Abstract
There are few published records of tardigrades (Phylum Tardigrada) from Oceania. Although terrestrial tardigrades have been collected from the Hawaiian islands of Oʻahu, Maui and Hawaiʻi, no species have been recorded from the northernmost main island, Kauaʻi. Samples of moss and lichen from Kaua'i and O'ahu yielded 302 tardigrade specimens and 35 eggs, representing nine genera and 16 species. Nine genera and 10 species were collected from Kaua'i, six genera and 10 species from O'ahu. A new species, Milnesium shilohae sp. nov., from O'ahu is described. The new species, a member of the ‘tardigradum’ group, has claw configuration [3–3]–[3–3]. It most closely resembles Milnesium bohleberi, from which it can be distinguished by its more posterior stylet support insertion point, narrower buccal tube standard width, more cylindrical buccal tube, and in having internal and anterior secondary claw spurs that are much larger than external and posterior spurs.
http://zoobank.org/urn:lsid:zoobank.org:pub:B33333AB-8E00-47FA-A856-27129AF25570
Introduction
Oceania is a biogeographic realm or ecozone consisting of the Papuan, Micronesian, Hawaiian, Southeastern Polynesian, Central Polynesian, New Caledonian and East Melanesian Biogeographic Provinces (Udvardy Citation1975). The tardigrade fauna (Phylum Tardigrada) of Oceania has received less attention than that of any other ecozone. To date, only 12 peer-reviewed papers have been published on terrestrial and freshwater water bears from Oceania, recording the presence of 19 genera and 56 species ( and ). These papers have addressed the tardigrade fauna of New Britain and New Guinea (Papuan Province), Eniwetok in the Marshall Islands and Palau in the Caroline Islands (Micronesian Province), Hawai'i and O'ahu (Hawaiian Province), Easter Island (Southeastern Polynesian Province), Samoan Islands (Central Polynesian Province), Vanuatu (New Caledonian Province) and Fiji (East Melanesian Province) (). Most sampling has been of lichen, moss and leaf litter (), but freshwater (Kaczmarek et al. Citation2012) and supralittoral sand (Mehlen Citation1972) have also been examined.
Table 1 Number of papers reporting tardigrades in Oceania and the number of freshwater and terrestrial tardigrade genera and species reported. The category species include species complexes only when no individual species within the complex have been reported.
Table 2 Terrestrial Tardigrada from Oceania (sensu Udvardy Citation1975). Records are from published papers and the results of this paper. Taxa are arranged in the order found in Degma et al. (Citation2009–2015). Species listed as cosmopolitan meet the criteria of Pilato & Binda (Citation2001); those listed as endemic have known distributions limited to Oceania.
In an unpublished dissertation Mehlen (Citation1971) recorded the following species on cryptogams and supralittoral sand in Oceania: Maui, Hawaiian Islands—Milnesium tardigradum Doyère, 1840, Hypsibius convergens (Urbanowicz, 1925), Hebesuncus conjungens (Thulin, 1911), Macrobiotus harmsworthi Murray, 1907, Macrobiotus hufelandi C.A.S. Schultze, 1834 and Dactylobiotus ambiguus (Murray, 1907); Palau, Caroline Islands—Hypsibius sp., M. harmsworthi and M. hufelandi; Ponape, Caroline Islands—Itaquascon umbellinae de Barros, 1939 and M. harmsworthi; Truk, Caroline Islands—Macrobiotus recens Cuénot, 1932 and Minibiotus hufelandioides (Murray, Citation1910), and Yap, Caroline Islands—Macrobiotus evelinae de Barros, 1938 and M. harmsworthi. Gon et al. (Citation1986) collected tardigrades from moss and lichen in the Central Crater District of Haleakala National Park on Maui. In a non-peer-reviewed conference proceedings, they reported the presence of Milnesium tardigradum; one species each of Dactylobiotus R.O. Schuster, 1980, Minibiotus R.O. Schuster, 1980, Calohpsibius Thulin, 1928, Diphascon Plate, 1888 and Hypsibius Ehrenberg, 1848; two species of Doryphoribius Pilato, 1969; five species of Macrobiotus C.A.S. Schultze, 1834, and 16 species of Echiniscus C.A.S. Schultze, 1840 (six from the ‘arctomys’ group and 10 others). Only Milnesium tardigradum was identified beyond genus or species group.
In this article I report the tardigrades found in terrestrial moss and lichen samples from O'ahu and Kaua'i, Hawaiian Islands and describe a new species of Milnesium Doyère, 1840. Fifteen species have been recorded from O'ahu (Richters Citation1908; Murray Citation1910; Binda & Pilato Citation1994). Pollock (Citation1995) described marine tardigrades found in beach sand from Anahola Bay, Kaua'i, but there are no published records of terrestrial water bears from the island.
