ABSTRACT
The energy expenditure of the tūī (Prosthemadera novaeseelandiae), a meliphagid endemic to New Zealand, was measured and compared with 20 species of honeyeaters (family Meliphagidae) to determine whether its expenditure is influenced either by life in a moist, temperate climate or an island residence. Body mass in the honeyeaters accounted for 91.5% of the variation in basal rate. The combination of body mass, climate and the maximal limit to an altitudinal distribution explained 98.6% of the variation in basal rate with tropical, low-altitude species having the highest mass-independent rate. The basal rates of meliphagids in tropical highlands are similar to those in temperate lowlands, which may reflect similar food supplies. The tūī mass-independent expenditure appears to reflect an active lifestyle in a temperate climate with no evidence that an island residence influenced its rate, whereas sedentary birds on New Zealand have responded to island life with a depressed basal rate. An effective analysis of the variation in energy expenditure requires the inclusion of the ecological and behavioural characteristics that distinguish species.
Introduction
The study of the energy expenditure of animals has many values. One is that it is a universal measure of the cost of living. All activities of a species require an expenditure of energy, but if its intake is limited, so too must be its expenditure. A further value of the rate of energy expenditure is that it is inherently quantitative, which theoretically permits a precise accounting of its variation.
An accounting of the variation in expenditures permits us to ask: why do some species have higher expenditures? The major factor influencing energy expenditure is body mass: large species have higher total rates than small species (Kleiber Citation1932; McNab Citation2009). All species of a given size, however, do not have the same rate of metabolism, which means that factors other than mass influence the quantitative variation in energy expenditure. What are these factors and what quantitative impact on energy expenditure do they have?
A difficulty in identifying the factors accounting for the variation in energy expenditure has been the inadequacy of the analytical methods used. The usual approach has been to make what might be called a Kleiberian analysis in which the rate of metabolism is described as a power function of body mass (Kleiber Citation1932). As valuable as this approach has been for more than 80 years, it is unable to account for all of the variation in energy expenditure, the magnitude of this inability depending on the range in mass and the behavioural and ecological diversity of the species examined. The greater this diversity, the less that mass can account for the variation in basal rate, which potentially implies that behaviour and ecology influence residual variation. How can the residual variation in a collection of species be quantitatively analysed? Various attempts have been made to improve the ability to account for the variation of energy expenditure in a group of endotherms. The one used here represents an extension of the Kleiber relationship by bringing in the behavioural and environmental characteristics of species that lead to energy expenditures unexpected from mass (McNab Citation2015).
The factors, other than body mass, that determine the quantitative variation in energy expenditure have been controversial. The two principal views are that it is determined either by: (1) phylogeny; or (2) the various ecological and behavioural factors that distinguish species, the complication being that the occurrence of behavioural characters is described by phylogeny, but their performance reflects conditions faced in the environment (McNab Citation2015). The argument in favour of the direct impact of character states is substantiated by the observation that the level of energy expenditure in convergent species is independent of phylogeny.
Variation in the energy expenditure of 20 species of honeyeaters (Passeriformes: Meliphagidae) is explored here (). This family consists of approximately 44 genera and 180 species (Higgens et al. Citation2008) limited in distribution to Australasia and Oceania. Two species, the tūī (Prosthemadera novaeseelandiae) and the bellbird (Anthornis melanura), are endemic to New Zealand. Data on the tūī are reported here, whereas data from 12 other species come from Australia (MacMillen Citation1985, 10 species; Vitali et al. Citation1999, three species) and seven from New Guinea (McNab Citation2013). One species, the white-naped honeyeater (Melithreptus lunatus), was measured both by MacMillen and Vitali.
Table 1. Energetics and ecological characteristics of meliphigid honeyeaters.
Unlike most meliphagids, the tūī is found in a moist, temperate environment, which raises the question whether this environment has influenced its energy expenditure. Furthermore, the tūī is restricted in distribution to islands (North, South and the Chathams) in New Zealand and evidence has indicated that some species endemic to New Zealand have lower energy expenditures than their continental relatives (McNab Citation1994, Citation1996, Citation2000, Citation2003, Citation2009; McNab & Ellis Citation2006). Does either a moist, temperate climate or island endemism influence the energetics of the tūī?
