ABSTRACT
We describe the upper portion of the bill sheath (rhinotheca) of the kākāpō (Strigops habroptilus) from three adult female specimens. The external buccal surface of the rhinotheca is deeply concave with a prominent palatal stop and hardened chevrons creating a ‘milling apparatus’ that the kākāpō uses to grind food. The palatal stop presents a working face of 40–50 mm2. The internal surface of the rhinotheca mirrors the overlying premaxilla and provides a distinct thickened abutment consistent with resistance against the increased workload of the mandibles (gnathotheca) due to the kākāpō’s fibrous diet and chewing style. Along the midline, the rhinotheca at the abutment is up to 5.6 mm thick, compared with as thin as 2.1 mm elsewhere on the midline. The closely related Nestor parrots have less developed palatal stops, chevrons and abutments on their rhinothecas consistent with their lower preference for fibrous plant material. The form of the rhinotheca agrees with the kākāpō’s feeding ecology as a generalist herbivore that grinds locally available fibrous material to assist digestion.
Introduction
Molecular studies confirming the position of the New Zealand endemic parrots Strigops and Nestor in a sister clade to all the other living parrots (Wright et al. Citation2008) led to them being placed together in their own family Strigopidae (Gill et al. Citation2010; Chambers & Worthy Citation2013). However, phylogenetic and morphological differences between the two genera argue for family rank separation (Joseph et al. Citation2012). Rheindt et al. (Citation2014) placed the divergence between Strigops and Nestor at c. 28–29 Ma. Worthy et al. (Citation2011) described the extinct early Miocene (19–16 Ma) fossils from Otago, New Zealand, in the genus Nelepsittacus (Strigopidae). It was shown to be similar to Nestor, but differs in several ways from Strigops. All these studies underline Strigops’ ancient lineage and exceptional characteristics.
The kākāpō (Strigops habroptilus) is the sole representative of its genus and has many features that make it an atypical parrot, including its large size, flightlessness, nocturnality and lek-breeding behaviour (Powlesland et al. Citation2006). The bill of the kākāpō also shows morphological divergence and adaptive change for mode of feeding. The avian bill (or beak) comprises the projecting jaw bones (premaxilla and mandibles) with their enveloping horny (keratinous) sheaths (Campbell & Lack Citation1985; Proctor & Lynch Citation1993). The horny sheath is termed the rhamphotheca with the upper and lower portions often named separately as the rhinotheca and gnathotheca, respectively.
The kākāpō is entirely herbivorous. It is a generalist feeder that consumes leaves, seeds, roots and fruits from a wide variety of plants. While ground-up plant material is digested, as confirmed by modern faecal analyses and coprolite research, mechanical ‘chewing’ performed by the bill reduces how much indigestible cellulose passes into the crop (Butler Citation2006). The kākāpō has a short, stout and strong beak with a short, thick tongue. The kākāpō gnathotheca and tongue work against the shaped palatal surface of the rhinotheca to act as a grinder for fibrous plant materials (McCann Citation1963). Buller (Citation1887–Citation1888, p. 179) noted that, ‘the upper mandible has a peculiar rasp-like character within’. Henry (Citation1903, p. 19) stated that, ‘there are diagonal grooves in the upper mandible, in contact with which the lower acts in the manner of a steel mill’.
McCann (Citation1963, p. 341) noted a distinctively shaped portion of the buccal surface of the rhinotheca of the kākāpō which he called, ‘a large “anvil” upon which the lower mandible operates’. He found that a similar structure in Nestor was less developed. McCann (1963, p. 341) summed up the kākāpō’s bill as, ‘a veritable “nut-cracker” or crusher, suitably adapted, mechanically, for dealing with hard food material’. The most recent study of the kākāpō’s beak morphology (in comparison with that of the kea and kākā) was by Kirk et al. (Citation1993, p. 58) who had available just one kākāpō fledgling and found that the gnathotheca, ‘fitted neatly against a ridged front pad at the front of the hard palate in the 12 mm wide crescent-shaped space between the sides of the upper mandible’. The buccal surface of the rhinotheca, ‘bore 14 small transverse ridges, spaced approximately 1 mm apart; the distal nine being smaller than the other five’ (Kirk et al. 1993, p. 57).