Materials and methods
Moss, foliose and fruticose lichen and leaf litter samples were collected 14–18 July 2011 from eight sites on the island of Kaua'i and five sites on O'ahu. Samples were stored in sealed paper envelopes. In the laboratory they were soaked overnight in tap water to rehydrate tardigrades. After soaking, the material was sieved and the residue retained by 42 μm mesh was examined with a dissecting microscope (Nikon SMZ-U Zoom 1:10). Tardigrade specimens were mounted on slides in polyvinyl lactophenol and examined using phase-contrast microscopy (Nikon Eclipse 50i).
Specimens of a new species of Milnesium were measured using imaging software (NIS-Elements D 2.30, SPI). The pt index is the ratio of the length of a given structure to that of the buccal tube expressed as a percentage (Pilato Citation1981). Morphometric analysis and terminology follow the recommendations of Tumanov (Citation2006) for claws and Michalczyk et al. (Citation2012a,Citationb) for the buccal tube and associated structures. Morphometric data were handled using the Apochela v1.0 template available from the Tardigrada Register (www.tardigrada.net/register) (Michalczyk & Kaczmarek Citation2013). Body length was measured from the mouth to the posterior end, excluding legs IV. Buccal tube length and the insertion point of the stylet supports were measured from the anterior margins of the stylet sheaths; the posterior end of the buccal tube was taken as the point where the wall had thinned to the extent that outer and inner buccal tube diameter were no longer distinguishable. Buccal tube width was measured at the external diameter at three points—anterior, standard (at the point of stylet support insertion) and posterior (at the point where the buccal tube wall starts to thin and curve inwards). Lengths of primary and secondary branches of claws were measured as illustrated in Tumanov (Citation2006).
Bartels et al. (Citation2011) demonstrated that morphometric traits in Tardigrada are often allometric (disproportionate growth) rather than isometric. They recommended that a normalisation technique be used to eliminate body size effects and that two metrics be included in species descriptions: b, the allometric exponent, and a*, the Y-intercept of the regression line of the Thorpe normalised trait versus body size (i.e. a* is the theoretical value of a given trait when adjusted to the mean body size of the population). Using the techniques described in Bartels et al. (Citation2011), b and a* were calculated for the new species of Milnesium; buccal tube length was used as an indicator of body size.
Tardigrade taxonomy and nomenclature follow Guidetti & Bertolani (Citation2005), Degma & Guidetti (Citation2007) and Degma et al. (Citation2009–2015). Comments on species distribution are based on McInnes (Citation1994), Meyer (Citation2013) and Kaczmarek et al. (Citation2014, Citation2015). Species or species groups are considered cosmopolitan if they meet the criterion of Pilato & Binda (Citation2001), namely that they have been reported from five or more ecozones.
Several papers on the Tardigrada of Oceania were written before the development of rigorous standards of specific diagnosis (Pilato Citation2013; Vicente & Bertolani Citation2013). Some species regarded in older literature as cosmopolitan have proven to belong to complexes of morphologically similar species. Such records must be regarded as tentative unless confirmed (Kaczmarek et al. Citation2011; Michalczyk et al. Citation2012a,Citationb). Nevertheless, in this article all species identifications are taken at face value unless amended by subsequent literature. Michalczyk et al. (Citation2012a,Citationb) stated that all earlier records of Milnesium tardigradum need confirmation; Nelson et al. (Citation1999) recommended the same for records of Thulinius augusti (Murray, 1907). In reports of these two species in Oceania are therefore qualified as sensu lato.
All specimens from this study are deposited in the W.A.K. Seale Museum, McNeese State University, Lake Charles, Louisiana, USA.
Collection sites
Site K1: Kōkeʻe Natural History Museum, Kōke'e State Park, Kaua'i; 22°7.81ʹN, 159°39.40ʹW, 1106 m; lichen on rocks at parking lot; slides SMLA14008–14013.
Site K2: Kalalau Lookout, Kōke'e State Park, Kaua'i; 22°9.01ʹN, 159°38.75ʹW, 1253 m.
K2a: fruticose lichen on tree; slide SMLA14017.
K2b: foliose lichen on tree; slides SMLA14018–14020.
Site K3: Fern Grotto, Wailua Falls, Wailua River State Park, Kaua'i; 22°02.03ʹN, 159°22.40ʹW, 53 m; moss on rock; slide SMLA14014.
Site K4: Pihea Trail, Kōke'e State Park, Kaua'i.