Methods
Species
Two captive tūī that had been caught in the field were measured during the day at Mt Bruce Wildlife Centre near Masterton, New Zealand. The number of individuals measured and time of measurement were controlled by the centre and the Department of Conservation. More than two individuals are preferred, but preoccupation with the number of individuals can be exaggerated when dealing with rare, endangered or protected species. The arbitrary requirement of measuring at least three individuals (McKechnie & Wolf Citation2004) would prohibit the inclusion of most of these species.
Measurements of metabolism
A post-absorptive tūī was placed into an appropriately sized dark chamber and exposed to a range of ambient temperatures. Room air was drawn through the chamber, the air exiting the chamber had CO2 and water vapour removed, flow rate measured by a Brooks flowmeter, and was delivered to a Beckman paramagnetic oxygen analyser, the electrical output of which was sent to a NGI strip chart recorder. The volume of oxygen consumed (mLO2/h), a measure of the rate of metabolism, was adjusted to standard conditions, i.e. 0 °C and 760 mmHg. The rate of metabolism is proportional to the product of the flow rate and the differential in oxygen tension between room and chamber air. A bird was kept in a chamber for 2 h or longer, depending on the presence or absence of activity; all reported measurements were from periods of inactivity. Data are reported as a mean ± SEM (n = number of measurements).
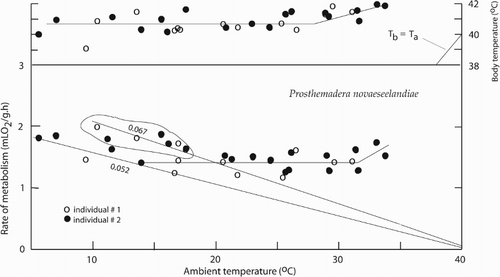
A common measure of energy expenditure in endotherms is the basal rate of metabolism (BMR), which is defined as the rate in post-absorptive individuals in the zone of thermoneutrality and in the absence of activity during the period of inactivity, while maintaining a normal body temperature (McNab Citation1997). Measurements of the tūī were made over a range in ambient temperatures so that the zone of thermoneutrality is clearly defined (). The tūī is diurnal, but could not be measured at night, so a question is whether its rate in thermoneutrality is basal. However, MacMillen (Citation1985) measured 10 Australian meliphagids during daylight hours, the results of which are thus comparable to my measurements on the tūī. Usually birds have a circadian rhythm in energy expenditure in which the minimal rate occurs during the inactive period (Aschoff & Pohl Citation1970); in meliphagids this would occur at night.
Statistics
Data on meliphagids were analysed by ANCOVA (JMP Pro 10.0, SAS Institute). A power function describes the relationship of rate of metabolism to body mass (MR = a [mass]b), where the power b < 1.00. Power functions are analysed by log10 rate as a function of log10 mass with and without other factors in the analysis (). The other factors examined in meliphagids are food habits, climate and maximal limits to an altitudinal distribution. The maximal limits to distribution were broken into four groups (). Species that were limited to altitudes <1000 m were assigned to category 1; those that have a maximal altitude of 2000 m were assigned to category 2; those found up to 3000 m to category 3; and those found at altitudes >3000 m to category 4 (altitude A). These are maximal limits and do not address minimal limits to distribution. Each category may not have an individual impact on basal rate but may when variously combined (see ), such as having a maximal altitude of 1000 m in contrast with all other species (altitude B). Another combination is that species might be divided into those found up to 2000 m vs species found at higher altitudes (altitude C). A fourth combination is that species found up to 3000 m might be different from those found at higher altitudes (altitude D). A similar situation exists with the other factors in the analysis, such as climate, either as temperate/ tropical (climate A) or as wet/dry (climate B). With reference to food habits, six categories were described (food A), or mixed diets could also be allocated to plant (food B) or animal diets (food C). Because 11 species were measured during the day, the effect of the time of measurement was also examined.
Results and discussion
The rate of metabolism and body temperature of the two tūī are plotted as a function of ambient temperature (). The data from the two tūī are in agreement. The zone of thermoneutrality extends from 14–32 °C within which the mean rate of metabolism equals 1.38 ± 0.024 mLO2/g h (n = 20) at a mass equal to 144.2 ± 3.63 g (n = 32). According to an all-bird curve (McNab Citation2009), the tūī basal rate is 107% of the basal rate expected from mass. The minimal thermal conductance that corresponds to the lower limit of thermoneutrality equals 0.052 ± 0.0007 mLO2/g h°C (n = 5) when mean body temperature equals 40.2 °C. A higher thermal conductance equals 0.067 ± 0.0016 mLO2/g h°C (n = 6) when mean body temperature equals 41.1 °C. The mean body temperature of the tūī at ambient temperatures between 5–28 °C equals 40.7 ± 0.12 °C (n = 21).