In 2014, Auckland Museum received three kākāpō specimens in poor condition (desiccated or decayed) which were subjected to a controlled rotting process (maceration) so that the bones could be extracted. During maceration of the first specimen the rhamphotheca came away from the underlying bone and was able to be saved and dried. This gave a rare opportunity to study the kākāpō’s rhinotheca including the hidden internal surface that contacts the premaxillary bone. The other two kākāpō and several kea (Nestor notabilis) and kākā (N. meridionalis) were then deliberately macerated in a way that obtained their rhamphothecas for examination.
Given the unusual adaptation of the kākāpō’s bill for grinding, and the interest attached to this atypical species, the purpose of this study is to describe and illustrate the kākāpō’s rhinotheca in finer detail than has been given before and compare the adaptations to observed feeding behaviour.
Methods
Three kākāpō specimens (all adult females) were obtained through the New Zealand Centre for Conservation Medicine (NZCCM) with permission from the Department of Conservation (DOC). All birds were from a managed translocated population. Kākāpō LB14794 (Auckland Museum's specimen registration number), known as ‘Fuchsia’ (bill length = 38.1 mm; this measurement is BCer of Eck et al. Citation2011) was found on Codfish Island on 22 March 2013 with the transmitter indicating death the prior day. LB14836 (bill length = 37.9 mm; ‘Sandra’) was found on Anchor Island on 29 January 2012. The transmitter signal indicated that death occurred on 16 January 2012. LB14838 (bill length = 38.6 mm; ‘Monoa’) was found on Anchor Island on 5 September 2011, approximately 3 months after death. For further details about these named individuals, see Appendix 1 of Ballance (Citation2010).
For comparison, we obtained rhinothecas from two kākā and one kea. Kākā LB14862 (bill length = 36.9 mm) was found on Great Barrier Island and died at NZCCM on 6 April 2013. Kākā LB14902 (bill damaged during maceration) was collected at Mangawhai on 24 August 2009. Kea LB14865 (bill length = 49.7 mm) was collected by DOC Hokitika and presented in November 2005. Another kea, LB13480 (bill length not recorded), had been processed for bones in 2007 and was found to have its rhinotheca intact. This specimen was collected by DOC Hokitika on 24 April 2000.
All specimens were removed from freezer storage and thawed. Any tissue and feathers were removed and the bodies placed into plastic drums for cold-water maceration to obtain the individual bones of the skeleton. The rhinotheca of the bill was removed from each specimen before completion of skeletal maceration and cleaned with hydrogen peroxide. Some rhinothecas were slightly damaged by the process due to the softening that occurs in water. For one kākāpō rhinotheca (LB14794) the dorsal part of the sheath was manually removed for closer inspection of the internal surface. After describing and photographing this structure it was sectioned longitudinally along the mid-line using a jeweller's saw to reveal the cross-sectional shape and thickness along its length.
To check the form of the premaxilla in non-strigopid parrots we used specimens in the Auckland Museum collection. We examined one cacatuid (Nymphicus hollandicus, LB563), one polyteline psittacid (Alisterus scapularis, LB14908) and numerous platycercine psittacids: one Melopsittacus undulatus (LB402), one Neophema pulchella (LB424), six Platycercus eximius (LB6487–9, 6639, 12706, 14259) and 14 Cyanoramphus of three species (LB737, 1394, 1605–6, 1638, 1669, 2030, 2047, 9253, 13469, 13965, 14631, 14712, 14806).
