the best methodology for observing the behaviour of this species. We conducted observations of L. barbicornis and report some notes on their aggregation, defence and feeding behaviour here.
In addition, during the same 3-year period, we conducted trips to 19 other locations around the North Island of New Zealand to record host plant associations. We also located L. barbicornis at one site in the South Island (Totaranui, Abel Tasman National Park). Only trees that hosted more than one individual (including a drilling female) are reported here, to ensure that only those trees likely to be larval hosts are listed, not those used as resting or adult feeding sites. All images were taken by CJ Painting, unless otherwise credited.
Results and discussion
Host trees and locating giraffe weevils
Giraffe weevil adults were commonly found by identifying trees that had clear signs of injury such as sap bleeds and piles of sawdust gathering at the bottom of the tree. Matuku Reserve is a predominantly coastal broadleaf forest (), with an abundance of karaka trees (Corynocarpus laevigatus J.R. Forst. et G. Forst.) (), which were the most common tree species found to host giraffe weevils. At Matuku Reserve, giraffe weevils were also found on māhoe (Melicytus ramiflorus J.R. Forst. et G. Forst.), rangiora (Brachyglottis repanda J.R. Forst. et G. Forst.), tī kōuka (Cordyline australis (G. Forst.f.) Endl.) and tarata (Pittosporum eugenioides A. Cunn.) trees.

Our collection trips around New Zealand revealed a total of 17 tree species from 16 families associated with giraffe weevils (), significantly increasing the record of previously known host trees (Kuschel Citation2003). These records show that L. barbicornis is associated with a large number of tree species, and specifically that karaka and māhoe were the most common hosts. Forest-dwelling brentid larvae are generally xylophagous (Sforzi & Bartolozzi Citation2004), but a dissection of the gut of an L. barbicornis specimen was found to contain only fungal material, suggesting that they rely on fungi rather than the dead wood itself for nutrition (May Citation1993). Therefore, the wide range of host plant associations is interesting, because it would suggest that the fungi that L. barbicornis larvae feed on also have widespread associations with different tree species or that they feed on a range of fungi.
Table 1 Lasiorhynchus barbicornis host tree associations.
The degree of host plant specificity is highly variable among other brentids, and probably relates in many cases to the extent to which the species has been collected in the past. Lasiorhynchus barbicornis does not seem unusual in being highly polyphagous in comparison to other brentids, although the other members of the tribe Ithystenini are recorded to be only associated with a single host species (Sforzi & Bartolozzi Citation2004).
Observation techniques
To ensure the success of conducting a large-scale study of mating and fighting behaviour of L. barbicornis, we first determined the possibility of observing this species in the wild. We found that observations of the behaviour of adult L. barbicornis in the field are relatively easy because large aggregations of both sexes can be found on dead fallen trees or dying standing trees throughout the reserve. Once a tree was identified as a host to L. barbicornis, one could make repeat visits to the tree and reliably find a population of weevils to observe. Observations were therein simple to conduct because copulation occurs on the trunks or branches of the trees while females drill holes to prepare for oviposition.
Standing karaka trees were found to be the most useful for behavioural observations, because they would reliably host aggregations of adult L. barbicornis throughout at least one breeding season. Standing trees were also best for ease of observation 360° around the tree, although occasionally a focal weevil would disappear into the canopy. While we regularly found dead, fallen trees hosting L. barbicornis, these dehydrated rapidly in dry years, and were only useful for observing adults over one breeding season. However, fallen trees are useful for short-term behavioural observations or collection. Observations were best undertaken on dry days, as L. barbicornis were very difficult to locate during heavy rain, particularly if the tree trunks became wet.