K4a: 22°8.91ʹN, 159°37.54ʹW, 1184 m; moss and fruticose lichen on rock; slide SMLA14022.
K4b: 22°9.10ʹN, 159°37.26ʹW, 1271 m; moss on tree; slide SMLA14023.
K4c: 22°9.15ʹN, 159°37.20ʹW, 1256 m; moss and fruticose lichen on tree; slide SMLA14021.
K4d: 22°8.97ʹN, 159°37.42ʹW, 1224 m; moss on trail; slides SMLA14015 and 14016.
Site K5: cave tunnel entrance, Grove Farm Plantation, Kaua'i; slide SMLA14007.
Site K6: Keʻe State Park, Kaua'i; 22°13.25ʹN, 159°34.98ʹW, 5 m.
K6a: 5 m; moss on tree; slide SMLA14006.
K6b: moss on rock; slides SMLA14001–14005.
K6c: moss on rock; slide SMLA14000.
Site O1: Kaʻena Point State Park, O'ahu; 21°34.73ʹN, 158°14.41ʹW, 21 m; lichen on rock.
O1a: slides SMLA15000 and 15001.
O1b: slide SMLA15010.
Site O2: Wahiawa Freshwater State Park, O'ahu; 21°29.51ʹN, 158°1.51ʹW, 273 m.
O2a: lichen on stone wall; slides SMLA15011–15013.
O2b: moss on broadleaf tree; slides SMLA15007–15009.
O2c: moss on pine; slides SMLA15025–15026.
O2d: lichen on pine; slide SMLA15002.
O2e: moss on pine; slide SMLA15003.
O3: Nuʻuanu Pali Lookout, Nu'uanu Pali State Wayside, O'ahu; 21°21.97ʹN, 157°47.62ʹN, 334 m.
O3a: moss on rock wall; slide SMLA15026.
O3b: moss on tree; slides SMLA15004–15006.
O4: Ahupuaʻa O Kahana State Park, O'ahu; 21°33.98ʹN, 157°52.78ʹW, 30 m; moss on trees.
O4a: slides SMLA15014–15016.
O4b: slides SMLA15017 and 15018.
Results
Samples of moss and lichen from Kaua'i and O'ahu yielded 302 tardigrade specimens and 35 eggs, representing nine genera and 16 species. Nine genera and 10 species were collected from Kaua'i, six genera and 10 species from O'ahu. The maximum number of species found in a single sample was five. Eight species are new records for Oceania, nine for the Hawaiian Islands and seven for O'ahu.
No tardigrades were found in samples of cryptogams from Waimea Canyon Lookout and P'uu o Kila Lookout, Kaua'i, and Heʻeia State Park, O'ahu. Leaf litter samples from Kōke'e State Park, Kaua'i and Wahiawa Freshwater State Park, O'ahu yielded no specimens.
Taxonomic account
Phylum Tardigrada Doyère, 1840
Class Heterotardigrada Marcus, 1927
Order Echiniscoidea Richters, 1926
Family Echiniscidae Thulin, 1928
Genus Echiniscus C.A.S. Schultze, 1840
Echiniscus cavagnaroi Schuster & Grigarick, 1966
Material examined: sites K2b, O2a, O2b. 21 specimens.
Echiniscus cavagnaroi has been reported from the Galápagos Islands, southeastern USA, Dominican Republic and China. This is its first record from Oceania.
Echiniscus nigripustulus Horning, Schuster & Grigarick, 1978
Material examined: sites K3, O2a. Two specimens.
This is the first record of E. nigripustulus outside New Zealand.
Echiniscus virginicus Riggin, 1962
Material examined: sites O1b, O2a. 10 specimens.
Echiniscus virginicus has not previously been recorded outside North and South America.
Genus Pseudechiniscus Thulin, 1911
Pseudechiniscus novaezeelandiae (Richters, 1908)
Material examined: sites O4a, O4b. 12 specimens.
Murray (Citation1910) found this cosmopolitan species in the vicinity of Honolulu, O'ahu.
Pseudechiniscus suillus (Ehrenberg, 1853)
Material examined: sites K1, K2a, K2b, K4a, K4b, K6b, O3b. 35 specimens.
Murray (Citation1910) found this cosmopolitan species in the vicinity of Honolulu, O'ahu.
Class Eutardigrada Richters, 1926
Order Apochela Schuster, Nelson, Grigarick & Christenberry, 1980
Family Milnesiidae Ramazzotti, 1962
Genus Milnesium Doyère, 1840
Milnesium sp.
Material examined: sites K2a, K2b, K4c, K6b. 16 specimens.
