Abstract
The New Zealand checklist of Collembola was prepared 10 years ago but only published in 2012 (Greenslade P 2012. Collembola. In: Gordon DP ed. The New Zealand inventory of biodiversity: a species 2000 symposium review. Volume 1, Animalia. Christchurch, Canterbury University Press. Pp. 237–243). Since 2004, a number of changes in the species list, both additions and deletions, have been published but they are scattered in disparate journals. Also, new synonymies and new combinations have become evident. Here, I gather these findings together in a single article, thus making them available. I synonymise Sminthurus multidentatus with S. viridis and remove the subfamily Uchidanurinae from the list, transferring its New Zealand and Australian species to the subfamily Pseudachorutinae. Other already published new records, corrected identifications and new combinations are listed here including some additions to the description of the type and only species Clavaphorura septemseta of its New Zealand endemic genus. The total number of species known from New Zealand is now 350, with 101 genera. The number of families remains at 18 but the number of subfamilies falls from six to five. I document them here with a new synonym and combination.
Introduction
Checklists and catalogues need to be continually revised and updated as changes in systematics are published, new species described and types representing anomalously named taxa are examined afresh. Updated lists also assist ecologists to correctly identify species they are studying. In the absence of local taxonomists, as is the case with New Zealand Collembola, this task is often carried out by specialists overseas. In order to make this task easier, and, in the absence of an online checklist of species which can be edited, formal publications make these changes publicly available.
Consequently, this article records recent additions and deletions from the published list of Collembola species from New Zealand (Greenslade Citation2012), moves three species into a different subfamily, removing the subfamily in which they had been placed from the list, describes a synonym of biogeographical importance and redescribes an endemic genus.
Sminthurus multidentatus (Salmon, Citation1943a) and Sminthurus viridis L.
Salmon (1943) erected S. multidentatus for a single specimen found on flowers of strawberry plants in Waimate, Auckland. The collection data and colour painting of the holotype indicate that the species is likely to be a synonym of S. viridis L. Salmon (Citation1943a) provided a key to the three species of Sminthurus known at that time from New Zealand. As well as S. viridis and S. multidentatus, the third species was Sminthurus denisi (Womersley, 1934), which has been transferred to the genus Temeritas Delamare-Deboutteville and Massoud, 1963 (Greenslade Citation1994). Salmon (Citation1943a) stated that S. viridis has no teeth on the empodial appendage and lacked external teeth to the claw. He figured the claw of multidentatus with two external teeth and with two or three minute extra internal teeth on inner lamella of the empodial appendage. He does not figure nor mention a tunica nor a toothed or fringed pseudonychia.
Stach's (Citation1956) detailed description of S. viridis recorded and figured teeth (one or, rarely, two) on the empodial appendage. Stach (Citation1956) also notes that the claw has a ‘dorsal’ tooth and inner tooth as well as a tunica and toothed and fringed pseudonychia. He figures the claw in detail showing these features in both lateral and dorsal view. Bretfeld (Citation1999) also noted that the claw of S. viridis has an inner tooth with a weak basal outer tooth and that empodial appendage on leg I lacks a tooth but on legs II and III there are teeth. He also notes that there is a long weakly serrate pseudonychia present. Australian specimens of S. viridis comply with Stach's and Bretfeld's descriptions. Salmon (Citation1943a) therefore was incorrect in his comment that S. viridis had no teeth on any empodial appendage nor external teeth to the claw. This correction to the claw characters removes all characters that separate S. multidentatus from S. viridis. Bretfeld (Citation1999) also recorded ‘specimens from India and New Zealand as having claws with 2 or more inner teeth’, and that ‘redetermination of new and old collections of S. viridis and related species is necessary’ (p. 2013). However this author did not examine Salmon's material of S. multidentatus.