Based on data from the 20 meliphagids (), log10 mass accounts for 96.4% of the variation in log10 thermoneutral rate (MR) (P < 0.0001):
Consequently:(1) which accounts for 91.5% of the variation in MR (). In the logged form of Equation (1), r2 is larger because it avoids the clumping of data at small masses in the non-logged form.
The impact of time of measurement must be examined because the strict definition of basal rate requires measurement during the inactive period and measurements in 11 meliphagids were made during the day and 10 at night. No difference was found in the daytime and night-time rates in the white-naped honeyeater (A), except as they reflected a difference in mass: the rate in daytime was 104% and at night 107% of the values expected from Equation (1). Furthermore, no difference in log10 rate was found in the collective of 20 species between measurements that were made during the day and those at night (P = 0.055) (). These data diminish the concern about the time of measurement.
Figure 2. A, Log10 basal rate of metabolism in meliphagids as a function of log10 body mass (Equation [1]): Log10 BMR = 0.729 (log10 mass) + 0.776. The two measurements on Melithreptus lunatus are connected. Some of the species that deviate from the fitted relationship are identified. The all-bird curve is derived from McNab (Citation2009). B, The basal rate of metabolism calculated from Equation (2) as a function of the measured basal rate of metabolism.
![Figure 2. A, Log10 basal rate of metabolism in meliphagids as a function of log10 body mass (Equation [1]): Log10 BMR = 0.729 (log10 mass) + 0.776. The two measurements on Melithreptus lunatus are connected. Some of the species that deviate from the fitted relationship are identified. The all-bird curve is derived from McNab (Citation2009). B, The basal rate of metabolism calculated from Equation (2) as a function of the measured basal rate of metabolism.](/cms/asset/8bae90cb-2d46-4fb8-94b8-db045a15ccde/tnzz_a_1148746_f0002_b.gif)
Table 2. Analysis of the factors determining the basal rate of metabolism in Meliphagids.
However, a temporal difference in basal rate appears to exist (P = 0.0020) in the eight species that weigh more than 50 g (log10 = 1.70), which includes the tūī (A). Contrary to expectations, the nocturnal mass-independent measurements in these species average 1.39 times the daytime measurements. This raises a question: do these rates reflect the time of measurement or the species measured? The conclusion here is that the difference reflects the species, given the similarity of diurnal and nocturnal measurements at masses <50 g (P = 0.51), and the similarity of rates in M. lunatus. Therefore, I consider the diurnal measurements in thermoneutrality in the tūī to be an acceptable estimate of its basal rate. Equation (1) indicates that the tūī has a BMR equal to 87% of the value expected from mass, a lower estimate than derived from the all-bird curve because the meliphagid curve is a higher standard (A).
Factors other than time of measurement were examined by regressing log10 basal rate as a function of log10 body mass when individually combined with climate, the maximal limit to an altitudinal distribution, or food habits (). Determining the effect of these factors is complex because several alternative combinations of the conditions in individual factors are available (). For example, log10 basal rate does not correlate with climate (), when divided into temperate and tropical (climate A; P = 0.20) or wet and dry (climate B; P = 0.34). Log10 basal rate did not correlate with any combination of altitudes () or with food habits () when in six categories (P = 0.59), or when mixed diets were designated either as plant (P = 0.54) or animal diets (P = 0.79).
At this point, the basal rate correlates only with body mass. Another possibility is that the rate of metabolism might correlate with factors other than mass when they were combined because of factor interactions. In fact, log10 basal rate correlated with the combination of altitude C (P = 0.014), when broken into two ranges, ≤2000 m and >2000 m; climate A (P = 0.0061), as represented by temperate and tropical; and log10 mass (P < 0.0001):
Therefore:(2) where C is a non-dimensional coefficient for climate equal to 0.68 in temperate species and 1.00 in tropical species and A is a non-dimensional coefficient for maximal altitude equal to 1.40 in species found at altitudes from sea level to 2000 m and 1.00 in species found at higher altitudes (). Equation (2) accounts for 98.6% of the variation in BMR (B) and for 84% ([7.1/8.5] × 100) of the variation missed by Equation (1). No combination of food habits is a significant addition to Equation (2). When time of measurement is added to Equation (2), it was insignificant (P = 0.14), which further justifies the conclusion that the daytime measurements were adequate estimates of basal rate in all species.