Results
The following description of the lower part of the kākāpō rhinotheca refers to two surfaces, external and internal. The external, or chewing surface, comes into contact with food and the lower bill (gnathotheca). The internal surface lies in contact with the premaxillary bone and is not visible on a live or intact dead bird. shows a kākāpō cranium and premaxilla with the rhinotheca in place (A) and placed beside the premaxilla (B). shows the isolated lower part of the kākāpō rhinotheca showing the external surface (A) and normally-hidden internal surface that contacts the premaxillary bone (B–D). – show comparative views of the rhinothecas of the kākāpō, kākā and kea.
Figure 1. Cranium and premaxilla of kākāpō (Strigops habroptilus; LB14836). A, Left lateral view with rhinotheca in place; B, ventral view with rhinotheca removed to show exposed premaxilla. Photograph: J Froggatt.
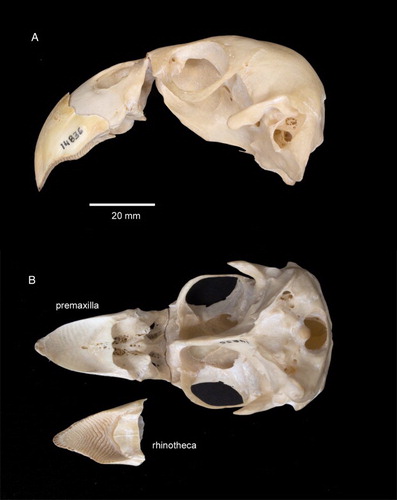
Figure 2. Part of rhinotheca of kākāpō (LB14794); the section that lies under the premaxilla (only) with dorsal section removed. ‘L’ is the ledge and ‘S' is the palatal stop against which the gnathotheca works during chewing, ‘a’ points to the vertical surface of the abutment. A, External buccal surface (i.e. ventral view); B, view of hidden internal surface that is normally in contact with the premaxilla; C, proximal end showing heavily-pitted inter-ridge areas; D, left lateral view (bill tip to left); E, view of cross-section of rhinotheca along the mid-line (bill tip to left) showing relative thickness of rhinotheca along its length. Photograph: P Quin.
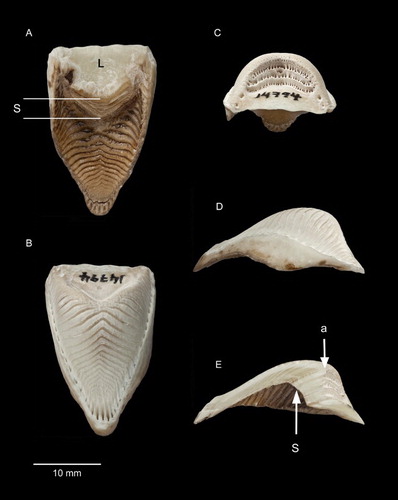
Figure 3. Rhinothecas of kākāpō (A, LB14838), kākā (Nestor meridionalis; B, LB14862) and kea (N. notabilis; C, LB14865). Left lateral view. Photograph: P Quin.

Figure 4. Rhinothecas of kākāpō (A, LB14838), kākā (Nestor meridionalis; B, LB14862) and kea (N. notabilis; C, LB14865). External buccal surface (i.e. ventral view). ‘S1’ is the palatal stop equivalent to that of kākāpō, ‘S2’ is the additional palatal stop of kākā. Photograph: P Quin.