Wise (Citation1977) listed S. multidentatus as a valid species, as did Greenslade (Citation2012). Bellinger et al. (Citation1996–Citation2014) also treat S. multidentatus as a good species. However, Betsch (Citation1980) suggested that S. multidentatus ‘is perhaps a common introduced Sminthurus as species in this genus are only found in the Northern Hemisphere, Palearctic and Nearctic’ (p. 167). In a list of types in Te Papa Tongarewa Museum of New Zealand, Palma et al. (Palma et al. Citation1989; R Palma, Te Papa Museum, pers. comm. 2012) states that Te Papa Museum considers ‘multidentatus a synonym of viridis’ based on a tentative unpublished identification by P. Greenslade in 1988. In order to definitively confirm or not the synonymy, the holotype of multidentatus was examined as suggested by Bretfeld (Citation1999). Palma carried this out in February 2013 (Palma in litt), and found Salmon's description of claw and empodial appendage was incorrect in that a fringed tunica is present on five visible legs. In spite of Bretfeld's (Citation1999) caution, from his and Stach's descriptions of viridis, it seems both of Salmon's (Citation1943a) distinguishing characters for S. multidentatus are not valid. These characters are very small, sometimes difficult to see. and can vary a little in size and with leg number. Also, it appears that Salmon was unaware of the existence of pseudonychia. On these grounds it seems probable that S. multidentatus is a synonym of S. viridis. DNA sequencing (three genes) of different New Zealand populations of S. viridis has been reported (Vink & Brown Citation2014) and these authors concluded only a single species is present in the country.
The genus Novokatianna Salmon, Citation1944
Background
This genus was erected by Salmon in 1944 with type species, Novokatianna cummyxa from Johnston's Hill, Karori near Wellington. Two other species were placed in this genus, Katianna venusta Salmon, Citation1943a from Number 2 Gully, Karori, Wellington, and Novokatianna radiata Salmon, 1946 from Homer Forks, Fjordland, South Island. Salmon placed the genus in Katiannidae.
Wise (Citation1977), in his checklist of the smaller orders of New Zealand insects, placed the genus with its three species in the family Bourletiellidae. He gave no explanation for this change. Betsch (Citation1980), in his revision of the world species of Symphypleona, moved the genus to the family Sminthuridae having viewed holotypes of two species and included figures of characters in his text (Betsch Citation1980, fig. 57A–G). He illustrated the chaetotaxy of the trochanter which is diagnostic for some genera in the family. He also noted the genus differed from other genera of Sminthuridae, especially Temeritas, in that it has a large number of macrochaetae arranged all along antennal segment III, not only in the basal half, antennal segment IV with 13 to 15 subsegments at most, five setae and a spine on trochanter III, spinose setae on the head, many long setae on the body and anterior setae of the dens, a dental seta, and a simple anal appendage, all distinguishing characters for Novakatianna.
Bretfeld (Citation1999) followed Betsch (Citation1980) in keying the genus to Sminthuridae, as did Greenslade in 2012 in a checklist of New Zealand Collembola updated from Wise (Citation1977). The genus appears currently to be endemic to New Zealand. A colour photograph of N. cummyxa was taken recently by A. Murray from Stewart Island, New Zealand ().
Sminthurus multidentatus and Novokatianna species
The possibility that S. multidentatus is a species of Novokatianna was also investigated. Both genera have macrochaetae on antennal segment III, a mucronal seta, subsegmented antennal segment IV and spines on vertex of head. The differences between the two genera according to Betsch (Citation1980) and Bretfield (Citation1999) are as follows. Sminthurus has 1 + 1 postantennal setae present with the trochanteral spine absent and Novokatianna has postantennal setae absent with the trochanteral spine present. Palma (in litt. 2014) recently examined anew the type of S. multidentatus and confirmed that it has 1 + 1 postantennal setae and no trochanteral spine. Therefore Sminthurus multidentatus is here confirmed as a junior synonym of Sminthurus viridis.
This synonym reduces the number of Collembola species recorded from New Zealand by one.
Stenacidia violaceus (Reuter, 1881) and Jeannenotia stachi (Jeannenot, 1955)
The characters separating these two species and genera have been shown to be invalid and they were therefore synonymised (Bretfeld Citation1999; Greenslade Citation2011). At the same time, Greenslade (Citation2011) showed that Arrhopalites coccineus Salmon, Citation1941, belonged to the species Stenacidia violaceus. It was not therefore included in the New Zealand checklist (Greenslade Citation2012). However, the checklist incorrectly listed the species Jeannenotia stachi. This species should have been replaced by Stenacidia stachi because Jeannenotia is a junior synonym of Stenacidia. Consequently this change is noted here.
This change does not alter the total number of species or genera of Collembola known from New Zealand.
Entomobryidae
Greenslade (Citation2012) used only two subfamilies, Entomobryinae and Orchesellinae, to classify species in this family. In a detailed analysis of the sensory setae (s setae or sensilla) on species in this family, Zhang & Deharveng (Citation2015) have revised the subfamily classification and four subfamilies are relevant to the Australian and New Zealand faunas. The Orchesellinae is unchanged but the Entomobryinae are now further divided into Lepidocyrtinae (Lepidocyrtus Bourlet, 1839, Pseudosinella Schäffer, 1897), Seirinae (Seira Lubbock, 1869, Lepidosira Schott, 1925, Lepidobrya Womersley, Citation1937) and Entomobryinae (Entomobrya Rondani, 1861, Sinella Brook, 1882, Coecobrya Yosii, 1956, Drepanura Schött, 1891). This change gives four subfamilies instead of two.
