Abstract
Burrow-nesting seabird populations are vulnerable to predation by introduced rats, because of their nesting habits and slow life histories. We investigated whether control of kiore (Pacific rats, Rattus exulans) by removal trapping, and during an unsuccessful community-led island-wide eradication attempt, had any effects on nest survival of grey-faced petrels (Pterodroma gouldi) on Ririwha (Stephenson Island), northeastern New Zealand. We compared nest survival between two plots at which rats were trapped and six un-trapped plots in 2010, as well as at all plots during and after the poisoning programme in 2011–2012. Neither mean rates of breeding burrow occupancy nor nest survival differed between trapped and un-trapped plots in 2010. We found no significant differences between years or between plots throughout the poisoning programme. Extrapolation of daily nest survival rates to the full 172-day combined egg and chick period gave an estimate of mean annual productivity for all plots combined of 0.285 (95% confidence interval 0.252–0.318), which is higher than on comparable predator-free islands. Although the absence of a detectable effect of kiore on breeding grey-faced petrels on Ririwha is reassuring, we can be less sure that smaller burrow-nesting seabirds on the island are secure.
Introduction
The effects of invasive species on populations of indigenous flora and fauna around the world are well documented (Clavero & García-Berthou Citation2005). Effects are particularly severe for species restricted to, or breeding on, islands. For example, most recor-ded avian extinctions have occurred on islands (Butchart et al. Citation2006; Keitt et al. Citation2011), with the impacts of introduced predatory mammals perhaps the most significant cause (Blackburn et al. Citation2004). High levels of endemism and relative naivety to terrestrial predators and their hunting behaviours make native species on islands especially vulnerable to the impacts of introduced predators (Courchamp et al. Citation2003; Cox & Lima Citation2006).
Rats (Rattus spp.) are arguably the most pervasive invasive mammals, having been introduced both accidentally and deliberately to most of the world's island groups (Atkinson Citation1985; Towns et al. Citation2006; Howald et al. Citation2007). Rats are omnivorous, generalist feeders and are capable of adapting to most environmental conditions. Population densities can increase rapidly under suitable conditions, leading to documented impacts on insular populations of invertebrates (Green et al. Citation2011), vascular plants (through seed predation; Campbell Citation1978; Campbell & Atkinson Citation2002), herpetofauna (Daltry et al. Citation2001; Towns et al. Citation2007) and birds, both terrestrial species (Bell Citation1978) and ground- or burrow-nesting seabirds (Pierce Citation2002; Imber et al. Citation2003; VanderWerf et al. Citation2011).
The behavioural and life-history traits of burrow-nesting procellariiform seabirds (petrels and shearwaters), in addition to their naivety to terrestrial predators, contribute to their vulnerability to rat predation (Moors & Atkinson Citation1984; Jones et al. Citation2008). Procellariids typically reproduce slowly, producing one chick per year without replacement, and adults may leave eggs or chicks unguarded for days or weeks at a time while foraging (Warham Citation1990). Despite the presence of introduced predators, breeding adults and returning juveniles continue to show strong fidelity to breeding colonies and even to specific burrows (Bried & Jouventin Citation2002). The use of burrows is likely to increase the encounter rate between eggs and chicks and rats as the latter use burrows extensively when foraging and as nest sites (Rutherford et al. Citation2009). The immediacy of rat impacts may vary with the size of the particular procellariiform species under threat; rats may prey on all life-history stages of smaller species, leading to rapid impacts on the population, but adults of larger species may be less vulnerable such that population impacts may not be apparent until reduced recruitment into the breeding population reduces growth rates (Imber Citation1975; Jouventin et al. Citation2003; Jones et al. Citation2008). Impacts on seabird populations are of particular concern because of seabirds’ role as ecosystem engineers (Sánchez-Piñero & Polis Citation2000; Fukami et al. Citation2006) and because procellariiforms also have significant nutritional, cultural, economic and biological value for many cultures (Skira Citation1996; Moller Citation2006). For example, pre-fledging chicks (‘muttonbirds’) are harvested by indigenous groups in Tasmania (short-tailed shearwater, Puffinus tenuirostris) and in both the North Island (grey-faced petrel, Pterodroma gouldi; Lyver et al. Citation2008) and South Island (sooty shearwater, Puffinus griseus) of New Zea-land (Skira Citation1996; Lyver Citation2002).
Programmes to eradicate introduced rats from islands have become increasingly common since the development of anticoagulant toxins and gradual improvements in strategy and delivery systems (Towns & Broome Citation2003; Howald et al. Citation2007; Parkes et al. Citation2011). These methodological advances have allowed islands of increasing size and habitat complexity to be cleared of rats, with a high success rate: the Database of Island Invasive Species Eradications reports 751 attempts at eradication of Rattus species from islands, of which 84% were successful (http://diise.islandconservation.org/, accessed 21 November 2014). As techniques have become more refined and expertise more widely available, increasing numbers of eradication and control programmes are being managed by community groups with support from management agencies (Towns et al. Citation2013). This is particularly true of the New Zealand archipelago where the risks to native species populations from the impacts of a range of introduced mammals have driven rapid advances in eradication methods.
Of the three rat species introduced to New Zealand, kiore (Rattus exulans) have special cultural status as one of the original species brought to the country by Māori over 700 years ago, and have traditional value as a food item and as indicators of environmental conditions (Haami Citation1994). Although kiore are often regarded as the least damaging of the three introduced rat species, partially by virtue of their being the smallest, there is evidence that they have negative impacts on indigenous plants (Campbell & Atkinson Citation1999), insects (Green et al. Citation2011), reptiles (McCallum Citation1986) and seabirds (Pierce Citation2002; Imber et al. Citation2003).