The habitus of these specimens is largely consistent with M. bohleberi Bartels et al. Citation2014 (i.e. smooth cuticle; wide, tapering buccal tube; [3–3]–[3–3] claw configuration); however, the buccal tube is somewhat narrower. Inadequate number of properly oriented specimens in good condition precluded conclusive identification.
Milnesium shilohae sp. nov. (–; )
Material examined: Holotype and nine paratypes—Ahupua'a O Kahana State Park, O'ahu, Hawaiian Islands; 21°33.98′N, 157°52.78ʹW, 30 m, moss on tree, slides SMLA15015 (with holotype), 15016. 84 additional specimens and 25 eggs—sites O1a, O2a, O3a, O3b, O4a, O4b.
Type repository: W.A.K. Seale Museum, McNeese State University, Lake Charles, Louisiana, USA.
Description of holotype: Morphometric measurements in . Female. Body white or transparent, cuticle smooth (A). Eyes not visible in mounted specimen. Two lateral and six peribuccal papillae present. Buccal apparatus of the Milnesium type (B). Six peribuccal lamellae. Buccal tube cylindrical (posterior/anterior width ratio 91%). Pharyngeal bulb elongated, pear-shaped, and without placoids or septulum.
Figure 1 Milnesium shilohae sp. nov. (phase-contrast microscopy). A, Habitus, ventral view, scale bar 100 μm; B, buccal tube, scale bar 10 μm.
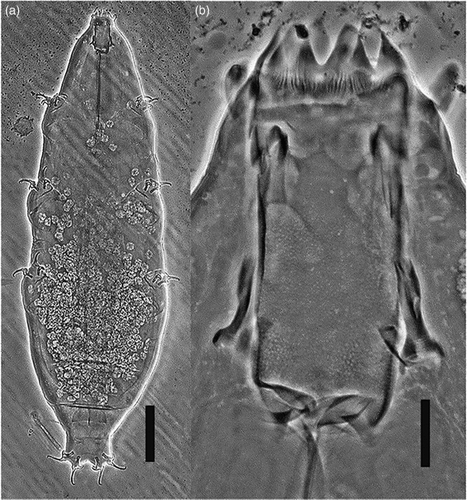
Figure 2 Milnesium shilohae sp. nov. (phase-contrast microscopy). A, Claws I with cuticular bar; B, claws II with cuticular bar; C, claws IV. Scale bar 10 μm.
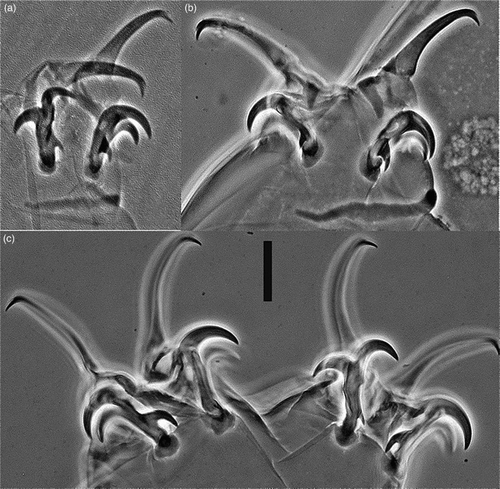
Table 3 Morphometric data and pt values of selected characters of the holotype and nine paratypes of Milnesium shilohae sp. nov. from O'ahu, Hawaii, USA. Range refers to the smallest and largest structure found among all measured specimens. All lengths in micrometres.
Claws of the Milnesium type (). Primary claw branches stout, with well developed accessory points. Secondary claw base + secondary branches slender, with round basal thickenings. All secondary claws with three points (i.e. with prominent basal spurs, claw configuration [3–3]–[3–3]). Internal and posterior spurs larger than external and anterior spurs. Long, wide, transverse cuticular bars on first three pairs of legs present (A–B).
Derivatio nominis: The specific epithet is a genitive noun honoring my niece Shiloh-Rose Harriet Jones in recognition both of her help with tardigrade collecting over the years and her abiding fascination with Hawaii.
Remarks: Eggs are oval with smooth surfaces and are deposited in the exuvia, as is characteristic of Milnesium. Two such exuviae were found, one with 10 eggs and one with 15.
Four characters out of 31, all from claws, were significantly allometric ().
This species is widely distributed in O'ahu. Four specimens collected from site K5, Kaua'i, may belong to this species, but conclusive identification was not possible because the stylet support insertion point could not be discerned in any of them. Differential diagnosis: Michalczyk et al. (Citation2012a,Citationb) divided species in the genus Milnesium into two groups, those with smooth cuticles (‘tardigradum’ group) and those with sculptured cuticle (‘granulatum’ group). The cuticle of two recently described species (M. beasleyi Kaczmarek, Jakubowska & Michalczyk, 2012, and M. berladnicorum Ciobanu, Zawierucha, Moglan & Kaczmarek, 2014) has pseudopores. The smooth cuticle of Milnesium shilohae sp. nov. places it in the tardigradum group, distinguishing it from species with sculptured cuticle or pseudopores. Claw configuration [3–3]–[3–3] distinguishes the new species from members of the tardigradum group with other configurations.