The product C·A ranks meliphagids by their mass-independent basal rates: a tropical, lowland species would have the highest rates (1.00 × 1.40 = 1.40); followed by tropical, highland species (1.00 × 1.00 = 1.00); temperate, lowland species (0.68 × 1.40 = 0.95); and temperate, highland species (0.68 × 1.00 = 0.68). Tropical, highland species have mean mass-independent basal rates similar to those of temperate, lowland species, which may reflect a similar availability of nectar and fruit in the two environments. The species with the highest mass-independent basal rate, the helmeted friarbird (Philemon buceroides), is found in the lowland tropics in New Guinea and Queensland, Australia.
Several studies indicated that tropical passerines average lower mass-independent basal rates than temperate species (McNab Citation2000, Citation2009; Wiersma et al. Citation2007; Londoño et al. Citation2015). However, the meliphagids in this study do not conform to this pattern. When both climate and altitude are entered into the analysis, the basal rates of tropical meliphagids are 1.47 (=1/0.68) times those of temperate species (P = 0.0061). This comparison has value because it is with relatives that have similar food habits. Furthermore, tropical birds of paradise have a mean basal rate that is 101% of the passerine mean and 124% of an avian mean (McNab Citation2005). In 274 passerines, 135 temperate species have a mean basal rate that averages only 1.08 times that of 139 tropical species (McNab Citation1998). This analysis does not invalidate the broader comparison of temperate and tropical species, but simply points out that a fauna is a collective containing species with a large diversity of characteristics that deny a uniform response to conditions in the environment (McNab Citation2016). This demonstrates the difficulty of considering one factor (here, climate) out of a broad context.
The lowest mass-independent expenditures of meliphagids would be expected in highland species living in a temperate climate (since C × A = 0.68). No species with this combination of characteristics was encountered and, given the geographic distribution of meliphagids, none may exist, the only potential location being New Zealand, where the highest altitude is on Mt Cook (Aoraki) at 3724 m. The two New Zealand meliphagids, however, are limited to altitudes <1500 m (Higgens et al. Citation2008), which is below the maximal altitude that appears to separate meliphagids with high and low basal rates. The potential adaptation of meliphagids to high altitude is unlikely in Australia because its highest altitude is on Mt Kosciuszko at 2228 m.
The multifactorial analysis (Equation [2]) improves the ability to account for the energy expenditures of species compared with the mass analysis (Equation [1]), as r2 increases from 0.915 to 0.986. The measured basal rate of the tūī, equals 199.0 mLO2/h and a rate estimated from Equation (2) equal to:
99% of the measured value, whereas the rate estimated from mass by Equation (1) equals 223.8 mLO2/h, 113% of the measured value. The estimate from mass, as expected, fails to take into consideration the impacts of climate and an altitudinal distribution. A similar improvement of estimate was found in P. buceroides (75% of the calculated value from Equation [1] and 97% from Equation [2]). However, the multifactorial analysis showed no improvement in predicting the two estimates of basal rate in M. lunatus (85% and 84%, respectively), possibly because some factor(s) important to its lifestyles is (are) missing.
Unfortunately for this analysis, only P. buceroides combines a tropical with a lowland distribution. A complication exists in that Lichmera indistincta, which along with Melidectes rufocrissalis has the second highest mass-independent basal rate (A), is widespread in dry, lowland, temperate Australia, which led it to be characterised as a temperate, lowland species. However, it is also found in tropical Queensland and marginally in wet, lowland, tropical New Guinea. This illustrates the difficulty of assigning some species to discrete climatic categories. If the analysis includes this species as tropical, lowland, L. indistincta then has a basal rate equal to 104% of the value expected from the multifactorial analysis, whereas its estimate from Equation (2) is 84%. With this allocation, the tūī has a predicted basal rate equal to 103% and P. buceroides 90%. Many more tropical, lowland species are needed to confirm high mass-independent basal rates in tropical, lowland meliphagids. In spite of the near equality of the two measurements on M. lunatus the individuals came from climatically different environments, Mediterranean (Perth) and rainforest (Brisbane). An important advancement of our knowledge of the factors influencing the expenditures of meliphagids would be to examine species over a wide geographical and climatic range, especially in Australia.
