Abstract
Squids in the family Mastigoteuthidae Verrill, 1881 are ecologically important, being prey to many apex predators, yet the diversity and systematics of the family remain poorly understood. Mastigoteuthid taxonomy has been controversial and unstable because species in this family are rarely caught and often damaged during capture due to their delicate nature. Out of 21 named species, recent reviews have accepted eight to 17 species. A complete taxonomic review of the New Zealand mastigoteuthids is undertaken here for the first time to identify and describe locally occurring species. Six species have been identified from New Zealand waters: Mastigoteuthis cf. dentata Hoyle, Citation1904; Mastigoteuthis psychrophila Nesis, Citation1977; Idioteuthis cordiformis (Chun, Citation1910); Mastigopsis hjorti (Chun, 1913); Magnoteuthis osheai sp. nov.; and Echinoteuthis famelica (Berry, Citation1909). A full review of this family is still required; an integrative taxonomic approach will be essential because there is often low interspecific and high intraspecific morphological variation.
http://zoobank.org/urn:lsid:zoobank.org:pub:FF4A64A5-7D13-4FAB-9DE9-B6FAD05831C7
Introduction
Squids of the mesopelagic and bathypelagic family Mastigoteuthidae are distinguished by their long whip-like tentacles. Although they appear to be one of the most common deep-sea squid taxa (Young Citation1972), specimens are rarely caught in large numbers, and are frequently damaged during collection, especially external characters that have previously been relied upon heavily for species identification (e.g. tentacles, photophores). As a result, there has been a great deal of confusion in mastigoteuthid taxonomy, confounded by the additional difficulties of many species being described in inadequate detail, and/or only from single life stages or damaged specimens. Higher taxonomy also remains unstable with several genera and subgenera named but currently debated (e.g. Salcedo-Vargas & Okutani Citation1994; Salcedo-Vargas Citation1997; Vecchione et al. Citation2007). Herein, the higher taxonomy follows Braid et al. (Citation2014) who reviewed the classification of this family using integrative taxonomy to distinguish five genera: Mastigoteuthis (Mt.) Verrill, 1881; Idioteuthis Sasaki, Citation1929; Mastigopsis (Mp.) Grimpe, Citation1922; Echinoteuthis Joubin, Citation1933; and Magnoteuthis (Mg.) Salcedo-Vargas & Okutani, Citation1994. An additional genus, Mastigotragus (Mr.) Young, Vecchione, & Braid Citation2014, has also been recently erected for the species Mr. pyrodes (Young, Citation1972).
Much of the previous work on the Mastigoteuthidae has focused on species found around the United States (e.g. Young Citation1972, Citation1991; Vecchione et al. Citation2007). In addition, Salcedo-Vargas (Citation1993) reviewed the Mastigoteuthidae from the northwestern Pacific. However, the taxonomy of the New Zealand mastigoteuthids has not been previously reviewed, and very little is known about local representatives of the family. They were first reported from local waters just 50 years ago by Dell (Citation1959), who identified three specimens from Cook Strait as Mt. flammea Chun, Citation1908. Unfortunately, his illustration of this material lacked detail and the specimens were not described. During a review of the biodiversity of New Zealand, Spencer et al. (Citation2009) compiled a list of mastigoteuthids in New Zealand identified from previous works consisting of: Idioteuthis cordiformis (Chun Citation1910); ‘I.' magna (Joubin, Citation1913); Mt. agassizii Verrill, 1881; Mt. sp. 1; and Mt. sp. 2. The taxonomic classifications were based on Salcedo-Vargas & Okutani (Citation1994) and these species were identified from specimens found in the collections of the Museum of New Zealand Te Papa Tongarewa (NMNZ) and the National Institute of Water and Atmospheric Research, Ltd (NIWA). A more recent list of New Zealand mastigoteuthids by Spencer et al. (Citation2011) reported three species: I. cordiformis and ‘I.’ magna (as cited in Spencer et al. Citation2009), and Mt. agassizii (which was synonymised with Mt. dentata Hoyle, Citation1904 and Mt. flammea following Salcedo-Vargas & Okutani Citation1994). In addition to identifying Mt. dentata as occurring in New Zealand, Nesis (Citation1987) identified ‘Mt.’ magna, ‘Mt.’ hjorti Chun, Citation1913, and Echinoteuthis sp. as occurring in the Tasman Sea, and Mt. psychrophila Nesis, Citation1977 as having a circumglobal Antarctic distribution.
Reviewing the taxonomy of the Mastigoteuthidae around New Zealand is essential in order to make informed decisions about the management of anthropogenic threats to their habitat. These are deep-sea species that have the potential to be affected by trawling activity (Freeman et al. Citation2010). The local population of one species, I. cordiformis (the largest, most easily recognisable species in this family), has already been classified as nationally critical (Freeman et al. Citation2010). However, the threat status for other locally occurring species has not been evaluated, although mastigoteuthids are known to be trophically important in New Zealand waters. They have been reported in the diets of a variety of marine predators, including sperm whales, Physeter macrocephalus macrocephalus Linnaeus, 1758 (Clarke & Roper Citation1998); orange roughy, Hoplostethus atlanticus Collett, 1889 (Rosecchi et al. Citation1988); hoki, Macruronus novaezelandiae (Hector, 1871) (Horn & Dunn Citation2010); and black oreo, Allocyttus niger James, Inada & Nakamura, 1988 (pers. obs., Mastigoteuthis sp., NIWA 84373). Interestingly, several local species of birds also consume mastigoteuthids, including sooty shearwaters, Puffinus griseus (Gmelin, 1789) (Cruz et al. Citation2001); Buller's mollymawk, Diomedea bulleri (Rothschild, 1893) (West & Imber Citation1986); Providence petrel, Pterodroma solandri (Gould, 1844) (Bester et al. Citation2010); southern Buller's albatross, Diomedea bulleri bulleri (Rothschild, 1893) (James & Stahl Citation2000); and the Snares penguin, Eudyptes robustus Oliver, 1953 (Mattern et al. Citation2009).
The present study therefore aims to resolve local mastigoteuthid systematics, an objective with several benefits. New Zealand's true marine biodiversity will be more accurately understood, our understanding of trophic dynamics in a variety of local marine habitats will be improved, and detailed descriptions of locally occurring taxa will provide an important comparative basis for researchers investigating the family in other regions.
Materials and methods
Materials examined
Original type descriptions for all mastigoteuthid species were reviewed. Type specimens from the National Museum of Natural History, Smithsonian Institution, USA (USNM) were examined for Mt. agassizii, Mt. dentata, ‘Chiroteuthoides’ hastula Berry, Citation1920, ‘Mt.’ microlucens Young, Lindgren, & Vecchione, 2008, and ‘Chiroteuthis’ famelica Berry, Citation1909. The entire national collections of mastigoteuthid specimens were loaned and examined from NMNZ and NIWA in Wellington. Species synonymies are limited to all citations that provide morphological species descriptions and/or images, and descriptions are based on type specimens.
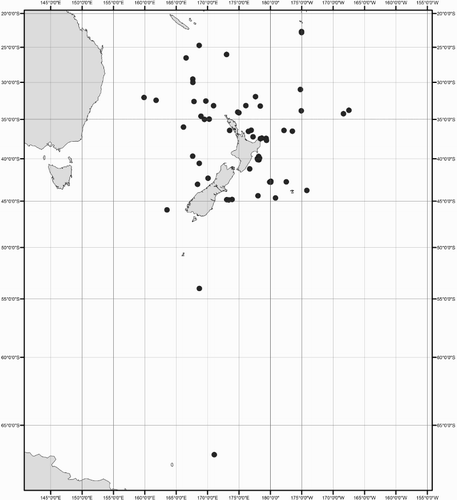
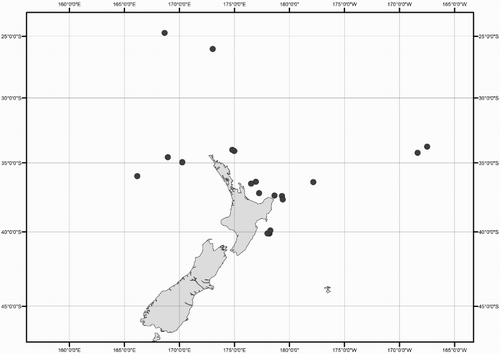
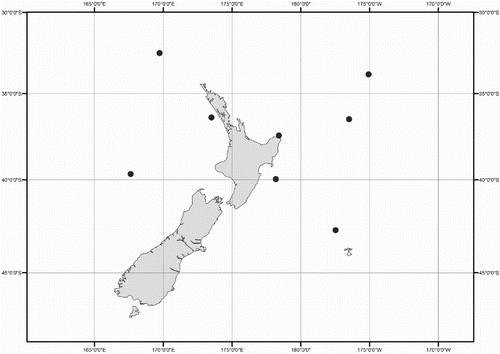
Collection data for some of the specimens were not available (mostly ex-gut-content material). A distribution map shows the location for all New Zealand specimens of Mastigoteuthidae used in this study (). Collection dates are listed as dd/mm/yyyy. Specimens are listed by order of decreasing latitude, and secondarily by dorsal mantle length (ML). Specimens were sexed except juveniles and those where the viscera were damaged (indicated as ‘sex indet.’). Specimens that were too damaged to be identified to species morphologically, and where no tissue was available for DNA analysis, were excluded from this study (four out of 98 available specimens).
Additional non-morphological abbreviations used are:
| BTT | = | bottom trawl |
| CAML | = | Census of Antarctic Marine Life |
| FV | = | fisheries vessel |
| IORAS | = | P. P. Shirshov Institute of Oceanology of the Russian Academy of Sciences |
| IPY | = | International Polar Year |
| MWT | = | midwater trawl |
| NIWA | = | National Institute of Water and Atmospheric Research, Ltd |
| NMNZ | = | Museum of New Zealand Te Papa Tongarewa |
| NORFANZ | = | New Zealand and Australia Norfolk Ridge and Lord Howe Rise Biodiversity Voyage |
| RNZFA | = | Royal New Zealand Fleet Auxiliary |
| RV | = | research vessel |
| Stn | = | station |
| USNM | = | Smithsonian National Museum of Natural History, USA |
| ZMB | = | Zoologisches Museum, Museum für Naturkunde der Humboldt-Universität, Berlin |
| ZMBN | = | University Museum of Bergen |
| ZIN | = | Zoological Institute, Russian Academy of Sciences |
Morphological examination
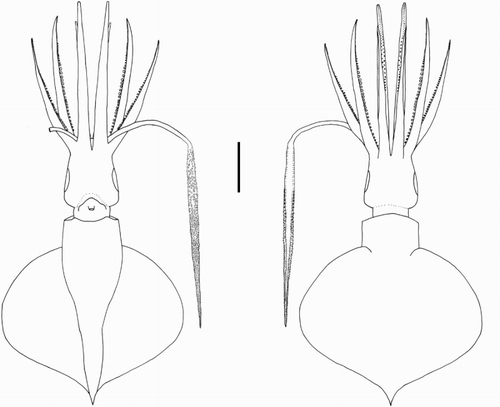
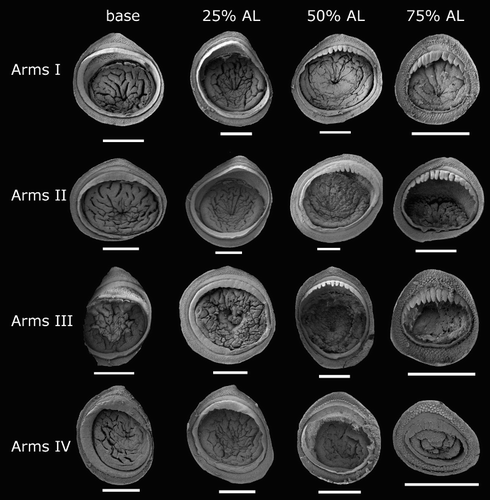
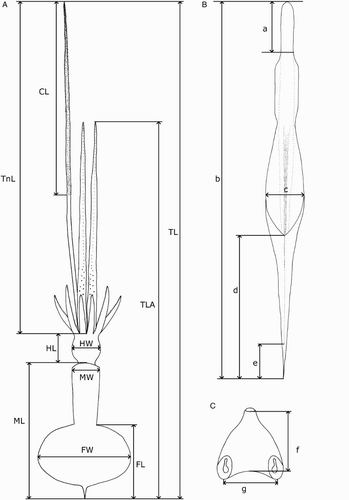
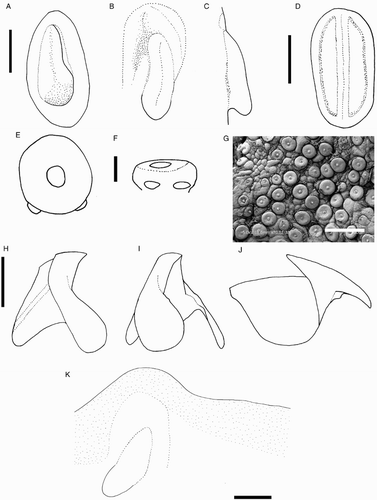
Species descriptions were made in accordance with the guidelines provided by Roper & Voss (Citation1983) as follows. Morphological descriptions focus on both internal anatomy (beak, gladius, radula, and palatine palps) and external anatomy (). The latter characters include: mantle shape; fin shape, width and length; head shape; funnel pocket (presence/absence); funnel-locking cartilage shape, tragus and antitragus (presence/absence) (A); mantle-locking cartilage shape; nuchal cartilage shape; arm length relative to ML; arm keels (presence/absence); arm-sucker and tentacle-sucker ultrastructure (B–C); and position of photophores (when present). The funnel pocket is a depression between the bridles of the funnel. The eye-sinus photophore is embedded in the tissue just ventral to the eye sinus, whereas the eye photophore is situated on the eye itself, and integumental photophores are situated on any external surface of the body (mantle, arms, fins, head, or funnel).
Figure 2 Terminology for mastigoteuthid funnel-locking cartilage and suckers. A, Funnel-locking cartilage; B, arm-sucker morphology; C, tentacle-sucker morphology.
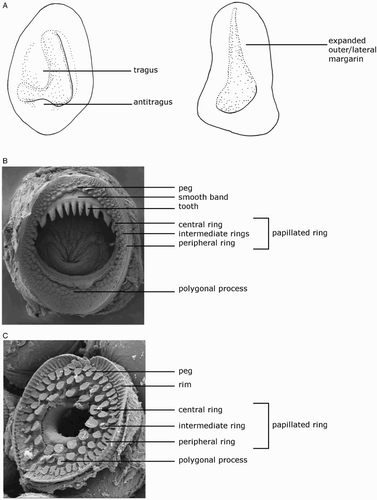
Table 1 Morphological characters for the mastigoteuthid species examined in this study.
Measurements () were taken from the more complete side of the specimen (indicated in the tables by R or L). Indices are based on the feature's dimension as a percentage of the mantle length; specific definitions are given below. Measurement ranges are given in the format of lowest value (X), mean (Y), and largest value (Z) in the format of X–Y–Z; where fewer than three specimens were available for a species, or when the range was less than 5% ML, only the mean is provided. Measurements of damaged features are indicated by an asterisk (*).
Figure 3 Mastigoteuthid measurements and acronyms as defined in text. A, Specimen measurements; B, gladius measurements: a, free rachis; b, gladius length; c, maximum width; d, secondary conus; e, rostrum; C, funnel measurements: f, funnel length; g, funnel width.
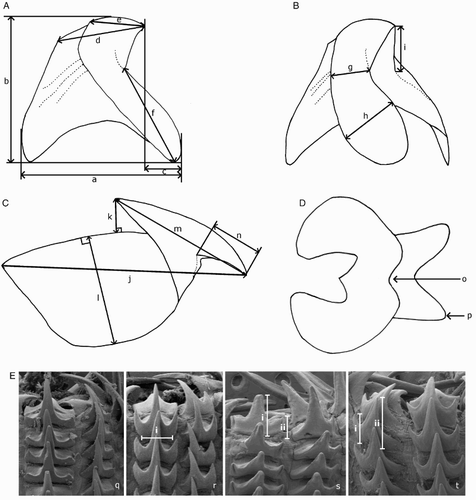
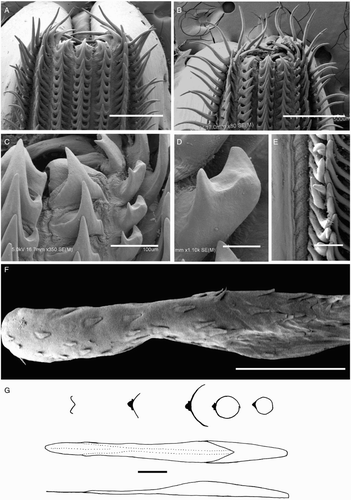
Beaks were described following Clarke (Citation1986), and drawn using a camera lucida (or when too large, from photographs; A–D). For scanning electron microscopy, specimens were critical-point dried at the University of Auckland, then platinum plated and imaged at the Auckland University of Technology. Sucker descriptions were based on Salcedo-Vargas (Citation1995) with some modifications (B–C). Palatine teeth on the lateral buccal palps are described following Bolstad (Citation2010). Radular tooth descriptions followed Bolstad (Citation2010) with some modifications (E).
Figure 4 Mastigoteuthid beak and radular tooth measurements. A, Lower beak, profile view: a, baseline; b, beak height; c, rostral tip behind leading edge of wing; d, crest length; e, hood length; f, wing length; B, lower beak, oblique view: g, minimum wing width; h, maximum wing width; i, lower rostral length; C, upper beak, profile view: j, beak length; k, hood height; l, beak width; m, hood length; n, upper rostral length; D, lower beak, ventral view: o, notch in hood; p, free corner; E, radular tooth measurements and types: q, rachidian with narrow, sharp, triangular mesocone and small lateral cusps, base rectangular and weakly bicuspid first lateral; r, rachidian with narrow, sharp, triangular mesocone and sharp lateral cusps, base concave and strongly bicuspid first lateral (i, base width); s, rachidian with broad, blunt, triangular mesocone and broad lateral cusps, base rectangular, bicuspid first lateral (i, mesocone height, ii lateral cusp height); t, rachidian with broad, sharp triangular mesocone and sharp lateral cusps, base concave; strongly bicuspid first lateral (i, first lateral outer cusp height; ii inner cusp height).
Specimen measurements used in text and tables were as follows:
| ML | = | mantle length (dorsal) |
| MW/MWI | = | mantle width/index |
| FL/FLI | = | fin length/index (measured from anterior edge of fin to end of tail) |
| FW/FWI | = | fin width/index |
| HL/HLI | = | head length/index (measured from anterior tip of nuchal cartilage to separation of Arms I) |
| HW/HWI | = | head width/index |
| ED/EDI | = | eye diameter/index |
| ALI | = | arm length index (arms measured from most proximal sucker to arm tip) |
| TL | = | total length including tentacles |
| TLA | = | total length from mantle tip to tip of longest arms |
| FLC | = | funnel-locking cartilage |
| MLC | = | mantle-locking cartilage |
| TnL/TnLI | = | tentacle length/index |
| CL/CLI | = | tentacle club length/index as % of ML |
| GL | = | gladius length |
| LRL | = | lower rostral length |
| URL | = | upper rostral length |
Histological examination
Formalin-fixed, ethanol-preserved museum specimens were used for histology. A piece of tissue was sourced from the ventral side of the eye for each species (except for Echinoteuthis famelica and Mastigopsis hjorti due to limited material). Tissue was embedded using a ThermoScientific Shandon Citadel 1000. Five-micrometre sections were cut and stained with Mallory's trichrome following Lormann (Citation2008). Acid fuchsin solution contained 0.5 g acid fuchsin in 100 mL tap water. Aniline blue and orange G contained 0.5 g water-soluble aniline blue, 2 g orange G, and 1 g phosphotungstic acid in 100 mL distilled water.
Genetic analyses
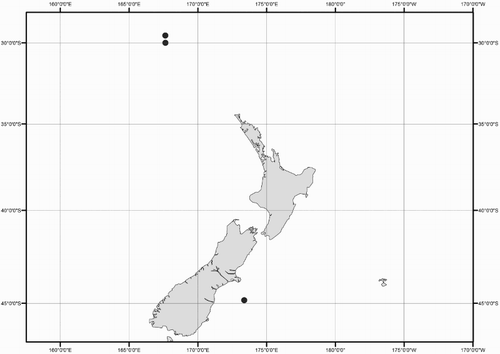
The outgroup species, Joubiniteuthis portieri (Joubin, 1916), was included following Young et al. (Citation2008) and Braid et al. (Citation2014) because it is a member of the chiroteuthid group of families (Young Citation1991). Tissue was obtained from five specimens of Mt. cf. dentata during sampling efforts around New Zealand (); two specimens were beaks only fixed in 100% ethanol, while the three others were whole specimens kept frozen at −80°C until analysis. Sequences were amplified for three mitochondrial genes—16S rRNA, 12S rRNA, and the DNA barcode region, cytochrome c oxidase subunit I (COI)—following Braid et al. (Citation2014) for DNA extraction, amplification, and sequencing. Additional sequences were obtained from GenBank for comparison with these sequences. These sequences represented Mt. agassizii, Mt. psychrophila, E. atlantica, Mp. hjorti, Mg. magna, Mg. microlucens, Mg. osheai, I. cordiformis, and the outgroup species J. portieri (). Sequences were uploaded to the Barcode of Life Data Systems (BOLD; Ratnasingham & Hebert Citation2007) public project titled ‘Integrative Taxonomy of New Zealand Mastigoteuthidae’ (project code: NZMTG) and subsequently submitted to GenBank. Sequences were checked for potential contamination by a BLAST search through GenBank.
Table 2 Specimen information for sequences used in this study.
Intraspecific and interspecific distances were calculated for COI (the barcode region) for Mt. cf. dentata and Mt. agassizii (two species that were not compared in Braid et al. Citation2014) in MEGA 6.06 (Tamura et al. Citation2013) using the Tamura-Nei model (Citation1993) with gamma correction. Sequences were aligned in Geneious 7.1.7 (Biomatters, Auckland, New Zealand) using the MAFFT algorithm (Katoh et al. Citation2002), and subsequently trimmed manually and concatenated. PartitionFinder 1.1.1 (Lanfear et al. Citation2012) was run on the concatenated alignment with all substitution models included under Bayesian Information Criterion. Each codon position for COI was searched separately, as well as each rRNA gene, which could lead to a maximum of five partitions. Different models were chosen for each codon position of COI as TrNef + 1, F81, and HKY + G, respectively. A single model, TrN + I + G, was chosen for both 16S rRNA and 12S rRNA. A maximum-likelihood combined phylogeny was created in GARLI 2.0.1 (Zwickl Citation2006) with 1000 bootstrap replicates. A single-gene phylogeny was created using the parameters specified above for COI only using GARLI with 1000 bootstrap replicates. This phylogeny was used to determine species delimitations using the Poisson tree processes model (Zhang et al. Citation2013). In addition, the Barcode Index Number (BIN) system was also used to test the species boundaries found in Mastigoteuthis species for COI (Ratnasingham & Hebert Citation2013).