Urewera Salmon, Citation1938
This genus was established for species of Entomobryidae similar in morphological details to Lepidosira Schött, 1925 or Lepidocyrtoides Schött, 1927, but with a lamella on the mucro and large basal lateral teeth to the claw. Salmon (Citation1964) described and recorded 17 species in the genus, all from New Zealand. I have examined most of the type specimens of species in the genus and the ‘lamella’ on the mucro appears to be an artefact. All Urewera species were placed in the genus Lepidosira in Greenslade (Citation2012) but the genus needs to be revised and all 17 species described by Salmon (1964 and included references) need to be examined.
Isotoma raffi Womersley, 1934
Parisotoma Bagnall, 1940 was originally described as a subgenus of Isotoma Bourlet, 1839. Potapov (2001) raised the subgenus to generic status. Fresh specimens of this species have been recollected from the type locality and the change of genus to Parisotoma found necessary. The record of Isotoma raffi in Greenslade (Citation2012) should consequently be changed to Parisotoma raffi (Womersley, 1934) so there is no change in the number of species known from New Zealand.
Isotopenola Potapov et al., Citation2009
It was noted in this publication (Potapov et al. Citation2009) that Isotopenola was not so far recorded from New Zealand but is likely to occur there. Some species of Cryptopygus Willem, 1901 described by Salmon may belong to the genus Isotopenola (Potapov et al. Citation2009). However, in Greenslade (Citation2012) the species Cryptopygus loftyensis Womersley, 1934, described from Australia, is recorded as present in New Zealand determined by Womersley (Citation1937). This species was redescribed and transferred to the new genus Isotopenola in Potapov et al. (Citation2009). The correct specific identity of Salmon's New Zealand specimens need to be confirmed but, for the present, Cryptopygus loftyensis should be listed as Isotopenola loftyensis (Womersley, 1934) in the New Zealand list.
Schoettella subcorta Salmon, Citation1941
This species was shown to belong to the genus Xenylla (Greenslade et al. Citation2011). Consequently, it should be recorded in the checklist as Xenylla subcorta (Salmon Citation1941).
This change does not alter the number of species known from New Zealand but it reduces the number of genera by one.
Ceratophysella denticulata (Bagnall, 1941)
Mesaphorura macrochaeta Rusek, 1976
Protaphorura sp. cf fimata (Gisin, 1952)
Isotomurus maculatus (Schäffer, 1896)
Brachystomella platensis Najt and Massoud, 1974
Folsomides parvulus Stach, 1922
Bourletiella viridescens Stach, 1920
These seven species were recorded from New Zealand for the first time in Greenslade et al. (Citation2014). This paper erroneously recorded Greenslade 2012 as Greenslade 2011 because of late changes to the publication year. A molecular analysis (CO1) of specimens of C. denticulata from New Zealand and Macquarie Island showed them to belong to a lineages which also occur in Canada (Porco et al. Citation2012).
The addition of these seven species to the New Zealand list increases the total number known by seven but does not change the number of genera.
Hypogastrura rossi Salmon, Citation1941
This species was shown to be a synonym of Hypogastrura vernalis (Carl, 1901) thereby both removing one species from the New Zealand list and replacing it with one new one (Greenslade et al. Citation2013).
This synonym entailed no change in numbers of species or genera in New Zealand.
Subfamily Uchidanurinae Cassagnau, 1980
Salmon (Citation1964, p. 109) established the family Uchidanuridae for the genus Uchidanura Yosii, 1954, type species Achorutes esakii Uchida, 1944. He defined the family on the following characters: absence of teeth on the mandibular and maxillary heads; mandible head sickle-shaped, scalpel-shaped or styliform; maxilla styliform, body plump and thick; and integument finely tuberculate or coarsely granulated and usually with bosses on dorsal surface. Two other monotypic genera were included by Salmon (Citation1964) in the family, Prospinanura kardosia Wray, 1953 and Wrayella oxfordi (Wray, 1950), both from North Carolina, USA. However, Mari Mutt (Citation1979) found both Uchidanura species have teeth on the mandible thereby doubting the validity of the family. Cassagnau redefined the group, reducing the family to subfamily level with seven genera (Cassagnau Citation1980). This author found only one character united the genera: the presence of digitations and/or lobes on the dorsal surface of the body. There are no other unifying characters and the character states of the mouthparts, ocelli, postantennal organ, pigment and plurichaetocity are variable. As well the genera are geographically strongly disjunct. Moreover, the defining characters of digitations and lobes on head, antennae and body are present in a number of other Neanuridae genera such as: Morulina, Borner, 1906; Coecoloba, Yosii, 1956; Bilobella Caroli, 1912; Digitanura Deharveng, 1987; and Rambutanura Deharveng, 1988.