A planned community-led attempt to eradicate the kiore population from a New Zealand offshore island presented us with the opportunity to examine how the productivity of a burrow-nesting seabird, the grey-faced petrel, might be affected by the control of kiore. We tested this by kill-trapping kiore at two plots prior to wide-spread control and by monitoring petrel productivity before, during and after control at these and six comparison plots. We therefore hypo-thesised that, if kiore had impacts on petrel productivity, nest survival rates would be higher at our kiore-controlled plots than at uncontrolled plots and at all sites subsequent to the control programme.
Methods
Study site and key species
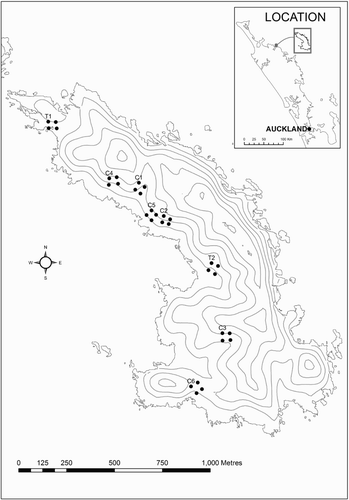
Ririwha (Stephenson Island or Mahinepua) is a 120 ha island lying approximately 5 km off the east coast of northern New Zealand (). The island has been highly modified by past land uses and by burning when it was leased for pastoral use. The leases have expired and the island's owners, a Māori trust, have expressed interest in managing the island to achieve their long-term vision of restoring the island's biocultural values, which includes both conservation and agricultural objectives. The island is approximately 2.4 km long with an elevation 132 m above sea level at its highest point. A smaller islet, Cone Island, lies approximately 50 m off the northwest tip of the main island. Although Ririwha is no longer used for commercial farming, around 400 sheep are retained largely to manage the dominant introduced grasses, particularly kikuyu grass (Pennisetum clandestinum), and provide a regular source of meat for the local community. Despite this modification, Ririwha retains remnant areas of native coastal forest around its coastal fringe. This is mostly pōhutukawa (Metrosideros excelsa) and native shrubs and herbs on the main island, but with more intact communities of tawāpou (Pouteria costata) and milk tree (Streblus spp.) on Cone Island. Bracken fern (Pteridium esculentum) and pōhuehue (Muehlenbeckia complexa) are recolonising some areas of kikuyu naturally (Conning & Miller Citation1999).
Figure 1 Map of Ririwha (Stephenson Island, Mahinepua), New Zealand, showing location of plots for monitoring grey-faced petrels (Pterodroma gouldi). Kiore (Rattus exulans) were controlled by kill-trapping in the 2010 petrel breeding season at plots T1 and T2 and monitored by index trapping at the same plots in 2011 and 2012. Inset shows the location of Ririwha relative to the northeastern coast of New Zealand.
The grey-faced petrel is a burrow-nesting gadfly petrel (adult body mass 460–750 g; Marchant & Higgins Citation1990). Breeding pairs produce a single egg during June and July (the Austral winter), with chicks fledging in December and early January (Imber Citation1976). The total New Zealand population of grey-faced petrels has been estimated at over 1 million birds and the species is classified by the New Zealand Department of Conservation accordingly as ‘Not Threatened’ (Robertson et al. Citation2013). The species has great cultural significance to northern Māori who traditionally harvested pre-fledging chicks. However, major harvests largely ceased due to prohibitions put in place by the earlier Wildlife Service and rahui (temporary harvest bans) imposed by iwi authorities following concerns over reported declines in the numbers of chicks in the late 1950s (Lyver et al. Citation2008).
Several other species of seabirds have been reported as nesting on the islands (Bell Citation1959; Conning & Miller Citation1999). In addition to grey-faced petrels, we observed the following species during field surveys: Pycroft's petrel (Pterodroma pycrofti); North Island little shearwater (Puffinus assimilis haurakiensis); northern diving petrel (Pelecanoides u. urinatrix); fluttering shearwater (Puffinus gavia); and little blue penguin (Eudyptula minor). The numbers of nesting pairs of these species have not been estimated formally, but Ririwha has been identified by the Department of Conservation as important breeding habitat for Pycroft's petrel and little shearwater populations, both of which are listed as ‘At Risk: Recovering’ (Robertson et al. Citation2013).
Study design
To investigate the predation impacts of kiore, we carried out both spatial and temporal comparisons of grey-faced petrel productivity before and during the rat control operations on Ririwha. Our spatial comparison in 2010, prior to the eradication programme, involved removal-trapping of kiore in and around two burrow survey plots and comparing nest survival at these with six un-trapped plots. We also monitored nest survival at all eight plots in 2011, when poisoning took place, and in 2012. This timeline is summarised in .
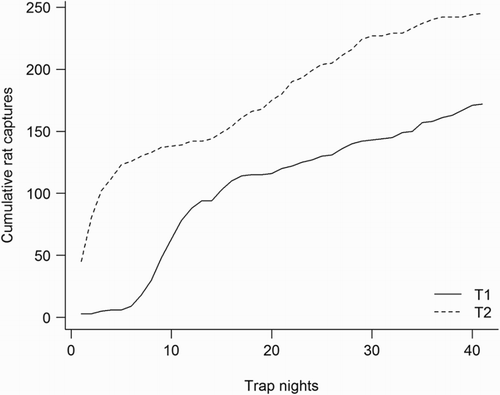
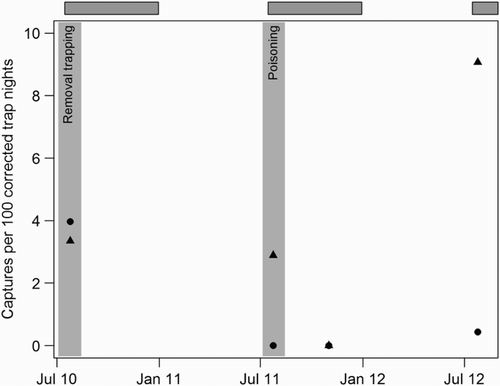
Figure 2 Timeline and capture rates of kiore (Rattus exulans) at two plots (T1—129 traps, ▴; T2—155 traps, ●) on Ririwha (Stephenson Island, Mahinepua), northeastern New Zealand. Rates are expressed as captures per 100 corrected trap nights (C/100 CTN) and are shown for the initial 41-night removal programme and for subsequent three-night index trapping. Widespread poisoning of rats also took place in June and July 2011(light grey shading). Grey-faced petrel (Pterodroma gouldi) nesting periods are indicated by darker grey horizontal bars. Note: no rats were captured at either site during the October 2010 index trapping.