Genetic results
The results found herein are consistent with the findings by Braid et al. (Citation2014), and add important information on the one locally occurring species for which no genetic information was previously available, Mt. cf. dentata. Herein, sequences were successfully recovered from five individuals of Mt. cf. dentata from New Zealand waters for COI, 16S rRNA, and 12S rRNA. The 658-base-pair sequences for COI did not contain indels or stop codons. 16S rRNA and 12S rRNA sequences contained 514 and 403 base pairs, respectively.
For each species represented by multiple individuals (I. cordiformis, Mg. magna, Mp. hjorti, Mt. agassizii, Mt. cf. dentata), a single distinct cluster was formed on the phylogeny (). The mean intraspecific distance within these species was 0.2%, with a maximum of 0.8% (observed in Mp. hjorti) and a minimum of 0% (observed in all species). Species that were represented by a single individual (Mt. psychrophila, E. atlantica, Mg. microlucens, and Mg. osheai) were separate and distinct from all other species. Magnoteuthis osheai was clearly distinguished from all other Magnoteuthis species, with an interspecific distance of 10.7% from Mg. microlucens, and a 10.6% divergence from Mg. magna. The recognition of Mg. osheai as a separate species from other Magnoteuthis species was previously recognised by Braid et al. (Citation2014) using the BIN system and through analysis of combined maximum likelihood and Bayesian phylogenies based on COI, 16S rRNA, and 12S rRNA. The mean interspecific distance observed was 15.3%, with a maximum of 22.9% observed between Mt. agassizii and Mg. microlucens, and a minimum of 1.5% observed between Mt. agassizii and Mt. cf. dentata.
Figure 5 Maximum-likelihood consensus tree for nine species of Mastigoteuthidae, based on a partitioned analysis of cytochrome c oxidase subunit I (COI), 12S rRNA, and 16S rRNA genes. Node labels are support values based on 1000 bootstrap samples. Sample names correspond to . Morphological character states are represented by symbols, which are defined in the legend.
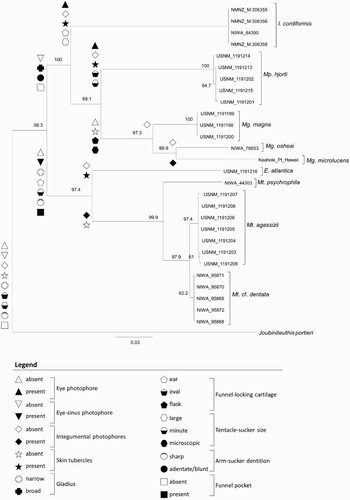
The BIN analysis suggested that the Mt. cf. dentata specimens collected in New Zealand represent a separate species from Mt. agassizii found in the North Atlantic; unfortunately, no tissue or sequences were available for Mt. dentata (s. str.) from the type locality (eastern tropical Pacific, near Panama). In contrast, the Poisson tree processes analysis suggested that Mt. cf. dentata from New Zealand and Mt. agassizii are a single species. In the combined phylogeny, the separation between Mt. cf. dentata from New Zealand and Mt. agassizii is well supported (bootstrap value of 97.9). The minimum intraspecific distance observed within both Mt. agassizii and Mt. cf. dentata is 0%. The maximum intraspecific distance within Mt. agassizii is 0.6% and Mt. cf. dentata is 0.2%. The minimum interspecific distance between Mt. agassizii and Mt. cf. dentata is 1.5%, and the maximum interspecific distance is 1.8% with a mean of 1.7%. Therefore, it is not currently clear whether the species found in New Zealand waters is genetically isolated from the specimens from the North Atlantic. The species found in New Zealand waters will be referred to as Mt. cf. dentata until specimens from the type locality of Mt. dentata and from the intermediate waters between the North Atlantic and New Zealand are available for genetic analysis. One specimen (USNM 1191205) that had thicker integument characteristic of one form of boreal Mt. agassizii (Vecchione & Young Citation2007a), but it was not genetically distinct from other Mt. agassizii specimens.
Systematics
Family Mastigoteuthidae Verrill, 1881
Diagnosis. Medium- to large-bodied squids (ML c. 130 mm to > 1 m at maturity). Ventral anterior margin produced into two points indicating underlying mantle-locking cartilage. Funnel-locking cartilage generally ovate, with tragus and/or antitragus in some species; buccal formula DDVV. Arm suckers arranged in two series (sometimes dorsoventrally compressed, appearing to merge into single series, midway along arm); oral faces of arms bordered by wide protective membranes. Arms IV generally longer and thicker than other arms, with strong lateral keels. Hectocotylus absent. Tentacles whip-like, often lost, club elongate and cylindrical; most tentacular suckers minute and densely set. Gladius with secondary conus.
Genus Mastigoteuthis Verrill, 1881
Mastigoteuthis Verrill, 1881: 100. Type species Mastigoteuthis agassizii Verrill, 1881, by original designation.
Chiroteuthopsis Pfeffer, Citation1900: 187. Type species Chiroteuthis grimaldii (= Mastigoteuthis grimaldii, fide Chun Citation1910) Joubin, Citation1895, by monotypy.
Diagnosis. Funnel-locking cartilage ear-shaped with tragus and antitragus present; funnel pocket present. Arm suckers with sharp teeth distally. Tentacular suckers minute. Small eye-sinus photophore present, diameter c. 9% eye diameter. Integumental photophores present, skin tubercles absent. Gladius narrow (greatest width c. 3% GL).
Description. Mantle length at maturity c. 130–200 mm. Fins together circular or elliptical in outline; fin length c. 65% ML. Funnel widely conical with recurved end. Eye photophore variably present. Mantle-locking cartilage nose shaped, posteriorly undercut in profile. Arm suckers arranged in two distinct series but appearing to merge into single series midway on Arms IV; Arms IV c. 140% ML, ‘minute trabeculate protective membrane is present’ (fide Young Citation1972). Tentacle club not expanded; protective membranes without trabeculae.
Mastigoteuthis cf. dentata Hoyle, 1904 (Tables 2–5, Figs 5–11)
Mastigoteuthis dentata Hoyle, Citation1904: 34, 35, pl. 6 Figs 8–11; Young (Citation1972): 66, pl. 26 T, S.
Mastigoteuthis sp. 1 Spencer et al. (Citation2009): 219.
Mastigoteuthis agassizii Spencer et al. (Citation2009): 219.
Type material examined. USNM 00574643, lectotype, ♂, ML 60* mm, 7.35°N, 79.58°W, 10/03/1891, RV Albatross, large beam trawl, Stn 3394.
Figure 7 Mastigoteuthis cf. dentata. A, NIWA 48885, ♀, ML 55 mm, photophore pattern on dorsal fins based on NMNZ M.091520, ♀, ML 58 mm; B, NIWA 71720, sex indet., ML 24 mm. Scale bars = 10 mm.
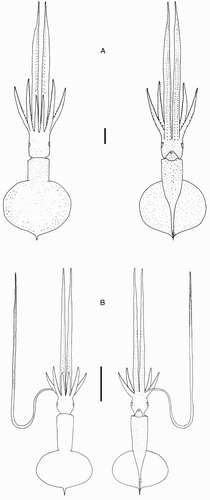
Figure 8 Mastigoteuthis cf. dentata. A–E, NIWA 48885, ♀, ML 55 mm; F, NIWA 75806, ♂, ML unknown (damaged; LRL 3.34 mm). A, left funnel-locking cartilage; B, left mantle-locking cartilage; C, left mantle-locking cartilage profile view; D, nuchal cartilage; E, proximal view of aboral photophores on Arms IV; F, internal mantle pigmentation. Scale bars = A–E, 1 mm; F, 5 mm.
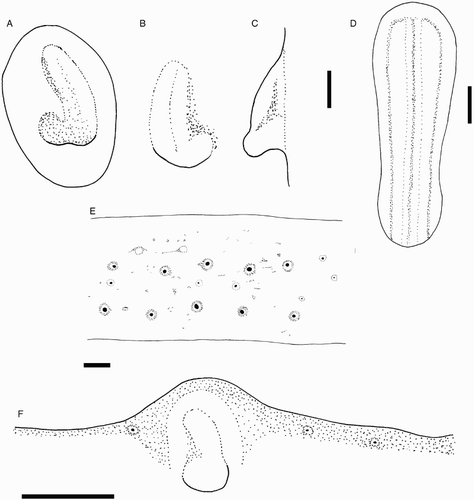
Figure 10 Mastigoteuthis cf. dentata lower beaks. A, F, NMNZ M.172963, ♂, ML 54 mm, LRL 2.11 mm; B, G, NMNZ M.287214, ♂, ML 85 mm, LRL 3.39 mm; C, H, M.172936, ♀, ML 81 mm, LRL 3.46 mm; D, I, NIWA 75806, ♂, ML unknown, LRL 3.34 mm; E, J, NMNZ M.091723, ♀, ML 92.3* mm, LRL 3.96 mm. A–E, lateral profile view; F–J, lateral oblique view. Scale bars = 5 mm.
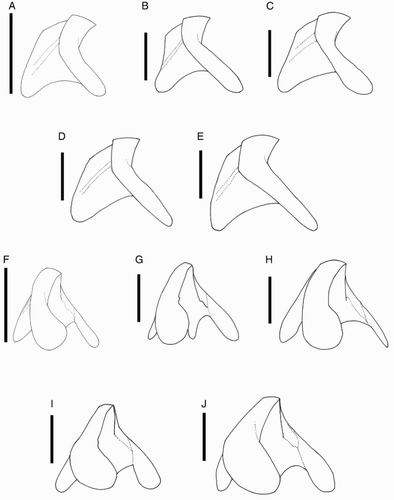
Figure 11 Mastigoteuthis cf. dentata. A, NMNZ M.172963, ♂, ML 54 mm; B, NMNZ M.287214, ♂, ML 85 mm; C, M.172936, ♀, ML 81 mm; D, NIWA 75806, ♂, ML unknown, LRL 3.34 mm; E, NMNZ M.091723, ♀, ML 92.3* mm. A–E, Upper beaks; F, regression equation for mantle length (ML) and lower rostral length (LRL). Scale bars = 5 mm.
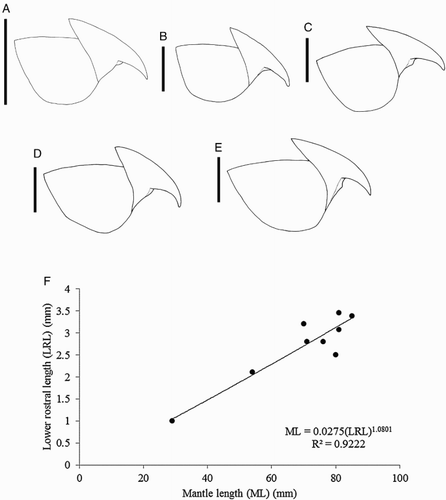
Table 3 Arm length index for mastigoteuthids examined in this study.
Table 4 Measurements (mm) of Mastigoteuthis cf. dentata of larger specimens (ML 46–85 mm), all taken on right side.
Table 5 Measurements (mm) of small Mastigoteuthis cf. dentata (ML 24–32 mm), all taken on left side except NMNZ M.287217.
Table 6 Measurements (mm) of Mastigoteuthis psychrophila all taken on right side except for NIWA 44277.
Additional material examined (24 specimens). NMNZ M.172934, 4 ♂, ML 43*–78 mm, 26.52°S, 166.57°E, 2000 m, 18/05/2003, RV Tangaroa, NORFANZ Stn 45; NMNZ M.074540, sex indet., ML 32 mm, 30.97°S, 172.21°W, 971 m over 5000 m, 15/12/1976, RV James Cook, MWT, Stn J17/76/76; NMNZ M.172936, ♀, ML 81 mm, 32.07°S, 159.88°E, 1920–1934 m, 24/05/2003, RV Tangaroa, NORFANZ Stn 71; NMNZ M.172963, ♂, ML 54 mm, 32.43°S, 161.79°E, 1132–1197 m, 24/05/2003, RV Tangaroa, NORFANZ Stn 73; NMNZ M.172944, ♀, ML 98 mm, ♂, ML 76 mm, sex indet., ML 70 mm, 32.61°S, 167.84°E, 1303–1313 m, 30/05/2003, RV Tangaroa, NORFANZ Stn 120; NMNZ M.074172, sex indet., ML 47 mm, 33.15°S, 176.10°E, 183 m over 1980–4000 m, 23/07/1962, RNZFA Tui, MWT; NMNZ M.091520, ♀, ML 58 mm, 33.17°S, 170.96°E, 600 m to over 1900 m, 25/10/1985, RV James Cook, MWT, Stn J16/28/85; NMNZ M.287218, ♀, ML 46 mm, 33.22°S, 178.40°E, 640–823 m, over 2213–2304 m, 02/08/1962, MWT, Stn Z87218; NMNZ M.172955, ♂, ML 71 mm, 34.98°S, 169.49°E, 1288–1294 m, 04/06/2003, RV Tangaroa, NORFANZ Stn 160; NMNZ M.287217, sex indet., ML 29 mm, 39.70°S, 178.13°E, 790–928 m, 11/10/1988, RV James Cook, Stn J12/31/88; NMNZ M.287214, ♂, ML 85 mm, 41.22°S, 176.70°E, 918–970 m, 24/10/1988, RV James Cook, BTT, Stn J12/56/88; NIWA 48885, ♀, ML 55 mm, 42.69°S, 179.93°W, 920 m, 06/07/1996, RV Tangaroa, Stn TAN9608/374, Stn Z9926; NIWA 71720, sex indet., ML 24 mm, 42.78°S, 179.90°E, 950 m, 08/07/1996, RV Tangaroa, Stn TAN9608/447; NIWA 95870, sex indet., ML 45 mm, 42.94°S, 174.56°E, 893–898 m, 25/01/2014, RV Tangaroa, ratcatcher BTT, Stn TAN1401/130; NIWA 95868, ♂, ML 70 mm, 42.92°S, 174.62°E, 908–911 m, 25/01/2014, RV Tangaroa, ratcatcher BTT, TAN1401/131; NIWA 95869, ♂, ML 73 mm, 42.92°S, 174.62°E, 908–911 m, 25/01/2014, RV Tangaroa, ratcatcher BTT, Stn TAN1401/131; NIWA 75806, ♂, fins missing, 44.80°S, 173.90°E, 899–973 m, 28/11/2011, TRIP3415/31; NMNZ M.091649, ♀, ML 81 mm, 45.96°S, 163.54°E, 480–80 m over 4570 m, 28/07/1985, RV Kaiyo Maru, Stn KM/110B/85; NMNZ M.091723, ♀, ML 93* mm, New Zealand, Macruronus novaezelandiae stomach content, FV Wanaka, years 1985–1986; CH-LIA-01 (not accessioned), sex indet., ML 69 mm, no data.
Beak only. NIWA 95872, LRL 3.82 mm, RV Tangaroa, Stn TAN1301/110/01; NIWA 95871, LRL 3.96 mm, RV Tangaroa, Stn TAN1003/81/06.
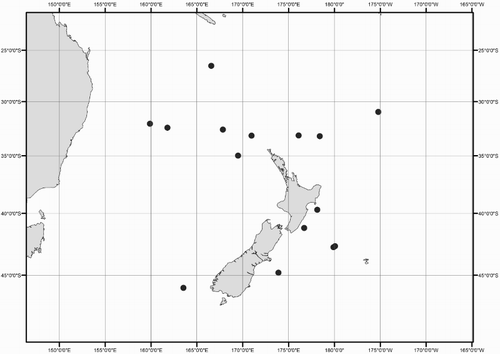
Distribution (). 26–46°S, 159°E to 172°W (near New Zealand); elsewhere reported from tropical and subtropical Indo-Pacific (except the California Current) (Nesis Citation1987).
Diagnosis. Arms IV with two main series of photophores along length; funnel-locking cartilage with weak tragus and antitragus; arm suckers with c. 7–15 long, sharp teeth distally; rostrum of gladius approximately triangular with rounded corners in cross section.
Description (ML 46–85 mm, , –). Mantle cone shaped anteriorly, with mantle cavity extending to c. 30% FL from anterior of fins (thereafter gladius and surrounding musculature continue as narrow cylinder) widest (c. 26% ML) at anterior margin; dorsal anterior mantle margin nearly straight. Fins together elliptical in outline, length 57–62–71% ML, width 59–70–80% ML; anterior lobes absent; posterior margin at tail concave. Photophores present on all external surfaces of head, fins, mantle, funnel, and aboral surface of arms. Arm IV photophore pattern with two distinct series of large photophores (E), with smaller photophores scattered; eye-sinus photophore diameter c. 9% ED.
Head cylindrical, length c. 31% ML, width c. 19% ML. Olfactory papilla cylindrical. Eye diameter c. 12% ML. Eye photophore absent. Funnel width c. 11% ML, length c. 9% ML; aperture approximately level with posterior margin of eye; funnel pocket present. Funnel-locking cartilage ear shaped (A), c. 6% ML; anterior groove slightly concave due to weak antitragus; tragus present along inner/medial margin, extending approximately to midline of groove; concave along outer/lateral margin. Mantle-locking cartilage nose shaped (B, C), c. 4% ML; posteriorly undercut in profile.
Arm formula IV>II>III>I; arm length 52–87–163% ML (–); Arms I–III all of subequal thickness with aboral keels present; Arms IV thicker with expanded lateral membrane. At ML c. 55–81 mm, 100–128 suckers present on each arm; largest suckers overall located at base of Arm IV (c. 30% arm width).
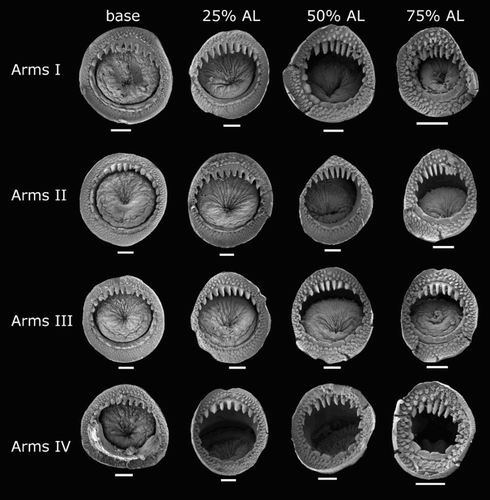
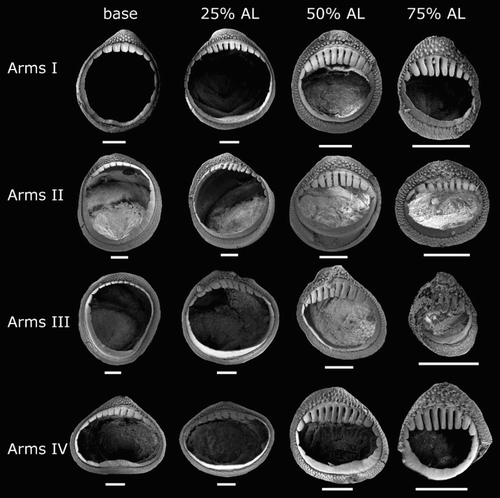
Arm-sucker rings () proximally with 8–14 short, blunt, rectangular teeth, distally with 7–15 sharp, conical teeth. Polygonal processes on oral surface of sucker in two to five concentric rings; distally, central and intermediate rings with cylindrical, pitted, porous pegs; proximally, central and intermediate rings nearly flat; peripheral ring with flat, rectangular processes.
At ML 24 mm (sole tentacle available for examination), tentacle length c. 308% ML, club c. 46% TnL (c. 142% ML), not expanded. Suckers minute, covering majority of tentacle surface apart from narrow aboral strip; where tentacle club begins, width of sucker-covered surface is less than half of stalk circumference, broadening distally to cover nearly entire stalk circumference. Tentacular suckers not examined due to lack of adult material.
Lower beak (), lateral profile: lower rostral length 30–37–52% wing length, rostral edge moderately curved, rostral tip without hook, rostral tip behind leading edge of wing by 9–30–38% of baseline; wing angle obtuse, jaw angle obscured by low wing fold, shoulder groove absent; beak height 83–88–97% baseline; hood close to crest, hood length 50–57–68% crest length, crest length 51–56–65% baseline, visible portion of crest nearly straight; lateral-wall fold present, thickened into ridge, lateral-wall fold extends to posterior edge of lateral wall; no notch in lateral wall. Lateral oblique view with wing narrowest level with jaw angle, 53–60–70% greatest wing width. Ventral view with broad notch in hood, free corners well separated. Wing entirely unpigmented at LRL 2.11 mm (ML 54 mm); midline of wing pigmented at LRL 3.08 mm (ML 81 mm); posterior edge, and anterior edge below shoulder of wing remains clear at LRL 3.65–3.87 mm. Regression equation (F) ML = 0.0275(LRL)1.0801 (R² = 0.9222) (n= 9).
Upper beak, lateral profile (A–E): upper rostral length c. 37% hood length; hood length 61–67–71% beak length; hood height c. 28% beak width. Lateral-wall fold absent; shoulder produced into point; jaw edge curved; jaw angle nearly 90°.
Radula (A) with tricuspid rachidian, base width c. 110% height, proximal margin of base rectangular, with narrow, sharp, triangular mesocone and small, pointed lateral cusps, laterally directed, their height c. 25% mesocone height. First lateral tooth weakly bicuspid; inner cusp narrow, triangular, slightly curved towards rachidian, its height c. 80% that of overall rachidian; outer cusp blunt, medially directed, its height c. 30% that of inner cusp. Second lateral tooth simple, curving slightly towards rachidian, c. 195% rachidian height. Marginal tooth simple, straight, c. 290% rachidian height. Marginal plate absent (B). Palatine palp (E) with c. 50 narrow, flat teeth, each c. 60–200% rachidian height, evenly distributed over palp.
Figure 12 Mastigoteuthis cf. dentata. A, B, NMNZ M.287214, ♂, ML 85 mm; C–E, NMNZ M.091649, ♀, ML 81 mm; F, CH-LIA-01, ♂, ML 70 mm. A, Radula; B, radula margin; C, asymmetrical radula; D, asymmetrical radula rachidian and first lateral teeth; E, palatine palp; F, gladius. Scale bars = A, C, 500 µm; B, 100 µm; D, 200 µm; E, 1 mm; F, 5 mm.
