Abstract
Stoats are significant predators of native fauna in New Zealand. They occur in many habitat types and consume a wide range of prey. The diet of stoats in the Tasman River, South Canterbury, was studied by analysis of scats and den contents. Analysis of 206 scats showed that stoats ate mainly lagomorphs, birds and invertebrates. Minor components included mice, lizards, fish and hedgehogs. Stoats ate more birds in spring than in autumn, and female stoats ate more invertebrates than did males. The contents of 219 dens collected in the same area at the same time provided further information. Birds and lagomorphs occurred at high frequency in dens, and other components were minor. Remains in dens were larger than in scats and allowed identification of many more prey items to species level. Den contents revealed a potentially substantial impact of stoats on threatened shorebirds locally; this impact was not detected by analysis of scats.
Introduction
Evidence that stoats (Mustela erminea) have been a major contributor to the decline of native fauna in New Zealand has accumulated in recent years (for summaries, see: McDonald & Murphy Citation2000; King & Powell Citation2007). Stoats can reproduce quickly and are capable of rapid, long-distance dispersal. They climb and swim readily, are often difficult to control, and can re-invade rapidly when control ceases (e.g. Murphy & Dowding Citation1995; King & Murphy Citation2005; Reardon et al. Citation2012).
Stoats are widespread in the North and South Islands and occur in virtually all habitat types, from the coastal zone to alpine areas. They are able to persist in this range of habitats partly because they can exploit a wide spectrum of prey. Lagomorphs and birds are common staples (King & Murphy Citation2005) and in forested areas, rodents often form a major component of the diet (e.g. King & Moody Citation1982; Murphy et al. Citation1998). In non-forested alpine areas, invertebrates are an important component of the diet (Smith et al. Citation2008). However, reports of stoat diet in non-forested systems are still relatively uncommon. Given the number of threatened bird species found in braided rivers, further information is required on the impacts of predators in that ecosystem. This paper describes the wide range of prey eaten by stoats in a South Island braided river valley, where there are no large areas of forest and rodent densities are low.
The braided rivers of New Zealand's South Island are an uncommon habitat type worldwide (Cromarty & Scott Citation1996). They are a dynamic and unstable system, characterised by mobile shingle banks, highly variable flow rates and regular flooding. These riverbeds provide vital breeding habitat for a number of threatened endemic shorebirds, including the black stilt (Himantopus novaezelandiae), banded dotterel (Charadrius bicinctus), wrybill (Anarhynchus frontalis), black-billed gull (Larus bulleri) and black-fronted tern (Chlidonias albostriatus). These and other bird species are specialised to varying degrees for breeding in braided riverbeds. Stoats are present in many braided rivers, but relatively little is known of their ecology in this habitat. In the Mackenzie Basin, they appear to be patchily distributed, with highest numbers in the Tasman and Godley Rivers (Keedwell & Brown Citation2001).
Rabbits (Oryctolagus cuniculus) are an important component of stoat diet in the Mackenzie Basin (Pierce Citation1987; Murphy et al. Citation2004), and there is a growing body of evidence suggesting that when rabbit densities are reduced, stoats (as well as ferrets, Mustela furo , and cats, Felis catus) prey on birds to a greater extent (Pierce Citation1987; Rebergen et al. Citation1998; Murphy et al. Citation2004; Norbury & Heyward Citation2008). Following its release in 1997, rabbit haemorrhagic disease reduced rabbit densities in South Canterbury dramatically, and the impacts on several shorebird species were considered to be potentially severe (Aikman Citation1997).
The diet study described here was part of a wider programme in which radio-telemetry was used to provide information on the ecology of stoats in a braided river ecosystem. The study aimed to record the range of prey species eaten by stoats, and to determine the relative importance of different prey classes in the diet. It also compared diet between spring–early summer (when substantial numbers of shorebirds breed in the area) and autumn–early winter (when most shorebirds are absent). In addition, the use of radio-collared stoats allowed many dens to be located, which enabled a comparison of scats and den contents as indicators of diet in this system.
Methods
Study area
The study area was located on the true right side of the Tasman Valley in the Mackenzie (Upper Waitaki) Basin, South Canterbury. It included the gravel riverbed, ponds and side-creeks, rough pasture and patches of scrub on the bank of the river, and pasture on the lower slopes of the western side of the valley. The area was about 17 km long and about 3 km wide in most places; it was bounded by Mount Cook airfield (43°46′S, 170°08′E) in the north and Twin Stream, Glentanner (43°54′S, 170°08′E) to the south. The eastern boundary ran roughly down the centre line of the riverbed or was dictated by the main channel of the Tasman River; the western boundary ran along the sloping foothills of the Ben Ohau Range 1–1.5 km west of State Highway 80. The total area was approximately 4700 ha. The riverbed rises from an altitude of about 530 m above sea level (a.s.l.) at its southern end near Lake Pukaki to about 640 m a.s.l. near Mount Cook airfield; the maximum altitude on the western side of the study area was about 800 m a.s.l.