Experimental manipulation
In the 2010 petrel breeding season, we removed kiore by kill-trapping for six weeks in and around two plots (designated T1 and T2), while the other six non-treatment plots (designated C1–C6) were left un-trapped (). Trapping grids at T1 and T2 were centred on 40 × 40 m burrow survey plots (see ‘Petrel nest monitoring’, below) and extended out 120 m, unless constrained by steep cliff edges. Trapping grids were at least 200 m from each other and from survey plots, to maintain independence (according to published home range estimates for kiore; Moller & Craig Citation1987; Atkinson & Towns Citation2005). Plot T1, containing 129 traps, was near the northwestern tip of the island and encompassed a small headland with native bush remnants and an adjacent cove; T2 (155 traps) was centred in a sheltered gully approximately 150 m in from the island's main beach. Snap-back rat traps in tunnels were set in a 20 × 20 m grid pattern at plots T1 and T2. Traps were left out unset for three nights before baiting. All traps were baited with a peanut butter and icing sugar composite on 4 July 2010 and were last checked on 14 August 2010. This period encompassed the early chick period of the breeding season during which burrow-nesting petrels are considered to be most vulnerable to predation by rats (Imber Citation1975). Traps were checked daily and, if triggered, cleared (if a rat was present), baited and reset. We also carried out three nights trapping using the same trapping grids in October 2010, July and October of 2011, and July 2012 as an index of relative rat abundance at the plots throughout the eradication programme. For each trapping period we report the number of captures per 100 trap nights (C/100 CTN), corrected for traps sprung empty and other failures, following Nelson & Clark (Citation1973).
We also attempted to monitor kiore relative abundance at our two treatment and three of the six non-treatment plots, using chew cards (Sweetapple & Nugent Citation2011) prior to trapping, but the high exposed terrain and strong winds meant that this technique was unsuitable because of card loss and likely neophobia of rats, as those cards remaining in place flapped or vibrated in the wind. In carrying out these initial surveys, we recorded characteristic rat-bite marks at all five of the monitored plots although there were insufficient data for reliable estimation of a relative abundance index.
Kiore eradication programme
The Ririwha Restoration Trust aims to restore Ririwha to reflect indigenous agro-ecological goals, with limited farming practices integrated with areas dominated by native vegetation and supporting growing populations of native seabirds (Lyver et al. unpubl. data). Management of weeds is via grazing to control kikuyu and more active control of woody species (e.g. lantana, Lantana camara). In consultation with a range of funding and management agencies, the Trust, in 2010, developed an operational plan for the eradication of kiore from the island. The Trust adopted a ground-based approach in preference to aerial application of toxic baits, in order to: 1. reduce the amount of toxin delivered to the environment; 2. minimise primary exposure to non-target species; 3. act as a self-monitoring programme with respect to rodenticide uptake; and 4. because of community concerns around potential contamination of the marine environment.
Toxic baits were deployed primarily in a grid of bait stations. The stations, each consisting of a 40 cm length of 11 cm diameter corrugated plastic pipe, were placed out in late May 2011 on a 30 × 30 m grid across all parts of the island accessible by foot. The stations were left un-baited for 2 weeks to allow rats to acclimatise to their presence before each was baited with two blocks of 0.2 g/kg brodifacoum (PestoffTM; Animal Control Products Ltd, Whanganui, New Zealand). On cliffs and steeper faces, bags of cereal baits (Pestoff 20RTM) were placed by hand. Bait stations were checked and baits replenished daily for 2 weeks after the initial deployment and then periodically until mid-July 2011. The same techniques were also used on Cone Island and the rocky stacks in between the two islands, although field staff noted that coverage was incomplete because of steep terrain and dense vegetation. Staff also observed that bait uptake was greatest around the fringe of Cone Island and on narrow causeways between it and some of the stacks (M. Sheehan, personal obs.). No formal monitoring of changes in rat abundance was carried out by the Trust. Spotlight searches during and after the main poisoning activities and irregular checks on bait stations in subsequent months described a marked decline in observations of rats from over 300 in 2 h of spotlighting in June 2011 to just two over a similar period in late July, and no sightings or evidence of bait-take in visits during December 2011 and January 2012.
Petrel nest monitoring
We established the eight 40 × 40 m burrow survey plots within petrel breeding areas. Within each plot we monitored grey-faced petrel productivity by surveying breeding burrows for eggs and chicks. Surveys were undertaken in both July and late October to early November, to coincide with the peak incubation period and mid chick-rearing periods, respectively. At each survey, we recorded the number of burrow entrances in each plot. Entrances were considered to be inside the plot if the burrow cavity was deeper than 20 cm and the centre of the burrow roof was within or overlapped the plot boundary. Each burrow entrance was labelled with a unique number using a plastic cattle tag and wire peg inserted into the ground. Burrows lost through collapse between surveys were considered a natural source of nest mortality.