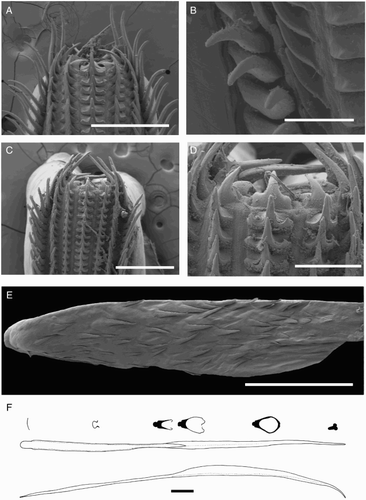
Gladius (F) with greatest width (c. 3% GL) attained at 5% GL; free rachis c. 4% GL; secondary conus c. 50% GL; rostrum c. 5% GL, approximately triangular with rounded corners in cross section.
Epidermis damaged on all examined material. Integumental photophores present. Colour (preserved) dark purple to pink; chromatophores overlying integumental photophores; pigmentation on interior mantle wall with some small photophores extending to the anterior margin of locking cartilage (F); scattered chromatophores present on aboral surface of arms, oral surface of arms darkly pigmented but individual chromatophores not readily discernible.
Smaller specimens (ML 24–32 mm, B, ) differ from above descriptions only as follows: fin length 54–57–63% ML, fin width 66–68–72% ML. Head length 21–28–34% ML. Arms I c. 30% ML, Arms II 38–54–63% ML, Arms III 33–45–55% ML, Arms IV 150–184–220% ML; with 44–100 suckers present on each arm.
Remarks. Two locally occurring species of Mastigoteuthis, Mt. cf. dentata and Mt. psychrophila, are approximately the same size at maturity (ML c. 140 mm), with many small integumental photophores, arm suckers with sharp teeth distally, and a funnel pocket. Mastigoteuthis psychrophila can be distinguished from Mt. cf. dentata by the photophore pattern on Arms IV, gladius cross sections, and the funnel-locking cartilage morphology. The photophore pattern on Mt. psychrophila Arms IV is widely spaced discrete clusters of larger photophores (A), whereas Mt. cf. dentata has two main series along the entire arm length (E). In Mt. psychrophila, the cross-section of the gladius rostrum is teardrop shaped (G); in Mt. cf. dentata, the cross section is approximately triangular with rounded corners (F). The funnel-locking cartilage of Mt. psychrophila (B) also has a much stronger antitragus than that of Mt. cf. dentata (A).
Figure 14 Mastigoteuthis psychrophila. A, NMNZ M.070954, ♀, ML 88 mm, tentacle based on NIWA 44301, ♀, ML 96 mm; B–E, NIWA 44301, ♀, ML 84 mm. A, Whole specimen; B, left funnel-locking cartilage; C, left mantle-locking cartilage; D, left mantle-locking cartilage, profile view; E, nuchal cartilage. Scale bars = A, 10 mm; B–E, 1 mm.
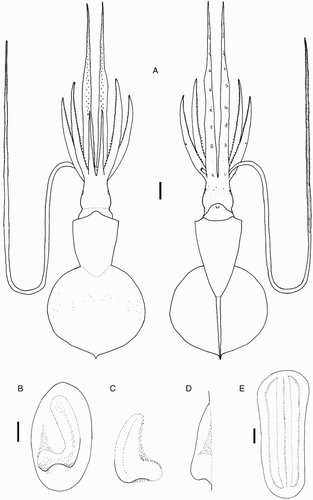
Figure 15 Mastigoteuthis psychrophila. A, NIWA 44301, ♀, ML 84 mm; B, NIWA 44277, ♀, ML 78 mm. A, Proximal view of aboral photophores on Arms IV; B, internal mantle pigmentation. Scale bars = 5 mm.
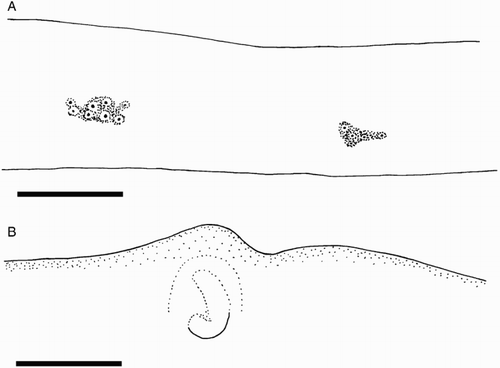
Figure 16 Mastigoteuthis psychrophila arm suckers, NIWA 44277, head only, LRL 3.07 mm. Scale bars = 100 µm.
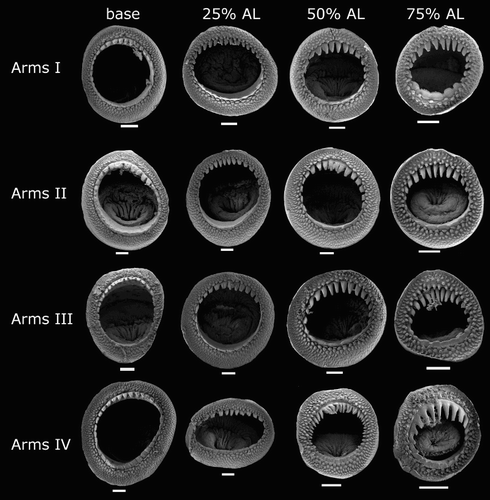
Figure 17 Mastigoteuthis psychrophila. A–F, NIWA 44301, ♀, ML 96 mm; G–I, NIWA 44301, ♀, ML 84 mm, LRL 2.67 mm; J–L, NIWA 44277, head only, LRL 3.07 mm. A–F, Tentacle suckers; G, J, lower beaks in lateral profile view; H, K, lower beaks in lateral oblique view; I, L, upper beaks. Scale bars = A, 500 µm; B, 100 µm; C, D, 50 µm; E, F, 10 µm; G–L, 5 mm.
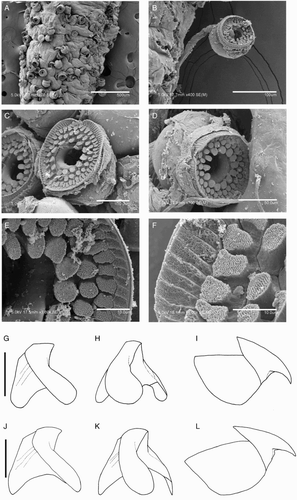
Figure 18 Mastigoteuthis psychrophila. A, B, M.070954, ♂, no fins, LRL 2.89 mm; C–F, NIWA 44277, sex indet., ML 86 mm; G, NIWA 44301, ♀, ML 96 mm. A, Radula; B, radula margin; C, asymmetrical radula; D, E, fused lateral teeth of asymmetrical radula; F, palatine palp; G, gladius. Scale bars = A, C, 500 µm; B, D, E, 100 µm; F, 1 mm; G, 5 mm.
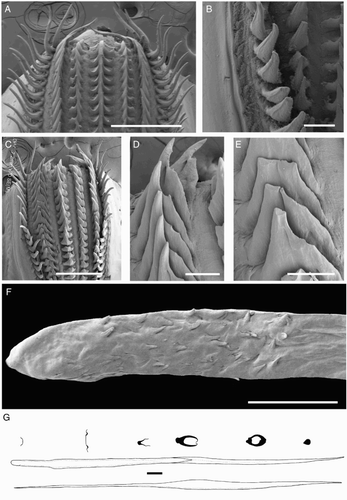
In addition to the material listed above, 14 specimens that were similar to (but morphologically distinct from) Mt. cf. dentata were reported by Braid (Citation2013) as Mt. sp. X. The best representatives are NMNZ M.074544 and NIWA 48864. These specimens were distinguished from Mt. cf. dentata by the funnel-locking cartilage morphology, photophore patterns on Arms IV, arm-sucker morphology, and beak morphology (see Braid Citation2013). However, examination of more individuals may reveal that this difference is part of continuous variation and that Mt. ‘sp. X’ individuals should be attributed to Mt. cf. dentata. Fresh material for genetic analysis will be necessary to resolve the status of Mt. sp. X. In addition, a single badly damaged specimen (NIWA 71721) identified as Mt. sp. Y by Braid (Citation2013) shares morphological characteristics with Mt. cf. dentata, apart from possessing a photophore around the circumference of each eye. However, due to the extensive damage of the sole known specimen, and no tissue available for genetic analysis, the specific status of this individual remains undetermined.
Hoyle (Citation1904) distinguished Mt. dentata (s. str.) from Mt. agassizii by the sucker dentition: Mt. dentata arm suckers possessed teeth. Verrill (Citation1881) had reported that the arm suckers of Mt. agassizii were adentate, but re-examination of the type specimen revealed that the suckers were degraded, but some dentition could be seen (Vecchione & Young Citation2006). In addition, specimens of Mt. agassizii from the type locality all have arm suckers with sharp teeth distally (Vecchione & Young Citation2006). The sucker illustrated by Hoyle (Citation1904) appears similar to those observed herein for Mt. cf. dentata. Young (Citation1972) suggested that Hoyle (Citation1904) may have described Mt. dentata from two specimens that belonged to different species because of the differences in fin length. Therefore, he declared the syntype from Cape Mala (male, ML 72 mm) as the lectotype. Hoyle (Citation1904) did not comment on the photophore pattern or draw the whole specimen; however, two main series of photophores on Arms IV are apparent on the lectotype. The FLI for the lectotype is within the range found herein, while the other specimen described had a much smaller FLI. Although Young (Citation1972) added information focused on the tentacles and tentacle-sucker ultrastructure, no tentacles from adult New Zealand material were available for examination, preventing comparison.
Within Mastigoteuthis, Mt. cf. dentata and Mt. dentata (s. str.) are most morphologically similar to Mt. agassizii. The type locality of Mt. agassizii is the North Atlantic (Verrill Citation1881), and Mt. dentata was described from the equatorial Pacific (Hoyle Citation1904). These species share the same photophore pattern on Arms IV, funnel-locking cartilage morphology, arm-sucker morphology, presence of a funnel pocket, and similar beak morphology; currently, there are no reliable morphological characters to separate these two species (Vecchione & Young Citation2007b). The current genetic evidence suggests that there is a divergence between Mt. agassizii and Mt. cf. dentata (). Unfortunately, specimens of Mt. dentata (s. str.) from the type locality were not available for analysis to confirm the identity of the species in New Zealand waters. In addition, there were no specimens from the South Atlantic to rule out continuous genetic variation between New Zealand and the North Atlantic. At present, it appears that there are two clades, one from New Zealand and one from the North Atlantic; this is supported by the BIN analysis. In addition, there was low interspecific divergence (maximum of 0.2% for Mt. cf. dentata and 0.6% for Mt. agassizii), and a higher divergence between the two species (minimum of 1.5%), which suggests that these two taxa are distinct. This distribution of species in this genus—Mt. agassizii in the Atlantic (Verrill Citation1881), Mt. dentata in the Pacific (Hoyle Citation1904), and Mt. psychrophila in the Southern Ocean (Nesis Citation1977)—would be consistent with the allopatric distributions found for Magnoteuthis—Mg. magna in the Atlantic (Joubin Citation1913), Mg. microlucens around Hawaii (Young et al. Citation2008), and Mg. osheai in New Zealand waters—and Echinoteuthis—E. atlantica in the Atlantic (Joubin Citation1933), E. famelica around Hawaii (Berry Citation1909), and Echinoteuthis glaukopis in the Indian Ocean (Chun Citation1908). Mastigopsis hjorti is the only species in this family that presently has a recognised cosmopolitan distribution, but it has been suggested that specimens in different locations may actually represent different species (Nesis Citation1987; Vecchione & Young Citation2007c).
Several other morphologically similar species have been described from the North Atlantic, which are not yet clearly distinguished from Mt. agassizii: Mt. grimaldii (Joubin, Citation1895); Mt. flammea Chun, Citation1908; and Mt. schmidti Degner, Citation1925 (Vecchione & Young Citation2007a). Because these specimens were described from the North Atlantic, and Mt. dentata is found in the Pacific, it is likely that these species are more closely related to (or synonymous with) Mt. agassizii than Mt. dentata. Vecchione & Young (Citation2007a) suggested that some boreal specimens have a thicker integument, but one such specimen analysed herein was not genetically distinct from other Mt. agassizii specimens (). Young (Citation1972) considered Mt. grimaldii a species dubium but maintained the validity of Mt. dentata, Mt. flammea, and Mt. schmidti. Salcedo-Vargas & Okutani (Citation1994) synonymised Mt. grimaldii, Mt. dentata, Mt. flammea, and Mt. schmidti with Mt. agassizii. In contrast, Salcedo-Vargas (Citation1997) maintained the validity of Mt. grimaldii, considering Mt. flammea a junior synonym, while Mt. schmidti remained a junior synonym of Mt. agassizii. However, due to the wide distribution of Mt. agassizii found herein, it is likely that these species are junior synonyms of Mt. agassizii.
Among the radulae examined (see Discussion), one appeared unusual (C–D) as follows: left first lateral tooth unicuspid; its height equal to that of rachidian. Right first lateral tooth bicuspid; its height equal to that of rachidian; outer cusp sharp.
Mastigoteuthis psychrophila Nesis, 1977 (, , 6, –)
Mastigoteuthis psychrophila Nesis, Citation1977: 835–841, .
Type material (not examined). ZIN 60034/1, holotype, ♂, ML 84 mm, 59.43–59.51°S, 158.6–158.48°E, 500–1500 m, 01–02/02/1976, RV Dmitry Mendeleyev, cruise 16 m Isaacs-Kidd MWT, Stn 1309; IORAS paratype, 2 ♂, ML 88, 143 mm, 54.95–54.87°S, 158.83–159.45°E, 1500 m, 25/01/1976, Dmitry Mendeleyev, pelagic trawl, Stn 1294; IORAS paratype, ♂, 124 mm, 57.18–57.13°S, 26.58–26.37°W, 650 m, Akademik Kurchatov, Isaacs-Kidd MWT, Stn 883.
Material examined (eight specimens). NMNZ M.070954, ♀, ML 88 mm, ♂, no fins, 54.02°S, 168.69°E, 955 m, 12/05/1979, RV Wesermunde, Stn W02B/137/79; NIWA 44277, 2 ♀, ML 86, 99 mm, sex indet., ML 68 mm, sex indet., head only, 66.91–66.99°S, 171.06–171.09°E, 1032–50 m, 12/03/2008, RV Tangaroa, Stn TAN0802/293. NIWA 44301, 2 ♀, ML 84, 96 mm, 67.01–66.94°S, 170.70–170.73°E, 1078–100 m, 14/03/2008, RV Tangaroa, Stn TAN0802/312.
Beak only. NIWA 86553, sex indet., LRL 3.65 mm, 43.76°S, 174.20°W, 43.81°S, 174.23°W, 1126–1150 m, 10/01/2011, RV Tangaroa, Stn TAN1101/43; NIWA 86557, sex indet., LRL 3.90 mm, 44.62°S, 179.19°W, 1272–1270 m, 14/01/2010, RV Tangaroa, Stn TAN1001/67.
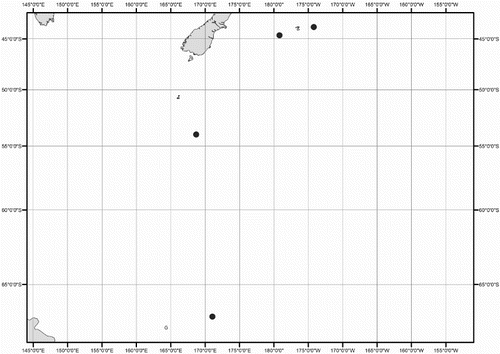
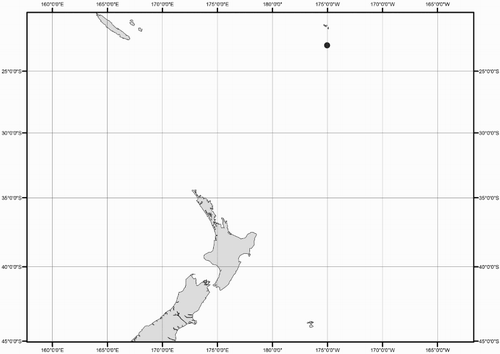
Distribution (). 43–67°S, 168°E to 174°W (near New Zealand); elsewhere reported from circumpolar sub-Antarctic waters (Nesis Citation1977, Citation1987).
Diagnosis. Arms IV with widely spaced discrete clusters of photophores; funnel-locking cartilage with strong tragus and antitragus; arm suckers with c. 8–30 long, sharp teeth distally; rostrum of gladius teardrop shaped in cross section.
Description (ML 42–99 mm, –18). Mantle cone shaped anteriorly, with mantle cavity extending to c. 30% FL from anterior of fins (thereafter gladius and surrounding musculature continue as narrow cylinder), widest (22–25–31% ML) at anterior margin; dorsal anterior mantle margin slightly convex. Fins together circular to elliptical in outline, length c. 63% ML, width 60–67–72% ML; anterior lobes absent; posterior margin at tail concave. Photophores present on all surfaces of head, funnel, mantle, dorsal surface of fins (ventral surface unknown), and aboral surface of arms; Arm IV photophore pattern with widely spaced discrete clusters of larger photophores distributed along the length of the arm (A); eye-sinus photophore diameter c. 10% ED.
Head cylindrical, length c. 29% ML, width c. 17% ML. Olfactory papilla cylindrical. Eye diameter c. 15% ML. Funnel width c. 13% ML, length c. 10% ML; aperture approximately level with posterior margin of eye; funnel pocket present. Funnel-locking cartilage ear shaped (B), c. 6% ML, anterior groove concave due to strong antitragus; strong tragus present along inner/medial margin, extending approximately to midline of groove; concave along outer/lateral margin. Mantle-locking cartilage nose shaped (C, D), c. 4% ML, posteriorly undercut in profile.
Arm formula IV>II>III>I; arm length 48–75–127% ML (, ); Arms I–III all of subequal thickness with aboral keels present; Arms IV thicker with expanded lateral membrane; oral faces of arms bordered by wide membranes. At ML c. 42–99 mm, 86–128 suckers present on each arm; suckers arranged in two distinct series, distally merging into one series on Arms IV; largest suckers overall located at base of Arm IV (sucker diameter c. 30% arm width).
Arm-sucker rings () proximally with 8–16 blunt, rectangular teeth, distally with 8–30 sharp, conical teeth. Polygonal processes on oral sucker surface in 4–7 concentric rings; distally, central and intermediate rings with cylindrical, pitted, porous pegs; proximally, central and intermediate rings nearly flat; peripheral ring with flat, rectangular processes.
At 96 mm ML (sole tentacle available for examination), tentacle length c. 242% ML, club c. 52% TnL (c. 125% ML), not expanded. Suckers minute, covering majority of tentacle surface apart from narrow aboral strip; proximally, width of sucker-covered surface is less than half of stalk circumference, broadening distally to cover nearly entire stalk circumference. Tentacular suckers (A–F) adentate; polygonal processes on oral sucker-ring surface in three concentric rings; central ring with 11–12 ovate to circular pegs; intermediate ring with 22–28 smaller, circular pegs; peripheral ring with 40–45 rectangular-faced pegs; rim processes rectangular, pitted, porous. Sucker diameter c. 100 µm (c. 4% club width).
Lower beak, lateral profile (G, J): lower rostral length c. 60% wing length, rostral edge moderately curved, rostral tip without hook, rostral tip behind leading edge of wing by c. 25% baseline; wing angle obtuse, jaw angle obscured by low wing fold, shoulder groove present; beak height c. 90% baseline; hood close to crest, hood length c. 60% crest length, crest length c. 55% baseline, visible portion of crest straight; broad lateral-wall fold present, thickened into ridge, lateral-wall fold extends to posterior edge of lateral wall; no notch in lateral wall. Lateral oblique view (H, K) with wing narrowest level with jaw angle, c. 70% of greatest width. Ventral view with broad notch in hood, free corners well separated. Wings nearly completely unpigmented at LRL 2.67 mm (ML 84 mm), shoulder remains clear at LRL 3.03 mm, entirely pigmented by LRL 3.07 mm.
Upper beak, lateral profile (I, L): upper rostral length c. 35% hood length; hood length c. 60% beak length; hood height c. 30% beak width. Lateral-wall fold absent; shoulder produced into point; jaw edge curved; jaw angle obtuse.
Radula (A) with tricuspid rachidian; base width c. 130% height; proximal margin of base concave, with broad, sharp, triangular mesocone and small, sharp lateral cusps, slightly laterally directed, their height c. 55% mesocone height. First lateral tooth weakly bicuspid; inner cusp broad, triangular, nearly straight, its height c. 80% that of overall rachidian; small, blunt outer cusp, laterally directed, its height c. 40% that of inner cusp. Second lateral tooth simple, straight, directed slightly towards rachidian, c. 120% rachidian height. Marginal tooth simple, curved proximally, straight distally, c. 225% rachidian height. Marginal plate absent (B). Palatine palp (F) with c. 40 narrow, flat teeth, each c. 40–75% rachidian height, evenly distributed over palp.
Gladius (G) with greatest width (c. 3% GL) attained at 13% GL; free rachis c. 12% GL; secondary conus c. 45% GL; rostrum c. 12% GL, teardrop shaped in cross section.
Epidermis damaged on all examined material. Integumental photophores present. Colour (preserved) pink-purple; aboral arms purple; head apparently without pigment; chromatophores overlying integumental photophores; pigmentation on interior mantle wall extending to the anterior margin of locking cartilage (B); scattered chromatophores present on aboral surface of arms; oral surface of arms darkly pigmented but individual chromatophores not readily discernible.
Remarks. Mastigoteuthis psychrophila inhabits sub-Antarctic waters of the Southern Ocean, and herein was found to have a northern limit of c. 44°S, which overlaps with the distributions of other local mastigoteuthids. The type description for Mt. psychrophila only included illustrations of the funnel-locking cartilage, arm suckers, a single tentacular sucker, and the terminal organ (Nesis Citation1977). Unfortunately, the photophore pattern on Arms IV, which is important for species identification, was not included. However, the specimens identified herein as Mt. psychrophila had characteristics consistent with this description as well as a distribution in sub-Antarctic waters. Nesis (Citation1977) used the strong antitragus in the funnel-locking cartilage of Mt. psychrophila to distinguish this species from other Mastigoteuthis species. Mastigoteuthis psychrophila can be differentiated from Mt. cf. dentata by its strong antitragus, gladius cross sections, and the Arm IV photophore pattern (see Remarks for Mt. cf. dentata). In addition, Mt. psychrophila is genetically distinct from Mt. cf. dentata and Mt. agassizii ().