Marking system
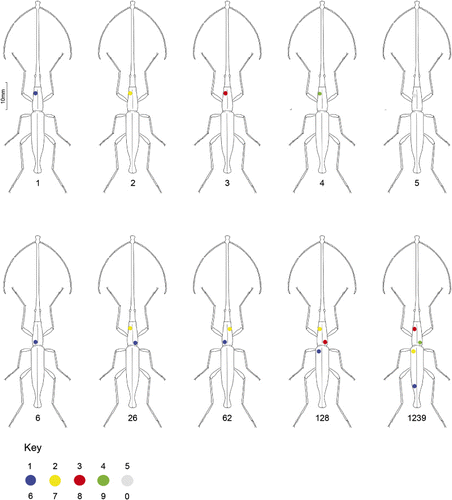
To enable a large field-based study to be developed, we designed a system to mark individual weevils so that they could be reliably and uniquely identified during observations on subsequent visits. This method was adapted from von Frisch (Citation1993), who originally developed a system to identify bees during his behavioural studies. Queen bee marking paints (Lega, Italy) were used as they are non-toxic, do not rub off, and last the life-time of the individual. Each of the five colours used (blue, yellow, red, green, white) was coded to two numbers from 0–9, in relation to where they were placed on the pronotum (). Numbers 1–99 were made on the pronotum, and hundreds and thousands were marked on the top and bottom of the elytra, respectively. During field observations this method was found to be an ideal way of distinguishing and identifying each individual, so that multiple individuals could be observed at one time ().
General behavioural observations
Although observing behaviour in the wild was relatively simple, it was possible to disturb giraffe weevils during observations. As previously observed in the brentid Arrenodes minutus (Sanborne Citation1983), if disturbed suddenly, both male and female L. barbicornis will fall backwards from the tree and feign death in the leaf litter, sometimes for more than an hour before climbing back up the tree. If an observer came too close to an aggregation, most of the group would ascend the tree and hide in the canopy or inside cracks in the tree. It was therefore important to develop a technique that ensured normal behaviour was not affected by observer presence. We ensured that all observations were made standing at least 2 m from the tree, using close-range binoculars (Pentax Papilio 8.5 × 21, focus to 0.5 m) to identify individuals and their behaviours.
Lasiorhynchus barbicornis were found to be diurnal, although mating pairs could still be found immediately after dusk. When it became very dark (at approximately 2200 h), L. barbicornis would climb into the canopy of the tree and hide on the underside of leaves. No pairs were observed copulating at this time; instead all individuals were motionless and solitary under the leaves. Pairs copulating around midnight were observed at Paengaroa Scenic Reserve near Taihape (A. Dennis pers. comm.), but we visited all the previously marked trees regularly after dark and did not observe any active pairs after 2200 h at Matuku Reserve.
On occasion, we observed both males and females gathered around sap deposits on dying karaka trees. It was assumed that they were feeding on the running sap because their mouthparts were pushed into the liquid (). Lasiorhynchus barbicornis have also previously been found feeding on the nectar of male nīkau (Rhopalostylis sapida H. Wendl. et Drude) and patē flowers (Schleffera digitata J.R. Forst et G. Forst) (Pritchard Citation1952; J. Waite pers. comm.), and we subsequently observed this occurring on nīkau flowers at Matuku Reserve.
Larval life-span experiment
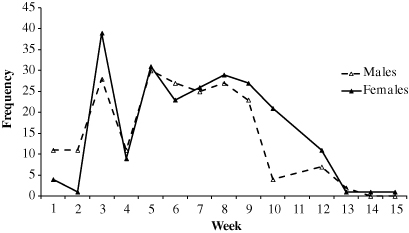
To determine the length of time taken for L. barbicornis larvae to emerge as adults, we set up an experiment in the field at Matuku Reserve. On 4 January 2011, a previously healthy karaka tree was felled and cut into 12 × 400 mm long pieces. Each log was left on the forest floor, spaced approximately 2 m apart. After approximately 3 weeks, adult L. barbicornis began arriving on the logs, including females that were observed ovipositing eggs into the wood. At the end of the breeding season, in April 2011, the logs were placed into mesh cages (Bioquip Rearing and Observation Cage, 90 × 60 × 60 cm; Bioquip, Rancho Dominguez, CA, USA) with three logs per cage. Air could pass freely through the cages, but the mesh was extremely fine to exclude any giraffe weevils from entering or exiting the cages. Although we did not measure environmental conditions within the cages, they were left on the forest floor in an attempt to match ambient humidity and temperature conditions. No adult L. barbicornis were found to emerge from the logs between December 2011 and April 2012. However, the following year from 11 December 2012, L. barbicornis were found to emerge from the logs, and the cages were checked weekly until 2 April 2013 (). Lasiorhynchus barbicornis therefore require at least 2 years as larvae before emerging as adults. Each week all weevils were counted, removed and released onto a nearby tree. A total of 430 adult weevils (206 male, 224 female) emerged from the logs over the 15-week period. The overall adult sex ratio was 0.52, which did not deviate significantly from a 1 : 1 ratio (binomial exact test, P = 0.4). These cage experiments, therefore, suggest that the sex ratio at emergence is equal, but further research is required to determine sex ratios in the field among aggregations of adults.