We used a Peeper 2000 (Sandpiper Technologies, Manteca, CA) flexible video-probe (henceforth ‘burrowscope’) with head-mounted display to determine the occupancy status of burrows. Occupied burrows were defined as those with a grey-faced petrel adult, egg or chick present (as appropriate for the survey period). The probe was eased beneath brooding adults to check for the presence of an egg. To minimise disturbance to the birds, and the risk of damaging eggs, the burrowscope was rarely pushed past the nesting site, particularly in narrow branches. Before analysis, we excluded burrows in which we observed other seabird species to ensure we only analysed data from burrows that were potentially available as breeding sites for grey-faced petrels.
Analyses
We estimated daily nest survival in our survey plots using the logistic-exposure method of Shaffer (Citation2004), which is essentially a general linear modelling framework but differs from standard logistic regression methods in the use of a link function that allows for variability in the intervals between nest visits. This approach requires no assumptions about dates of nest failures and requires only information on the length of the intervals between nest checks and the fate of the surveyed nests. In estimating survival, we used data from all burrows that contained eggs in July and were subsequently checked in the following October–November. We constructed a series of simple models in which the response was a binary variable indicating the presence or absence of a chick in an individual burrow during the November nest check. We first tested for differences in nest survival across all plots and between rat-trapped and un-trapped plots in 2010 only. We then used a fully interactive model to estimate and compare daily nest survival in all plots and years to see if there was a relationship with the effects of the rat eradication programme. All analyses were conducted in R v2.15.2 (R Development Core Team Citation2012). We report estimates of daily nest survival rates and period survival, which we assume represents annual productivity, obtained by extrapolating these rates to the full 172-day combined egg and chick period reported for grey-faced petrels (Imber Citation1976; Marchant & Higgins Citation1990).
Results
Rat trapping
During 41 nights of trapping to control kiore at plots T1 and T2 in July 2010 we removed 417 rats. Plots of cumulative captures over time at the two sites revealed different patterns of captures (). At plot T2, offtake rates were consistently higher than at T1 where they remained low for the first week of trapping before following a similar pattern to that at T2. In spite of these differences, over the full 41 nights of trapping the indices were more similar (). Over the first 10 days of trapping, approximately twice as many males were trapped as females. During the poisoning operation in 2011, our index trapping detected no rats at plot T2, with only two captures 12 months later. At T1, however, rats remained during the initial poisoning and had recovered to a greater extent than at T2 during the final trapping session ().
Seabird monitoring
We surveyed a total of 1131 burrow entrances during the July egg checks across all survey periods. Of these, 587 burrows were occupied by eggs of grey-faced petrels (). Mean occupancy did not vary between trapped and un-trapped plots in 2010 (t = 0.645; df = 2; P = 0.58) or across all plots between years (F = 0.80; df = 2; P = 0.47).
Table 1 Numbers of burrow entrances and rates of burrow occupancy by breeding grey-faced petrels on Ririwha (Stephenson Island, Mahinepua), New Zealand. Plots are 40 × 40 m and were monitored from 2010 to 2012. Estimated daily nest survival rates are for all plots combined. Period survival is over the full 172-day combined egg and chick period. Values in parentheses are 95% confidence intervals.
We detected no difference in daily nest survival between trapped and un-trapped plots in 2010 (omnibus test χ2 = 0.051; df = 1; P = 0.82). Similarly, when testing for any temporal changes in daily survival associated with the island-wide poisoning programme, we found no statistically significant differences between years (range, χ2 = 1.33; df = 2; P = 0.51) or between plots (χ2 = 6.29; df = 7; P = 0.51). Extrapolation of estimated daily nest survival rate to the full 172-day combined egg and chick period gave a mean annual productivity estimate for all plots combined of 0.285 (95% confidence interval 0.252–0.318; annual estimates in ).
Discussion
If kiore on Ririwha had an impact on the productivity of grey-faced petrels, we would have predicted two likely responses to the trapping and poisoning of the rats during our study. First, we would have expected nest survival to be higher at our two trapped plots than at those plots where rats were not controlled during the 2010 breeding season. Second, we would have expected an increase in productivity at the un-trapped plots commensurate with the poisoning programme. We detected neither. There are two possible explanations for this result: 1. that kiore prey on petrel eggs and chicks but neither control method was effective in reducing this predation; or 2. kiore do not have a significant impact on nest survival of grey-faced petrels. We consider the support for these hypotheses below.
Although the kiore eradication programme on Ririwha was ultimately unsuccessful, there are indications that both our trapping and the poisoning programme reduced rat numbers during the petrel breeding seasons. As shows, the removal rate of kiore from both trapped plots declined markedly and appeared to reach an asymptote, albeit briefly, after around 14 days of trapping. Rats continued to be trapped beyond this, but at a much reduced rate. We suggest that this indicates the core population at both plots was removed after 2 weeks of trapping and that later-trapped individuals may have been animals from outside or peripheral areas of the grids. The initially higher trap rate of males than females is likely to reflect the greater tendency of male kiore to move around their foraging range compared with the more sedentary females (Atkinson & Towns Citation2005). The lag in offtake at plot T1 suggests a possible neophobic response to the traps similar to that described for kiore by Yackel Adams et al. (Citation2011). There was no lag in capture rates at T2 and we speculate that the difference may be due either to reduced neophobia (this plot was within 100 m of the only house on the island) or to differences in the local habitats at the two plots. Plot T2 was a sheltered gully with a small creek and areas of introduced and native bush; T1 was more open and exposed and dominated by short kikuyu grassland. It is therefore likely that T2 was able to support a greater density of kiore through greater availability of food and shelter (Roberts & Craig Citation1990). In addition, the more complex habitat might have facilitated greater ranging behaviour (Harper et al. Citation2005; Bramley Citation2014) and thus higher initial encounter rates with traps than at T1.
Limited resourcing, coupled with the expense and logistics of working on an offshore island, meant that we were unable to monitor kiore abundance at both treatment and non-treatment plots. Although we are confident that kiore were present and abundant across the island in 2010, we were reliant on a combination of index trapping at our treatment plots and anecdotal observations by field staff elsewhere to infer temporal changes in kiore abundance subsequent to poisoning.