Among the radulae examined (see Discussion), one appeared unusual (C–E) as follows: unicuspid to weakly tricuspid rachidian, base c. 65% height, with small, blunt lateral cusps, their height c. 40% that of mesocone height. Right first and second lateral teeth fused, triangular, broad sharp, slightly curved; height c. 135% that of rachidian; width 120% rachidian. Left first and second lateral teeth separate; first lateral tooth nearly unicuspid with low, rounded outer cusp, its height c. 55% that of inner cusp.
Genus Idioteuthis Sasaki, 1916
Idioteuthis Sasaki, Citation1916: 108. Type species Idioteuthis latipinna Sasaki, Citation1916, by monotypy.
Iridioteuthis [sic] Sasaki, Citation1929: 310.
Diagnosis. Fin length c. 80% ML. Funnel-locking cartilage ear shaped, tragus and antitragus present; funnel pocket absent. Arm suckers adentate or with blunt teeth; arm length subequal. Tentacular suckers proximally large, distally minute. Eye-sinus photophore absent. Single crescent-shaped photophore present on ventral surface of eye. Integumental photophores absent, skin tubercles present. Gladius broad (greatest width c. 10% GL).
Description. Mantle length at maturity c. 500 mm (in males) to > 1 m. Fins together heart shaped or elliptical in outline. Funnel bulbous posteriorly, cylindrical anteriorly. Mantle-locking cartilage nose shaped, posteriorly undercut in profile. Arm suckers arranged in two distinct series, largest suckers of all located mid Arms II; Arms II (c. 75% ML) often slightly longer than Arms IV (c. 75% ML); trabeculae present on arms’ protective membranes. Club slightly expanded with trabeculate membrane.
Figure 21 Idioteuthis cordiformis. A, NIWA 71660, ♀, ML 503 mm; B, NIWA 71659, ♀, ML 520 mm; C–E, NMNZ M.181333, ♂, ML 181 mm; F–H, NIWA 71652, ♂, ML 248 mm; I, NMNZ M.171893, ♀, ML 299 mm. A–C, Left funnel-locking cartilage; D, left mantle-locking cartilage; E, left mantle-locking cartilage profile view; F, nuchal cartilage; G, right eye; H, ventral view of right eye; I, ventral mantle skin tubercles. Scale bars = A, B, G, H, 10 mm; C–F, 5 mm; I, 1 mm.
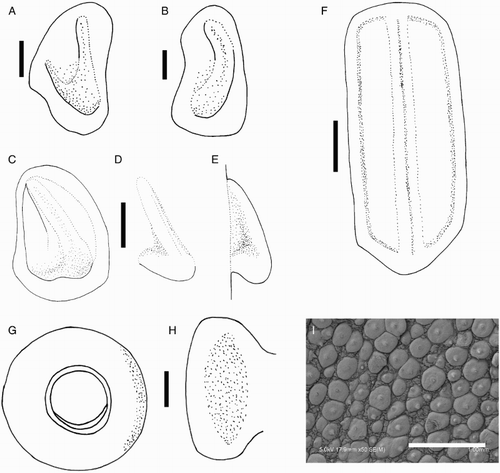
Figure 22 Cross section through ventral surface of Idioteuthis cordiformis eye photophore stained with Mallory's trichrome; NIWA 71655, ♀, ML 320 mm; p, indicates photophore tissue (in orange); i, indicates iris. Scale bar = 5 mm.
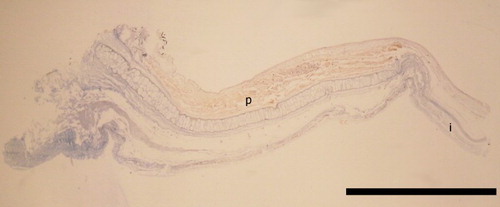
Figure 24 Tentacle suckers of Idioteuthis cordiformis, NMNZ M.171893, ♀, ML 299 mm. A, Proximal sucker, c. 10% CL; B, large proximal sucker, c. 20% CL; C, mid-club sucker, c. 50% CL; D, distal sucker, c. 90% CL; E, tentacle tip suckers; F–H, minute tentacle tip suckers with ‘cushions’. Scale bars = A, B, E, 1 mm; C, D, 500 µm; G, 300 µm; F, H, 200 µm.

Figure 25 Idioteuthis cordiformis lower beaks, profile view. A, NMNZ M.181333, ♂, ML 181 mm, LRL 6.36 mm; B, NIWA 71652, ♂, ML 248 mm, LRL 8.31 mm; C, NIWA 71655, ♀, ML 320 mm, LRL 11.1 mm; D, NIWA 71666, ♀, ML 380* mm, LRL 16.98 mm; E, NIWA 71653, ♂, ML 428 mm, LRL 13.78 mm; F, NMNZ M.306358, ♂, ML 549 mm, LRL 16.28 mm; G, NIWA 71437, ♂, ML 608 mm, LRL 20.69 mm; H, NMNZ M.118004, ♀, ML 715 mm, LRL 18.99 mm; I, NIWA 84390, ♀, ML 820 mm, LRL 19.28. Scale bars = 10 mm.
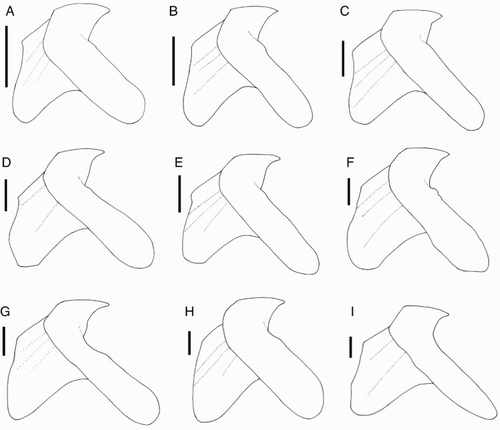
Figure 26 Idioteuthis cordiformis lower beaks, lateral oblique view. A, NMNZ M.181333, ♂, ML 181 mm; B, NIWA 71652, ♂, ML 248 mm; C, NIWA 71655, ♀, ML 320 mm; D, NIWA 71666, ♀, ML 380* mm; E, NIWA 71653, ♂, ML 428 mm; F, NMNZ M.306358, ♂, ML 549 mm; G, NIWA 71437, ♂, ML 608 mm; H, NMNZ M.118004, ♀, ML 715 mm; I, NIWA 84390, ♀, ML 820 mm. Scale bars = 10 mm.
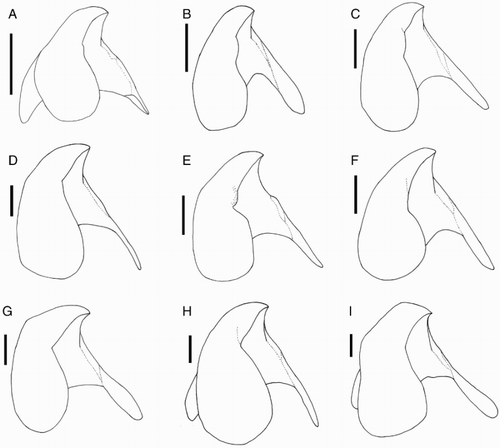
Figure 27 Idioteuthis cordiformis. A, NMNZ M.181333, ♂, ML 181 mm; B, NIWA 71652, ♂, ML 248 mm; C, NIWA 71655, ♀, ML 320 mm; D, NIWA 71666, ♀, ML 380* mm. E, NIWA 71653, ♂, ML 428 mm; F, NMNZ M.306358, ♂, ML 549 mm; G, NIWA 71437, ♂, ML 608 mm; H, NMNZ M.118004, ♀, ML 715 mm; I, NIWA 84390, ♀, ML 820 mm. A–I, Upper beaks, profile view; J, regression equation for ML and LRL; K, regression equation for ML and LRL without outlier (NIWA 71437). Scale bars = 10 mm.
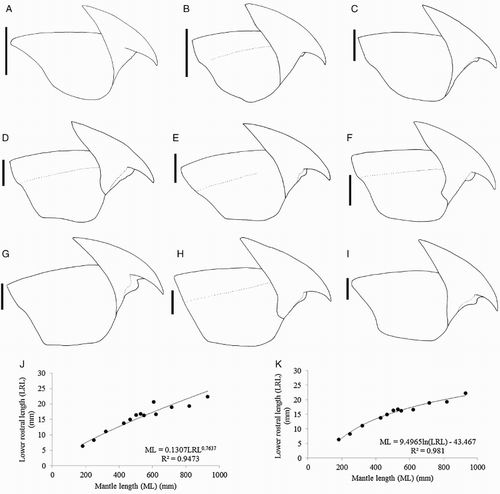
Figure 28 Idioteuthis cordiformis. A, NIWA 71437, ♂, ML 608 mm; B–D, NMNZ M.118004, ♀, ML 715 mm; E, NIWA 71652, ♂, ML 248 mm. A, B, Radula; C, marginal plates of radula; D, palatine palp; E, gladius. Scale bars = A–D, 1 mm; E, 10 mm.
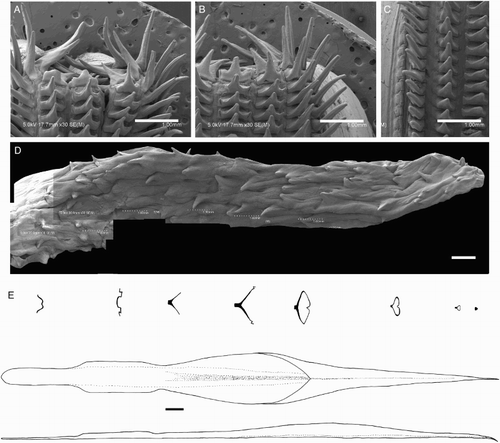
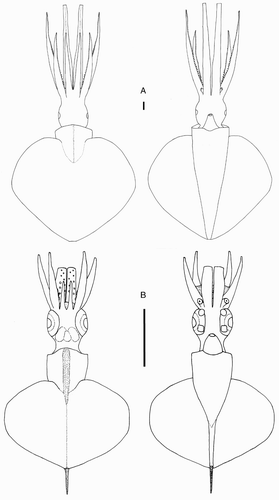
Figure 30 Mastigopsis hjorti. A, NMNZ M.172921, ♀, ML 142* mm; B, NIWA 48868, sex indet., ML 25 mm. Scale bars = 10 mm.
Idioteuthis cordiformis (Chun, Citation1908) (, , , –)
Mastigoteuthis cordiformis Chun, Citation1908: 88; Chun (Citation1910): 177–180, pl. 34, 35 Figs 1, 5, 6, 8, 10–14, pl. 36 Figs 3–5, pl. 37 Fig. 5; Sasaki (Citation1929): 310–312, pl. 24 Figs 15–20; Adam (Citation1954): 159–161, Fig. 25, pl. 2 Fig. 1; Voss (Citation1963): p. 140–142, Fig. 30B–H; Young (Citation1972): 67.
Idioteuthis cordiformis (Chun Citation1908)—Salcedo-Vargas (Citation1993): 155–170, Figs 169–222; Salcedo-Vargas & Okutani (Citation1994): 124, Figs 1, 2, 5.
Type material (not examined). ZMB/Moll 46096, holotype, ♂, ML 83 mm, 0.25°N, 98.13°E, 614 m.
Material examined (28 specimens). NMNZ M.181333, ♂, ML 181 mm, 24.72°S, 168.65°E, 18/11/1996, 720–1048 m, RV Tangaroa, Stn HALIPRO2 BT62; NMNZ M.274092, ♀, ML 205 mm, 26.04°S, 173.03°E, 956 m, 20/06/2007, FV Ocean Fresh with Sanford Limited; NMNZ M.306358, ♂, ML 549 mm, 33.78°S, 167.49°E, 29/05/2003, RV Tangaroa, Stn TAN308/103; NIWA 71655, ♀, ML 320 mm, 34.02°S, 174.80°E, 780 m, 10/07/1999, Stn Z9784; NIWA 71651, ♂, ML 251* mm, 34.10°S, 174.91°E, 862 m, 08/07/1999, Stn Z9785; NMNZ M.306356, sex indet., ML 405 mm, 34.24°S, 168.35°E, 03/06/2003, RV Tangaroa, Stn TAN308/146; NMNZ M.306355, ♂, ML 513 mm, 34.57°S, 168.94°E, 03/06/2003, RV Tangaroa, Stn TAN308/151; NIWA 71437, ♂, ML 608 mm, 34.94°S, 170.25°E, 820 m, 17/01/2002, Stn Z11003; NMNZ M.174313, ♀, ML 654 mm, 34.95°S, 170.25°E, 1105 m, 08/10/2003, FV Seamount Explorer with Thickpenny, Stn 1828/11; NIWA 71653, ♂, ML 428 mm, 35.98°S, 166.17°E, 1000 m, 07/03/1999, Stn Z9723; NIWA 71661, ♀, ML 620 mm, 36.40–36.40°S, 176.95–176.94°E, 940–1175 m, 17/06/1995, Stn Z8292; NMNZ M.298936, ♀, ML 620* mm, 36.42°S, 177.83°E, 1177 m, FV Seamount Explorer with Rattray, Stn 2268; NIWA 71665, ♂, ML 286 mm, 36.54°S, 176.51°E, 909 m, 24/06/1998, Stn Z9158; NIWA 71664, ♀, ML 344 mm, 36.54°S, 176.51°E, 899 m, 19/06/1998, Stn Z9157; NIWA 71659, ♀, ML 520 mm, 36.54°S, 176.52°E, 913–920 m, 25/06/1995, Stn Z8291; NIWA 71663, ♀, ML 286 mm, ♂, ML 342 mm, 37.24°S, 177.23°E, 800 m, 28/03/2000, Stn Z10079; NIWA 71656, ♀, ML 434 mm, 37.40°S, 178.64°E, 37.41°S, 178.64°E, 783–1050 m, 19/05/1995, Stn Z8415; NMNZ M.118004, ♀, ML 715 mm, 37.44°S, 179.32°E, 1095–1193 m, 25/03/1992, RV Tangaroa, Stn TAN9203/138; NMNZ M.274094, ♀, ML 284 mm, 37.68°S, 179.38°E, 778 m, 21/06/2007, FV San Rakaia; NIWA 71666, ♀, ML 380* mm, ♂, ML 463* mm, 39.90°S, 178.27°E, 1187 m, 20/09/1995, Stn Z8380; NIWA 71654, ♀, ML 530 mm, 40.00°S, 178.15°E, 40.08°S, 178.12°E, 730–1350 m, 20/09/1995, Stn Z8381; NIWA 71658, ♀, ML 466 mm, 40.10°S, 178.00°E, 750 m, 19/08/1995, Stn Z8324; NMNZ M.117572, ♀, ML 840* mm, 40.11°S, 178.18°E, 857 m, 10/04/1993, RV Tangaroa, Stn TAN9303/220; NMNZ M.299012, ♀, ML 930 mm, New Zealand, no data; NIWA 71668, ♀, ML 475* mm, New Zealand, no data; NIWA 84390, ♀, ML 820 mm, New Zealand, no data; NIWA 71652, ♂, ML 248 mm, New Zealand, no data, Stn Z10282; NIWA 71660, ♀, ML 503 mm, New Zealand, no data, Stn Z1972.
Distribution (). 24–40°S, 166–170°E (near New Zealand); elsewhere reported from the Pacific Ocean near the Philippines (Voss Citation1963), Japan (Salcedo-Vargas Citation1993), Indonesia (Adam Citation1954), and the Indian Ocean near Sumatra (Chun Citation1910; Sasaki Citation1929).
Diagnosis. As for genus.
Description (ML 181–930 mm, –). Mantle goblet shaped, tapering gradually towards tail, widest (29–37–43% ML) around viscera; dorsal anterior mantle margin slightly convex. Fins together heart shaped to elliptical in outline, length 66–81–86% ML, width 72–90–105% ML; rounded anterior lobes present; posterior margin at tail concave. Photophores absent from integument; skin tubercles () present on dorsal surface of fins, aboral surface of arms, and all external surfaces of mantle, head, and funnel (absent from collar).
Head boxy, length 23–32–52% ML, width 17–23–30% ML. Olfactory papilla cylindrical. Eye diameter 10–15–23% ML. One crescent-shaped photophore on ventral surface of eye (, ). Funnel bulbous posteriorly, cylindrical anteriorly, width c. 18% ML, length c. 12% ML; aperture approximately level with posterior margin of eyelid; funnel pocket absent. Funnel-locking cartilage ear shaped (), c. 7% ML; anterior groove slightly concave due to weak antitragus; weak tragus along inner/medial margin that extends approximately to midline of groove; outer/lateral margin nearly straight, angled towards midline. Mantle-locking cartilage nose shaped (), c. 5% ML; posteriorly undercut in profile.
Arm formula IV≥II>III≥I; arm length 68–73–77% ML (, ); Arms IV thickest followed by Arms II; oral faces of arms bordered by wide trabeculate membranes, trabeculae originating level with bases of sucker pedicels; aboral keels present on Arms I–III; expanded lateral membrane present on Arms IV. Each arm with 56–78 suckers in two series; largest suckers of all located on Arms II (sucker diameter c. 100% arm width) at about row 13–15 (c. 30–50% arm length).
Table 7 Measurements (mm) of Idioteuthis cordiformis, all taken on right side except for NIWA 71659 and NMNZ M.299012.
Arm-sucker rings () proximally adentate, distally adentate or with 6–14 blunt, rectangular teeth; proximal suckers adentate, distal suckers with teeth. Polygonal processes on oral surface of sucker in 4–8 concentric rings; distally, central and intermediate rings with rounded, pitted pegs, separated from teeth by smooth, unstructured band; proximally, central and intermediate rings nearly flat; peripheral ring with flat, rectangular processes.
Tentacle length 153–240–326% ML, club 41–52–63% TnL (84–123–164% ML), expanded to 125–187–300% stalk width; wide trabeculate membrane bordering dorsal and ventral margins of club, distally decreasing in width, nearly absent distally. Proximal suckers in two series for c. 12–23 pairs, three series for c. 12–16 rows, then series number progressively increases distally; proximal c. 50–60% CL with large suckers (diameter c. 30% club width), gradually decreasing in size, while increasing in density distally, with minute suckers covering majority of tentacle surface apart from narrow aboral strip. Proximal tentacle suckers (A–C) proximally adentate, distally with 7–22 blunt, conical teeth; polygonal processes on oral surface of sucker in 7–12 concentric rings; distally, central and intermediate rings with cylindrical pegs; proximally, central and intermediate rings nearly flat; peripheral ring with flat, rectangular processes. Distal tentacle suckers (D) minute, proximally adentate, distally with c. 6 long, conical teeth; polygonal processes on oral surface of suckers in c. 6 concentric rings; distally, central and intermediate rings with long, cylindrical to conical pegs; proximally, central and intermediate rings conical to nearly flat; peripheral ring with flat rectangular processes. Distal-most tentacle suckers (E–H) with c. 12 blunt, conical teeth, ‘cushions’ (F–H), polygonal processes on oral face of suckers in 3–4 concentric rings; central ring with c. 22 round pegs; intermediate rings with c. 35–55 smaller, round pegs; peripheral ring with flat, rectangular-faced processes.
Lower beak, lateral profile (): lower rostral length c. 35% wing length, rostral edge variably nearly straight, curved, or S-shaped, rostral tip often slightly hooked, rostral tip behind leading edge of wing by 26–32–40% baseline; wing angle obtuse, jaw angle variably obscured by low wing fold, shoulder groove variably present; height 74–85–100% baseline; hood well above crest, hood length c. 50% crest length, crest length 57–67–73% of baseline, visible portion of crest nearly straight; broad lateral-wall fold extending to posterior edge of lateral wall; no notch in lateral wall. Lateral oblique view () with wing narrowest level with jaw angle, 53–61–70% of greatest width. Ventral view with deep, broad notch in hood, free corners separated slightly. Wings remain unpigmented at ML 248 mm, darkening in middle of wing at ML c. 320 mm, edges (c. 5 mm) of wings remain clear at ML 428 mm, fully pigmented in male at ML 608 mm, posterior edge of wing remains clear in female at ML 930 mm. Regression equation (J): ML = 0.1307(LRL)0.7637 (R² = 0.9473) (n = 13); regression (K) excluding outlier NIWA 71437 (G), which is morphologically distinct from all other beaks: ML = 9.4965 ln(LRL) – 43.467 (R² = 0.981) (n = 12).
Upper beak, lateral profile (A–I): upper rostral length c. 25% hood length; hood length c. 70% beak length; hood height c. 40% beak width. Lateral-wall fold variably present; shoulder produced into point; jaw edge slightly curved; jaw angle nearly 90°.
Radula (A–B) with tricuspid rachidian, base width 130% height, proximal margin of base rectangular, with broad, blunt, triangular mesocone and broad, rounded lateral cusps, slightly laterally directed, their height c. 45% mesocone height. First lateral tooth bicuspid; inner cusp broad, triangular, slightly curved towards rachidian, its height c. 130% that of overall rachidian; small, pointed outer cusp, slightly medially directed, its height c. 40% that of inner cusp. Second lateral tooth simple, straight, c. 215% rachidian height. Marginal tooth simple, straight, c. 220% rachidian height. Marginal plate present (C). Palatine palp (D) with c. 70 broad, conical teeth, each c. 25–125% rachidian height, evenly distributed over palp.
Gladius (E) with greatest width (c. 10% GL) attained at c. 50% GL; free rachis c. 10% GL; secondary conus c. 35% GL; rostrum c. 6% GL, triangular in cross section.
Integumental photophores apparently absent, skin often damaged. Colour (preserved and fresh) dark purple, chromatophores densely and evenly distributed on all exterior surfaces, sparsely set on internal mantle and funnel surfaces. Subcutaneous chromatophores present on all surfaces, larger and less dense than overlying chromatophores.
Remarks. Three mature males were observed (ML 513–608 mm), while the largest female examined herein was still immature (and unmated) at ML 930 mm. Other I. cordiformis specimens have been observed at greater than 1 m ML (S O'Shea, pers. comm.). Salcedo-Vargas (Citation1993) examined a mature male that was 318 mm ML, which is far smaller than the mature males examined in this study. The differences in size at maturity observed in different regions (New Zealand and Japan) could indicate either different species or that separate populations within this species reach different sizes, as in Dosidicus gigas (Nigmatullin et al. Citation2001). However, because Salcedo-Vargas (Citation1993) only observed a single mature male specimen, it is possible that this specimen was an anomaly.