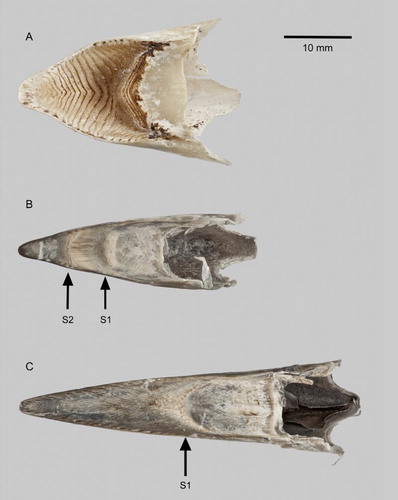
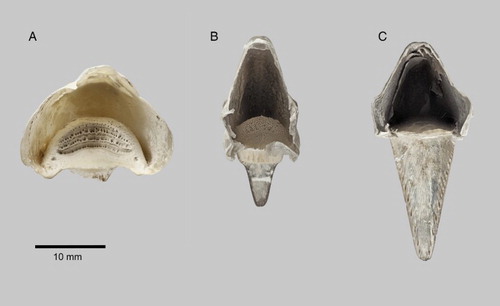
Figure 5. Rhinothecas of kākāpō (A, LB14838), kākā (Nestor meridionalis; B, LB14862) and kea (N. notabilis; C, LB14865). View of proximal end showing cavity after removal of premaxilla. Photograph: P Quin.
Kākāpō
External buccal surface of rhinotheca
The rhinotheca of the kākāpō is broad and deeply concave (, 4A) with the proximal third forming a smooth, slightly concave, ledge (A, ‘L’). The surface then rises abruptly towards the premaxilla at an angle of about 55° from the horizontal (when placed on a flat surface), forming an overhang (see cross-section, E). This acts as a stop (A ‘s’, E ‘s’) against which the gnathotheca works. The surface of the stop has four parallel ridges in a chevron pattern. The first ridge follows the leading (distal) margin of the ledge and the next three continue along the face of the stop. The palatal stop has a grinding face 40–50 mm2 in area (c. 12 mm wide × c. 3.5 mm; n = 3).
From the stop, the rhinotheca surface continues distally following the curve of the beak. This area has a complex pattern of ridges (, 4A). Centred on the midline are at least 10 complete chevrons pointing proximally. The chevrons decrease in size and become closer together along the length of the rhinotheca as it tapers towards the tip. Proximal to the complete chevrons on either side of the stop are a row of some 10–12 broken chevrons with the same orientation as the row of complete chevrons. At the tip of the rhinotheca the chevrons stop short of the end and give way to a region with short longitudinal ridges.
Internal surface of rhinotheca in contact with premaxillary bone
The internal surface of the rhinotheca (B–D) mirrors the external buccal surface in that, moving distally, there is a smooth, slightly convex ledge (B, 2C; where the museum number is written), followed by a ridged abutment and then a long, curved section with complex ridges. Compared to the external surface, the ledge on the internal surface is shorter. The abutment on the inner surface is vertical to the horizontal (c. 90°; see arrow ‘a’ in E). It has four ridges and the three inter-ridge areas are heavily pitted (, 5A). The internal surface of the long curved section has numerous chevrons (B, 2D) broadly mirroring the pattern on the external surface. This inner surface is smooth (whereas the external surface has ridges that can be felt with the fingertip) and the chevrons are the result of alternating rows of material of slightly different colour. At the distal end of the rhinotheca, the ends of the chevrons point forward to form a pattern of longitudinal ridges up to 3 mm long (B).
E shows the cross-section of the rhinotheca at the mid-line. The thickness is not uniform throughout the cross-section, the maximum thickness being 5.6 mm at the region of the stop/abutment, and the minimum being 2.1 mm further towards the tip of the bill. The surface of the overlying premaxilla (B, upper) has an abutment and pattern of chevron marks that approximately mirror those on the internal surface of the rhinotheca (B). However, the premaxilla's surface features are smoothed out and less pronounced.
Kākā
Compared to the kākāpō rhinotheca, that of the kākā (B) is narrower and the form of the external buccal surface is very different, with two palatal stops against which the mandible and tongue work (B). The main stop (B, ‘S2’) is formed at a different position to that of the kākāpō, and is where the kākāpō has its concave palate with prominent chevron-shaped ridges. The counterpart of the kākāpō’s stop (B, ‘S1’) is greatly reduced in the kākā. S2 seems to have two working surfaces orientated forwards and backwards. The working surface of the kākāpō’s palatal stop is directed only forwards. The positions of the kākā’s two palatal stops correspond with the two notches evident on each side of its bill (B).