Characters of proven value in distinguishing genera of Neanuridae are the form of the mouthparts as well as presence and number of ocelli and form of postantennal organ if present. Two genera of Uchidanurinae have similar mouthparts, ocelli 7 + 7 or 8 + 8 and complex postantennal organs, Acanthanura Börner, 1906 from Australia and Holocanthella Börner, 1906 from New Zealand, but differ in other characters such as presence or absence of seta L’ on the labium, normally considered a character of generic value. Two other genera have mouthparts that might place them within the Neanurinae, this includes includes species in Uchidanura as well as Caledonimeria Delamare Deboutteville and Massoud, 1963 (Stevens et al. Citation2007). Although Wise (Citation1977) did not use the subfamily Uchidanurinae, Greenslade (Citation2012) placed five New Zealand species in the genus Holocanthella in the subfamily Uchidanurinae. Because species in Holocanthella have no characters currently known in common with the genotype of this subfamily, except the presence of digitations, they are hereby moved to the subfamily Pseudachorutinae and the subfamily Uchidanurinae is removed from the 2012 list. At the same time and for the same reasons the Australian monotypic genera Acanthanura, Megalanura Ellis and Bellinger, 1973 and Womersleymeria Stach, 1949 and their included species, are hereby moved from the subfamily, Uchidanurinae, to the subfamily Pseudachorutinae sensu Deharveng 2004. The genus Caledonimeria should be moved to the subfamily Neanurinae Börner, 1901 as noted by D'Haese (Citation2003). The affinities of the genus, Uchidanura Yosii, 1954 is still not clear as the type species, U. esakii has not been barcoded. So the subfamily Uchidanurinae remains a valid taxon with three genera, Uchidanura as genotype with Assamanura Cassagnau, 1980 and Denisimeria Massoud, 1965 also included. This change reduces the number of subfamilies known from New Zealand by one.
Vitronura Yosii, 1969
This genus was recorded for the first time from New Zealand in Phillips et al. (Citation2015) but without described species.
Zealandmeria Stach, 1949 and Ceratrimeria Börner, 1926
Two species, Ceratrimeria harrisi Salmon, 1942 and Pseudachorutes novazealandiae Womersley, 1936 were listed twice in the 2012 checklist under two different genera, both Ceratrimeria and Zealandmeria. Stach (1949) separated Zealandmeria from Ceratrimeria on the basis of abdomen VI in the former genus being visible dorsally while it was not in Ceratrimeria. He included three species in the genus, Ceratrimeria aurea Salmon, 1942 as type species, as well as Ceratrimeria harrisi and Pseudachorutes novaezealandiae. Massoud (Citation1967) synonymised Zealandmeria with Ceratrimeria after examining type specimens but without giving any justification. Although it is possible that Zealandmeria is a good genus, Massoud's synonymy, being the most recent, should be retained. Consequently, the entries of Z. harrisi and Z. novaezealandiae must be removed from the 2012 checklist, as they are already recorded in the genus Ceratrimeria, so resulting in two fewer species records.
Clavaphorura Salmon, Citation1943b
There are two monobasic genera of Tullbergiidae that key out first and second respectively in the recent key to the world genera of the family because of the exceptional characters they are recorded as possessing (Dunger & Schlitt Citation2011). Both are from temperate rainforest in southern regions. The first is Clavaphorura Salmon, Citation1943b from New Zealand, which possesses, according to the original and only description of the genus, seven clavate tenent hairs on all legs and an antennal III organ consisting of four clubs instead of two or three. The second is Tasphorura Greenslade and Rusek, 1996 from Tasmania, which is pink in life and possesses an enlarged apical bulb on the tip of antennal segment IV. None of these four characters are found in other genera of Tullbergiidae except for a species of Neonaphorura Bagnall, 1935, which has three slightly clavate hairs, and Tullbergia alcirae Palacios-Vargas and Martinez, 2014, which has two. It is timely to re-examine the types of the genotype and redescribe it giving particular attention to the chaetotaxy as I do below. This is because Clavaphorura was described before chaetotaxy was widely accepted as a stable character of value not only in species determinations but also in phylogenetic analysis.