Acknowledgements
We thank Forest and Bird Waitakere Branch, particularly John Staniland, for providing funding and logistical support for this study. We are grateful to Megan Friesen, Cor Vink, Richard Leschen and an anonymous reviewer for helpful comments on the manuscript. Thank you to Thomas Buckley, Alice Dennis, Grace Hall, Robert Hoare, Richard Leschen, Shelley Myers, Christina Rowe, David Seldon and Jenny Waite for their observations and host plant association records, and the many volunteers who have assisted us in the field over the course of this project. Specimens were collected under the DoC permits (CA-31615-OTH, NO-23262-FAU), an ARC permit (WS 302), and with permission from Maungatautari Ecological Island Trust and the Stubbs Family, Waitomo.
References
- Broun T 1880. Descriptions of the larva and pupa of Lasiorhynchus barbicornis. Transactions of the New Zealand Institute XIII: 228–230.
- Brockmann HJ, Oliveira RF, Taborsky M 2008. Integrating mechanisms and function: prospects for future research. In: Oliveira RF, Taborsky M., Brockmann HJ eds. Alternative reproductive tactics – an integrative approach. Cambridge, Cambridge University Press. Pp. 471–489.
- Gadgil M 1972. Male dimorphism as a consequence of sexual selection. The American Naturalist 106: 574–580. 10.1086/282797
- Hudson GV 1934. New Zealand beetles and their larvae: an elementary introduction to the study of our native Coleoptera. Wellington, Ferguson & Osborn Limited. 236 p.
- Kuschel G 2003. Nemonychidae, Belidae, Brentidae (Insect: Coleoptera: Curculionoidea). Fauna of New Zealand Number 45. Lincoln, Manaaki Whenua Press. 100 p.
- May BM 1993. Larvae of Curculionoidea (Insecta: Coleoptera): a systematic overview. Fauna of New Zealand 28. Lincoln, Manaaki Whenua Press. 226 p.
- Meads MJ 1976. Some observations on Lasiorhynchus barbicornis (Brentidae: Coleoptera). The New Zealand Entomologist 6: 171–176.
- Painting CJ, Holwell GI 2013. Exaggerated trait allometry, compensation and trade-offs in the New Zealand giraffe weevil (Lasiorhynchus barbicornis). PLoS ONE 8: e82467. doi:10.1371/journal.pone.0082467
- Pritchard ED 1952. Random notes. New Zealand Entomologist 1: 12–14.
- Sanborne M 1983. Some observations on the behaviour of Arrhenodes minutus (Drury) (Coleoptera: Brentidae). The Coleopterists Bulletin 37: 106–113.
- Sforzi A, Bartolozzi L 2004. Brentidae of the world (Coleoptera, Curculionoidea). Torino, Museo Regionale di Scienze Naturali. 976 p.
- Shuster SM, Wade MJ 2003. Mating systems and mating strategies. Princeton, Princeton University Press. 520 p.
- von Frisch K 1993. The dance language and orientation of bees. Cambridge, Harvard University Press. 566 p.