Kiore index trapping rates declined markedly at plot T2 as the poisoning programme progressed (). Even allowing for the seasonally predictable zero trap-rate in October 2011 (see Moller & Craig 1987 and Bunn & Craig Citation1989 for comparisons) this suggests that rat numbers had been reduced significantly by the control efforts. Furthermore, only two were caught in over 460 corrected trap nights in 2012, a year after the operation had ceased. Anecdotal reports of spotlight searches from the programme's manager support this conclusion. We suspect that the continued presence of kiore at T1 represents insufficient bait coverage during the control programme by untrained volunteers. The failure rate for rodent eradication programmes using ground-based brodifacoum is around 24% (Parkes et al. Citation2011) and, in ground-based programmes generally, failures to deploy bait adequately or maintain bait stations are key causes of these failures (Howald et al. Citation2007; VanderWerf et al. Citation2011). If each individual in the targeted population is not put at risk, populations may be suppressed but not eradicated (Keitt et al. Citation2011). Thus, although eradication was not achieved, kiore were suppressed both by our trapping and by the poisoning, at least at plot T2. Suppression of rat populations can lead to positive outcomes for breeding petrels, although the need to continue intensive control may make this impractical and cost-ineffective in the longer term (Jouventin et al. Citation2003). The fact that we detected no difference in nest survival in 2010 between trapped and un-trapped plots and no temporal change through the control programme suggests, therefore, that kiore did not have an impact on grey-faced petrel productivity on Ririwha.
The lack of an impact is further supported by comparing our estimates of nest survival rates on Ririwha with those from other grey-faced petrel breeding colonies in the region. Extrapolation of daily rates to the full combined egg and chick period suggests that annual productivity on Ririwha ranged between 26% and 32% across all plots, although we acknowledge that daily survival is likely to vary within this period, with rates for established chicks likely to be higher than for eggs and newly hatched chicks (Ricklefs Citation1969; Warham Citation1990; Jones et al. Citation2003; Minguez & Oro Citation2003). These values are higher than recent estimates of nest survival for four other New Zealand grey-faced petrel populations on islands that are free of introduced mammalian predators. Using the same methodology, Jones et al. (Citation2015) estimated period survival from 11% to 28% over periods of up to 4 years. Both this study and Jones et al. (Citation2015) overlapped in 2010; our estimate of 27% period survival for the Ririwha population is markedly higher than the combined estimate across the other four islands of just over 16% (Jones et al. Citation2015; C. Jones, unpubl. data).
To put the apparent lack of risk to grey-faced petrel nests into context, we surveyed published evidence of kiore impacts on procellariid seabirds (). This appears to conform to the reported trend of increased rodent predation risk to burrow-nesting species of smaller adult body mass compared with larger, surface-nesting species (Atkinson Citation1985; Jones et al. Citation2008; Cuthbert et al. Citation2013a). Documented changes in the distributions of smaller seabirds following the historical spread of kiore further support this pattern (Holdaway Citation1999). However, local ecological conditions may also serve to mode-rate or exacerbate impacts on some species. For example, Imber et al. (Citation2003) noted that kiore im-pacts on Cook's petrel appeared to fluctuate with the availability of alternative foods, driven, in turn, by climatic events. Similarly, Rayner et al. (Citation2007) proposed that elevation linked changes in resource availability moderated predation on the same species in northern New Zealand. In contrast, kiore predation on surface-nesting Murphy's petrel chicks was lower on Ducie Island (Pitcairn Islands) where poorer vegetation quality maintained kiore at lower density and constrained adult body mass compared with Henderson Island where larger, more abundant rats had a dramatic impact with no chicks from a sample of 59 surviving more than 5 days (Brooke Citation1995).
Table 2 Documented impacts of kiore on a range of procellariiform seabirds relative to seabird adult body mass. Adult body mass estimates are mean values except, where a range was given, the median.
Although grey-faced petrels in our study were at low risk of nest predation from kiore, in part by virtue of their size, there are examples of kiore and other small rodents preying on seabirds of moderate size (> 600 g) and larger. Kiore were documented attacking Laysan albatrosses by Kepler (Citation1967) and there has been much recent attention on reports of mouse (Mus musculus) predation on a range of petrel and albatross species on isolated Gough Island (Jones & Ryan Citation2010; Cuthbert et al. Citation2013a,Citationb). This raises the possibility that our study may have failed to detect significant levels of chick predation during the seasonal peak in kiore numbers in December, a month later than our chick surveys. We suggest that this is unlikely given both the favourable burrow occupancy rates throughout our study in comparison to other recent local surveys (Whitehead et al. Citation2014; Jones et al. Citation2015) and lack of any detectable population response by grey-faced petrels in the decade following the complete eradication of kiore from Korapuki (Towns et al. Citation2006). Grey-faced petrels themselves are vulnerable to predation from larger rats; negligible fledging rates on Moutohora (Whale Island, Bay of Plenty, New Zealand) were observed during the 1970s when Norway rats (R. norvegicus) were abundant on the island before their eventual eradication in the 1980s (Imber et al. Citation2000).
This study exemplifies the importance of a null result in ecological field studies (Merrill Citation2014); the absence of a detectable effect of kiore on breeding grey-faced petrels on Ririwha will be reassuring for the island's management trust as the bird has great cultural and food value to the local Ngātikahu ki Whangaroa community. We can be less sure that smaller burrow-nesting seabirds recorded on the island are secure. Evidence of the variability in rat impacts with changes in resources suggests that the island's managers also need to be aware that proposed management of the island, including replanting of native plants and staged release of areas of the island from grazing, are likely to change the prevailing ecological conditions with as yet unknown effects on the kiore population and, in turn, on breeding seabirds.