Collection and analysis of samples
The study was undertaken between September 2000 and June 2002. Fieldwork was carried out in four 3-month sessions: Session 1, mid-September to mid-December 2000; Session 2, mid-March to mid-June 2001; Session 3, mid-September to mid-December 2001; Session 4, mid-March to mid-June 2002.
Stoats were caught in Mk 3 Edgar live-traps (King & Edgar Citation1977) baited with fresh rabbit and a hen egg. Traps were set in a range of habitat types (scrub/forest, pasture, stabilised riverbed, bare gravel riverbed) throughout the study area at the start of each session. Stoats were anaesthetised, weighed, sexed and fitted with a numbered ear-tag and a two-stage radio-transmitter (Sirtrack Ltd, Havelock North, New Zealand). They were subsequently located on foot using a Telonics TR4 receiver (Telonics Inc., Mesa, AZ, USA) and a hand-held three-element Yagi antenna. In total, 48 individual stoats were fitted with collars; 40 of them were trapped and tracked in one of the four sessions only, seven in two sessions, and one in three sessions. Thirteen animals were tracked in Session 1, 15 in Session 2, 11 in Session 3, and 18 in Session 4. Manipulation of animals was undertaken with the approval of the Department of Conservation's Animal Ethics Committee (Approval AEC 69).
Scats were collected from live traps and from latrines outside dens. When several scats were collected from the same place on the same date, they were treated as a single sample (see King & Powell Citation2007). When bait (egg and/or rabbit) had been eaten in a trap, egg and/or lagomorph were not recorded as prey items in scats collected from that trap. Twenty-nine scats were excluded from analysis because they contained only trap bait (n = 26), because they contained nothing identifiable (n = 2), or because the den was also known to have been used by a female ferret (n = 1). A total of 206 scats contained identifiable remains that were not bait. Scats were collected from 44 stoats (average 4.7 scats per stoat). Scats were identified as being from stoats by size and shape (see King & Murphy Citation2005), and the fact that almost all were collected either from traps (and could not have been from any other species) or from outside dens containing radio-collared stoats (very likely to have been from stoats). In a few cases, large scats (which could have been from male stoats or female ferrets) were not collected. Old dried scats were not collected.
When radio-collared stoats were tracked to dens, the locations were recorded using GPS and the dens were marked. At the end of each session, dens were excavated (where possible) and the contents were collected. Den contents were collected from 36 stoats (average 6.1 dens per stoat). We recorded no cases where excavated dens were re-used by other stoats in subsequent sessions.
Scats and den contents were stored frozen until analysis. Scats were washed through a 1.0 mm sieve and the resulting material was sorted by low-power microscopy into the following broad categories: bird (which included eggshell), lagomorph, rodent, lizard, invertebrate, fish, hedgehog. Identification of feathers and mammalian hairs was carried out following Day (Citation1966). Invertebrate remains were further identified by A. Evans and P. Johns. Den contents were identified by comparison with frozen reference material.
Results are presented as percent frequencies of occurrence of each prey type. Except where noted, the significance of differences in frequency of occurrence between categories was assessed using two-tailed Fisher's Exact tests. As almost all bird species in the study area breed only in spring–summer, frequencies of occurrence of eggshell were expected to be higher in spring samples of scats and den contents than in autumn samples; in those cases, one-tailed Fisher's Exact tests were used to test for seasonal differences. When multiple comparisons were made, probability values were adjusted using the stepwise Holm–Bonferroni correction (Holm Citation1979).
Nomenclature of mammals follows King (Citation2005), and that of birds follows Checklist Committee (Citation2010). Scientific names of prey species are included in .
Table 1 Prey taxa identified in stoat scats and dens, Tasman Valley 2000–2002.
Results
A list of all taxa identified in scats and dens is shown in .