Regarding the internal surface of the rhinotheca (in contact with the premaxilla), that of the kākā (B) has an abutment that is well developed but not as greatly developed as in the kākāpō (A).
Kea
The form of the external buccal surface of the kea rhinotheca (C), though narrow like the kākā’s, is similar to that of the kākāpō (A), and differs from that of the kākā, in having only one palatal stop (S1). However, the kea is more similar to the kākā in lacking the pronounced concavity and ridging of the kākāpō’s palate. Internally (C), the abutment is undeveloped compared with the condition in the other two species (A, 5B).
Non-strigopid parrots
The buccal surface of the premaxilla (that contacts the internal surface of the rhinotheca) was concave and perfectly smooth, lacking any abutment or pattern of ridges, in all 24 premaxillas (of eight species) that we examined.
Discussion
The features of the kākāpō’s bill structure described here agree with observations by Henry (Citation1903), McCann (Citation1963), Kirk et al. (Citation1993), Higgins (Citation1999) and Gray (in Butler Citation2006). The kākāpō has a shorter and wider bill when compared with the Nestor parrots (McCann Citation1963) and its bill shows pronounced adaptation for grinding (Kirk et al. Citation1993). Field observations show that kākāpō feed exclusively on seasonally available plants and fungi (Higgins Citation1999) with preferences for fruit and new leaf growth. Faecal and coprolite studies confirm that kākāpō have a generalist herbivorous diet that includes trees, shrubs, grasses, ferns mosses and liverworts (Butler Citation2006; Horrocks et al. Citation2008). Gray (in Butler Citation2006) describes in detail the characteristic sign, or ‘chews’, of kākāpō feeding—chewed leaves are compressed between the rhinotheca and gnathotheca into a tight ball which is worked against the ridges of the rhinotheca's buccal surface, crushing the material and removing the available nutrients. This chewed section of the leaf is rejected as a pellet and often left attached to the eaten leaf blade (Butler Citation2006).
In contrast, kākā are omnivorous, with invertebrates, nectar, seeds and fruit making up most of their diet (O'Donnell & Dilks Citation1994; Moorhouse Citation1997; Higgins Citation1999). Their use of the bill varies from excavating and stripping bark in a search for invertebrates, to chiselling to obtain sap, to plucking fruits and pulling flowers from plants (Higgins Citation1999). The reduced, narrower abutment and undeveloped grooves of the kākā’s rhinotheca support field observations of a low preference for fibrous plant material in the diet.
The kea, while similarly omnivorous like the kākā, is more reliant on plants (roots, seeds, leaves, flowers) which comprise 70%–96% of its diet (Higgins Citation1999). The rhinotheca of the kea is longer, narrower and more curved than that of the kākāpō. The kea's preference for plant food, which is broken up and ground with the sharp edge of the gnathotheca against the inside of the rhinotheca, is supported by the presence of grooves on the rhinotheca's buccal surface (Higgins Citation1999). Although the kea is more similar to the kākāpō than the kākā in having these grooves, they are reduced and the internal surface abutment is undeveloped confirming that kea consume less fibrous plant material than kākāpō.
The adaptations of the kākāpō’s rhinotheca enable it to grind food finely within the confines of the bill. In turn the gizzard is much less muscled for a parrot this size with a ‘chewy’ diet (Powlesland et al. Citation2006; Kirk et al. Citation1993), and corresponds with the increase in the gnathotheca's and premaxilla's workload. The rhinotheca has greater enhancement of the ridges on the buccal surface and a greater abutment surface area compared with the Nestor parrots. The near vertical rise of the internal abutment, and chevron-pattern of palatal ridges, are matched by the shape of the ventral premaxillary surface. No such manifestations of a modified buccal surface of the rhinotheca were seen in the premaxillas of eight species of non-strigopid parrots.