Eight species in the tardigradum group (Milnesium antarcticum Tumanov, Citation2006; M. asiaticum Tumanov, Citation2006; M. barbadosense Meyer & Hinton, 2012; M. bohleberi, M. brachyungue Binda & Pilato, 1990; M. eurystomum Maucci, 1991; M. longiungue Tumanov, Citation2006 and M. zsalakoae Meyer & Hinton, 2010) have claw configuration [3–3]–[3–3]. Milnesium shilohae sp. nov. has a more posterior insertion of the stylet supports than all of these species (mean pt 76.3 in the new species; 60.0–72.4 in the others), and wider standard buccal tube width than M. antarcticum, M. asiaticum, M. barbadosense, M. brachyungue and M. zsalakoae (mean standard width to buccal tube length ratio in the new species 0.51; 0.35–0.44 in the others). Milnesium shilohae sp. nov. has a narrower standard buccal tube width than M. bohleberi and M. eurystomum (mean standard width to buccal tube length ratio 0.59 in M. bohelberi, 0.62 and 0.65 for the holotype and one paratype of M. eurystomum).
Milnesium shilohae sp. nov. most closely resembles M. bohleberi in habitus (i.e. smooth cuticle, wide buccal tube) and [3–3]–[3–3] claw configuration (Bartels et al. Citation2014). In addition to its much more posterior stylet support insertion point and narrower buccal tube standard width, the buccal tube of M. shilohae sp. nov. is more cylindrical than M. bohleberi (range of posterior to anterior buccal tube width ratio 90%–107% in the new species, 80%–88% in M. bohleberi). Moreover, M. shilohae sp. nov. differs from M. bohleberi in the relative sizes of internal and anterior secondary claw spurs compared to external and posterior. In M. bohleberi there is little difference in size among spurs; in M. shilohae sp. nov. internal and anterior spurs are about twice the length of external and posterior spurs (see a* values in ).
Order Parachela Schuster, Nelson, Grigarick & Christenberry, 1980
Superfamily Hypsibioidea Pilato, 1969
Family Hypbsibiidae Pilato, 1969
Subfamily Diphasconinae Dastych, 1992
Genus Diphascon Plate, 1888
Diphascon sp.
Material examined: sites K4a, K4b. Two specimens.
These specimens have the ‘drop’ diagnostic of the genus (Bertolani et al. Citation2014).
Genus Ramazzottius Binda & Pilato, 1986
Family Microhypsibiidae Pilato, 1998
Ramazzottius cf. oberhaeuseri (Doyère, 1840)
Material examined: site K1. Three specimens.
Murray (Citation1910) found this cosmopolitan species in the vicinity of Honolulu, O'ahu. Definitive identification requires eggs, which were not found.
Superfamily Isohypsibioidea Sands, McInnes, Marley, Goodall-Copestake, Convey & Linse, 2008
Family Isohypsibiidae Sands, McInnes, Marley, Goodall-Copestake, Convey & Linse, 2008
Genus Doryphoribius Pilato, 1969
Doryphoribius flavus (Iharos, 1966)
Material examined: site O2d. One specimen.
This is the first record of this cosmopolitan species in Oceania.
Superfamily Macrobiotoidea Thulin, 1928
Family Macrobiotidae Thulin, 1928
Genus Macrobiotus C.A.S. Schultze, 1834
Macrobiotus cf. echinogenitus Richters, 1903
Material examined: site O1b. 16 specimens.
The specimens belong to the cosmopolitan ‘echinogenitus’ species group, but as no eggs were recovered they could not be identified to species.
Macrobiotus hufelandi C.A.S. Schultze, 1834
Material examined: sites K1, K2a, K2b, K6c, O3a, O3b. 47 specimens, six eggs.
Murray (Citation1910) found this cosmopolitan species in the vicinity of Honolulu, O'ahu.
Genus Minibiotus R.O. Schuster, 1980
Minibiotus furcatus (Ehrenberg, 1859)
Material examined: site K2b. Two eggs.
This is the first record of this cosmopolitan species in Oceania.
Minibiotus intermedius (Plate, 1888)
Material examined: sites K2a, K2b, K4b, K4d, K6a, K6b, O2b, O2c, O2e. 37 specimens, two eggs.