Scats
The percent frequencies of occurrence of the main prey categories in 206 scats are shown in . Birds (36.4%), lagomorphs (56.8%) and invertebrates (49.0%) were present at high frequencies and all other components combined were relatively minor (20.4%). Without the use of DNA analyses, specific identification of bird remains in scats is usually difficult; feathers are readily recognisable but are usually present as small, degraded fragments that cannot be classified beyond Order. Similarly eggshell, which was present in 16.0% of scats, is usually present as small fragments that cannot be identified to species. Categories of invertebrate prey eaten by stoats are shown in . Orthopterans (grasshoppers, locusts, wētās, crickets) were the largest group represented, being identified in 53 (52.5%) of the 101 scats containing invertebrates and in 27.0% of all scats. The most common orthopteran identified to species was the mountain stone wētā Hemideina maori. The most common minor components in scats were lizards (9.2%) and rodents (6.8%). Lizard remains consisted of scales or parts of toes/claws that could not be identified to species; nearly all were skinks, but one gecko was recorded. All rodents identified in scats were house mice (Mus musculus). The ‘Other’ category included stoat hair (probably from grooming, n = 4) and sheep wool (n = 1).
Figure 1 Percent frequencies of occurrence of major prey categories in 206 stoat scats, Tasman Valley 2000–2002. Lago = lagomorph, Invert = invertebrate, Hhog = hedgehog.
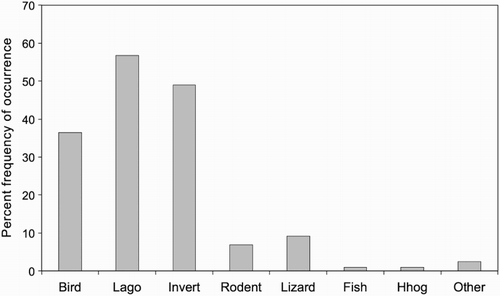
Table 2 Percent frequencies of occurrence of invertebrate taxa in 206 stoat scats, Tasman Valley 2000–2002.
Seasonal differences in the frequencies of occurrence of major prey categories are shown in . The proportion of scats containing all bird remains (eggshell and/or feathers) was significantly higher in spring (55.4%) than in autumn (21.1%) (P < 0.0001). The proportions of scats containing lagomorph remains and invertebrate remains did not differ significantly between seasons (lagomorph P = 0.180, invertebrate P = 0.153).
Figure 2 Percent frequencies of occurrence of prey categories in stoat scats in spring (filled columns, Sessions 1 and 3 combined, n = 92) and autumn (open columns, Sessions 2 and 4 combined, n = 114), Tasman Valley 2000–2002. Lago = lagomorph, Invert = invertebrate, Minor = minor components combined.
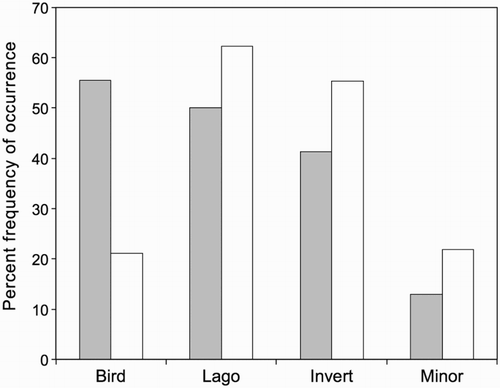
Eggshell occurred significantly more often in spring (29.3%) than in autumn (5.3%) (one-tailed Fisher's Exact test, P < 0.0001), as did bird remains excluding eggshell (spring 35.9%, autumn 17.5%; P = 0.0038).
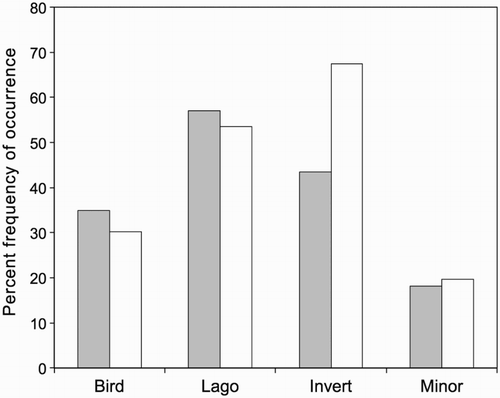
Of the 206 scats analysed, 200 were from traps, latrines or dens that could be assigned to individual male or female stoats (four were from unknown stoats, and two were outside dens known to have been used by a male and a female stoat at different times). The percent frequencies of occurrence of the main prey categories in scats designated as male or female are shown in . There were no significant differences in occurrence of birds, lagomorphs, or minor components, but invertebrate remains were present in a significantly higher proportion of scats from female stoats (P = 0.0272).
Figure 3 Percent frequencies of occurrence of prey categories in scats of male stoats (filled columns, n = 154) and female stoats (open columns, n = 46), Tasman Valley 2000–2002. Lago = lagomorph, Invert = invertebrate, Minor = minor components combined.