Clavaphorura Salmon, 1943b
Type species
Clavaphorura septemseta Salmon, 1943b
Diagnosis (modified from Salmon 1943b)
Antennal III organ with two minute hammer-shaped clubs bent towards each other, two slightly longer rods, one on either side, curved towards the clubs and one longer bent club, slightly more ventral and basal, flanking the organ,Footnote1 all rods ‘sausage-shaped’, behind a distinct, crenulated fold of cuticle; one much smaller curved club ventrally and slightly more basal; seven distinctly clavate tenent hairs on each leg, three apically and externally and four internally and anteapically; pseudocelli of type I (Weiner & Najt Citation1991), star shaped with 10 rays; postantennal organ long with just over 30 simple tubercles in two rows; abdomen I with pseudocelli; macrochaetae numerous, longer than segment lengths on all segments; abdomen VI with two anal spines in m1 position.
Clavaphorura septemseta Salmon, Citation1943b
(A‒I)
Figure 2 Line drawings of the holotype of Clavaphorura septemseta Salmon, 1943b. A, Head and thorax 1, II, dorsal chaetotaxy; B, C. septemseta, antennal III organ; C, pseudocelli of head; D, C. septemseta, apical bulb on antenna IV; E, C. septemseta, dorsal chaetotaxy of abdomen I, II and III; F, C. septemseta, postantennal organ; G, C. septemseta, ventral chaetotaxy of abdomen I, II and III; H, C. septemseta, dorsal chaetotaxy of abdomen IV, V and VI; I, C. septemseta, ventral chaetotaxy of abdomen IV, V and VI.
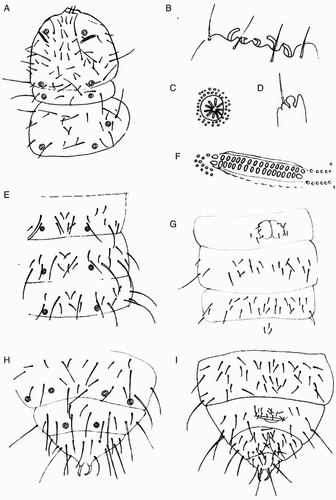
Type locality
Karori Hills, under bark of old logs on exposed hillsides, 500‒800 ft (c. 200 m a.s.l.) North Island, New Zealand.
Material examined
Paratype female, slide 3/1221, deposited Te Papa Museum, Wellington, New Zealand, A1.032517.Footnote2 Same locality and collection data as holotype.
Other material examined
One female, one unknown, 3/1348, Karori Hills, under Fuschia bark, 11.5.1941,3/1348 A1-032895; one immature, 3/1242, Kaori Hills, under bark of log, 24.ii.1943, A1-032517.
Description
Body
Pigment absent in mounted specimen; granulation fine and even over all body; ant length a third to half length of head diagonal; pseudocelli round with indistinct rim and 10 ribs radiating from centre (C), arranged as 1,1/1,2,2/1,1,1,2,1 in positions as shown in the A,E,H.
Dorsal chaetotaxy
Heterochaetocity marked, macrochaetae numerous, well differentiated, long, even on antennae with ant I with 1, ant II with 2, ant III with 3, ant IV with 8. Total number of Mc setae on abd I as 5, abd II as 10, abd III as 15; ms present on Th II and III as normal for family. Axial setae present on segments: abd I (po), abd II (po), abd III (po), abd IV (po), abd V(absent), abd VI (ao, po), th II and III lacking both m2 and m3; dorsal chaetotaxy as in A,E,H. S setae not distinct on abdomen but fine and pointed on thorax. Ventral chaetotaxy as in G,I.
Head and antennae
Antennal segment IV with five well developed S chaetae d, b, c, e, f, all equal in size; apical bulb simple, largish; antennal III organ in irregular groove with large cuticular granules as in diagnosis for genus (B); ant I with six setae, ant II with 10 setae, ant III with 15 seta; ant III apical bulb with two clubs and three rods behind two cuticular folds.
Labrum with 5, 5, 4 setae from posterior to anterior, the three mid setae and two mid setae in p and m rows respectively longer than the others, a row setae curved inwards and thickish; PAO elongate with about 30 tubercles arranged in two parallel rows (F); ventral head papillae 4 + 4, large; grinding mandibular plate well developed; maxilla with two large teeth and three shorter very finely fringed lamellae. Other lamellae not seen.