Murray (Citation1910) found this cosmopolitan species in the vicinity of Honolulu, O'ahu.
Genus Paramacrobiotus Guidetti et al., 2009
Paramacrobiotus cf. areolatus (Murray, 1907)
Material examined: sites K4b, K4d. Two specimens.
The adult specimens resemble P. areolatus, a cosmopolitan species, but in the absence of eggs the identification could not be confirmed.
Paramacrobiotus cf. richtersi (Murray, 1911)
Material examined: sites K4d, O2d. Three specimens.
The specimens belong to the cosmopolitan ‘richtersi’ species group. Because no eggs were recovered they could not be identified to species.
Discussion
Many islands in Oceania have seen little or no tardigrade collecting. Even in better-studied islands, the high level of small-scale spatial variability in tardigrade distribution (Meyer Citation2006; Degma et al. Citation2011) makes it likely that species have been missed. Accurate assessment of regional variation would be facilitated if consistent standards of specific diagnosis were used. For example, the ‘Macrobiotus hufelandi’ group was once considered one of a few cosmopolitan species, but systematic revision has shown that it includes a large number of non-cosmopolitan species (Biserov Citation1990a,Citationb; Bertolani & Rebecchi Citation1993).
A relatively high percentage (> 68%) of freshwater and terrestrial tardigrade species are limited to a single biogeographic ecozone (Pilato & Binda Citation2001). The currently published distributions of 10 species are restricted to Oceania (18% of Oceania Tardigrada). Twenty-eight species (50%) found in Oceania are cosmopolitan. However, how many (if any) tardigrade species are truly cosmopolitan is uncertain. The existence of cryptic species that are genetically but not morphologically distinguishable has been reported (Faurby et al. Citation2008, Schill et al. Citation2010). Integrative taxonomic methodology, combining morphological and molecular techniques, can also identify hitherto unsuspected synonymous species (Vicente et al. Citation2013, Bertolani et al. Citation2014). Heger et al. (Citation2009) demonstrated that taxonomic uncertainty undermines biogeographic studies of testate amoebae; the same uncertainty undermines tardigrade studies.
Guil & Carbrero-Sañudo (Citation2007) remarked on the preponderance of tardigrade studies carried out by scientists from the Palearctic and Nearctic ecozones (e.g. very few of the papers on tardigrades from the Afrotropical and Indomalayan ecozones are authored by workers from those areas themselves). All papers on the water bears of Oceania have been published by scientists from North America and Europe.
This study increases considerably our knowledge of the Tardigrada of the Hawaiian Islands. Among the components of Oceania, only the Papuan Province has a comparable number of recorded species. Even so, the tardigrade fauna of Hawai'i and Maui are poorly known, and there are no records from the islands of Molakaʻi, Lānaʻi, Niʻihau and Kahoʻolawe. There is much scope for further tardigrade research in the Hawaiian Islands and elsewhere in Oceania.
Associate Editor: Dr Cor Vink.
Acknowledgements
I thank Paul Bartels for confirming that Milnesium specimens from O'ahu differ significantly from Milnesium bohleberi.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Bartels PJ, Nelson DR, Exline RP 2011. Allometry and the removal of body size effects in the morphometric analysis of tardigrades. Journal of Zoological Systematics and Evolutionary Research 49: 17–25. doi: 10.1111/j.1439-0469.2010.00593.x
- Bartels PJ, Nelson DR, Kaczmarek Ł, Michalczyk Ł 2014. The genus Milnesium (Tardigrada: Eutardigrada: Milnesiidae) in the Great Smoky Mountains National Park (North Carolina and Tennessee, USA), with the description of Milnesium bohleberi sp. nov. Zootaxa 3826: 356–368. doi: 10.11646/zootaxa.3826.2.5
- Bertolani R, Guidetti R, Marchioro T, Altiero T, Rebecchi L, Cesari M 2014. Phylogeny of Eutardigrada: new molecular data and their morphological support lead to the identification of new evolutionary lineages. Molecular Phylogenetics and Evolution 76: 110–126. doi: 10.1016/j.ympev.2014.03.006
- Bertolani R, Rebecchi L 1993. A revision of the Macrobiotus hufelandi group (Tardigrada, Macro biotidae) with some observations on the taxonomic characters of eutardigrades. Zoologica Scripta 22: 127–152. doi: 10.1111/j.1463-6409.1993.tb00347.x
- Binda MG, Pilato G 1994. Notizie sui Tardigradi delle Isole Hawaii con descrizione di due specie nuove. Animalia 21: 57–62.