There were no significant differences in the frequencies of occurrence of birds, lagomorphs, invertebrates, or minor components in scats from Year 1 (Sessions 1 and 2 combined) and Year 2 (Sessions 3 and 4 combined).
Den contents
Identifiable prey items were found in 219 dens. Dens contained birds and lagomorphs at high frequency and in similar proportions and all other components were minor (). Twenty-three bird taxa were identified (), with most belonging to three Orders: waterfowl (Anseriformes, n = 4), shorebirds (Charadriiformes, n = 6) and passerines (Passeriformes, n = 11). The 10 bird species most commonly identified in stoat dens are listed in . Three of the species were threatened native shorebirds. Most lagomorph remains identified to species were from rabbits, but hare remains were also present. Minor components (rodent, lizard, invertebrate, hedgehog and fish) were present at less than 7.0% each. All the fish remains large enough to identify were trout. The only lizard remains found in dens (n = 2) were pieces of skink foot, too small to identify to species. The ‘Other’ category included brushtail possum (n = 5), ferret (n = 3), sheep wool (n = 3) and stoat hair (n = 2).
Figure 4 Percent frequencies of occurrence of prey categories in 219 stoat dens, Tasman Valley 2000–2002. Lago = lagomorph, Invert = invertebrate, Hhog = hedgehog.
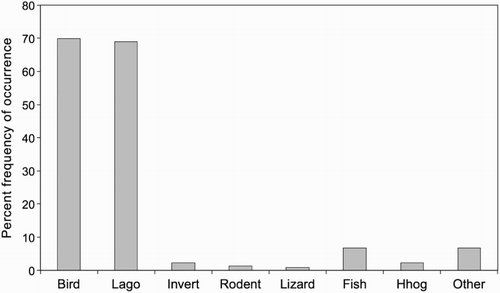
Table 3 The 10 bird species most commonly identified in 219 stoat dens, Tasman Valley 2000–2002.
There were 94 ‘spring’ dens (used by stoats in Sessions 1 and 3 combined) and 125 ‘autumn’ dens (used in Sessions 2 and 4 combined). The frequency of occurrence of all bird remains (eggs, chicks and adults combined) in dens was significantly higher in spring (80.9%) than in autumn (61.6%) (P = 0.0084). Eggshell was present in 46 (21%) of the 219 dens, and also occurred significantly more often in dens excavated in spring than in autumn (one-tailed Fisher's Exact test, P = 0.0008). There were no significant seasonal differences in frequency of occurrence of lagomorphs (spring 66.0%, autumn 71.2%; P = 0.461) or minor categories combined (spring 14.9%, autumn 24.8%; P = 0.182).
Of the 219 dens, 10 were known from radio-telemetry to have been used by a male and a female stoat at different times. The remaining 209 dens were assigned to a gender (males n = 153, females n = 56), although we cannot rule out the possibility that some had been used by a stoat of the other gender. No significant gender-related differences were detected in frequency of occurrence of birds (P = 0.184), lagomorphs (P = 0.134), or minor components combined (P = 0.243).
There were no significant differences in the frequencies of occurrence of birds, lagomorphs or minor components in dens between Year 1 and Year 2.
A number of dens excavated during the study were of particular interest, either because they contained a wide prey spectrum, a large number of one species of prey, or banded birds. Dens that contained notable numbers of shorebirds are shown in .
Table 4 Contents of stoat dens in the Tasman Valley that contained notable shorebird remains.
Banded birds killed by stoats
Colour bands fitted to seven wrybills in the Tasman River between September 1998 and December 1999 were recovered from stoat dens during the study. Remains of four wrybills also had metal bands that had been fitted in the North Island at various times. Two colour-banded wrybills found in the B0932/B0933 den were both 1 year old, and unlikely to have been breeding. A den used by a male stoat in April 2002 contained the remains of a banded dotterel carrying a metal band and a plastic leg flag. The bird had been banded 100 km southeast of Melbourne, Australia, in July 2000.
Comparison of scats and den contents
Percent frequencies of occurrence of the seven major prey categories differed considerably between scats and dens (). Bird, lagomorph and fish remains were more common in dens, whereas mice, lizards and invertebrates were more common in scats.
Table 5 Differences in frequencies of occurrence of prey categories in 206 scats and 219 stoat dens in the Tasman Valley, 2000–2002.