Voss (Citation1963) described several small I. cordiformis specimens (36–92 mm ML) from the Philippines, with some apparent morphological differences from the material reported here. Voss (Citation1963) and Sasaki (Citation1929) found head width slightly greater than mantle width (HW c. 115% MW), contrary to what was found herein (HW c. 62% MW). They also both noted a distinct eye sinus, which was not found here. The tentacle suckers that Sasaki (Citation1929) examined bore teeth around the circumference, while teeth on tentacle suckers in the New Zealand material were only observed on the distal margin of the sucker ring. Sasaki (Citation1929) found the basal portion of the club to have eight series, while here only two or three were found. Sasaki (Citation1929) also found that the teeth on the tentacle suckers were much smaller than those illustrated by Chun (Citation1910). Size difference and damage could account for these discrepancies, but it is also possible that these descriptions refer to different taxa.
Arm suckers have been reported in varying numbers and dentition for I. cordiformis. Herein, arms had 56–78 pairs of suckers, in two series, and were adentate or sometimes possessed 6–14 blunt, rectangular teeth; the sucker shape was globular, similar to the suckers reported by Sasaki (Citation1929). Chun (Citation1910) described one specimen of 83 mm ML with 57–59 pairs of ‘acorn-shaped’ suckers on the ventral arms, and 50 pairs on Arms III. Sasaki (Citation1929) found arm suckers ranging from adentate to possessing 4–11 quadrangular teeth distally. Young (Citation1972) described arm suckers with 30 small, blunt teeth distally, while Adam (Citation1954) found ‘irregular’ teeth on the arm suckers. Approximately half the specimens examined herein had Arms II that exceeded the length of Arms IV, which was also found by Salcedo-Vargas (Citation1993). Voss (Citation1963) reported five or six basal arm suckers on Arms IV and III were in a single series, while specimens examined here had two distinct series on all arms. These differences could be due to size or may also indicate different species. However, because of the wide range of morphological variation found within New Zealand material, it is clear that integrative taxonomy will be necessary to determine whether the differences found between these specimens and previous studies represent the same taxa.
The radula illustrated by Adam (Citation1954) is similar to the radula scanning electron micrographs by Salcedo-Vargas (Citation1993); however, the mesocones for these specimens were relatively much taller than in the specimens examined herein. This difference could be due to growth or geographic area. The specimen examined by Adam (Citation1954) was 96 mm ML from Indonesia (7°S, 117°E) and the specimens illustrated by Salcedo-Vargas (Citation1993) were 109 and 318 mm ML from Japan (32–33°N, 133°E). Two specimens with different beak morphologies (G–H) were found herein to have similar radular morphologies (A–B). Although cephalopod radulae have been used widely in taxonomic work, much intraspecific variation has been found (Voss Citation1977). In addition, serial repetition in the lateral cusps of the rachidian and asymmetry has been found within single specimens of cephalopods by Nixon (Citation1998), who suggested that radulae should be examined from a full ontogenetic series in a species to determine the degree of intraspecific variation. Unfortunately, this was not possible in the current study because small specimens were not available.
The presence of a photophore on the eye of I. cordiformis was first recognised by Salcedo-Vargas (Citation1993); its presence was supported herein with histology (). Salcedo-Vargas (Citation1993) also reported photophores embedded in the protective membrane of the tentacle, which were not found in specimens in this study, although histological examinations were not undertaken. Young (Citation1972) stated that I. cordiformis is covered in small light organs because Chun (Citation1910, pl. 36) wrote ‘luminous organs?’ within the figure caption of a cross section of an I. cordiformis tubercle. However, Chun (Citation1910) stated that he believed that there were no photophores in the integument of I. cordiformis. No integumental photophores were observed for I. cordiformis herein, but histology was not attempted.
Idioteuthis cordiformis is morphologically similar to I. latipinna, which was described from a single specimen from the Sagami Sea in Japan, from the largest mastigoteuthid specimen known at the time (238 mm ML). The eyes of I. latipinna are differentiated from I. cordiformis by the presence of an eye sinus, and the left eye being approximately twice the size of the right. While this size difference was supported by Salcedo-Vargas (Citation1997), Young & Vecchione (Citation2004) presumed that Sasaki (Citation1929) believed this difference was due to damage because the uneven eyes were not discussed in his comparison with I. cordiformis. The tentacles of I. latipinna have four series of suckers proximally (Sasaki Citation1929), which contrasts with the three proximal series observed herein. The skin of I. latipinna is smooth (Sasaki Citation1929), while I. cordiformis is covered in skin tubercles. Although Sasaki (Citation1929) found that the fins of I. latipinna were larger and rounder than those of I. cordiformis, this study found that the fin shape of I. cordiformis is quite variable. Sasaki (Citation1929) found that the tragus and antitragus were weaker in I. latipinna, but here it appears that the funnel- and mantle-locking cartilages are variable in morphology and easily deformed, especially in larger specimens (A–C). Sasaki (Citation1929) found 65–70 suckers on each arm, which falls within the range found herein for I. cordiformis. Because of the similarities between the two species, and the potential for the observed differences to result from damage, some authors have considered I. latipinna a junior synonym of I. cordiformis (Salcedo-Vargas & Okutani Citation1994; Young & Vecchione Citation2004). This seems likely to be due to the commonalities between these species, and the extent of variation found within I. cordiformis herein; however, genetics will be essential in resolving the status of I. latipinna.
There are also some morphological similarities between I. cordiformis and Mp. hjorti, but these two taxa can be readily distinguished. Both species have heart-shaped fins, nearly the length of the mantle. The skin tubercles of both species have similar structure (Roper & Lu Citation1990). Eye photophores are present in both species, but Mp. hjorti has two hemispherical photophores (one postero-ventral, one antero-ventral; E–F), while I. cordiformis has a single, flat, crescent-shaped photophore on the ventral surface of the eye (G–H). The proximal arm suckers of I. cordiformis are adentate (), while all arm suckers of Mp. hjorti have long, blunt rectangular teeth distally (). Idioteuthis cordiformis has trabeculae on the protective membranes of the arms, while trabeculae are absent from the protective membranes of Mp. hjorti arms. In addition, these species are genetically distinct from each other ().
Figure 31 Mastigopsis hjorti. A–C, E–G, K, NMNZ M.172921, ♀, ML 142* mm; D, H–J, M.172986, ♂, ML 78* mm, LRL 4.00 mm. A, Left funnel-locking cartilage; B, left mantle-locking cartilage; C, left mantle-locking cartilage, profile view; D, nuchal cartilage; E, right eye; F, ventral view of right eye; G, skin tubercles on ventral surface of mantle; H, lower beak, profile view; I, lower beak, lateral oblique view; J, upper beak; K, internal mantle pigmentation. Scale bars = A–D, H–K, 5 mm; E, F, 10 mm; G, 500 µm.
Extreme morphological variation was found in the beaks of I. cordiformis (), although the individuals with different beak shapes were nearly genetically identical (). Variation was found in both upper and lower beaks (A–I). Upper beaks of some specimens possessed a lateral-wall groove while others lacked this feature. In the lower beak, the jaw edge varied from slightly curved to S-shaped, and sometimes the rostral tip was slightly hooked. The shoulder varied from a smooth surface leading to the jaw edge in a gentle curve, to a pronounced bump at the wing fold. No consistent differences were found between beak variation and size, sex, or location. The regression equation (K) that was calculated without the LRL of NIWA 71437 (G), which is morphologically distinct from the other beaks, had a higher R2 value (R2 = 0.981) than the equation (J) that included this beak (R2 = 0.9473). Clarke (Citation1980) described beaks of ‘?Mastigoteuthis B’ from stomach contents of sperm whales from South Africa, which could belong to I. cordiformis. He noted that the beaks were similar to other mastigoteuthid beaks, but were much larger (LRL 8–10 mm). His description for the ‘?Mt. B’ beak is mostly consistent with the I. cordiformis beaks described herein. However, ‘?Mt. B’ beaks did not have the deep notch in the hood-wing structure, the free corners were closer together, and the rostral tip was closer to the leading edge of the wing. Due to the wide range of morphological variation found herein for this species, it is likely that these differences are intraspecific and that ‘?Mt. B’ is synonymous with I. cordiformis.
Genus Mastigopsis Grimpe, 1922
Mastigopsis Grimpe, Citation1922: 50. Type species Mastigoteuthis hjorti Chun, Citation1913, by monotypy.
Diagnosis. Fin length c. 90% ML. Funnel narrowly conical. Funnel-locking cartilage oval with convex protrusion along outer/lateral margin, tragus and antitragus absent; funnel pocket absent. Arm suckers with blunt teeth distally; Arms IV c. 80% ML. Tentacular suckers minute; club not expanded. Eye-sinus photophore absent. Two round photophores on each eye (one postero-ventral, one antero-ventral). Integumental photophores absent, skin tubercles present. Gladius broad (greatest width c. 10% GL).
Description. Mantle length at maturity c. 180–235 mm. Fins together heart shaped in outline; mantle-locking cartilage ovate, posteriorly undercut in profile. Arm suckers arranged in two distinct series but appearing to merge into single series midway on Arms IV; largest arm suckers overall located proximally on Arms III; protective membranes on arms without trabeculae. Tentacles with trabeculate protective membrane (fide Chun Citation1913).
Mastigopsis hjorti (Chun, Citation1913) (, , 8; –)
Mastigoteuthis hjorti Chun, Citation1913: 6–8, Fig. 1, pl. 2; Rancurel (Citation1973): 27–32, Figs 1–8; Vecchione et al. (Citation2001): 34–36, Fig. 26.
Mastigoteuthis (Mastigopsis) hjorti (Chun, Citation1913): Grimpe (Citation1922): 50.
Idioteuthis (Idioteuthis) hjorti (Chun, Citation1913): Salcedo-Vargas & Okutani (Citation1994): 124.
Idioteuthis hjorti (Chun Citation1913): Salcedo-Vargas (Citation1997): 107.
Mastigoteuthis sp. 2 Spencer et al. (Citation2009): 219.
Type material (not examined). ZMBN 25906, syntype, 3 sex indet., ML 71–95 mm, 31.40°N, 34.78°W, 06–07/06/1910, RV Michael Sars, Stn 52; ZMBN 25910, syntype, sex indet., ML 55 mm, 36.08°N, 43.97°W, 22/06/1910, RV Michael Sars, Stn 63; ZMBN 25909, syntype, sex indet., ML 68 mm, 36.87°N, 39.92°W, 20–21/06/1910, RV Michael Sars, Stn 62.
Material examined (five specimens). NMNZ M.172986, ♂, ML 78 mm, 29.53°S, 167.63°E, 200–1200 m, 15/05/2003, RV Tangaroa, NORFANZ Stn 23, 2003023; NMNZ M.172921, ♀, ML 145 mm, 29.99°S, 167.65°E, 1245–1285 m, 14/05/2003, RV Tangaroa, NORFANZ Stn 16, 2003016; NIWA 48868, 3 sex indet., ML 24–26 mm, 44.84°S, 173.37°E, 44.70°S, 175.38°E, 1000–983 m, 16/04/1997, Stn Z8795 TAN9101.
Figure 33 Mastigopsis hjorti. A, E, NMNZ M.172921, ♀, ML 142* mm; B–D, F, G, H, M.172986, ♂, ML 78* mm. A, Radula; B, asymmetrical radula; C, asymmetrical radula rachidian, first lateral tooth, and additional tooth; D, additional tooth of asymmetrical radula; E, radula margin; F, palatine palp; G, gladius. Scale bars = A, B, 500 µm; C, E, 100 µm; D, 20 µm; F, 1 mm; G, 10 mm.
Distribution (). 29–45°S, 167–175°E (near New Zealand); elsewhere reported from North Atlantic (Chun, Citation1910; Vecchione et al. Citation2002), Central Pacific (Vecchione & Young Citation2007c), off South Africa (Roper & Lu Citation1990), Sulu Sea in the Indian Ocean (Nesis Citation1987).
Diagnosis. As for genus.
Description (ML c. 145 mm, , –). Mantle cone shaped, tapering gradually towards tail, widest (c. 32% ML) at anterior margin; dorsal anterior mantle margin convex. Fins together heart shaped, length c. 89% ML, width c. 106% ML; rounded anterior lobes present; posterior margin at tail convex. Photophores absent from integument; skin tubercles (G) present on ventral surface of mantle and fins (dorsal surfaces unknown), and all surfaces of head; overlying gelatinous coating present on posterior half of mantle and on arms.
Arm formula unknown due to damage; Arms II c. 75% ML, Arms III c. 63% ML (, ); Arms I–III all of subequal thickness; Arms IV thicker, with expanded lateral membrane. Trabeculae absent. Arm I suckers in two series (number unknown); Arms II with about 240 suckers: proximal 30% of arms with 10 rows in two series, next c. 25% with 20 rows in three series, distal portion with 78 rows in two series; Arms III with 200 suckers in two series; Arm IV suckers in two series (number unknown), distally merging into one series; largest suckers located on Arms III (c. 30% arm width) at about second pair.
Table 8 Measurements (mm) of Mastigopsis hjorti, all taken on right side.
Arm-sucker rings () proximally adentate, distally with 8–21 blunt, rectangular teeth; teeth relatively shorter in proximal suckers. Polygonal processes on oral surface of sucker in 3–4 concentric rings; distally, central and intermediate rings with cylindrical, pitted pegs; proximally, central and intermediate rings nearly flat; peripheral ring with flat, rectangular processes.
Tentacles absent from all specimens examined.
Lower beak, lateral profile (H): lower rostral length c. 60% wing length, rostral edge nearly straight, rostral tip slightly hooked, rostral tip behind leading edge of wing by c. 30% baseline; wing angle obtuse, jaw angle obscured by low wing fold, shoulder groove present; beak slightly taller than length of baseline; hood slightly above crest, hood length c. 65% crest length, crest length c. 55% of baseline, visible portion of crest straight; lateral-wall fold with solid ridge extending to posterior edge of lateral wall; no notch in lateral wall. Lateral oblique view (I) with wing narrowest level with jaw angle, breadth c. 70% of greatest wing width. Ventral view with narrow notch in hood, free corners separated slightly. Posterior and anterior edges of wing remain clear through at least ML 78* mm (LRL 4.00 mm), beak entirely pigmented at ML 142* mm (LRL 5.26 mm).
Upper beak, lateral profile (J): upper rostral length c. 30% hood length; hood length c. 70% beak length; hood height c. 30% beak width. Lateral-wall fold absent; shoulder produced into point; jaw edge curved; jaw angle nearly 90°.
Radula (A) with tricuspid rachidian, base width c. 75% height, proximal margin of base concave, with broad, sharp triangular mesocone and sharp lateral cusps, slightly laterally directed, their height c. 50% mesocone height. First lateral tooth strongly bicuspid; inner cusp broad, triangular, slightly curved towards rachidian, its height c. 80% that of overall rachidian; outer cusp sharply pointed, medially directed, its height c. 60% that of inner cusp. Second lateral tooth simple, curved slightly towards rachidian, c. 150% rachidian height. Marginal tooth simple, straight, c. 190% rachidian height. Poorly defined marginal plate present (E). Palatine palp (F) with c. 40 short, flat teeth, each 50–100% rachidian height, sparsely distributed over palp.
Gladius (G) missing rostrum in best available specimen; greatest width (12% GL) attained at 67% (damaged) GL; free rachis 15% GL; secondary conus 22% GL.
Epidermis damaged on all examined material. Integumental photophores not apparent on intact portion of skin in examined material. Colour (preserved) purple, chromatophores densely and uniformly distributed on mantle, fins, and arms; pigmentation on interior mantle wall extending from anterior margin to approximate level of locking cartilage (K), area around locking cartilages without chromatophores and remainder of inner mantle wall not pigmented; chromatophores present on all surfaces of arms; subcutaneous chromatophores on arms of similar same size and density as overlying chromatophores.
Smaller specimens (ML 24–26 mm, B) differ from above only as follows: mantle width c. 26% ML, fin length c. 79% ML, fin width c. 92% ML. Head length c. 33% ML, head width c. 23% ML; eye diameter c. 14% ML; MLC c. 5% ML, funnel-locking cartilage c. 6% ML. Arms c. 20–25% ML, suckers in two series; 12–14 pairs of suckers present on each arm.
Remarks. This species appears to have a circum-global distribution; its type locality is the North Atlantic (Chun Citation1910), and specimens have also been reported from the Pacific and Indian Oceans (Nesis Citation1987). However, some authors suggest that specimens from different geographic locations may belong to different species (Nesis Citation1987; Vecchione & Young Citation2007c). The specimens in New Zealand collections all had characteristics that correspond to Mp. hjorti s. str. (Chun Citation1913); however, they did not have the skin sculpture that Chun (Citation1913) described, which Rancurel (Citation1973) determined to be impressions left by the nets during capture, and the triserial suckers on Arms II in the New Zealand material are an unusual feature. It is not clear whether this arrangement is an illusion resulting from live fixation, or characteristic of specimens from this location, since only one specimen with complete Arms II was available for study. No fresh material was available from New Zealand for genetic comparison with Mp. hjorti from the North Atlantic.
Mastigopsis hjorti shares some morphological traits with I. cordiformis: fin shape, fin length greater than 80% ML, and skin tubercles. However, Mp. hjorti beaks are entirely pigmented at LRL 5.26 mm, whereas I. cordiformis beaks are still nearly entirely unpigmented at LRL 6.36 mm, indicating that I. cordiformis attains much greater sizes than Mp. hjorti. Additionally, Mp. hjorti can be distinguished by the two round eye photophores (I. cordiformis has a single ventral photogenic band), oval funnel-locking cartilage (lacking tragus and antitragus), the lack of trabeculae on the protective membranes of the arms, the presence of largest suckers at the arm bases, and beak morphology. The beak of Mp. hjorti (H) is taller and narrower than that of I. cordiformis (). Mastigopsis hjorti and Mg. osheai are similar in size and both have funnel-locking cartilage that lacks an antitragus; however, Mp. hjorti can be readily distinguished by the larger fin, two eye photophores, and skin tubercles. Magnoteuthis magna—the closest genetic relative, but genetically distinct from North Atlantic Mp. hjorti ()—can be also be easily distinguished; Mg. magna lacks eye photophores and generally has smaller fins (FL c. 67%, FW c. 80% ML) than Mp. hjorti (FL c. 90%, FW c. 92% ML).
Among the radulae examined (see Discussion), one appeared unusual (B–D) as follows: rachidian mesocone with small, sharp lateral cusps, their height c. 45% that of mesocone height. Left first lateral tooth unicuspid, broad, triangular, straight, its height c. 75% that of rachidian. Additional tooth present between left first lateral tooth and second lateral tooth, elongate with two or three irregular cusps, height c. 50% of rachidian, with small sharp lateral cusps slightly anteriorly directed, their height approximately equal to that of mesocone (B–E).
Genus Echinoteuthis Joubin, 1933
Echinoteuthis Joubin, Citation1933:13. Type species Echinoteuthis danae Joubin, Citation1933, by monotypy.
Diagnosis. Funnel-locking cartilage ear shaped, tragus present, antitragus absent; funnel pocket present. Mantle-locking cartilage C-shaped, not posteriorly undercut in profile. Arm suckers with sharp teeth distally. Tentacular suckers minute. Large eye-sinus photophore present, diameter c. 25% eye diameter. Eye photophore absent. Integumental photophores absent, skin tubercles variably present. Gladius narrow (fide Salcedo-Vargas & Okutani Citation1994).
Description. Mantle length at maturity c. 240 mm. Fins together lanceolate; fin length c. 65% ML. Funnel widely conical with recurved end. Arm suckers arranged in two distinct series but appearing to merge into single series midway along Arms IV; Arms IV c. 75% ML; protective membranes without trabeculae. Tentacle club not expanded, with trabeculate membrane present in some species (fide Vecchione & Young, Citation2007c).
Echinoteuthis famelica (Berry, 1909)
(, , , )
Chiroteuthis famelica Berry, Citation1909: 414, 415, .
Mastigoteuthis famelica (Berry, Citation1909)—Young (Citation1991): 176–178, .
Type material examined. USNM neotype, ♀, ML 241 mm, off Oahu, FIDO X, Stn 9.
Additional material examined (two specimens). NMNZ M.074535, 2 sex indet., ML 38, 40 mm, 22.83°S, 175.03°W, 377 m over 4000 m, RV James Cook, 12/12/1976, MWT, Stn J17/60/76.
Distribution (). 22–23°S, 175–176°W (near New Zealand); elsewhere reported from the Pacific Ocean, near Hawaii (Berry Citation1909; Young Citation1991).
Diagnosis. As for genus, except tubercles present (fide Young Citation1991) and tentacular protective membranes barely detectable in larger paralarvae and subadults.
Description (ML c. 40 mm, ). Mantle cone shaped anteriorly, with mantle cavity extending to c. 20% FL from anterior of fins (thereafter gladius and surrounding musculature continue as narrow cylinder), widest (c. 18% ML) at anterior margin; ventral anterior mantle margin slightly convex with medial notch; dorsal anterior mantle margin pointed. Fins together lanceolate, length c. 55% ML, width c. 46% ML; anterior lobes absent; posterior margin at tail convex. Integumental photophores absent; eye-sinus photophore diameter c. 24% ED. Skin tubercles present only on mantle.
Figure 35 Echinoteuthis famelica. A, NMNZ M.074535, sex indet., ML 38 mm; B–E, NMNZ M.074535, sex indet., ML 40 mm. A, Whole specimen; B, nuchal cartilage; C, left funnel-locking cartilage; D, left mantle-locking cartilage; E, left mantle-locking cartilage, profile view. Scale bars = A, 5 mm; B–E, 1 mm.
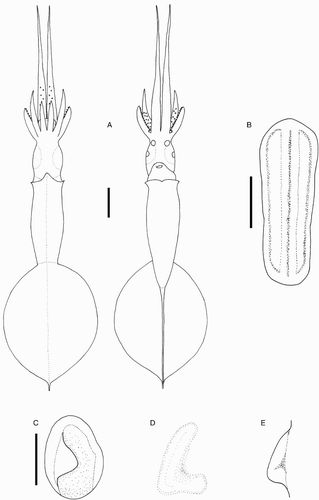
Head rounded, length c. 21% ML, width c. 13% ML. Olfactory papilla cylindrical. Eye diameter c. 10% ML. Funnel width c. 8% ML, length c. 6% ML, aperture approximately level with posterior margin of eye; funnel pocket present. Funnel-locking cartilage ear shaped (C), c. 4% ML, groove bulbous posteriorly, antitragus absent; strong tragus present along inner/medial margin, extending to midline of groove; concave along outer/lateral margin. Mantle-locking cartilage C-shaped (D–E), c. 3% ML; not posteriorly undercut in profile.