Associate Editor: Associate Professor Jim Briskie.
Acknowledgements
We thank the Ririwha Restoration Trust and the Ngātikahu ki Whangaroa community for collaborating with us and granting access to Ririwha. Field sampling contributions from M Coleman, K Drew, B Karl, S Leucht, R Mapp and EJ Thompson were gratefully appreciated. We also thank C Bezar for editorial assistance and J Willoughby for .
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Atkinson IA 1985. The spread of commensal species of Rattus to oceanic islands and their effects on island avifaunas. In: Moors PJ ed. Conservation of island birds. ICBP technical publication No. 3. Cambridge, UK, International Council for Bird Preservation. Pp. 35–81.
- Atkinson IAE, Towns DR 2005. Kiore. In: King CM ed. The handbook of New Zealand mammals. 2nd edition. Melbourne, Oxford University Press. Pp. 159–174.
- Bell BD 1959. The birds of Stephenson Island, Whangaroa. Notornis 8: 255–256.
- Bell BD 1978. The Big South Cape Islands rat irruption. In: Dingwall PR, Atkinson IAE, Hay C eds. The ecology and control of rodents in New Zealand nature reserves. Information series 4. Wellington, Department of Lands and Survey. Pp. 33–40.
- Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ 2004. Avian extinction and mammalian introductions on oceanic islands. Science 305: 1955–1958. doi: 10.1126/science.1101617
- Booth AM, Minot EO, Fordham RA, Innes JG 1996. Kiore (Rattus exulans) predation on the eggs of the little shearwater (Puffinus assimilis haurakiensis). Notornis 43: 147–153.
- Bramley GN 2014. Home ranges and interactions of kiore (Rattus exulans) and Norway rats (R. norvegicus) on Kapiti Island, New Zealand. New Zealand Journal of Ecology 38: 328–334.
- Bried J, Jouventin P 2002. Site and mate choice in seabirds: an evolutionary approach. In: Schreiber EA, Burger J eds. Biology of marine birds. Boca Raton, FL, CRC Press. Pp. 263–305.
- Brooke MDL 1995. The breeding biology of the gadfly petrels Pterodroma spp. of the Pitcairn Islands: characteristics, population sizes and controls. Biological Journal of the Linnean Society 56: 213–231. doi: 10.1111/j.1095-8312.1995.tb01086.x
- Bunn TJ, Craig JL 1989. Population cycles of Rattus exulans: population changes, diet, and food availability. New Zealand Journal of Zoology 16: 409–418. doi: 10.1080/03014223.1989.10422907
- Butchart SHM, Stattersfield AJ, Brooks TM 2006. Going or gone: defining ‘Possibly Extinct’ species to give a truer picture of recent extinctions. Bulletin of the British Ornithologists Club 126A: 7–24.
- Campbell DJ 1978. The effects of rats on vegetation. In: Dingwall PR, Atkinson IAE, Hay C eds. The ecology and control of rodents in New Zealand nature reserves. Information series 4. Wellington, Department of Lands and Survey. Pp. 99–120.
- Campbell DJ, Atkinson IAE 1999. Effects of kiore (Rattus exulans) on recruitment of indigenous coastal trees on northern offshore islands of New Zealand. Journal of the Royal Society of New Zealand 29: 265–290. doi: 10.1080/03014223.1999.9517597
- Campbell DJ, Atkinson IAE 2002. Depression of tree recruitment by the Pacific rat (Rattus exulans Peale) on New Zealand's northern offshore islands. Biological Conservation 107: 19–35. doi: 10.1016/S0006-3207(02)00039-3
- Clavero M, García-Berthou E 2005. Invasive species are a leading cause of animal extinctions. Trends in Ecology & Evolution 20: 110. doi: 10.1016/j.tree.2005.01.003
- Conning L, Miller N 1999. Natural areas of Kerikeri Ecological District. Reconnaissance survey report for the Protected Natural Areas Programme. New Zealand PNA programmes 42. Whangarei, New Zealand, Department of Conservation.
- Courchamp F, Chapuis JL, Pascal M 2003. Mammal invaders on islands: impact, control and control impact. Biological Reviews 78: 347–383. doi: 10.1017/S1464793102006061
- Cox JG, Lima SL 2006. Naiveté and an aquatic–terrestrial dichotomy in the effects of introduced predators. Trends in Ecology & Evolution 21: 674–680. doi: 10.1016/j.tree.2006.07.011
- Cuthbert RJ, Louw H, Lurling J, Parker G, Rexer-Huber K, Sommer E et al. 2013a. Low burrow occupancy and breeding success of burrowing petrels at Gough Island: a consequence of mouse predation. Bird Conservation International 23: 113–124. doi: 10.1017/S0959270912000494
- Cuthbert RJ, Louw H, Parker G, Rexer-Huber K, Visser P 2013b. Observations of mice predation on dark-mantled sooty albatross and Atlantic yellow-nosed albatross chicks at Gough Island. Antarctic Science 25: 763–766. doi: 10.1017/S0954102013000126
- Daltry JC, Bloxham Q, Cooper G, Day ML, Hartley J, Henry M et al. 2001. Five years of conserving the ‘world's rarest snake’, the Antiguan racer Alsophis antigue. Oryx 35: 119–127.
- Fukami T, Wardle DA, Bellingham PJ, Mulder, CPH, Towns DR, Yeates GW et al. 2006. Above- and below-ground impacts of introduced predators in seabird-dominated island ecosystems. Ecology Letters 9: 1299–2006. doi: 10.1111/j.1461-0248.2006.00983.x
- Green CJ, Gibbs GW, Barrett PA 2011. Wetapunga (Deinacrida heteracantha) population changes following Pacific rat (Rattus exulans) eradication on Little Barrier Island. In: Veitch CR, Clout MN, Towns DR eds. Island invasives: eradication and management. Proceedings of the international conference on Island invasives. Gland, Switzerland, IUCN, and Auckland, The Centre for Biodiversity and Biosecurity. Pp. 305–308.