Thorax
Thorax II and thorax III with 1 + 1 setae ventrally; claw without teeth; empodial appendage lacking except for minute pointed lobe; clavate hairs long, moderately clavate arranged as given for genus as A7, A1, A2 and B3, B4, B5, B6; chaetotaxy of legs shown in . No trace of furca or remnant.
Table 1 Leg chaetotaxy.
Abdomen
Ventral tube with 4 + 4 chaetae laterally; 2 + 2 setae lateral to VT; Abd VI without crescentic ridges; a1 as mesochaeta, anal spines long, curved, on distinct papillae as long as inner claw; male genital plate not seen, female genital plate with 15 setae, anterior anal lobes with 14 setae each.
Ratio of lengths
Body length: head diagonal: antenna = 8:1.3:1
Head diagonal: antennal length = 1:0.5
Anal spine: inner claw length = 1:1.
Lateral macrochaetae abd V: mesochaeta a1 = 2:1
Comment
As the only diagnostic characters reported by Salmon (Citation1943b) are now the clavate tenent hairs as a result of the findings in the redescription above, the status of the genus is in doubt. The genus most similar to Clavaphorura is now the monobasic genus Boudinotia Weiner and Najt, Citation1991 from New Caledonia, because of similar form of antennal III organ, PAO and pseudocelli except for the absence of clavate tenent hairs in the latter genus. Also, the type of Boudinotia has two pseudocelli on abdominal segments I to III unlike Clavaphorura that has only one. However, the number of pseudocelli is considered to be a specific and not a generic character. Other characters of Boudinotia that differ from Clavaphorura are: ventral rod of antennal III organ more basal and ventral; abdomen V mid pseudocelli at extreme posterior of segment; postantennal organ with over 35 tubercles and pseudocelli with apparently only five radiations. The presence of clavate tenent hairs in C. septemseta may be an adaptive character associated with an epigaeic habitat.
Schaller (Citation1949) described a species from Austria as Tullbergia septemspina. It presumably had seven spines or spine-like processes distally on abdomen VI. Although it is a nomen nudum, because no description was provided, nor holotype or type locality designated. Salmon (Citation1964, p 147) placed this species in synonymy with C. septemseta suggesting Schaller (Citation1949, p. 147) made an orthographic error. This synonymy is unlikely as C. septemseta has only two spines. However, in a footnote, Salmon noted that T. septemspina was a ‘spec. indet., possibly Dinaphorura diversispina (Wahlgren, 1906)’. On the next page, Salmon (Citation1964, p 148) places T. septemspina also in ‘?’ synonymy with D. diversispina. Since the genus Dinaphorura Bagnall, 1935 is currently only known from the extreme south of the Southern Hemisphere and D. diversispina is described from Tierra del Fuego, the synonymy of a species from Austria is unlikely. Schaller's specimens, if they exist, need to be re-examined to correctly determine the generic placement of T. septemspina. Bellinger et al. (Citation1996–Citation2014) consider T. septemspina to be a possible nomen nudum as did Hüther Citation1961, Christian Citation1987 and Mihelcic Citation1952. It is likely that Schaller (Citation1949) placed his specimens in Tullbergia, although they probably belong to the genus Neonaphorura Bagnall, 1935. This genus was revised by Arbea (Citation1991) which is the Northern Hemisphere analogue genus to the Southern Hemisphere Dinaphorura. Christian (1987) notes that it is possibly identical with Neonaphorura novemspina Gisin, 1961.
A further confusion arises in the differentiating characters given by Dunger & Schlitt (Citation2011) in their key to world genera of Tullbergiidae. These authors use the character (couplet 9) of one pair of spines on abdomen VI for Dinaphorura and two pairs of spines for Neonaphorura. In fact both genera have only one pair of spines with a varying number of short spine-like processes on the dorsal surface of this segment. As the form of the pseudocelli is similar in both genera, the only currently known character to separate the genera is the postantennal organ said to be complex in Neonaphorura and simple in Dinaphorura.
An additional correction to Greenslade (Citation2012) is made here in that the type species of the genus was incorrectly placed in the family Onychiuridae in the list of New Zealand Collembola (Greenslade Citation2012) although Wise (Citation1977) placed it in Tullbergiidae. This error was possibly because of some doubt over the accuracy of the characters described by Salmon (Citation1943b).