Arm formula IV>II>III≥I; arm length 13–25–54% ML (, ); Arms I–III all of subequal thickness, Arms IV thickest; oral faces of arms bordered by wide membranes; expanded lateral membrane present on Arms IV. Trabeculae absent. Arms I–III with 34–44 suckers arranged in two distinct series; Arms IV damaged on all available material.
Table 9 Measurements (mm) of Echinoteuthis famelica.
Tentacles absent from all specimens examined.
Arm suckers, radula, gladius, and beak not dissected due to limited material.
Colour (preserved) yellow-beige; gladius visible through dorsal surface of fin; chromatophores present but damaged.
Remarks. This description is limited because only two damaged, juvenile specimens were found in the New Zealand collections. Berry's (Citation1909) description was based on a single specimen of a similar size to those examined herein (ML 39 mm) and characterised by the lanceolate fins, relatively narrow head, and slender mantle; the specimens examined herein agree with this description. Although the original description did not mention the relatively large eye-sinus photophores, they are present on the specimens examined herein, including the E. famelica neotype. In addition, this species can be readily distinguished from other local mastigoteuthid species by the lanceolate fin shape, ear-shaped funnel-locking cartilage without an antitragus, C-shaped mantle-locking cartilage, and large eye-sinus photophores (). However, no similarly sized I. cordiformis specimens were available for comparison.
The three Echinoteuthis species are believed to occupy different oceans: E. glaukopis Chun, Citation1908, Indian Ocean; E. famelica, Pacific Ocean; and E. atlantica Joubin, Citation1933, Atlantic Ocean (Vecchione & Young Citation2007d). However, E. famelica has only been previously identified from Hawaiian waters, and is known to occur at latitudes as low as 3°S (Young Citation2010). Echinoteuthis glaukopis is differentiated from E. atlantica and E. famelica by a rhomboidal fin, the presence of an antitragus in the funnel-locking cartilage, and an undercut mantle-locking cartilage (Chun Citation1910). Echinoteuthis atlantica also possesses a trabeculate membrane on the tentacles, which further distinguishes it from E. famelica (Vecchione & Young Citation2007d).
The characteristics of E. famelica are similar to those in published descriptions for E. danae; however, they appear to occupy different geographic areas because E. danae has been reported from the North Atlantic (Joubin Citation1933) and the Indian Ocean (Salcedo-Vargas Citation1997), whereas E. famelica is only presently known from the Pacific Ocean (Berry Citation1909; Young Citation1991). In addition, the funnel-locking cartilage of E. danae has an antitragus, which is not present in E. famelica. However, Salcedo-Vargas & Okutani (Citation1994) considered E. danae to be a junior synonym of E. famelica, while Vecchione & Young (Citation2007e) suggested that E. danae may be synonymous with E. atlantica. Because very few specimens have been collected, this issue remains unresolved. Genetics indicate that E. atlantica is distinct from other mastigoteuthids (), but tissue samples for E. famelica were not available.
Echinoteuthis famelica shares some characteristics with E. tyroi (Salcedo-Vargas, Citation1997) (known only from a single paralarva collected from the Indian Ocean, near Somalia): lanceolate fin shape, and skin tubercles. Echinoteuthis tyroi was originally described under Idioteuthis, but has been recently reclassified under Echinoteuthis (Salcedo-Vargas & Young Citation2014). Salcedo-Vargas (Citation1997) noted the similarities between E. danae, E. famelica, and E. tyroi and placed them in the same ‘group’ in the type description of E. tyroi. Echinoteuthis tyroi possesses a lanceolate fin and skin tubercles (Salcedo-Vargas Citation1997), which are characteristic of this genus. Although Echinoteuthis species have ear-shaped funnel-locking cartilage (with a tragus but lacking an antitragus), ovate funnel-locking cartilage was reported for E. tyroi (Salcedo-Vargas Citation1997). However, the tragus of E. famelica does not develop until ML 25 mm (Young Citation1991) and it is possible that the funnel-locking cartilage of the ML 15 mm specimen of E. tyroi was not fully developed. In addition, no photophores were reported for E. tyroi (Salcedo-Vargas Citation1997), but all other Echinoteuthis species have eye-sinus photophores. In the original description of E. danae, Joubin (Citation1933) stated that no photophores were found on his specimens, but suggested that there may be some near the eyes. In paralarval mastigoteuthid specimens (such as the type, ML 15 mm), these structures are easily damaged during collection, or overlooked; it is also possible that they develop during later ontogenetic stages (Young Citation1991). The tentacle club described for E. danae is similar to the club described for E. tyroi: slightly expanded relative to the tentacle-stalk width, the club tip is elongated and narrow, and the aboral side has bands of chromatophores.
Genus Magnoteuthis Salcedo-Vargas & Okutani, 1994
Magnoteuthis Salcedo-Vargas & Okutani, Citation1994: 125. Type species Mastigoteuthis magna Joubin, Citation1913, by original designation.
Diagnosis. Funnel-locking cartilage flask shaped, tragus present, antitragus absent, convex protrusion along outer/lateral margin; funnel pocket absent. Mantle-locking cartilage flask shaped, posteriorly undercut in profile. Arm suckers adentate or possess blunt teeth distally; Arms IV c. 110% ML. Tentacular suckers microscopic; club not expanded, protective membranes without trabeculae. Eye-sinus photophore absent. Eye photophore absent. Integumental photophores microscopic or absent, skin tubercles absent. Gladius broad (greatest width c. 10% GL).
Description. Mantle length at maturity c. 220 mm. Fins together heart shaped; fin length c. 65% ML. Funnel bulbous posteriorly, cylindrical anteriorly. Arm suckers arranged in two series but appearing to merge into single series midway on Arms IV; largest arm suckers overall located in the middle of arms or basal. Tentacular protective membranes without trabeculae.
Magnoteuthis osheai sp. nov. (, , 10; –)
Figure 37 Magnoteuthis osheai. A, NMNZ M.287221, ♀, ML 163 mm; B, NMNZ M.172945, sex indet., ML 42 mm; C, NMNZ M.287233, sex indet., ML 26 mm. Scale bars = A, 20 mm; B, C, 10 mm.
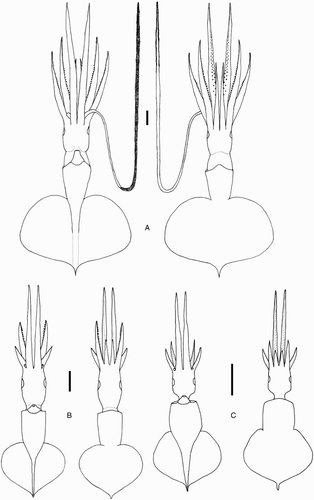
Figure 38 Magnoteuthis osheai. A–C, H, NIWA 48866, ♀, ML 160 mm; D, NMNZ M.287216, sex indet., head only, LRL 3.09 mm; E–G, NIWA 76653, ♂, ML 180 mm, LRL 4.76 mm. A, Left funnel-locking cartilage; B, left mantle-locking cartilage; C, left mantle-locking cartilage, profile view; D, nuchal cartilage; E, lower beak, profile view; F, lower beak, lateral oblique view; G, upper beak, profile view; H, internal mantle pigmentation. Scale bars = A–C, 1 mm; D, 2 mm; E–H, 5 mm.
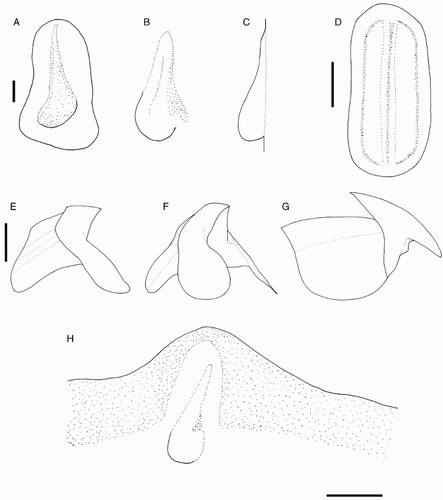
Figure 39 Magnoteuthis osheai arm suckers, NMNZ M.287216, sex indet., head only, LRL 3.09 mm. Scale bars = 100 µm.
Figure 40 Magnoteuthis osheai. A, B, NMNZ M.091708, ♀, ML 73 mm; C–E, NMNZ M.287216, sex indet., head only; F, NIWA 76653, ♂, ML 180 mm. A, Mid-tentacle club; B, degraded microscopic tentacle sucker; C, radula; D, marginal plates of radula; E, palatine palp; F, gladius. Scale bars = A, C, 500 µm; B, 30 µm; D, 100 µm; E, 1 mm; F, 10 mm.
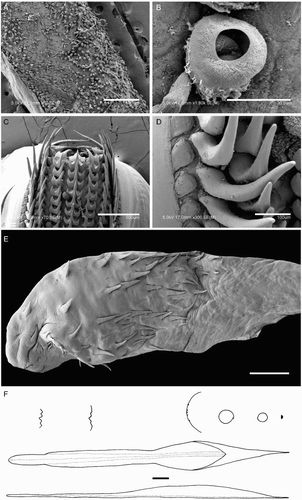
Type material examined. NIWA 48866, holotype, ♀, ML 160 mm, 36.52°S, 176.50°W, 904–1086 m, 24/06/1995, FV Seamount Enterprise, Stn Z8289; NMNZ M.287221, paratype, ♀, ML 163 mm, 39.67°S, 167.63°E, 1072-1102 m, 20/07/1990, FV Will Watch; NIWA 76653, paratype, ♂, ML 180 mm, 39.96°S, 178.18°E, 906 m, 23/03/2010, RV Tangaroa, Stn TAN1003/39; NMNZ M.091708, paratype, ♀, ML 73 mm, 42.76°S, 177.47°W, 116–1164 m, 09/07/1984, FV Otago Buccaneer, Stn B1/6/84.
Additional material examined (five specimens). NMNZ M.172975, sex indet., ML 47 mm, 32.54°S, 169.73°W, 15/05/2003, RV Tangaroa, NORFANZ Stn 10; NMNZ M.287233, sex indet., ML 26 mm, 33.84°S, 175.08°E, 277 m over 5000 m, RV James Cook, 16/12/1976, MWT, Stn J17/85/76; NMNZ M.091715, sex indet., ML 123 mm, 36.42°S, 173.50°E, 1024–1045 m, FV Wanaka, 23/03/1986, Stn WK4/14/86; NMNZ M.287216, sex indet., head only, 37.47°S, 178.40°E, 339 over 1350 m, 14/12/1975, RV James Cook, MWT, Stn J17/69/75; NMNZ M.091721, ♀, ML 105 mm, year 1985–1986, FV Wanaka, stomach content of Hoplostethus atlanticus.
Distribution (). 32–43°S, 167°E–169°W (near New Zealand).
Diagnosis. Locally, as for genus (sole representative in New Zealand waters). Distinguished from other Magnoteuthis species by absence of integumental photophores and largest arm suckers overall are located midway on Arms II.
Description (ML c. 120–180 mm, , –). Mantle cone shaped anteriorly, with mantle cavity terminating beneath midpoint of fins (thereafter gladius and surrounding musculature continue as narrow cylinder), widest (c. 21% ML) at anterior margin; dorsal anterior mantle margin convex. Fins heart shaped in outline when considered together, length 60–64–70% ML, width 71–79–82% ML; rounded anterior lobes present; posterior margin at tail concave. Photophores and tubercles absent from integument; gelatinous coating present overlying posterior half of mantle.
Head cylindrical, length 25–30–36% ML, width c. 16% ML. Olfactory papilla cylindrical. Eye diameter c. 13% ML. Funnel bulbous posteriorly, cylindrical anteriorly, width c. 14% ML, length c. 11% ML; aperture approximately level with posterior margin of eye; funnel pocket absent. Funnel-locking cartilage flask shaped (A), c. 7% ML, groove bulbous posteriorly; concave along inner/medial margin (due to tragus); convex along outer/lateral margin, with anterior portion narrower, sides nearly parallel. Mantle-locking cartilage flask shaped (B, C), c. 6% ML, approximately hemispherical posteriorly and hemiconical anteriorly; posteriorly undercut in profile.
Arm formula IV>II>III≥I; arm length 98–116–138% ML (, ); Arms IV thickest, followed by Arms II; aboral keels present on Arms I–III, expanded lateral membrane present on Arms IV. Trabeculae absent. At ML 160 mm, 114–122 suckers present on each arm, arranged in two distinct series, distally merging into one series on Arms IV; largest suckers located midway along Arms II (c. 100% arm width) at about pair 17.
Table 10 Measurements (mm) of Magnoteuthis osheai, all taken on right side except for NMNZ M.172975 and NMNZ M.287221.
Arm-sucker rings () proximally adentate, distally adentate or with 6–18 indistinct teeth; at c. 75% arm, infundibular ring with seven blunt, conical teeth. Polygonal processes on oral surface of sucker in 4–8 concentric rings; distally, central and intermediate rings with rounded, pitted, porous pegs; proximally, central and intermediate rings nearly flat; peripheral ring with flat, rectangular processes.
At ML 105 mm (sole tentacle available for examination), tentacle length c. 280% ML, club c. 64% TnL (c. 180% ML), not expanded; stalk without keel. Suckers microscopic (A), covering majority of tentacle surface apart from narrow aboral strip; where tentacle club begins, width of sucker-covered surface is less than half of stalk circumference, broadening distally to cover nearly entire stalk circumference. Tentacle suckers degraded, infundibular rings lost (B); sucker diameter c. 40 µm, c. 3% club width.
Lower beak, lateral profile (E): lower rostral length c. 50% wing length, rostral edge moderately curved, tip without hook, rostral tip behind leading edge of wing by c. 25% of baseline; wing angle obtuse, jaw angle obscured by low wing fold, shoulder groove present; beak height c. 60% baseline; hood close to crest, hood length c. 50% crest length, crest length c. 65% baseline, visible portion of crest slightly curved; broad lateral-wall fold extends to posterior edge of lateral wall; no notch in lateral wall. Lateral oblique view (F) with wing narrowest level with jaw angle, c. 70% of greatest width. Ventral view with narrow notch in hood, free corners separated slightly. Anterior edge of wing below shoulder clear at ML 123 mm (LRL 4.00 mm), pigmented by ML 180 mm (LRL 4.76 mm).
Upper beak, lateral profile (G): upper rostral length c. 30% hood length; hood c. 70% beak length; hood height c. 25% beak width. Lateral-wall fold extending to posterior edge of lateral wall; shoulder produced into point; jaw edge nearly straight; jaw angle obtuse.
Radula (C) with tricuspid rachidian, base width c. 60% height, proximal margin of base concave, with narrow, sharp, triangular mesocone and sharp lateral cusps, slightly laterally directed, their height c. 55% mesocone height. First lateral tooth strongly bicuspid; inner cusp narrow, triangular, slightly curved towards rachidian, its height c. 80% rachidian height; outer cusp sharply pointed, medially directed, its height c. 65% that of inner cusp. Second lateral tooth simple, curved slightly towards rachidian, c. 130% rachidian height. Marginal tooth simple, straight, c. 180% rachidian height. Marginal plate present (D). Palatine palp (E) with c. 60 narrow, flat teeth, each 30–90% rachidian height, sparsely distributed over palp.
Gladius (F) with greatest width (c. 10% GL) attained at c. 70% GL; free rachis c. 13% GL; secondary conus c. 25% GL; rostrum c. 4% GL, oval in cross section.
Epidermis damaged on all examined material. Integumental photophores absent from available skin. Colour (preserved) pink or purple, dark red or purple on mantle and fins; head white, with pronounced darkening around eye sinus; chromatophores more densely distributed on fin than head; pigmentation on anterior edge of mantle interior extending approximately to level of locking cartilage (H), area around locking cartilages without chromatophores and remainder of inner mantle wall not pigmented; chromatophores present on aboral surface of arms, arm oral surface darkly pigmented but individual chromatophores not readily discernible; subcutaneous chromatophores on mantle, fins, and funnel less dense than overlying chromatophores. Colour (fresh) of liver bright red.
Smaller specimens (ML 42–73 mm, B) differ from above only as follows: mantle width c. 30% ML; fin length c. 67% ML, fin width c. 74% ML. Head length c. 47% ML, head width c. 24% ML; mantle-locking cartilage c. 8% ML, funnel-locking cartilage c. 8% ML. Arms I c. 42% ML, Arms II c. 64% ML, Arms III c. 41% ML, Arms IV c. 113% ML. At ML 42 mm, 24–36 pairs of suckers present on each arm; largest sucker c. 75% arm width, appearing in pair c. 17 at c. 70% arm length in Arms II.
Paralarval specimens (ML 25 mm, C) differ from adult and ‘smaller’ specimens (as described above) only as follows: mantle width c. 40% ML; fin width c. 86% ML. Head width c. 27% ML; eye diameter c. 20% ML; mantle-locking cartilage c. 10% ML, funnel-locking cartilage c. 11% ML. Arms I c. 31% ML, Arms II c. 39% ML, Arms III c. 27% ML, Arms IV c. 102% ML; 14–35 pairs of suckers present on each arm; largest sucker c. 50% arm width, appearing in pair 15 at c. 70% arm length in Arms II.
Remarks. The two locally occurring species that most closely resemble Mg. osheai are Mp. hjorti and I. cordiformis, but they can be differentiated as follows. Magnoteuthis osheai (FL c. 64% ML, ALI4 c. 118% ML) has a relatively smaller fin and longer Arms IV than Mp. hjorti (FL, c. 89% ML, ALI4 c. 80% ML) and I. cordiformis (FL c. 81% ML, ALI4 c. 77% ML). Additionally, Mp. hjorti has two prominent eye photophores (E–F) and the funnel-locking cartilage lacks a tragus (A). Idioteuthis cordiformis reaches a larger size than Mp. osheai and the arms have trabeculate membranes.
Magnoteuthis osheai is also morphologically similar to Mg. magna, Mg. inermis Rancurel, Citation1972, Mg. ‘type beta’ (fide Young & Vecchione Citation2007), and Mg. microlucens. These species all share the same flask-shaped funnel-locking cartilage, largest arm suckers in the middle of the arms, and microscopic tentacular suckers. The distributions of these species presently appear disjunct: Mg. magna is known from the Western North Atlantic; the type locality of Mg. inermis is in the Eastern North Atlantic, south of Vridi (Abidjan, Ivory Coast); Mg. microlucens is known from the North Pacific, south of Hawaii (Young et al. Citation2008); and Mg. osheai is found in the South Pacific around New Zealand, where Young and Vecchione (Citation2007) reported a possible additional taxon, Mg. ‘type beta’. Morphologically, Mg. microlucens is distinguished from Mg. osheai by the presence of microscopic integumental photophores on the aboral side of the arms, ventral surface of funnel, and dorsal and ventral sides of the mantle, fins, and head. There are several characters that separate Mg. inermis from Mg. osheai. The fins of Mg. osheai have anterior lobes and FW c. 79% ML, whereas the fins of Mg. inermis lack anterior lobes and are FW 53% ML (Rancurel, Citation1972). Magnoteuthis inermis has more suckers on the arms (c. 150 suckers at ML 142 mm, ♀) (Rancurel Citation1972), than similar-sized specimens of Mg. osheai (c. 114–122 suckers at ML 160 mm, ♀). In addition, the largest suckers are basal in Mg. inermis and found midway along the arms in Mg. osheai. Because these differences could result from treatment history or damage, some authors consider Mg. inermis to be a junior synonym of Mg. magna (Salcedo-Vargas & Okutani Citation1994; Young & Vecchione Citation2004). Both Mg. osheai and Mg. ‘type beta’ are found at the same longitude, but Mg. ‘type beta’ is known from sub-Antarctic waters. Magnoteuthis ‘type beta’ was recognised from a single unusual specimen from the Southern Ocean, distinguished from other taxa by an apparent complete lack of chromatophores and by the dentition on the arm suckers, which have small teeth distally that appear morphologically intermediate between the smooth suckers of Mg. magna, and the broad, round teeth of Mg. microlucens (Young et al. Citation2008). It is possible that Mg. osheai and Mg. ‘type beta’ are the same species; however, all specimens examined for Mg. osheai possessed chromatophores. In Mg. magna, the inside of the mantle is darkly pigmented, while Mg. osheai lacks this pigmentation (H). The most distinctive feature of Mg. osheai is that the largest suckers are located on Arms II, and are twice as large as the largest suckers on other arms, while the largest arm suckers of Mg. magna are located on Arms IV and are only slightly larger than those on other arms (Vecchione & Young Citation2010). In addition, Mg. magna, Mg. microlucens, and Mg. osheai are genetically distinct ().
Etymology. This species is named for Dr Steve O'Shea, who has made significant contributions to cephalopod research and conservation, and inspired young taxonomists to pursue similar aspirations.
Discussion
These results suggest that New Zealand mastigoteuthid diversity is higher than has been previously reported. Checklists of local species have listed three (Spencer et al. Citation2011) or five (Spencer et al. Citation2009) species. However, in this review six species were identified: five previously named species, and one new species. In addition, some valid taxa may not yet have been recognised from this region (Braid Citation2013). Many characters were evaluated for their utility in distinguishing species. A subset of these were also deemed useful for separating genera; their systematic value is discussed below, and comparisons with other chiroteuthid families (Young Citation1991) are used to polarise character states to make inferences about higher-level relationships. The chiroteuthid families consist of the Chiroteuthidae Gray, 1849, the Mastigoteuthidae, the Joubiniteuthidae Naef, 1922, the Promachoteuthidae Naef, 1912, the Batoteuthidae Young & Roper, 1968, and the Magnapinnidae Vecchione & Young, Citation1998 (Young Citation1991). These families share morphological similarities, which has historically led to a great deal of taxonomic confusion (Young Citation1991; Braid Citation2013). These families are also genetically closely related, but the relationships within this group remain unclear (Lindgren Citation2010). The Mastigoteuthidae and the Chiroteuthidae share many morphological features such as enlarged fourth arms, ear-shaped funnel-locking cartilage, and photophores, and have, at times, been classified as a single family (see Braid et al. Citation2014). Therefore, characters are compared among the families, specifically between the Chiroteuthidae and the Mastigoteuthidae, and among locally occurring mastigoteuthids found in New Zealand waters.
Mantle and fins
The shape and musculature of the mantle and fins are variable among the chiroteuthid families, although species in this clade often have fins that are posterior to the mantle musculature (Young Citation1991). A variety of fin lengths are found in other chiroteuthid families: large fins are present in the Magnapinnidae (Vecchione & Young Citation1998) and the Promachoteuthidae (Young & Vecchione Citation2003); small fins (c. 20% ML) are present in the Batoteuthidae (Guerra et al. Citation2012); and very long, often ‘decorated’, tails are present in the Chiroteuthidae (Young & Roper Citation2011a), and the Joubiniteuthidae (Young & Roper Citation1969).