No evidence indicates that island life or a temperate environment influenced the energy expenditure of the tūī when compared with meliphagids in temperate Australia (P = 0.50). The absence of an adjustment in the tūī implies an active lifestyle, which also appears to be the case in five species of New Zealand parrots (McNab & Salisbury Citation1995) and in the kererū, the New Zealand pigeon (Hemiphaga novaeseelandiae) (McNab Citation2000). In contrast, the energetics of some other birds endemic to New Zealand appear to reflect a sedentary lifestyle, including three species of kiwis (McNab Citation1996), two flightless rails (McNab & Ellis Citation2006) and nine ducks (McNab Citation2003), two of which are flightless. Whether island size or sedentary habits influence the energy expenditure of meliphagids can best be determined by examining species endemic to small, oceanic islands in Polynesia, Melanesia and Micronesia, where an appreciable diversity is found. Alternatively, since the tūī has a subspecies, P. n. chathamensis, endemic to the Chatham Islands, the potential adjustment to a small island could be examined there.
Three phylogenies have described the relationships of the species included in this study (Driskell & Christidis Citation2004; Anderson et al. Citation2004; Joseph et al. Citation2014). They indicated a similar pattern of relationships with the exception of the position of Acanthorhynchus tenuirostris. When the physiological diversity and altitudinal limits to distribution in these 20 meliphagids are put into an evolutionary context (), several points can be made. First, the evolution of these meliphagid subclades involves an extensive interchange between Australia and New Guinea (Joseph et al. Citation2014) without any change in basal rate. The mass-independent basal rates of species within a genus are similar, with the exception of Melidectes. A concern that this similarity represents phylogenetic ‘inertia’ is inappropriate. It reflects the similarity of altitudes and climate in relatives. Phylogeny cannot override environmental factors in the determination of energy expenditure (B. McNab, in prep.). The basis for the variable basal rates in Melidectes is unknown. Equally, the basis for the high basal rate in L. indistincta compared with the low rates of the two species of Phylidonyris is unclear.
Figure 3. Partial phylogeny of meliphagids (Joseph et al. Citation2014). BMR, percentage of the value expected from an all-bird curve (Equation [1]). Climate: TE, temperate; TR; tropical; D, dry; W, wet. Maximal altitude: 1, 1000 m; 2, 2000 m; 3, 3000 m; 4, >3000 m. NG, New Guinea; AU, Australia; NZ, New Zealand.
![Figure 3. Partial phylogeny of meliphagids (Joseph et al. Citation2014). BMR, percentage of the value expected from an all-bird curve (Equation [1]). Climate: TE, temperate; TR; tropical; D, dry; W, wet. Maximal altitude: 1, 1000 m; 2, 2000 m; 3, 3000 m; 4, >3000 m. NG, New Guinea; AU, Australia; NZ, New Zealand.](/cms/asset/b84b6a96-c45a-44cf-93fe-065e17da1be3/tnzz_a_1148746_f0003_b.gif)
Conclusions
The mass-independent energy expenditures of meliphagids vary with several factors, including body mass, climate and maximal limit to an altitudinal distribution. These correlations indicate that their principal determinants are the character states, as modified by conditions encountered in the environment. No evidence exists that indicates that evolutionary history can override the impact of the behaviour of species and the influence of the environment. The most effective way to account for the quantitative variation in energy expenditure in endotherms is to extend a scaling relationship by the introduction of behavioural and environmental factors that lead to basal rates unexpected from an unmodified scaling relationship.
Acknowledgements
Measurements on the tūī were accomplished at Mt Bruce Wildlife Centre, New Zealand with the help of Martin Bell, whose cooperation I greatly appreciate. Permission to work on the tūī required the use of two captive individuals that were caught in the field. The species measured in Papua New Guinea were made at sea level at Madang, 2100 m (Ambua Lodge) and 2860 m (Kamul Lodge). I thank Frank Bonaccorso for his extensive help in Papua New Guinea.
Associate Editor: Associate Professor Jim Briskie.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Anderson MJ, Naikatini A, Moyle RG. 2014. A molecular phylogeny of Pacific honeyeaters (Aves: Meliphagidae) reveals extensive paraphyly and a isolated Polynesian radiation. Mol Phylogenet Evol. 71:308–315. doi: 10.1016/j.ympev.2013.11.014
- Aschoff J, Pohl H. 1970. Rhythmic variations in energy expenditure. Fed Proc. 29:1541–1552.