Discussion
Lagomorphs, rodents, birds and invertebrates are common to the diet of stoats in many parts of New Zealand, but there are often local differences related to habitat type, season, gender and other variables (King & Murphy Citation2005). Stoats are flexible in their diet; they are able to take a wide range of prey, but may specialise on one type when it is locally abundant (e.g. Murphy & Dowding Citation1995; Cuthbert et al. Citation2000) or when prey options are limited (e.g. Smith et al. Citation2008). They are also opportunistic and able to switch between types of prey as circumstances change (Murphy & Bradfield Citation1992; Murphy et al. Citation1998). Results from diet studies that differ temporally and spatially are therefore expected to vary, and in many cases the differences probably reflect prey availability.
The bulk of the diet of stoats in the Tasman Valley appeared to be made up of birds and lagomorphs, which both occurred at high frequencies in scats and den contents. Invertebrates were common in scats but were probably a relatively minor component of the diet by weight (King & Moody Citation1982). The fact that female stoats ate more invertebrates than males is consistent with the general observation that female stoats typically prefer smaller prey when they have a choice (e.g. Erlinge Citation1981; McDonald et al. Citation2000). Rodents in non-forested habitat in New Zealand are eaten by stoats (Fitzgerald Citation1964; Alterio & Moller Citation1997; Smith et al. Citation2005). However, mice were scarce in the Tasman Valley during our study and rats were not detected (JED & MJE, unpubl. data), and rodents formed a small part of the diet.
Differences between contents of scats and dens
Most studies of stoat diet in New Zealand have relied on an analysis of scats or gut contents (King & Murphy Citation2005, table 54). The few studies that have examined den contents have been based on relatively few dens (Fitzgerald Citation1964; Murphy & Dowding Citation1995; Dowding & Murphy Citation1996). Radio-collared stoats allowed us to supplement our analysis of scats with an examination of a large sample of den contents, collected in the same area and at the same time as the scats, and from many of the same stoats.
Birds, lagomorphs and fish were more common in dens, whereas mice, lizards and invertebrates were more common in scats. This suggests that stoats generally eat smaller prey items as they find them, and cache larger items (which constitute more than one meal) in dens. Alternatively, stoats may also carry small items to dens, but eat them entirely and leave few remains.
Large species (e.g. sheep, Canada goose) were identified in a few dens, indicating that in addition to killing prey, stoats were also scavenging. Because stoats are not capable of moving such large carcasses, it seems likely that when they find one, they may establish a den nearby. Male (but not female) stoats were recorded denning near and scavenging road-killed brushtail possums (and a Canada goose) in the Eglinton Valley (Murphy & Dowding Citation1994, Citation1995). The importance of scavenged items in the diet of stoats in New Zealand is not clear, and probably varies with location. Although carrion is normally present at low frequencies of occurrence in scats or guts (King & Murphy Citation2005), stock carcasses and goose carcasses from population culls (both present in the Tasman River during our study) potentially provided large quantities of food for stoats. It is possible that carrion could increase stoat survival over winter in some situations.
There are limitations to the use of den contents in quantitative assessments of diet. In our study, dens probably provided an overestimate of the abundance of lagomorphs in stoat diet; some dens were in rabbit burrows, some of which may have contained the remains of rabbits that died of rabbit haemorrhagic disease and were not killed or scavenged by stoats. In addition, dens usually contained multiple prey items, which had often been gathered over an extended period. The occasional presence of wrybill and black-fronted tern remains in ‘autumn’ dens (i.e. at a time when those species are entirely absent from the study area) suggests that some dens were used in an earlier ‘spring’ session. However, the fact that expected seasonal differences (e.g. in birds and eggshell) were detected and were significant, suggests that most remains were relatively recent.
One major advantage of examining den contents is that the remains are nearly always much larger and less degraded than those in scats or guts, allowing easier identification of prey to species level. Stoats commonly left entire wings, heads and feet of birds in dens, and pieces of eggshell were much larger than in scats. As a result, it was possible to identify 23 bird species among den contents and only two from scats. Although not strictly comparable with frequencies of occurrence in scats or guts, frequencies of occurrence of prey in dens can provide an indication of which species are being targeted over a longer period.
Our study suggests that scat (or gut content) analyses and den content analysis are complementary techniques; scats or guts provide a better quantitative, short-term assessment of stoat diet, whereas den contents allow the identification of more prey species and over a longer period. However, locating useful numbers of dens without radio-collared stoats is difficult (King & Powell Citation2007), and it seems likely that most studies of stoat diet will continue to rely on analysis of scats or gut contents from trapping programmes.