The cross-section of the partial rhinotheca of the kākāpō (E) shows that the thickness is greatest at the abutment, where most grinding pressure will occur. These adaptations increase the rhinotheca's robustness for an animal that is able to chew its food, but lacks teeth. Similarly, the developed and hardened ridges on the buccal surface of the rhinotheca act as teeth to break up tough plant material. The kākāpō’s rhinotheca has become modified to cope with increased chewing and fibre in the diet over that of the Nestor parrots, and this allows material to be expelled or more easily digested. Gray's analysis of kākāpō droppings revealed lower levels of grass cuticles and grass seeds than were expected, as kākāpō had been observed stripping and chewing this material in the field. This is most likely because the fibrous content was expelled as chews or broken up into unidentifiable material (Butler Citation2006; Horrocks et al. Citation2008).
Pitted areas visible on the internal surface of the rhinotheca where it contacts the premaxillary bone (B, 2C) may be provision for abundant nerve endings to enable the bird to sense pressure and/or taste while grinding. Demery et al. (Citation2011, pp. 3687–3688) noted a bill-tip organ in Senegal parrots (Poicephalus senegalus) that seemed to comprise, ‘groups of mechanoreceptors embedded in pits at the tip and along the inner ventral edges’ of the rhinotheca.
The unique characteristics seen here in the kākāpō rhinotheca emphasise the role that diet has in adaptive changes in bill morphology and are consistent with its feeding ecology. A factor affecting variability in kākāpō diet is its sedentary habits limiting its ability to source seasonally available plants over different geographic areas (Livezey Citation1992; Horrocks et al. Citation2008). During breeding years, field observations confirm that plant fruits, when they are available, are preferentially eaten over other staples (Powlesland et al. Citation2006; Wilson et al. Citation2006). The evolution of flightlessness in the kākāpō would have increased the selective pressure to adapt to food sources that were locally available year-round (Livezey Citation1992). This could explain the adaptations seen in the rhinotheca and premaxilla to a diet heavy on fibrous plant material, and their reduced presence in the volant Nestor parrots.
Auckland Museum has four fossil Strigops specimens (from caves and probably Holocene in age) where the skull or premaxilla is present (LB4, LB5, LB6079 from Waikato, LB6 from Fiordland). None includes the rhinotheca, confirming that it does not survive decomposition over time as well as bone. This seems to rule out using Holocene material, even from caves, as a source of information on the kākāpō’s rhinotheca and helps to explain why the details we report have not been noticed before.
Preparing bone specimens for a natural history museum collection gave an unexpected opportunity to examine the fine structure of the rhinothecas of New Zealand's large parrots. We have been able to describe and illustrate the kākāpō’s rhinotheca in close detail. In particular, examination of the midline cross-section stresses the importance of a grossly thickened abutment, contacting part of the overlying premaxillary bone at a 90° angle, as a key element in providing strength and robustness to the kākāpō’s grinding apparatus.
Acknowledgements
We thank the NZCCM and DOC for providing the kākāpō specimens used in this study; Peter Quin for producing images; Chris Sheehan for assisting in the sectioning of a segment of rhinotheca; and Matt Rayner, Trevor Worthy and an anonymous referee for helpful comments on the manuscript.
Associate Editor: Associate Professor Jim Briskie.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ballance A. 2010. Kakapo: rescued from the brink of extinction. Nelson: Craig Potton Publishing.
- Buller WL. 1887–1888. A history of the birds of New Zealand. 2nd ed. London: The author.
- Butler DJ. 2006. The habitat, food and feeding ecology of kakapo: in Fiordland: a synopsis from the unpublished MSc thesis of Richard Gray. Notornis. 53:55–79.
- Campbell B, Lack E, editors. 1985. A dictionary of birds. Calton, Staffordshire, UK: T. & A.D. Poyser.
- Chambers GK, Worthy TH. 2013. Our evolving view of the kakapo (Strigops habroptilus) and its allies. Notornis. 60:197–200.