Discussion
Greenslade (Citation2012) gave the total known number of species from New Zealand to be 346 with 34 subspecies, of which 266 species and 29 subspecies were, at that time, endemic. The total number of genera was 103 with 20 being endemic. With the additions and synonymy reported here there are an additional eight new records of species, two new combinations and three deletions making 350 species with one new genus added and two deleted.Footnote3 I exclude the new record of Vitronura as it is not confirmed by descriptions of New Zealand species. The number of endemic species is reduced by one but there is no change in endemic genera. New Zealand Collembola are very poorly known with numerous undescribed species in collections, as well as earlier described species being in need of re-examination.
Associate Editor: Dr Rob Cruickshank.
Acknowledgements
Thanks are due to Ricardo Palma for loan of specimens from Te Papa Museum, Wellington, New Zealand, and for advice on the morphology of Sminthurus multidentatus type.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes
1. The single paratype which was cleared, has only three rods and two clubs not four rods and two clubs as described and illustrated by Salmon (Citation1943b). This is an arrangement found in other genera of Tullbergiidae. Salmon's (Citation1943b) other figures (pseudocelli arrangement, tip of tibiotarsus, postantennal organ and tip of antennal segment IV are essentially correct.
2. Specimens on two other paratype slides were not in good enough condition to include in the redescription.
3. A new species recently described for New Zealand increased the known species to 351 and genera to 102 (Babenko & Minor Citation2015).
References
- Arbea J 1991. A revision of the genus Neonaphorura Bagnall, 1935 (Collembola, Onychiuridae, Tullbergiidae). Spixiana 14(2): 175–188.
- Babenko A, Minor M 2015. Austrodontella monticola sp. nov., a new species of Collembola from montane New Zealand. Zootaxa 3974: 122–128.
- Bellinger PF, Christiansen KA, Janssens F 1996–2014. Checklist of the Collembola of the world. http://www.collembola.org (accessed 8 October 2014).
- Betsch JM 1980. Eléments pour une monographie des Collemboles Symphypléones Hexapodes, Aptérygotes. Memoires du Museum National d'Historire naturelle Serie A, Zoologie 116: 1–227.
- Bretfeld G 1999. Synopses on Palaearctic Collembola, Volume 2. Symphypleona., Abhandlungen und Berichte des Naturkundemuseums Görlitz, Band 71, Heft 1, 1999. 318 p.
- Cassagnau P 1980. Sur le genre Assamanura n. g. du nord-est de l'Inde et sur la lignée Uchidanurienne (Collemboles). Travaux du Laboratoire d’Écobiologie des Arthopodes Édaphique, Toulouse 2(3): 1–7.
- Christian E 1987. Catalogus Faunae Austriae. Part XIIa: Collembola (Springschwänze). Wien [Vienna], Austria, Verlag der Österreicheischen Akademie der Wissenschaftern. 80 p.
- D'Haese C 2003. Morphological appraisal of Collembola phylogeny with special emphasis on Poduromorpha and a test of the aquatic origin hypothesis. Zoologica Scripta 32: 563–586.
- Dunger W, Schlitt B 2011. Tullbergiidae. Synopses on Palaearctic Collembola. Volume 6. 1st ed. Senckenberg, Museum of Natural History Görlitz. 168 p.
- Greenslade P 1994. Collembola. In Houston WWK ed. Zoological catalogue of Australia. Volume 22. Protura, Collembola, Diplura. Melbourne, CSIRO Australia. Pp. 19–138.
- Greenslade P 2011. Consideration of the genera Stenacidia and Jeannenotia Collembola: Sminthurididae. Zootaxa 2996: 66–68.
- Greenslade P 2012. Collembola. In Gordon DP ed. The New Zealand inventory of biodiversity: a species 2000 symposium review. Volume 1, Animalia. Christchurch, Canterbury University Press. Pp. 237–243.
- Greenslade P, Boyer S, Wratten S 2013. New records of springtail Collembola species in New Zealand pasture: how well do we know our pastoral invertebrate fauna? New Zealand Journal of Agricultural Research 56: 93–101.
- Greenslade P, Ireson JE, Skarzynski D 2014. Hypogastrura and Ceratophysella species in Australia. Austral Entomology 53: 53–74.
- Greenslade P, Stevens MI, Torricelli G, D'Haese C 2011. Redescription of Gomphiocephalus hodgsoni Carpenter, 1908 (Collembola: Hypogastruridae): morphology and molecular data restore an ancient Antarctic endemic genus. Systematic Entomology 36: 223–240.
- Hüther W 1961. Őkologishe Untersuchungen uber die Fauna pfälzischer Weinbergsböden mit besonderer Burücksichtigung der Collembolen und Milbern. Zoologische Jahrbücher Systematik 89: 243–368.