Potential impacts on birds
Analysis of scats or guts provides a short-term ‘snapshot’ of stoat diet, and is therefore likely to reveal what stoats are eating most frequently and most recently. Apart from where rare or threatened species are locally abundant (e.g. Cuthbert et al. Citation2000), they are less likely to appear in diet analyses than common species simply because of their rarity. This can obscure potential impacts. For example, no Okarito brown kiwi (Apteryx rowi) remains were found in 871 stoat gut samples from Okarito collected over 3 years (Murphy et al. Citation2008), although stoats are known to be the main predator of the species (McLennan et al. Citation1996). Similarly, Lewis et al. (Citation2010) recorded predation by the small Indian mongoose (Herpestes auropunctatus) on hatchlings of the Critically Endangered Jamaican iguana (Cyclura collei), but did not detect that species in an analysis of more than 200 mongoose gut samples from the same area. Impacts on threatened species may be further hidden by the difficulty of identifying prey remains to species level in gut samples or scats.
The ability to identify a wide range of bird prey in den contents to species level may therefore provide information on the potential impact of stoats on local populations of native birds that may not be afforded by a standard gut or scat analysis. Three of the ten most common bird species identified in Tasman River stoat dens were threatened endemic shorebirds; it is noteworthy that two of them (wrybills and black-fronted terns) were not detected in scats, but were relatively common in dens.
The banded dotterel is currently classified as Threatened (Nationally Vulnerable) by Robertson et al. (Citation2013). Remains were present in 15% of all dens excavated, the highest frequency for any bird species in our study. The banded dotterel was the second most common bird species found in stoat dens on Kaitorete Spit by Fitzgerald (Citation1964).
The wrybill is also classified as Threatened (Nationally Vulnerable) (Robertson et al. Citation2013). The number of wrybills in the whole Tasman River in 1992–1994 was estimated at about 150 (Maloney et al. Citation1997); our surveys suggested that in 2000–2002, there were probably 100 or fewer within our study area. It is worth noting that the 24 adults we found in stoat dens must be an underestimate of numbers killed by stoats, because we are unlikely to have collared all the stoats in the study area, and almost certainly did not find all dens of stoats that were collared.
The black-fronted tern is classified as Threatened (Nationally Endangered) (Robertson et al. Citation2013). Numbers in the Tasman River were estimated at 175 in 1992–1994 (Maloney et al. Citation1997); we estimated that there were fewer than 100 in our study area by 2000. Again, the minimum of 25 adults counted in stoat dens must be an underestimate of actual mortality. In a contemporary study in the Ohau River (about 50 km from our study area), stoats were not recorded as predators of black-fronted terns (Keedwell Citation2005), but the Ohau River was known to have very few or no stoats (Keedwell & Brown Citation2001). A more recent study in the Wairau River, Marlborough, also recorded stoats as predators of adult black-fronted terns (Steffens et al. Citation2012).
Population trends in long-lived birds are usually most sensitive to changes in adult survival (e.g. Croxall & Rothery Citation1991; Saether & Bakke Citation2000). Stoat den contents in the Tasman River suggested that substantial numbers of adult wrybills and black-fronted terns were being killed; this will have had a greater impact on trends of those species locally than losses of similar numbers of eggs or chicks. Without local data on survival and immigration, it is impossible to determine the impact on population trends of the levels of mortality (particularly of adults) that we observed over a 2-year period. However, the numbers of dead adult wrybills and terns found in relation to local population sizes of those species in the Tasman study area appears high, and is cause for concern. We suggest that where stoats are common in braided rivers, they must be considered a significant threat to local populations of wrybills and black-fronted terns, and probably to banded dotterels.
There was no obvious indication that stoats were affecting any particular shorebird species disproportionately. Maloney et al. (Citation1997) found banded dotterels to be the most common shorebird in the Tasman River, followed by wrybills, black-fronted terns and pied oystercatchers. The frequency of remains in the dens we examined mirrored this availability, except that spur-winged plovers (which were not recorded by Maloney et al. Citation1997) ranked second among shorebird species.
A video study of predators at shorebird nests in the Mackenzie Basin showed that only three (3.9%) of 77 lethal events were caused by stoats, and did not show any predation of adult birds by stoats (Sanders & Maloney Citation2002). However, that study was undertaken mainly in four rivers with few or no stoats (Keedwell & Brown Citation2001). The den contents examined in our study suggest that in a riverbed where stoats were more numerous, they were significant predators of all life stages of a range of ground-nesting shorebirds. This difference within a limited geographical area (the Mackenzie Basin) and a single habitat type (braided rivers) reinforces the need to use detailed, site-specific information when assessing threatening processes.
Conclusions
Our study confirms that stoats in New Zealand are flexible in their diet and capable of taking a wide range of prey. This explains in part why they can persist in a broad range of habitat types throughout the country.