- Demery ZP, Chappell J, Martin GR. 2011. Vision, touch and object manipulation in Senegal parrots Poicephalus senegalus. P Roy Soc B-Biol Sci. 278:3687–3693. doi: 10.1098/rspb.2011.0374
- Eck S, Fiebig J, Fiedler W, Heynen I, Nicolai B, Töpfer T, van den Elzen R, Winkler R, Woog F. 2011. Measuring birds. Wilhelmshaven: Deutsche Ornithologen Gesellschaft.
- Gill BJ, Bell BD, Chambers GK, Medway DG, Palma RL, Scofield RP, Tennyson AJD, Worthy TH. 2010. Checklist of the birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th ed. Wellington: Te Papa Press.
- Henry R. 1903. The habits of the flightless birds of New Zealand; with notes on other New Zealand birds. Wellington: Government Printer.
- Higgins PJ, editor. 1999. Handbook of Australian, New Zealand and Antarctic birds. 4. Parrots to Dollarbird. Melbourne: Oxford University Press.
- Horrocks M, Salter J, Braggins J, Nichol S, Moorhouse R, Elliott G. 2008. Plant microfossil analysis of coprolites of the critically endangered kakapo (Strigops habroptilus) parrot from New Zealand. Rev Palaeobot Palynol. 149:229–245. doi: 10.1016/j.revpalbo.2007.12.009
- Joseph L, Toon A, Schirtzinger EE, Wright TF, Schodde R. 2012. A revised nomenclature and classification for family-group taxa of parrots (Psittaciformes). Zootaxa. 3205:26–40.
- Kirk EJ, Powlesland RG, Cork SC. 1993. Anatomy of the mandibles, tongue and alimentary tract of kakapo, with some comparative information from kea and kaka. Notornis. 40:55–63.
- Livezey BC. 1992. Morphological corollaries and ecological implications of flightlessness in the kakapo (Psittaciformes: Strigops habroptilus). J Morphol. 213:105–145. doi: 10.1002/jmor.1052130108
- McCann C. 1963. External features of the tongues of New Zealand Psittaciformes. Notornis. 10:326–328, 341–345.
- Moorhouse RJ. 1997. The diet of the North Island kaka (Nestor meridionalis septentrionalis) on Kapiti Island. New Zeal J Ecol. 21:141–152.
- O'Donnell CF, Dilks PJ. 1994. Foods and foraging of forest birds in temperate rainforest, South Westland, New Zealand. New Zeal J Ecol. 18:87–107.
- Powlesland RG, Merton DV, Cockrem JF. 2006. A parrot apart: the natural history of the kakapo (Strigops habroptilus), and the context of its conservation management. Notornis. 53:3–26.
- Proctor NS, Lynch PJ. 1993. Manual of ornithology. Avian structure and function. New Haven: Yale University Press.
- Rheindt FE, Christidis L, Kuhn S, de Kloet S, Norman JA, Fidler A. 2014. The timing of diversification within the most divergent parrot clade. J Avian Biol. 45:140–148. doi: 10.1111/j.1600-048X.2013.00200.x
- Wilson DJ, Grant AD, Parker N. 2006. Diet of kakapo in breeding and non-breeding years on Codfish Island (Whenua Hou) and Stewart Island. Notornis. 53:80–89.
- Worthy TH, Tennyson AJD, Scofield RP. 2011. An Early Miocene diversity of parrots (Aves, Strigopidae, Nestorinae) from New Zealand. J Vertebr Paleontol. 31:1102–1116. doi: 10.1080/02724634.2011.595857
- Wright TF, Schirtzinger EE, Matsumoto T, Eberhard JR, Graves GR, Sanchez JJ, Capelli S, Mueller H, Scharpegge J, Chambers GK, Fleischer RC. 2008. A multilocus molecular phylogeny of the parrots (Psittaciformes): support for a Gondwanan origin during the Cretaceous. Mol Biol Evol. 25:2141–2156. doi: 10.1093/molbev/msn160