Within the Mastigoteuthidae, large fins are present and long tails are absent. In contrast, species in the Chiroteuthidae have long tails during the characteristic doratopsis stage (Young Citation1991). The morphological similarities shared between the Chiroteuthidae and the Mastigoteuthidae indicate a common ancestor, but the presence of a tail could be ancestral because it is also present in the Joubiniteuthidae, and this suggests that the lack of a tail in the Mastigoteuthidae may be derived. The presence of a secondary fin retained into adulthood in two genera in the Chiroteuthidae, Asperoteuthis Nesis, 1980 and Grimalditeuthis Joubin, 1898, has been suggested as a potential indication of a close relationship between these genera (Arkhipkin & Laptikhovsky Citation2008).
Mantle shape along with fin size and shape have some value for species identification within local mastigoteuthids. While most local mastigoteuthids have fins that are wider than long, E. famelica can be distinguished by its lanceolate fins, which are longer than wide (A). Within the Mastigoteuthidae, the lanceolate fin shape is present only in Echinoteuthis, while the fin shape varies from heart to elliptical in other mastigoteuthids. Mastigopsis hjorti and I. cordiformis are distinguished from other species by both having fins that are nearly as long as the mantle. In addition, both Mp. hjorti and I. cordiformis have similarly shaped mantles that gradually narrow towards the fin. This could suggest a common ancestor between Idioteuthis and Mastigopsis, although the mantle of I. cordiformis is widest around the viscera, whereas in Mp. hjorti it is widest at the anterior edge. However, genetically Mp. hjorti is more closely related to Magnoteuthis species () than to I. cordiformis.
Locking cartilages
The locking-cartilage morphology is quite variable within the chiroteuthid families. In general, the funnel-locking cartilage is oval in outline, often with a tragus and/or antitragus. There are three families within this clade that all have an oval funnel-locking cartilage that lacks a tragus or an antitragus: the Magnapinnidae (Vecchione & Young Citation1998), the Promachoteuthidae (Nesis Citation1987), and the Joubiniteuthidae (Nesis Citation1987). The funnel-locking cartilage of the Batoteuthidae also lacks an antitragus, but is distinctly curved (Guerra et al. Citation2012). A morphological analysis by Lindgren et al. (Citation2004) indicated a close relationship of the Chiroteuthidae, the Mastigoteuthidae, the Batoteuthidae, and the Joubiniteuthidae, which all shared similar funnel-locking cartilages. However, their combined analysis of morphology along with nuclear and mitochondrial genes resulted in polyphyly; it was noted that the family Promachoteuthidae had not been included in the analysis, which could help resolve the relationships between these families. Young and Roper (Citation1969) suggested that the funnel-locking cartilage showed a close relationship between the Chiroteuthidae, the Mastigoteuthidae, and the Promachoteuthidae.
Within the chiroteuthid families, the funnel-locking cartilage lacks a tragus and antitragus, except for some species within the Chiroteuthidae and the Mastigoteuthidae. This suggests a close relationship between these two families, and a common ancestor that also possessed funnel-locking cartilage with a tragus and antitragus. Within the Chiroteuthidae, there is a wide range of locking-cartilage morphology. In Grimalditeuthis bonplandi (Verany, 1839), paralarvae have funnel-locking cartilage with a straight groove and nose-shaped mantle-locking cartilage that fuses during maturity (Young Citation1991). Within Asperoteuthis, the funnel-locking cartilage is ear-shaped, inverted Y-shaped, or a curved groove (Arkhipkin & Laptikhovsky Citation2008). Planctoteuthis Pfeffer, 1912 species have oval funnel-locking cartilage that has an antitragus but lacks a tragus (Young Citation1972). Chiroteuthis d'Orbigny, 1841 species all have ear-shaped funnel-locking cartilage (Salcedo-Vargas Citation1996), which is similar to Mastigoteuthis and Idioteuthis. Within the Mastigoteuthidae, funnel-locking and mantle-locking cartilages can be helpful in species identification and provide additional support for the generic divisions. Mastigoteuthis and Idioteuthis have ear-shaped funnel-locking cartilage, which suggests that they have retained the potential ancestral form of funnel-locking cartilage that is common to mastigoteuthids and chiroteuthids. Although the tragus has been retained in Echinoteuthis, the antitragus has been lost (C). The unique flask-shaped funnel-locking cartilage in Magnoteuthis (A) is synapomorphic, being present only in this genus. Mastigopsis can be distinguished from the other genera in the family by its oval funnel-locking cartilage.
Among locally occurring mastigoteuthids, funnel-locking and mantle-locking cartilage is one of the most useful characters for species identification due to interspecific differences. Local species with this ear-shaped funnel-locking/nose-shaped mantle-locking cartilage arrangement are I. cordiformis, Mt. cf. dentata, and Mt. psychrophila. Mastigoteuthis psychrophila has a strong antitragus (B), which distinguishes it from Mt. cf. dentata (A). Echinoteuthis famelica also has ear-shaped funnel-locking cartilage (C), but it lacks an antitragus and the mantle-locking cartilage is C-shaped (D). Magnoteuthis osheai can be differentiated from other local mastigoteuthids by its flask-shaped funnel-locking cartilage (A). Mastigopsis hjorti can be differentiated from other local species by its oval funnel-locking cartilage (A); however, the locking cartilage of damaged/degraded specimens of other species may look approximately oval. Although this is a very useful character, it is not always reliable for species identification because the morphology of the cartilages can be damaged or degraded (e.g. I. cordiformis; A–C).
Arm suckers
Although variation was found in sucker dentition both along the length of a single arm and among arms, some consistencies were observed to be useful for species identification and separating the genera. Arm-sucker dentition has been previously suggested as a useful generic character (Salcedo-Vargas & Okutani Citation1994; Salcedo-Vargas Citation1995): the arm suckers of Mastigoteuthis and Echinoteuthis bear sharp teeth distally, while the arm suckers of Idioteuthis, Mastigopsis, and Magnoteuthis are adentate or possess blunt teeth distally. Within the chiroteuthid families, arm-sucker dentition varies from adentate to sharp teeth, and it is not clear which form is ancestral. The same range of sucker dentition found herein for mastigoteuthids is also found in the Chiroteuthidae (Young & Roper Citation2011a).
Sucker morphology can also be helpful in species identification. Sucker dentition can be used to separate Mg. osheai from the all other species in Magnoteuthis; the indistinct teeth of Mg. osheai are intermediate between the blunt teeth of Mg. microlucens and the adentate suckers of Mg. magna. In addition, a smooth, unstructured band, between the teeth and polygonal processes, was only found in I. cordiformis (). The number of papillated rings varies both within and among species, but Mp. hjorti has only three or four (), while other species have up to eight (e.g. I. cordiformis; ). However, the number of papillated rings is also known to vary ontogenetically (Salcedo-Vargas Citation1995). Therefore, arm-sucker ultrastructure seems useful for species-level identification in some genera, but appears less useful for higher taxonomy within the Mastigoteuthidae.
Tentacles
The chiroteuthid families are united by the absence of a primary tentacle club, and presence of a secondary tentacle club that is straight and unexpanded (Young Citation1991), except Idioteuthis. Young et al. (Citation1998) suggested that the tentacles in these families have undergone radial modification. The tentacles of the Magnapinnidae, in contrast to the fragile mastigoteuthid tentacles, are more robust than the arms and have eight series of suckers (Vecchione & Young Citation1998). Similarly, the Promachoteuthidae also have robust tentacles with 6–20 series of suckers (Nesis Citation1987). The tentacles of the Joubiniteuthidae are laterally compressed, with a protective membrane distally, and 8–12 series of suckers (Young & Roper Citation1969). Similar to the morphological changes that occur in the Chiroteuthidae during development, the tentacles of the Batoteuthidae change drastically during ontogeny and have suckers in six series (Guerra et al. Citation2012).
Although there appear to be many morphological similarities between the Chiroteuthidae and the Mastigoteuthidae, their tentacle morphology is quite different. The Chiroteuthidae have a tentacle club with up to six series of suckers, bordered by wide protective membranes (Young & Roper Citation2011a). In contrast, all mastigoteuthid species have a tentacle club with multiple irregular series of suckers and greatly reduced protective membranes. The tentacles of the Chiroteuthidae undergo drastic changes during ontogeny, and the adult club develops along the stalk of the tentacle, while the larval club is either lost or resorbed (Young et al. Citation1998). The tentacular suckers of Chiroteuthis (Young et al. Citation1998) and Asperoteuthis (Young et al. Citation2007) species are attached by long stalks, and there are photophores along the length of the stalk and at the distal tip of the tentacle. The proximal half of the tentacles in Asperoteuthis lack suckers (Young et al. Citation1998), while Grimalditeuthis tentacles lack suckers entirely and are used as a lure to attract prey (Hoving et al. Citation2013).
Tentacles and tentacle-sucker morphology have been relied on in the past for mastigoteuthid taxonomy and identification. Locally, however, these characters are generally unreliable for species identification because tentacles are usually lost during capture. Out of the six species examined here, tentacles were only available for three, and then only from a single specimen (except I. cordiformis, where most specimens retain their tentacles); of these, only two possessed intact tentacular sucker rings. Despite the degraded nature of the tentacular sucker rings, the microscopic size of Mg. osheai sucker rings (A) distinguishes this species from other local mastigoteuthids, and unites it with other species in Magnoteuthis. Unlike other mastigoteuthid genera, Idioteuthis has an expanded tentacle club and large proximal suckers (B). The large proximal suckers closely resemble arm suckers, with several rows of flat polygonal processes, and teeth on the infundibular ring. Distally, the sucker rings are minute and similar to the tentacle suckers of other mastigoteuthids (E–H). Unlike I. cordiformis, the tentacle suckers of Mt. psychrophila (A–D) are adentate.
Salcedo-Vargas (Citation1995) proposed that ‘cushions’ be included in evaluations of sucker ultrastructure. He recognised two types of cushions, the ‘spongy type’ and the ‘horny type’. Herein, the horny type was observed only in I. cordiformis tentacle suckers (F–H) and the arm suckers of larger specimens. It is possible that these cushions are, as proposed by Young & Vecchione (Citation2004) for the ‘irregular horny lumps’ described for the sucker rings of I. latipinna by Sasaki (Citation1929), a preservation artefact. However, Salcedo-Vargas (Citation1995) argued that other scientists have overlooked cushions, considering them pathological malformations, when they are an adaptation to deep-sea life. Cushions were found in all large I. cordiformis specimens examined herein. Four specimens examined before preservation also had uneven, potentially degraded suckers, indicating that this malformation is not necessarily caused by preservation, but may be due to either freezing, or the ammonia in the arm tissue; or they may truly represent an adaption as suggested by Salcedo-Vargas (Citation1995).
Beaks
Both upper and lower beaks of I. cordiformis displayed extreme variation (–27), even in individuals that were genetically identical (). Upper beaks appear to have limited use for identifying species because of the lack of clearly distinguishing characteristics, combined with high intraspecific variation; those of some I. cordiformis individuals have a lateral groove, which is absent in others regardless of sex or size; the same variability was found in Mg. osheai. The lower beaks of I. cordiformis show great variation in the jaw edge and shoulder in particular, which does not appear to be correlated with growth or sex (). Locally, variation has been found in the beaks of another species in the chiroteuthid families, Chiroteuthis veranyi Ferussac, 1835 (Mensch Citation2010). Similarly, Voss (Citation1977) found that intraspecific variability can exceed interspecific variability in cephalopods, which may limit beak value for species identification.
Variation in beak morphology has implications in particular for ecological studies that rely on morphology to identify species from stomach contents. Intraspecific variation was also found in the lower beaks of Mt. cf. dentata (). However, the beaks of I. cordiformis (above at least ML 181 mm, the smallest specimen observed herein), due to their large size, are not likely to be confused with those of any other mastigoteuthid. Locally, the beaks of I. cordiformis are most similar to those of Mg. osheai, but they are easily distinguished even at small sizes by the larger rostral-length-to-wing-length ratio in Mg. osheai, and the higher beak-height-to-baseline ratio in I. cordiformis. Other large squid have been identified from stomach contents of sperm whales stranded in New Zealand waters (Architeuthis dux Steenstrup, 1857, Kondakovia longimana Filippova, 1972, Lepidoteuthis grimaldii Joubin, 1895, Mesonychoteuthis hamiltoni Robson, 1925, and Taningia danae Joubin, 1931 [Gomez-Villota, 2007]), but I. cordiformis beaks can be readily distinguished from the beaks of these species based on morphological differences.
Radulae
Herein, asymmetry of the radula has been found in individuals of half of the species examined. In Mt. cf. dentata, the first lateral tooth was bicuspid on one side and unicuspid on the other (C–D). A similar morphology was found in one individual of Mp. hjorti (B); however, in addition to the asymmetry of the first lateral tooth, beside the unicuspid tooth, there was an additional series of small, elongate teeth with two or three irregular cusps (C–D). In contrast, one individual of Mt. psychrophila had a fusion of the first and second lateral teeth on one side (C–E). This minor and non-directional deviation from symmetry in a bilaterally symmetrical animal is known as fluctuating asymmetry (Palmer & Strobeck Citation1986), which is an epigenetic process that is induced by environmental and genetic stress (Parsons Citation1992). Jokela et al. (Citation2001) conducted a study on fluctuating asymmetry in the radulae of freshwater snails (Potamopyrgus antipodarum Gray, 1843). Measuring both sides of the middle radular teeth, they found that the level of fluctuating asymmetry was higher within populations than among populations. In another gastropod genus, Peristernia Mörch, 1852, Taylor & Lewis (Citation1995) found radular tooth variation among rows on the same radula, and suggested that it may be a form of intra-individual fluctuating asymmetry. Similarly, some taxa in the Octopodidae d'Orbigny, 1839 show asymmetry of the lateral cusps of the rachidian tooth, or serial repetition of asymmetry between rows (Nixon Citation1998).
All species examined herein (excluding the asymmetrical radula of a single Mp. hjorti specimen with an additional series) had seven series of teeth, and tricuspid rachidians. Variation among species was found in the tooth shape and relative length, and relative cusp length. Marginal plates were variably present among, but consistent within, genera: absent in Mt. cf. dentata and Mt. psychrophila; present in I. cordiformis and Mg. osheai; and poorly defined in Mp. hjorti. Although differences in the morphology of the radulae of all species were found, due to limited material, the consistency of the radula morphology and occurrence of asymmetry in the Mastigoteuthidae remains unknown. Voss (Citation1977) found great variation in the number and morphology of radular teeth within a single species of octopus, and suggested that there is limited value in the radula for species identification. Therefore, intraspecific variation as well as fluctuating asymmetry in the mastigoteuthid radula should be investigated in the future.
Gladius structure
It appears that the ancestral form of the chiroteuthid family gladius was long and narrow because this trait is present in the Magnapinnidae (Vecchione & Young Citation1998), the Promachoteuthidae (Young & Vecchione Citation2003), the Batoteuthidae (Guerra et al. Citation2012), the Chiroteuthidae (Young & Roper Citation2011a), and the Joubiniteuthidae (Young & Roper Citation1969). Therefore, it is likely that the most recent common ancestor between the Chiroteuthidae and the Mastigoteuthidae had a long, narrow gladius, as is found in Mastigoteuthis and Echinoteuthis, which each have a narrow gladius (width c. 3% GL) and a long secondary conus (c. 50% GL). The gladius structure of Idioteuthis, Mastigopsis, and Magnoteuthis appears to be more derived and suggests that they may have a more recent common ancestor; they have a wider gladius (c. 10% GL) and a shorter secondary conus (c. 20–35% GL).
The structure of the gladius is also very useful in differentiating some species. Among local mastigoteuthids, the gladius structure, and especially the cross sections, can be used for species identification. Although I. cordiformis, Mp. hjorti, and Mg. osheai all have a broad gladius, they are all distinct in cross section. The rachis of I. cordiformis thickens into a square keel (E), while that of Mp. hjorti is rounded (G), and Mg. osheai does not appear to have a thickened keel (F). Unfortunately, the differences in gladius structure of Mg. osheai and the closely related species Mg. magna and Mg. microlucens could not be compared because sufficient material was not available. Therefore, although the gladius can be used locally for some identifications, it is not known whether any two closely related species can be differentiated in this way. Less variation was observed in the gladii of Mastigoteuthis species, although, in cross-section, the rostrum of Mt. cf. dentata appears approximately triangular with rounded corners (F), whereas in Mt. psychrophila it is teardrop shaped (G). Overall, they share a similar structure, which may limit the use of this character for species-level identification within this genus.
Photophores
Photophores are variably present in the chiroteuthid families (Lindgren et al. Citation2012). Several of the chiroteuthid families presently appear to lack photophores entirely: the Magnapinnidae (Vecchione & Young Citation1998), the Promachoteuthidae (Young & Vecchione Citation2003), and the Joubiniteuthidae (Young & Roper Citation1969). The single batoteuthid species, Batoteuthis skolops Young & Roper, 1968, has photophores only on the tips of Arms IV (Guerra et al. Citation2012). Within the Chiroteuthidae, photophores appear absent in Planctoteuthis (Young Citation1972) and Grimalditeuthis bonplandi, except for the arm tips on mature G. bonplandi females (Young & Roper Citation2011b). Chiroteuthis species are distinguished from other chiroteuthids by the presence of Arm IV photophores (Young Citation1972). Eye, tentacle, and tentacle-stalk photophores are present in both Asperoteuthis (Young et al. Citation2007) and Chiroteuthis (Roper & Young Citation2013).
Some mastigoteuthids have integumental, eye-sinus, and/or eye photophores, whereas photophores appear entirely absent in others. The presence of similar types of photophores may indicate a recent common ancestor between some species, while differences in photophore structure may indicate that they arose independently. Lindgren et al. (Citation2012) suggested that photophores were likely present in a common ancestor of pelagic squids, but are rapidly lost and gained. Integumental photophores are present in Mg. microlucens (but appear absent from all other Magnoteuthis species), all Mastigoteuthis species, and Mr. pyrodes (the sole species in this genus). However, these three groups have different photophore sizes and structures, which could indicate that they arose independently, or were present in a distant common ancestor, lost in the most recent common ancestor for this family, and then redeveloped in some lineages. Although the distribution of integumental photophores is similar in Mastigoteuthis and Mastigotragus—with photophores appearing on all external surfaces of the mantle, head, fins, funnel, and aboral surface of arms—the integumental photophores of Mr. pyrodes are morphologically distinct. Mastigoteuthis species have vacuole-like cells surrounding the photocytes, which are absent in Mr. pyrodes (Young et al. Citation2014). Eye photophores are present in Idioteuthis and Mastigopsis. As eye photophores are also present in some chiroteuthid species, this could indicate that their most recent common ancestor possessed eye photophores, which were then lost in some genera and species. The presence of eye-sinus photophores indicates a recent common ancestor shared between Mastigoteuthis, Echinoteuthis, and Mastigotragus because these structures are absent in Idioteuthis, Mastigopsis, and Magnoteuthis. However, the eye-sinus photophores in Echinoteuthis and Mastigotragus are relatively large compared with those in Mastigoteuthis.
Local mastigoteuthid species can be distinguished based on photophore location, size, and patterns. Eye photophores are found in two forms in mastigoteuthids: hemispherical (Mp. hjorti, I. okutanii, fide Salcedo-Vargas Citation1997) and a photogenic patch (I. cordiformis). Although Salcedo-Vargas (Citation1993) first recognised the presence of the photophore on the eye of I. cordiformis, its presence has not been acknowledged by subsequent authors (e.g. Young & Vecchione Citation2014). However, the presence of the I. cordiformis eye photophore was supported herein with histology (). The relatively large size of the eye-sinus photophore can be used to distinguish E. famelica from locally occurring Mastigoteuthis species, which have integumental photophores of a similar size to their eye-sinus photophore. Locally, integumental photophores are present in Mt. cf. dentata and Mt. psychrophila. The photophore pattern on Arms IV can be used to differentiate Mt. cf. dentata (E) and Mt. psychrophila (A). Unfortunately, photophore presence and location should not be heavily relied upon for species identification because mastigoteuthids are so frequently damaged during capture.
Skin tubercles
Most squid species have smooth skin, lacking dermal structures; however, dermal structures with a wide variety of tissue types have been reported within some cephalopod taxa (Roper & Lu Citation1990). Within the chiroteuthid families, skin tubercles have been reported in the Chiroteuthidae and the Mastigoteuthidae (Roper & Lu Citation1990). Dermal structures can be used to determine relationships within families, such as within the Mastigoteuthidae; the skin tubercles of both Idioteuthis and Mastigopsis are conical tubercles of elastic or fibrocartilage tissue with overlying epidermis (Roper & Lu Citation1990). In contrast, the skin tubercles found in A. acanthoderma (Lu, 1977) are morphologically distinct from those found in the mastigoteuthids, and are composed of hyaline-like tissue (Roper & Lu Citation1990).
Within the Mastigoteuthidae, skin tubercles have been used in the past for classifications. These structures are present in Idioteuthis, Mastigopsis, mature Mastigotragus, and juvenile Echinoteuthis. The genus Echinoteuthis was originally distinguished from Mastigoteuthis based on the presence of skin tubercles. The classification by Salcedo-Vargas & Okutani (Citation1994) used skin-tubercle morphology to distinguish four subgenera within the Mastigoteuthidae and included E. famelica within Idioteuthis due to the presence of tubercles. However, the skin tubercles present in Echinoteuthis are morphologically unique because they are small and multicuspid (Young Citation1991). In Mastigotragus, tubercles have only been reported in mature females (mature males are unknown) and occur on all external surfaces and appear small and rounded (Young & Vecchione Citation2015), which is distinct from the tubercles of other mastigoteuthids.
Locally, three species have tubercles: I. cordiformis (), Mp. hjorti (G), and E. famelica. Skin tubercles appear absent from species of Magnoteuthis and Mastigoteuthis. The similar structure of Idioteuthis and Mastigopsis tubercles may indicate a recent common ancestor; however, it appears that the skin tubercles present in other genera arose independently based on their different morphology. Skin tubercles can be helpful during species identification within New Zealand waters, but due to ontogenetic changes and the damaged nature of most specimens their value is limited.