- Biserov VI 1990a. On the revision of the genus Macrobiotus. The subgenus Macrobiotus s. str.: a new systematic position of the hufelandi group (Tardigrada, Macrobiotidae). Communication 1. Zoologichesky Zhurnal 69: 5–17. [in Russian with English abstract]
- Biserov VI 1990b. On the revision of the genus Macrobiotus. The subgenus Macrobiotus s. str.: a new systematic position of the hufelandi group (Tardigrada, Macrobiotidae). Communication 2. Zoologichesky Zhurnal 69: 38–50. [in Russian with English abstract]
- Degma P, Bertolani B, Guidetti R 2009–2015. Actual checklist of Tardigrada species (2009–2015, Ver. 28: 31-03-2015). http://www.tardigrada.modena.unimo.it/miscellanea/Actual%20checklist%20of%20Tardigrada.pdf (accessed 27 April 2015).
- Degma P, Guidetti R 2007. Notes to the current checklist of Tardigrada. Zootaxa 1579: 41–53.
- Degma P, Katina S, Sabatovičová L 2011. Horizontal distribution of moisture and Tardigrada in a single moss cushion. Journal of Zoological Systematics and Evolutionary Research 49: 71–77. doi: 10.1111/j.1439-0469.2010.00602.x
- Faurby S, Jönsson KI, Rebecchi L, Funch P 2008. Variation in anhydrobiotic survival of two eutardigrade morphospecies: a story of cryptic species and their dispersal. Journal of Zoology 275: 139–145. doi: 10.1111/j.1469-7998.2008.00420.x
- Gon III SM, Kimsey RA, Schuster RO, Willis MA 1986. A prodromus for the water bear fauna of Haleakala National Park. In: Smith CW, Stone CP eds. Proceedings of the Sixth Conference in Natural Sciences Hawaii Volcanoes National Park. Manoa, Cooperative National Park Resources Studies Unit, University of Hawaii at Manoa. Pp. 15–22.
- Guidetti R, Bertolani R 2005. Tardigrade taxonomy: an updated checklist of the taxa and a list of characters for their identification. Zootaxa 845: 1–46.
- Guil N, Carbrero-Sañudo FJ 2007. Analysis of the species description process for a little-known invertebrate group: the limnoterrestrial tardigrades (Bilateria, Tardigrada). Biodiversity and Conservation 16: 1063–1086. doi: 10.1007/s10531-006-9069-y
- Heger TJ, Mitchell EAD, Ledeganck P, Vincke S, Van de Vijver B, Beyens L 2009. The curse of biogeographical uncertainty in biogeographical studies of free-living terrestrial protists: a case study of testate amoebae from Amsterdam Island. Journal of Biogeography 36: 1551–1560. doi: 10.1111/j.1365-2699.2009.02094.x
- Iharos G 1967 Beitrage zur Kenntnis der Tardigradenfauna von Neuguinea. Opuscula Zoologica Budapest 7: 113–135.
- Iharos G 1973. Neuere Daten zur Kenntnis der Tardigraden-Fauna von Neuguinea. Opuscula Zoologica Budapest 11: 63–73.
- Kaczmarek Ł, Diduszko D, Michalczyk Ł. 2011. New records of Mexican Tardigrada. Revista Mexicana de Biodiversidad 82: 1324–1327.
- Kaczmarek Ł, Michalczyk Ł, McInnes SJ 2014. Annotated zoogeography of non-marine Tardigrada. Part I: Central America. Zootaxa 3763: 1–62. doi: 10.11646/zootaxa.3763.1.1
- Kaczmarek Ł, Michalczyk Ł, McInnes SJ 2015. Annotated zoogeography of non-marine Tardigrada. Part II: South America. Zootaxa 3923: 1–107. doi: 10.11646/zootaxa.3923.1.1
- Kaczmarek Ł, Schabetsberger R, Litwin M, Michalczyk Ł 2012. A new freshwater eutardigrade from Fiji and Vanuatu (Oceania), with remarks on the genus Dactylobiotus. New Zealand Journal of Zoology 39: 311–318. doi: 10.1080/03014223.2012.693511
- Kristensen RM, Michalczyk Ł, Kaczmarek Ł 2010. The first record of the genus Bryodelphax (Tardigrada: Heterotardigrada: Echiniscidae) from Easter Island, Rapa Nui (Pacific Ocean, Chile) with the description of a new species, Bryodelphax aaseae. Zootaxa 2343: 45–56.
- McInnes SJ 1994. Zoogeographic distribution of terrestrial/freshwater tardigrades from current literature. Journal of Natural History 28: 257–352. doi: 10.1080/00222939400770131
- Mehlen RH 1971. Eutardigrada from the Central Pacific. Ecdysis, morphology, taxonomy and zoogeography. Unpublished PhD Thesis. College Station, TX, Texas A&M University. 109 p.