- Driskell AC, Christidis L. 2004. Phylogeny and evolution of the Austro-Papuan honeyeaters (Passeriformes, Melaphagidae). Mol Phylogenet Evol. 31:943–960. doi: 10.1016/j.ympev.2003.10.017
- Higgens PJ, Christidis L, Ford HA. 2008. Family Meliphagidae (honeyeaters). In: del Hoyo J, Elliott A, Christie D, editors. Handbook of the birds of the world. Volume 13. Penduline-tits to shrikes. Barcelona, Spain: Lynx Edicions; p. 498–691.
- Joseph LA, Toon A, Nyári ÁS, Longmore NW, Rowe KMC, Haryoko TH, Trueman J, Gardner L. 2014. A new synthesis of the molecular systematics and biogeography of honeyeaters (Passerformes: Meliphagidae) highlights biogeographical and ecological complexity of a spectacular avian radiation. Zool Scr. 43:235–248. doi: 10.1111/zsc.12049
- Kleiber M. 1932. Body size and metabolism. Hilgardia. 6:315–353. doi: 10.3733/hilg.v06n11p315
- Londoño GA, Chappell MA, Castañeda MR, Jankowski LE, Robinson SK. 2015. Basal metabolism in tropical birds: latitude, altitude, and the ‘pace of life’. Funct Ecol. 29:338–346. doi: 10.1111/1365-2435.12348
- MacMillen RE. 1985. Energetic patterns and lifestyle in the Meliphagidae. New Zeal J Zool. 12:623–629. doi: 10.1080/03014223.1985.10428311
- McKechnie AE, Wolf BO. 2004. The allometry of avian basal metabolic rate: good predictions need good data. Physiol Biochem Zool. 77:502–521. doi: 10.1086/383511
- McNab BK. 1994. Resource use and the survival of land and freshwater vertebrates on oceanic islands. Am Naturalist. 144:643–660. doi: 10.1086/285698
- McNab BK. 1996. Metabolism and temperature regulation of kiwis (Apterygidae). Auk. 113:687–692. doi: 10.2307/4088996
- McNab BK. 1997. On the utility of uniformity in the definition of basal rate of metabolism. Physiol Zool. 70:718–720. doi: 10.1086/515881
- McNab BK. 1998. Ecological factors affect the level and scaling of avian BMR. Comp Biochem Phys A. 152:22–45. doi: 10.1016/j.cbpa.2008.08.021
- McNab BK. 2000. The influence of body mass, climate, and distribution on the energetics of South Pacific pigeons. Comp Biochem Phys A. 137:309–329. doi: 10.1016/S1095-6433(00)00268-3
- McNab BK. 2003. The energetics of New Zealand's ducks. Comp Biochem Phys A. 135:229–247. doi: 10.1016/S1095-6433(03)00085-0
- McNab BK. 2005. Food habits and the evolution of energetics in birds of paradise (Paradisaeidae). J Comp Physiol B. 175:117–132. doi: 10.1007/s00360-004-0468-7
- McNab BK. 2009. Ecological factors affect the level and scaling of avian BMR. Comp Biochem Phys A. 152:22–45. doi: 10.1016/j.cbpa.2008.08.021
- McNab BK. 2013. The ecological energetics of birds in New Guinea. Bull Uni Florida Mus Nat Hist. 52:95–159.
- McNab BK. 2015. Behavioral and ecological factors account for variation in the mass-independent energy expenditures of endotherms: a review. Comp Physiol B. 84:1–13. doi: 10.1007/s00360-014-0850-z
- McNab BK. 2016. Avian energetics: the passerine/non-passerine dichotomy. Comp Biochem Phys A. 191:152–155. doi: 10.1016/j.cbpa.2015.10.005
- McNab BK, Ellis HI. 2006. Flightless rails endemic to islands have lower energy expenditures and clutch sizes than flighted rails on islands and continents. Comp Biochem Phys A. 145:295–311. doi: 10.1016/j.cbpa.2006.02.025
- McNab BK, Salisbury CA. 1995. Energetics of New Zealand's temperate parrots. New Zeal J Zool. 22:339–349. doi: 10.1080/03014223.1995.9518050
- Vitali SD, Withers PC, Richardson KC. 1999. Standard metabolic rate of three nectarivorous meliphagid passerine birds. Aust J Zool. 47:385–391. doi: 10.1071/ZO99023
- Wiersma P, Muñoz-Garcia A, Wallace A, Williams JB. 2007. Tropical birds have a slow pace of life. Proc Natl Acad Sci USA. 104:9340–9345. doi: 10.1073/pnas.0702212104