- Mari Mutt JA 1979. A review of the genus Uchidanura with description of a new species (Collembola Neanuridae). Pacific Insects 20: 53–58.
- Massoud Z 1967. Monographie des Neanuridae, Collemboles Poduromorphes a pèeces buccales modifiées. Thèse de Faculté des Sciences, Université de Paris.
- Mihelcic F 1952. Beitrag sur Kenntnis der Oribatei und Collembola der Humusboden. Archivio Zoologico Italiano 37(1): 93–105.
- Palma R, Lovis PM, Tither C 1989. An annotated list of primary types of the phyla Arthropoda except Crustacea and Tardigrada held in the National Museum of New Zealand. National Museum of New Zealand Miscellaneous Series 20: 1–49.
- Phillips CB, Brown SJ, Greenslade P, Reay S, Allen RB, Easdale TA, et al. 2015. Collembola in southland beech litter and soil. New Zealand Entomologist 38: 79–87.
- Porco D, Bedos A, Greenslade P, Janion C, Skarzinsky D, Stevens M, Deharveng L 2012. Species delimitation in Collembola: cryptic diversity cases among common springtails unveiled by DNA barcoding. Invertebrate Systematics 26: 470–477.
- Potapov M 2001. Isotomidae. In: Dunger W ed. Synopses on Palearctic Collembola. Abhandlungen und Berichte des Naturkundemuseums. 73: 1–603.
- Potapov M, Babenko A, Fjellberg A, Greenslade P 2009. Taxonomy of the Proisotoma complex. II A revision of the genus Subisotoma and a description of Isotopenola gen. nov. (Collembola: Isotomidae). Zootaxa 2314: 1–40.
- Salmon JT 1938. Supplement to the collembolan fauna of New Zealand. The genus Ceratrimeria Börner in New Zealand and a new genus Novacerus to replace the genus Neocerus (preoccupied). Transactions of the Royal Society of New Zealand 68: 349–361.
- Salmon JT 1941. The collembolan fauna of New Zealand including a discussion of its distribution and affinities. Transactions and Proceedings of the Royal Society of New Zealand 70: 282–446.
- Salmon JT 1943a. New records of Collembola from New Zealand with descriptions of new species. Transactions of the Royal Society of New Zealand 73: 1–12.
- Salmon JT 1943b. New records of Collembola from New Zealand with descriptions of new genera and species. Transactions and Proceeding of the Royal Society of New Zealand 72: 373–388.
- Salmon JT 1944. New genera, species and records of New Zealand Collembola and a discussion of Entomobrya atrocincta Schött. Dominion Museum Records in Entomology 1(Part 1): 123–182.
- Salmon JT 1964. An index for Collembola. Bulletin of the Royal Society of New Zealand 7: 1–144.
- Schaller F 1949. Biologische Beobachtunger an humusbildenede Bodentieren insbesondere an Collembolen. Zoologishe Jahrbucher Systematik 78: 263–293.
- Stach JS 1956. The Apterygotan fauna of Poland in relation to the world-fauna of this group of insects. Family: Sminthuridae. Polskie Akademii Nauk. Instytut. Zoologii. Krakowie. Pp. 1–287.
- Stevens MI, Winter DJ, Morris R, Greenslade P 2007. New Zealand's giant Collembola: new information on distribution and morphology for Holacanthella Börner, 1906 Neanuridae: Uchidanurinae. New Zealand Journal of Zoology 34: 63–78.
- Vink CJ, Brown SDJ 2014. High mitochondrial DNA sequence divergence in Sminthurus viridis (Linnaeus) (Collembola: Sminthuridae) from New Zealand. New Zealand Journal of Entomology 37: 29–34.
- Weiner W, Najt J 1991. Collemboles Poduromorpha de Nouvelle-Calédonie. 6. Onychiuridae Tullbergiinae. Mémoirs du Museum National d'Histoire Naturelle A 149: 119–130.
- Womersley H 1937. On the collembolan fauna of New Zealand. Transactions and Proceedings of the Royal Society of New Zealand 66: 316–328.
- Wise KAJ 1977. A synonymic checklist of the Hexapoda of the New Zealand sub-region. The Smaller Orders. Bulletin of the Auckland Institute and Museum 1: 1–176.
- Zhang F, Deharveng L 2015. Systematic revision of Entomobryidae (Collembola) by integrating molecular and new morphological evidence. Zoologica Scripta 44: 298–311.