- Mehlen RH 1972 Eutardigrada: distribution at Eniwetok Atoll, Marshall Islands. Pacific Science 26: 223–226.
- Meyer HA 2006. Interspecific association and substrate specificity in tardigrades from Florida, southeastern United States. Hydrobiologia 558: 133–139. doi: 10.1007/s10750-005-1412-x
- Meyer HA 2013. Terrestrial and freshwater Tardigrada of the Americas. Zootaxa 3747: 1–71. doi: 10.11646/zootaxa.3747.1.1
- Michalczyk Ł, Kaczmarek Ł 2013. The Tardigrade register: a comprehensive online data depository for tardigrade taxonomy. Journal of Limnology 72: 175–181. doi: 10.4081/jlimnol.2013.s1.e22
- Michalczyk Ł, Wełnicz W, Frohme M, Kaczmarek Ł 2012a. Corrigenda of Zootaxa, 3154: 1–20 Redescriptions of three Milnesium (Doyère, 1840) taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 3393: 66–68.
- Michalczyk Ł, Wełnicz W, Frohme M, Kaczmarek Ł 2012b. Redescriptions of three Milnesium (Doyère, 1840) taxa (Tardigrada: Eutardigrada: Milnesiidae), including the nominal species for the genus. Zootaxa 3154: 1–20.
- Murray J 1910. Tardigrada. British Antarctic expedition 1907–1909. Reports on the scientific investigations. 1 Biology (Part V): 83–187.
- Nelson DR, Marley NJ, Bertolani R 1999. Re-description of the genus Pseudobiotus (Eutardigrada, Hypsibiidae) and of the new type species Pseudobiotus kathmanae sp. n. Zoologischer Anzeiger 238: 311–317.
- Pilato G 1981. Analisi di nuovi caratterri nello studio degli Eutardigrada. Animalia 8: 51–57.
- Pilato G 2013. The past, the present and the future of eutardigrade taxonomy. Journal of Limnology 72: 1–7. doi: 10.4081/jlimnol.2013.s1.e1
- Pilato G, Binda MG 2001. Biogeography and limno-terrestrial tardigrades: are they truly incompatible binomials? Zoologischer Anzeiger 240: 511–516. doi: 10.1078/0044-5231-00061
- Pilato G, Fontoura P, Lisi O 2008. New description of Echiniscus viridis Murray, 1910 and remarks on the viridis group. New Zealand Journal of Zoology 35: 85–92. doi: 10.1080/03014220809510105
- Pollock LW 1995. New marine tardigrades from Hawaiian beach sand and phylogeny of the family Halechiniscidae. Invertebrate Zoology 114: 220–235. doi: 10.2307/3226877
- Rahm G 1936. Tardigraden der Osterinsel (Rapa-nui). Zoologischer Anzeiger 115: 27–28.
- Ramazzotti G, Maucci W 1983. Il Phylum Tardigrada (III. edizione riveduta e aggiornata). Memorie dell'Istituto Italiano di Idrobiologia 41: 1–1012.
- Richters F 1908. Beitrag zur Kenntnis der Moosfauna Australiens und der Inseln des Pazifischen Ozeans. Zoologischer Jahrbücher, Jena 26: 196–213.
- Schill RO 2013. Life-history traits in the tardigrade species Paramacrobiotus kenianus and Paramacrobiotus palaui. Journal of Limnology 72: 160–165. doi: 10.4081/jlimnol.2013.s1.e20
- Schill RO, Förster F, Dandekar T, Wolf M 2010. Using compensatory base change analysis of internal transcribed spacer 2 secondary structures to identify three new species in Paramacrobiotus (Tardigrada). Organism, Diversity & Evolution 10: 287–296. doi: 10.1007/s13127-010-0025-z
- Tumanov DV 2006. Five new species of the genus Milnesium (Tardigrada, Eutardigrada, Milnesiidae). Zootaxa 1112: 1–23.
- Udvardy MDF 1975. A classification of the biogeographical provinces of the World. IUCN Occasional Paper No. 18: 1–48.
- Vicente F, Bertolani R 2013. Considerations on the taxonomy of the Phylum Tardigrada. Zootaxa 3636: 235–248. doi: 10.11646/zootaxa.3646.3.3
- Vicente F, Fontoura F, Cesari M, Rebecchi L, Guidetti R, Serrano A et al. 2013. Integrative taxonomy allows the identification of synonymous species and erection of a new genus of Echiniscidae (Tardigrada, Heterotardigrada). Zootaxa 3613: 557–572. doi: 10.11646/zootaxa.3613.6.3