- Haami BJTM 1994. The kiore rat in Aotearoa: a Maori perspective. In: Morrison J, Geraghty T, Crowl L eds. Science of the Pacific Island Peoples, vol. 3: Fauna, flora, food and medicine. Suva, Fiji, University of the South Pacific, Institute of Pacific Studies. Pp. 65–76.
- Harper GA, Dickinson KJM, Seddon PJ 2005. Habitat use by three rat species (Rattus spp.) on Stewart Island/Rakiura, New Zealand. New Zealand Journal of Ecology 29: 251–260.
- Heather BD, Robertson HA 2005. The field guide to the birds of New Zealand. Auckland, Viking. 440 p.
- Holdaway RN 1999. Introduced predators and avifaunal extinctions in New Zealand. In: MacPhee RDE ed. Extinctions in near time. New York, Kluwer Academic/Plenum. Pp. 189–238.
- Howald G, Donlan CJ, Galvan JP, Russell JC, Parkes J, Samaniego A et al. 2007. Invasive rodent eradication on islands. Conservation Biology 21: 1258–1268. doi: 10.1111/j.1523-1739.2007.00755.x
- Imber MJ 1975. Petrels and predators. Bulletin of the International Council for Bird Preservation 12: 260–263.
- Imber MJ 1976. Breeding biology of the grey-faced petrel Pterodroma macroptera govldi. Ibis 118: 51–64. doi: 10.1111/j.1474-919X.1976.tb02010.x
- Imber M, Harrison M, Harrison J 2000. Interactions between petrels, rats and rabbits on Whale Island, and effects of rat and rabbit eradication. New Zealand Journal of Ecology 24: 153–160.
- Imber MJ, West JA, Cooper WJ 2003. Cook's petrel (Pterodroma cookii): historic distribution, breeding biology and effects of predators. Notornis 50: 221–230.
- Jones C, Bettany S, Moller H, Fletcher D, Lyver P, de Cruz J 2003. Burrow occupancy and productivity at coastal sooty shearwater (Puffinus griseus) breeding colonies, South Island, New Zealand: can mark recapture be used to estimate burrowscope accuracy? Wildlife Research 30: 377–388. doi: 10.1071/WR01050
- Jones CJ, Lyver PO'B, MacLeod CJ, Whitehead AL, Forrester GJ 2015. Variation in productivity of Grey-faced Petrels (Pterodroma macroptera gouldi) with local burrow density and breeding Island. Emu—Austral Ornithology 115: 20–28.
- Jones HP, Tershy BR, Zavaleta ES, Croll DA, Keitt BS, Finkelstein, ME et al. 2008. Severity of the effects of invasive rats on seabirds: a global review. Conservation Biology 22: 16–26. doi: 10.1111/j.1523-1739.2007.00859.x
- Jones MGW, Ryan PG 2010. Evidence of mouse attacks on albatross chicks on sub-Antarctic Marion Island. Antarctic Science 22: 39–42. doi: 10.1017/S0954102009990459
- Jouventin P, Bried J, Micol T 2003. Insular bird populations can be saved from rats: a long-term experimental study of white-chinned petrels Procellaria aequinoctialis on Ile de la Possession (Crozet archipelago). Polar Biology 26: 371–378.
- Keitt BS, Campbell A, Saunders A, Clout M, Wang YW, Tershy B 2011. The global islands invasive vertebrate eradication database: a tool to improve and facilitate restoration of island ecosystems. In: Veitch CR, Clout MN, Towns DR eds. Island invasives: eradication and management. Proceedings of the International Conference on Island Invasives. Gland, Switzerland, and Auckland, IUCN, The Centre for Biodiversity and Biosecurity. Pp. 74–77.
- Kepler CB 1967. Polynesian rat predation on nesting Laysan albatross and other Pacific seabirds. The Auk 84: 426–430. doi: 10.2307/4083097
- Lyver PO'B 2002. The use of traditional knowledge by Rakiura Maori to guide Sooty Shearwater (Puffinus griseus) harvests. Wildlife Society Bulletin 30: 29–40.
- Lyver PO'B, Davis J, Ngamane L, Anderson A, Clarkin P 2008. Hauraki Māori mātauranga for the conservation and harvest of tītī (Pterodroma macroptera gouldi). Papers and Proceedings of the Royal Society of Tasmania 142: 149–159.
- Marchant S, Higgins PJ 1990. Handbook of Australian, New Zealand and Antarctic birds. Volume 1: Ratites to ducks; Part A: Ratites to petrels. Melbourne, Oxford University Press. 735 p.
- McCallum J 1986. Evidence of predation by kiore upon lizards from the Mokohinau Islands. New Zealand Journal of Ecology 9: 83–88.
- Merrill E 2014. Should we be publishing more null results? The Journal of Wildlife Management 78: 569–570. doi: 10.1002/jwmg.715
- Minguez E, Oro D 2003. Variations in nest mortality in the European storm petrel Hydrobates pelagicus. Ardea 91: 113–117.
- Moller H 2006. Are current harvests of seabirds sustainable? Acta Zoologica Sinica 52: 649–652.
- Moller H, Craig JL 1987. The population ecology of Rattus exulans on Tiritiri Matangi Island, and a model of comparative population dynamics in New Zealand. New Zealand Journal of Zoology 14: 305–328. doi: 10.1080/03014223.1987.10423001
- Moors PJ, Atkinson AE 1984. Predation on seabirds by animals, and factors affecting its severity. In: Croxall JP, Evans PGH, Schreiber RW eds. Status and conservation of the world's seabirds. Technical publication 2. Cambridge, International Council for Bird Preservation. Pp. 667–690.