Our results provide further evidence that without DNA techniques, diet studies based on analysis of scats or gut contents are unlikely to reveal the full extent of potential impacts of a predator on rare or threatened taxa. Examination of a large sample of den contents permitted the identification of many more prey items to species level, and suggested that when stoats are common in braided river systems, they are probably having a substantial local impact on threatened shorebird species.
Acknowledgements
We thank Ross Ivey, Glentanner Station, for permission to work on his property. We are grateful to Fraser Maddigan (DOC) for assistance with analysis of scats, and to Alison Evans and Peter Johns for identification of invertebrate remains in scats. Thanks also to staff of DOC's Twizel and Aoraki Area Offices for advice and support during fieldwork. Manipulation of stoats was undertaken with the approval of the DOC Animal Ethics Committee (Approval AEC 69). We thank the referees and editor for comments that improved the draft manuscript.
Associate Editor: Dr Grant Norbury.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aikman H 1997. Department of Conservation response plan for use in the event of an outbreak of rabbit calicivirus disease (RCD). Threatened Species Occasional Publication 10. Wellington, Department of Conservation.
- Alterio N, Moller H 1997. Diet of feral house cats Felis catus, ferrets Mustela furo and stoats M. erminea in grassland surrounding yellow-eyed penguin Megadyptes antipodes breeding areas, South Island, New Zealand. Journal of Zoology (London) 243: 869–877. doi: 10.1111/j.1469-7998.1997.tb01987.x
- Checklist Committee 2010. Checklist of the birds of New Zealand. 4th edition. Wellington, Te Papa Press.
- Cromarty P, Scott DA 1996. A directory of wetlands in New Zealand. Wellington, Department of Conservation.
- Croxall JR, Rothery P 1991. Population regulation of seabirds: implications of their demography for conservation. In: Perrins CM, LeBreton JD, Hirons GM eds. Bird population studies: relevance to conservation and management. Oxford, Oxford University Press. Pp. 272–296.
- Cuthbert R, Sommer E, Davis LS 2000. Seasonal variation in the diet of stoats in a breeding colony of Hutton's shearwaters. New Zealand Journal of Zoology 27: 367–373. doi: 10.1080/03014223.2000.9518246
- Day MG 1966. Identification of hair and feather remains in the gut and faeces of stoats and weasels. Journal of Zoology (London) 148: 201–217. doi: 10.1111/j.1469-7998.1966.tb02948.x
- Dowding JE, Murphy EC 1996. Predation of Northern New Zealand dotterels (Charadrius obscurus aquilonius) by stoats. Notornis 43: 144–146.
- Erlinge S 1981. Food preference, optimal diet and reproductive output in stoats Mustela erminea in Sweden. Oikos 36: 303–315. doi: 10.2307/3544627
- Fitzgerald BM 1964. Ecology of mustelids in New Zealand. Unpublished MSc thesis. Christchurch, University of Canterbury. 164 p.
- Holm S 1979. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 6: 65–70.
- Keedwell RJ 2005. Breeding biology of black-fronted terns (Sterna albostriata) and the effects of predation. Emu 105: 39–47. doi: 10.1071/MU04010
- Keedwell RJ, Brown KP 2001. Relative abundance of mammalian predators in the upper Waitaki Basin, South Island, New Zealand. New Zealand Journal of Zoology 28: 31–38. doi: 10.1080/03014223.2001.9518254
- King CM ed. 2005. The handbook of New Zealand mammals. 2nd edition. Melbourne, Oxford University Press. 610 p.
- King CM, Edgar RL 1977. Techniques for trapping and tracking stoats (Mustela erminea); a review, and a new system. New Zealand Journal of Zoology 4: 193–212. doi: 10.1080/03014223.1977.9517953
- King CM, Moody JE 1982. The biology of the stoat (Mustela erminea) in the National Parks of New Zealand. II Food habits. New Zealand Journal of Zoology 9: 49–55. doi: 10.1080/03014223.1982.10423837
- King CM, Murphy EC 2005. Stoat. In: King CM ed. The handbook of New Zealand mammals. 2nd edition. Melbourne, Oxford University Press. Pp. 261–287.
- King CM, Powell RA 2007. The natural history of weasels and stoats: ecology, behavior, and management. 2nd edition. New York, Oxford University Press. 446 p.