Conclusion
The mastigoteuthid fauna of New Zealand appears to comprise five genera: Mastigoteuthis, Idioteuthis, Mastigopsis, Echinoteuthis, and Magnoteuthis. This has been supported by the present, and also previous, genetic analysis (Braid et al., Citation2014); further comparison among the morphological characters of these genera, and those of other chiroteuthid families, also supports this conclusion. It seems likely that the most recent common ancestor for the Chiroteuthidae and the Mastigoteuthidae possessed integumental photophores, eye photophores, ear-shaped funnel-locking cartilage, and a narrow gladius, and lacked skin tubercles. It is likely that integumental photophores were lost early in the evolution of the Mastigoteuthidae, and subsequently redeveloped in some genera. Some character states show a close relationship between Idioteuthis, Mastigopsis, and Magnoteuthis: broad gladius, absence of eye-sinus photophore, absence of funnel pocket, and arm suckers that are adentate or possess blunt teeth distally. In contrast, Echinoteuthis and Mastigoteuthis share the following characters: narrow gladius, presence of an eye-sinus photophore, presence of a funnel pocket, and arm suckers with sharp teeth distally. Therefore, it is possible that a recent common ancestor was shared by Idioteuthis, Mastigopsis, and Magnoteuthis, and another by Mastigoteuthis and Echinoteuthis.
New Zealand waters host a high diversity of mastigoteuthids. Five previously known species have been identified: Mt. cf dentata, Mt. psychrophila, I. cordiformis, Mp. hjorti, and E. famelica. In addition, a new species, Mg. osheai, has been identified and described herein. These species have been identified using a combination of genetic data () and morphological characters (), and can be reliably distinguished using the latter. Tentacles and photophore pattern, which have been heavily relied upon in the past for species identification, are the two characters most often damaged during capture. Herein, beak morphology and funnel-locking cartilage have also been found to be helpful for species identifications, although some intraspecific variation was observed.
Associate Editor: Dr Rob Cruickshank.
Acknowledgements
We would like to thank Steve O'Shea for taxonomic advice. We are very grateful to: the Museum of New Zealand Te Papa Tongarewa, particularly Bruce Marshall; the National Institute of Water and Atmospheric Research, Ltd. (NIWA), particularly Sadie Mills, Kareen Schnabel, Diana Macpherson, and Dean Stotter for access to specimens; and to Museum Victoria, particularly Chris Rowley and Melanie Mackenzie. Thanks to Darren Stevens and the crew of the RV Tangaroa for the specimens of Mastigoteuthis cf. dentata used for genetic analysis. Thanks to Dick Young, Mike Vecchione, and Annie Lindgren for their contributions of other sequences to GenBank used herein. Many thanks to Paul McBride for assistance with phylogenies. We would like to thank the Auckland University of Technology (AUT) for funding assistance with this research. We would like to thank Adrian Turner at the Auckland University for assistance with critical-point drying, and Patrick Conor at AUT for platinum plating, and imaging assistance. Histology would not have been possible without Adam Rusk and Karin Lohrmann. From AUT, many thanks to Aaron Boyd Evans, Jesse Kelly, and Bríd Lorigan. Many thanks to the anonymous reviewers for their helpful comments. We are grateful to Bob Hanner at the University of Guelph, for laboratory space. And to the squids that were used in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adam W 1954. Cephalopoda. 3E Partie. IV—Céphalopodes à l'exclusion des genres Sepia, Sepiella et Sepioteuthis. Siboga Expedition 55: 121–199.
- Arkhipkin AI, Laptikhovsky VV 2008. Discovery of the fourth species of the enigmatic chiroteuthid squid Asperoteuthis (Cephalopoda: Oegopsida) and extension of the range of the genus to the South Atlantic. Journal of Molluscan Studies 74: 203–207. doi: 10.1093/mollus/eyn007
- Berry SS 1909. Diagnoses of new cephalopods from Hawaiian Islands. Proceedings of the United States National Museum 37: 407–419. doi:10.5479/si.00963801.37-1713.407
- Berry SS 1920. Preliminary diagnosis of new cephalopods from the western Atlantic. Proceedings of the United States National Museum 58: 293–300. doi: 10.5479/si.00963801.58-2335.293
- Bester AJ, Priddel D, Klomp NI 2010. Diet and foraging behaviour of the Providence petrel Pterodroma solandri. Marine Ornithology 39: 163–172.
- Bolstad KSR 2010. Systematics of the Onychoteuthidae Gray, 1847 (Cephalopoda: Oegopsida). Zootaxa 9626: 1–186.
- Braid HE 2013. Systematics and ecology of the New Zealand Mastigoteuthidae (Cephalopoda, Oegopsida). MAppSc thesis. Auckland, Auckland University of Technology.
- Braid HE, McBride PD, Bolstad KSR 2014. Molecular phylogenetic analysis of the squid family Mastigoteuthidae (Mollusca, Cephalopoda) based on three mitochondrial genes. Hydrobiologia 725: 145–164. doi: 10.1007/s10750-013-1775-3
- Chun C 1908. Über Cephalopoden der Deutschen Tiefsee-Expedition. Zoologischer Anzeiger 33: 86–89.
- Chun C 1910. Die cephalopoden. I. Oegopsida. Wissenschaftliche Ergebnisse der Deutschen Tiefsee-Expedition auf dem Dampfer ‘Valdivia’ 1898–1899. [English translation] Israel Program for Scientific Translations, 1975 18. 552 p. + 92 plates.
- Chun C 1913. Cephalopoda. Report on the Scientific Results of the ‘Michael Sars’ North Atlantic Deep-Sea Expedition, 1910 3: 1–28.
- Clarke MR 1980. Cephalopoda in the diet of sperm whales of the southern hemisphere and their bearing on sperm whale biology. Discovery Reports 37: 1–324.
- Clarke MR 1986. A handbook for the identification of cephalopod beaks. Oxford: Clarendon Press. 220 p.
- Clarke MR, Roper CFE 1998. Cephalopods represented by beaks in the stomach of a sperm whale stranded at Paekakariki, North Island, New Zealand. South African Journal of Marine Science 20: 129–133. doi: 10.2989/025776198784126601
- Cruz JB, Lalas C, Jillett JB, Kitson JC, Lyver POB, Imber M et al. 2001. Prey spectrum of breeding sooty shearwaters (Puffinus griseus) in New Zealand. New Zealand Journal of Marine and Freshwater Research 35: 817–829. doi: 10.1080/00288330.2001.9517044
- Degner E 1925. Cephalopoda. Report on the Danish Oceanographical Expeditions 1908–1910 to the Mediterranean and Adjacent Seas 2: 1–94.
- Dell RK 1959. Some additional New Zealand cephalopods from Cook Strait. Zoology Publications from Victoria University of Wellington 25: 1–12.
- Freeman DJ, Marshall BA, Anyong ST, Wing SR, Hitchmough RA 2010. Conservation status of New Zealand marine invertebrates, 2009. New Zealand Journal of Marine and Freshwater Research 44: 129–148. doi: 10.1080/00288330.2010.495373
- Gómez-Villota F 2007. Sperm whale diet in New Zealand. MAppSc thesis. Auckland, Auckland University of Technology. 242 p.
- Grimpe G 1922. Systematische Übersicht der europäischen Cephalopoden. Sitzungsberichte der Naturforschenden Gesellschaft zu Leipzig 45: 36–52.
- Guerra Á, Portela JM, Roura Á, Río JLD, Vecchione M 2012. Morphological variability of the rare bush-club squid Batoteuthis (Cephalopoda, Batoteuthidae)? Neues Jahrbuch für Geologie und Paläontologie-Abhandlungen 266: 77–83. doi: 10.1127/0077-7749/2012/0267
- Horn PL, Dunn MR 2010. Inter-annual variability of the diets of hoki, hake, and ling on the Chatham Rise from 1990 to 2009. Wellington, Ministry of Fisheries. 57 p.
- Hoving HJT, Zeidberg LD, Benfield MC, Bush SL, Robinson BH, Vecchione M 2013. First in situ observations of the deep-sea squid Grimalditeuthis bonplandi reveal unique use of tentacles. Proceedings of the Royal Society B: Biological Sciences 280: 1–5. doi: 10.1098/rspb.2013.1463
- Hoyle WE 1904. Reports on the Cephalopoda. Bulletin of the Museum of Comparative Zoology, Harvard 43: 1–72.
- James GD, Stahl JC 2000. Diet of southern Buller's albatross (Diomedea bulleri bulleri) and the importance of fishery discards during chick rearing. New Zealand Journal of Marine and Freshwater Research 34: 435–454. doi: 10.1080/00288330.2000.9516946
- Jokela J, Niederegger S, Negovetic S, Mutikainen P 2001. Mode of reproduction, ploidy and fluctuating asymmetry: comparison of co-existing sexual and asexual freshwater snails. Evolutionary Ecology Research 3: 969–984.
- Joubin L 1895. Contribution à l'étude des céphalopodes de l'Atlantique nord. Résultats des Campagnes Scientifiques Accomplies sur Yacht par Albert 1er Prince Souverain de Monaco 9: 1–63.
- Joubin L 1913. Éstudes préliminaires sur les céphalopodes recueillis au cours des croisières de S.A.S. le Prince de Monaco. 3e Note: Mastigoteuthis magna nov. sp. Bulletin de l'Institut Océanographique 275: 1–11.
- Joubin L 1933. Notes préliminaires sur les Céphalopodes des croisières du Dana (1921–1922), 4e Partie. Annals de l'Institut Océanographiques 13: 1–49.
- Katoh K, Misawa K, Kuma K, Miyata T 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. doi: 10.1093/nar/gkf436
- Lanfear R, Calcott B, Ho SYW, Guindon S 2012. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29: 1695–1701. doi: 10.1093/molbev/mss020
- Lindgren AR 2010. Molecular inference of phylogenetic relationships among Decapodiformes (Mollusca: Cephalopoda) with special focus on the squid Order Oegopsida. Molecular Phylogenetics and Evolution 56: 77–90. doi: 10.1016/j.ympev.2010.03.025
- Lindgren AR, Giribet G, Nishiguchi MK 2004. A combined approach to the phylogeny of Cephalopoda (Mollusca). Cladistics 20: 454–486. doi: 10.1111/j.1096-0031.2004.00032.x
- Lindgren AR, Pankey MS, Hochberg FG, Oakley TH 2012. A multi-gene phylogeny of Cephalopoda supports convergent morphological evolution in association with multiple habitat shifts in the marine environment. BMC Evolutionary Biology 12: 129.
- Lormann KB 2008. Subcutaneous photophores in the jumbo squid Dosidicus gigas d'Orbigny, 1835) (Cephalopoda: Ommastrephidae). Revista de Biología Marina y Oceanografía 43: 275–284.
- Mattern T, Houston DM, Lalas C, Setiawan AN, Davis LS 2009. Diet composition, continuity in prey availability and marine habitat—keystones to population stability in the Snares Penguin (Eudyptes robustus). Emu 109: 204–213. doi: 10.1071/MU08001
- Mensch R 2010. A systematic review of the squid genus Chiroteuthis (Mollusca: Cephalopoda) in New Zealand waters. MAppSc thesis. Auckland, Auckland University of Technology. 121 p.
- Nesis KN 1977. Mastigoteuthis psychrophila sp. n. (Cephalopoda, Mastigoteuthidae) from the Southern Ocean. Zoologichesky Zhurnal 56: 835–842.
- Nesis KN 1980. Taxonomic position of Chiroteuthis famelica Berry (Cephalopoda, Oegopsida). Byulleten Moskovskogo Obshchestva Ispytatelei Prirody (Otdel Biologicheskii) 85: 59–66.
- Nesis KN 1987. Cephalopods of the world [English translation]. Neptune City, NJ, Tropical Fish Hobbyist (T.F.H.) publications. 352 p.
- Nigmatullin CM, Nesis KN, Arkhipkin AI 2001. A review of the biology of the jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae). Fisheries Research 54: 9–19. doi: 10.1016/S0165-7836(01)00371-X
- Nixon M 1998. The radulae of cephalopoda. Smithsonian Contributions to Zoology 586: 39–53.
- Palmer AR, Strobeck C 1986. Fluctuating asymmetry: measurement, analysis, patterns. Annual Review of Ecology and Systematics 17: 391–421. doi: 10.1146/annurev.es.17.110186.002135
- Parsons PA 1992. Fluctuating asymmetry: a biological monitor of environmental and genomic stress. Heredity 68: 361–364. doi: 10.1038/hdy.1992.51
- Pfeffer G 1900. Synopsis der oegopsiden Cephalopoden. Mitteilungen aus dem Naturhistorischen Museum Hamburg 17: 147–198.
- Rancurel P 1972. Mastigoteuthis inermis espèce nouvelle de Chiroteuthidae du Golfe de Guinée (Cephalopoda, Oegopsida). Extrait du Bulletin de la Société Zoologique de France 97: 25–34.
- Rancurel P 1973. Mastigoteuthis hjorti Chun 1913 description de trios échantillons provenant du Golfe de Guiée (Cephalopoda, Oegopsida). Cahiers ORSTOM. Série Océanographie 11: 27–32.
- Ratnasingham S, Hebert PDN 2007. BOLD: the Barcode of Life Data System (http://www.barcodinglife.org). Molecular Ecology Notes 7: 355–364. doi: 10.1111/j.1471-8286.2007.01678.x
- Ratnasingham S, Hebert PDN 2013. A DNA-based registry for all animal species: the barcode index number (BIN) system. PLoS One 8: e66213. doi: 10.1371/journal.pone.0066213
- Roper CFE, Lu CC 1990. Comparative morphology and function of dermal structures in oceanic squids (Cephalopoda). Smithsonian Contributions to Zoology 493: 1–40. doi: 10.5479/si.00810282.493
- Roper CFE, Voss GL 1983. Guidelines for taxonomic descriptions of cephalopod species. Memoirs of the National Museum Victoria 44: 48–63.
- Roper CFE, Young RE 2013. Chiroteuthis Orbigny, 1841. Tree of Life web project. http://tolweb.org/Chiroteuthis/19462 (accessed 28 September 2013).
- Rosecchi E, Tracey DM, Webber WR 1988. Diet of orange roughy, Hoplostethus atlanticus (Pisces: Trachichthyidae) on the Challenger Plateau, New Zealand. Marine Biology 99: 293–306. doi: 10.1007/BF00391992
- Salcedo-Vargas MA 1993. Revision of the squid family Mastigoteuthidae (Mollusca: Cephalopoda) from the Northwest Pacific. PhD thesis. Tokyo, Japan, Tokyo University of Fisheries. 253 p.
- Salcedo-Vargas MA 1995. Systematic value of the ultrastructure of the sucker surface in the squid family Mastigoteuthidae (Mollusca: Cephalopoda). Contributions to Zoology 65: 65–77.
- Salcedo-Vargas MA 1996. Cephalopods from the Netherlands Indian Ocean Programme (NIOP)—1. Chiroteuthis spoeli n. spec. and Chiroteuthis picteti somaliensis n. subspec. Beatfortia 46: 11–26.
- Salcedo-Vargas MA 1997. Cephalopods from the Netherlands Indian Ocean Programme (NIOP)-II. Mastigoteuthid lineage and related forms. Beaufortia 47: 91–108.
- Salcedo-Vargas MA, Okutani T 1994. New classification of the squid family Mastigoteuthidae (Cephalopoda: Oegopsida). Venus 53: 119–127.
- Salcedo-Vargas MA, Young RE 2014. Echinoteuthis tyroi Salcedo-Vargas, 1997. http://tolweb.org/Echinoteuthis_tyroi/19526 (accessed 9 March 2015).
- Sasaki M 1916. Notes on oegopsid cephalopods found in Japan. Annotationes Zoologicae Japonenses 9: 89–120.
- Sasaki M 1929. A monograph of the dibranchiate cephalopods of the Japanese and adjacent waters. Journal of the College of Agriculture 20: 1–357.
- Spencer HG, Marshall BA, Willan RC 2009. Phylum Mollusca: clams, snails, chitons, squids, octopuses and their kin. In: Gordon DP ed. The New Zealand inventory of biodiversity: a species 2000 symposium review. Christchurch, University of Canterbury Press. Pp. 196–219.
- Spencer HG, Willan RC, Marshall BA, Murray TJ 2011. Checklist of the recent Mollusca recorded from the New Zealand exclusive economic zone. http://www.molluscs.otago.ac.nz/ (accessed 30 April 2013).
- Tamura K, Nei M 1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution 10:512–526.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S 2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. doi: 10.1093/molbev/mst197
- Taylor JD, Lewis A 1995. Diet and radular morphology of Peristernia and Latirolagena (Gastropoda: Fasciolariidae) from Indo-Pacific coral reefs. Journal of Natural History 29: 1143–1154. doi: 10.1080/00222939500770481
- Vecchione M, Roper CFE, Widder EA, Frank TM 2002. In situ observations on three species of large-finned deep-sea squids. Bulletin of Marine Science 71: 893–901.
- Vecchione M, Roper CF, Sweeney MJ, Lu CC 2001. Distribution, relative abundance and developmental morphology of paralarval cephalopods in the Western North Atlantic Ocean. NOAA Technical Report NMFS 152. A scientific paper of the Fishery Bulletin. Seattle, WA, US Department of Commerce. 52 p.
- Vecchione M, Young RE 1998. The Magnapinnidae, a newly discovered family of oceanic squid (Cephalopoda: Oegopsida). South African Journal of Marine Science 20: 429–437. doi: 10.2989/025776198784126340
- Vecchione M, Young RE 2006. Mastigoteuthis agassizii: type description with notes on the syntype and squid from type locality. http://tolweb.org/notes/?note_id=4004 (accessed 4 September 2014).
- Vecchione M, Young RE 2007a. Mastigoteuthis agassizii Verrill, 1881. http://tolweb.org/Mastigoteuthis_agassizii/19508 (accessed 16 April 2013).
- Vecchione M, Young RE 2007b. Mastigoteuthis dentata Hoyle, 1904. http://tolweb.org/Mastigoteuthis_dentata/19512 (accessed 16 April 2013).
- Vecchione M, Young RE 2007c. Mastigoteuthis hjorti Chun, 1913. http://tolweb.org/Mastigoteuthis_hjorti/19517 (accessed 17 May 2013).
- Vecchione M, Young RE 2007d. Mastigoteuthis atlantica Joubin, 1933. http://tolweb.org/Mastigoteuthis_atlantica/19509/2007.11.19 (accessed 5 April 2013).
- Vecchione M, Young RE 2007e. Mastigoteuthis danae (Joubin, 1933). http://tolweb.org/Mastigoteuthis_danae/19511/2007.11.19 (accessed 5 April 2013).
- Vecchione M, Young RE 2010. Mastigoteuthis magna: description continued. http://tolweb.org/notes/?note_id=3917 (accessed 5 April 2013).
- Vecchione M, Young RE, Lindgren A 2007. Mastigoteuthidae Verrill, 1881. Mastigoteuthis Verrill, 1881. Whip-lash squid. http://tolweb.org/Mastigoteuthis/19453/2007.11.19 (accessed 5 April 2013).
- Verrill AE 1881. Report on the cephalopods and on some additional species dredged by the U.S. Fish Commission Steamer ‘Fish-hawk’, during the season of 1880. Bulletin of the Museum of Comparative Zoology, Harvard 8: 99–116.
- Voss GL 1963. Cephalopods of the Philippine Islands. United States National Museum Bulletin 234: 1–180. doi: 10.5479/si.03629236.234.1
- Voss GL 1977. Present status and new trends in cephalopod systematics. Symposia of the Zoological Society of London 38: 49–60.
- West JA, Imber MJ 1986. Some foods of Buller's mollymawk Diomedea bulleri. New Zealand Journal of Zoology 13: 169–174. doi: 10.1080/03014223.1986.10422659
- Young RE 1972. The systematic and areal distribution of pelagic cephalopods from the seas off southern California. Smithsonian Contributions to Zoology 97: 1–159. doi: 10.5479/si.00810282.97
- Young RE 1991. Chiroteuthid and related paralarvae from Hawaiian waters. Bulletin of Marine Science 49: 162–185.
- Young RE 2010. Mastigoteuthis famelica (Berry, 1909). http://tolweb.org/Mastigoteuthis_famelica/19513 (accessed 15 April 2013).
- Young RE, Lindgren A, Vecchione M 2008. Mastigoteuthis microlucens, a new species of the squid family Mastigoteuthidae (Mollusca: Cephalopoda). Proceedings of the Biological Society of Washington 121: 276–282. doi: 10.2988/07-40.1
- Young RE, Roper CFE 1969. A monograph of the Cephalopoda of the North Atlantic: the family Joubiniteuthidae. Smithsonian Contributions to Zoology 15: 1–10.
- Young RE, Roper CFE 2011a. Chiroteuthidae Gray, 1849. http://tolweb.org/Chiroteuthidae/19451/2011.11.22 (accessed 5 April 5 2013).
- Young RE, Roper CFE 2011b. Grimalditeuthis Joubin, 1898. Grimalditeuthis bonplandi (Verany, 1839). http://tolweb.org/Grimalditeuthis/19463 (accessed 28 September 2013).
- Young RE, Vecchione M 2003. Promachoteuthidae Naef, 1912. Promachoteuthis Hoyle, 1885. http://tolweb.org/Promachoteuthidae/19454 (accessed 28 September 2013).
- Young RE, Vecchione M 2004. Mastigoteuthidae: doubtful and questionable species. http://tolweb.org/notes/?note_id=2643 (accessed 15 April 2013).
- Young RE, Vecchione M 2007. Mastigoteuthis type beta. http://tolweb.org/Mastigoteuthis_type_beta/65357 (accessed 15 April 2013).
- Young RE, Vecchione M 2014. Idioteuthis Sasaki, 1916. Idioteuthis cordiformis (Chun, 1908). http://tolweb.org/Idioteuthis_cordiformis/19510 (accessed 9 March 2015).
- Young RE, Vecchione M 2015. Mastigotragus pyrodes (Young, 1972). http://tolweb.org/Mastigotragus_pyrodes/19523 (accessed 9 March 2015).
- Young RE, Vecchione M, Braid HE 2014. Mastigotragus, a new generic name for Mastigoteuthis pyrodes Young, 1972 (Cephalopoda: Mastigoteuthidae). European Journal of Taxonomy 105: 1–6.
- Young RE, Vecchione M, Donovan DT 1998. The evolution of coleoid cephalopods and their present biodiversity and ecology. South African Journal of Marine Science 20: 393–420. doi: 10.2989/025776198784126287
- Young RE, Vecchione M, Roper CFE 2007. A new genus and three new species of decapodiform cephalopods (Mollusca: Cephalopoda). Reviews in Fish Biology and Fisheries 17: 353–365. doi: 10.1007/s11160-007-9044-z
- Zhang J, Kapli P, Pavlidis P, Stamatakis A 2013. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29: 2869–2876. doi: 10.1093/bioinformatics/btt499
- Zwickl DJ 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. PhD dissertation. Austin, TX, The University of Texas. 115 p.