- Nelson L, Clark FW 1973. Correction for sprung traps in catch/effort calculations of trapping results. Journal of Mammalogy 54: 295–298. doi: 10.2307/1378903
- Parkes J, Fisher P, Forrester G 2011. Diagnosing the cause of failure to eradicate introduced rodents on islands: brodifacoum versus diphacinone and method of bait delivery. Conservation Evidence 8: 100–106.
- Pierce RJ 2002. Kiore (Rattus exulans) impact on breeding success of Pycroft's petrels and little shearwaters. DOC Internal Science Series 39. Wellington, Department of Conservation. 24 p.
- R Development Core Team 2012. R: A language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing. http://www.R-project.org/ (accessed 13 February 2013).
- Rayner MJ, Hauber ME, Imber MJ, Stamp RK, Clout MN 2007. Spatial heterogeneity of mesopredator release within an oceanic island system. Proceedings of the National Academy of Sciences (USA) 104: 20862–20865. doi: 10.1073/pnas.0707414105
- Ricklefs RE 1969. An analysis of nesting mortality in birds. Smithsonian Contributions to Zoology 9: 1–48. doi: 10.5479/si.00810282.9
- Roberts M, Craig JL 1990. The demography of kiore, Rattus exulans in three habitats. New Zealand Journal of Zoology 17: 43–53. doi: 10.1080/03014223.1990.10422583
- Robertson HA, Dowding JE, Elliott GP, Hitchmough RA, Miskelly CM, O'Donnell, CFJ et al. 2013. Conservation status of New Zealand birds, 2012. New Zealand threat classification series 4. Wellington, Department of Conservation. 22 p.
- Rutherford M, Harper GA, Moller H 2009. Denning behaviour of ship rats (Rattus rattus) on Taukihepa, a seabird breeding island. New Zealand Journal of Zoology 36: 343–353. doi: 10.1080/03014220909510159
- Sánchez-Piñero F, Polis GA 2000. Bottom-up dynamics of allochthonous input: direct and indirect effects of seabirds on islands. Ecology 81: 3117–3132. doi: 10.1890/0012-9658(2000)081[3117:BUDOAI]2.0.CO;2
- Shaffer TL 2004. A unified approach to analyzing nest success. The Auk 121: 526–540. doi: 10.1642/0004-8038(2004)121[0526:AUATAN]2.0.CO;2
- Skira IJ 1996. Aboriginal people and muttonbirding inTasmania. In: Bomford M, Caughley J eds. Sustainable use of wildlife by Aboriginal peoples and Torres Strait Islanders. Canberra, Australian Government Publishing Service. Pp. 167–175.
- Spear LB, Ainley DG 1998. Morphological differences relative to ecological segregation in petrels (Family: Procellariidae) of the Southern Ocean and tropical Pacific. The Auk 115: 1017–1033. doi: 10.2307/4089519
- Spear LB, Howell SN, Ainley DG 1992. Notes on the at-sea identification of some Pacific gadfly petrels (Genus: Pterodroma). Colonial Waterbirds 15: 202–218. doi: 10.2307/1521454
- Sweetapple P, Nugent G 2011. Chew-track-cards: a multiple-species small mammal detection device. New Zealand Journal of Ecology 35: 153–162.
- Thoresen AC 1967. Ecological observations on Stanley and Green Islands, Mercury Group. Notornis 14: 182–200.
- Towns DR, Atkinson IA, Daugherty CH 2006. Have the harmful effects of introduced rats on islands been exaggerated? Biological Invasions 8: 863–891. doi: 10.1007/s10530-005-0421-z
- Towns DR, Broome KG 2003. From small Maria to massive Campbell: forty years of rat eradications from New Zealand islands. New Zealand Journal of Zoology 30: 377–398. doi: 10.1080/03014223.2003.9518348
- Towns DR, Parrish GR, Tyrrell CL, Ussher GT, Cree A, Newman DG et al. 2007. Responses of tuatara (Sphenodon punctatus) to removal of introduced Pacific rats from islands. Conservation Biology 21: 1021–1031. doi: 10.1111/j.1523-1739.2007.00742.x
- Towns DR, West CJ, Broome KG 2013. Purposes, outcomes and challenges of eradicating invasive mammals from New Zealand islands: an historical perspective. Wildlife Research 40: 94–107. doi: 10.1071/WR12064
- VanderWerf EA, Mosher SM, Burt MD, Taylor PE, Sailer D 2011. Variable efficacy of rat control in conserving O'ahu ‘Elepaio populations. In: Veitch CR, Clout MN, Towns DR eds. Island invasives: eradication and management. Proceedings of the International Conference on Island Invasives. Gland, Switzerland, and Auckland, IUCN, The Centre for Biodiversity and Biosecurity. Pp. 124–130.
- Warham J 1977. Wing loadings, wing shapes, and flight capabilities of Procellariiformes. New Zealand Journal of Zoology 4: 73–83. doi: 10.1080/03014223.1977.9517938
- Warham J 1990. The petrels, their ecology and breeding systems. London, Academic Press. 448 p.
- Whitehead AL, Lyver PO, Jones CJ, Macleod CJ, Bellingham PJ, Coleman M et al. 2014. Establishing accurate baseline estimates of breeding populations of a burrowing seabird, the grey-faced petrel. Establishing accurate baseline estimates of breeding populations of a burrowing seabird, the grey-faced petrel (Pterodroma macroptera gouldi) in New Zealand. Biological Conservation 169: 106–116.
- Yackel Adams AA, Stanford JW, Wiewel AS, Rodda GH 2011. Modelling detectability of kiore (Rattus exulans) on Aguiguan, Mariana Islands, to inform possible eradication and monitoring efforts. New Zealand Journal of Ecology 35: 145–152.