- Lewis DS, van Veen R, Wilson BS 2010. Conservation implications of small Indian mongoose (Herpestes auropunctatus) predation in a hotspot within a hotspot: the Hellshire Hills, Jamaica. Biological Invasions 13: 25–33. doi: 10.1007/s10530-010-9781-0
- Maloney RF, Rebergen AL, Nilsson RJ, Wells NJ 1997. Bird density and diversity in braided river beds in the Upper Waitaki Basin, South Island, New Zealand. Notornis 44: 219–232.
- McDonald RA, Murphy EC 2000. A comparison of the management of stoats and weasels in Great Britain and New Zealand. In: Griffiths HI ed. Mustelids in a modern world. Leiden, Backhuys Publishers. Pp. 21–40.
- McDonald RA, Webbon C, Harris S 2000. The diet of stoats (Mustela erminea) and weasels (Mustela nivalis) in Great Britain. Journal of Zoology (London) 252: 363–371. doi: 10.1111/j.1469-7998.2000.tb00631.x
- McLennan JA, Potter MA, Robertson HA et al. 1996. Role of predation in the decline of kiwi, Apteryx spp., in New Zealand. New Zealand Journal of Ecology 20: 27–35.
- Murphy EC, Bradfield P 1992. Change in diet of stoats following poisoning of rats in a New Zealand forest. New Zealand Journal of Ecology 16: 137–140.
- Murphy EC, Dowding JE 1994. Range and diet of stoats (Mustela erminea) in a New Zealand beech forest. New Zealand Journal of Ecology 18: 11–18.
- Murphy EC, Dowding JE 1995. Ecology of the stoat in Nothofagus forest: home range, habitat use and diet at different stages of the beech mast cycle. New Zealand Journal of Ecology 19: 97–109.
- Murphy EC, Clapperton BK, Bradfield PMF, Speed HJ 1998. Effects of rat-poisoning operations on abundance and diet of mustelids in New Zealand podocarp forests. New Zealand Journal of Zoology 25: 315–328. doi: 10.1080/03014223.1998.9518161
- Murphy EC, Keedwell RJ, Brown KP, Westbrooke I 2004. Diet of mammalian predators in braided river beds in the central South Island, New Zealand. Wildlife Research 31: 631–638. doi: 10.1071/WR03033
- Murphy E, Maddigan F, Edwards B, Clapperton K 2008. Diet of stoats at Okarito kiwi sanctuary, South Westland, New Zealand. New Zealand Journal of Ecology 32: 41–45.
- Norbury G, Heyward R 2008. Predictors of clutch predation of a globally significant avifauna in New Zealand's braided river ecosystems. Animal Conservation 11: 17–25. doi: 10.1111/j.1469-1795.2007.00142.x
- Pierce RJ 1987. Predators in the Mackenzie Basin: their diet, population dynamics, and impact on birds in relation to the abundance and availability of their main prey (rabbits). Wellington, Department of Internal Affairs. 110 p.
- Reardon JT, Whitmore N, Holmes KM et al. 2012. Predator control allows critically endangered lizards to recover on mainland New Zealand. New Zealand Journal of Ecology 36: 141–150.
- Rebergen A, Keedwell R, Moller H, Maloney R 1998. Breeding success and predation at nests of Banded Dotterel (Charadrius bicinctus) on braided riverbeds in the central South Island, New Zealand. New Zealand Journal of Ecology 22: 33–41.
- Robertson HA, Dowding JE, Elliott GP et al. 2013. Conservation status of New Zealand birds, 2012. New Zealand threat classification series 4. Wellington, Department of Conservation. 22 p.
- Saether B-E, Bakke Ø 2000. Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81: 642–653 doi: 10.1890/0012-9658(2000)081[0642:ALHVAC]2.0.CO;2
- Sanders MD, Maloney RF 2002. Causes of mortality at nests of ground-nesting birds in the Upper Waitaki Basin, South Island, New Zealand: a 5-year video study. Biological Conservation 106: 225–236. doi: 10.1016/S0006-3207(01)00248-8
- Smith DHV, Jamieson IG, Peach RME 2005. Importance of ground weta (Hemiandrus spp.) in stoat (Mustela erminea) diet in small montane valleys and alpine grasslands. New Zealand Journal of Ecology 29: 207–214.
- Smith DHV, Wilson DJ, Moller H, Murphy EC, Pickerell G 2008. Stoat density, diet and survival compared between alpine grassland and beech forest habitats. New Zealand Journal of Ecology 32: 166–176.
- Steffens KE, Sanders MD, Gleeson DM, Pullen KM, Stowe CJ 2012. Identification of predators at black-fronted tern Chlidonias albostriatus nests, using mtDNA analysis and digital video recorders. New Zealand Journal of Ecology 36: 48–55.
