ABSTRACT
Larvae of the invasive mosquito Culex quinquefasciatus Say are morphologically similar to those of the native Culex pervigilans Bergroth, yet distinguishing these species can be hampered by morphological variations in Cx. quinquefasciatus. We present detail about the extent of these variations in an urban population of Cx. quinquefasciatus in Auckland. To aid in identification of this exotic species, we provide images of key diagnostic characters and some observed exceptions to these. Details regarding variation in diagnostic characters for < 3rd instar and 3rd/4th instar larvae are given. Of the nine characters used for identification, three were highly consistent (dorsal papillae, mantle plate, pecten teeth); each observed in > 90% of larvae, although these characters were not always visible. Other characters were less reliable, for instance, the expected position of seta 1a-S in relation to the pecten teeth was observed in < 10% of larvae. Further exploration of regional morphological variation in both Cx. quinquefasciatus and Cx. pervigilans is recommended, ideally with associated molecular characterisation.
Introduction
Morphological characters are crucial in the classification and grouping of different organisms (Oliveira et al. Citation2010) in a process known as alpha taxonomy. These characters are compiled into identification keys used to distinguish between species. When using such keys it is often assumed that discriminating characters are well-defined and fixed (Oliveira et al. Citation2010). Yet, in nature, morphological traits can vary considerably within species. For instance, the adult dung beetle (Onthophagus acuminatus Harold) can display a range of body sizes (Emlen Citation1997). Also, some cryptic species such as the hoverflies Merodon atratus (Oldenberg), Merodon virgatus Vujić et Radenković and Merodon balkanicus Šašić, Ačanski et Vujić, can only be identified through subtle morphological variations (Šašić et al. Citation2016). Producing an accurate identification key can therefore be difficult.
Despite these challenges, keys are fundamentally important for mosquito surveillance for biosecurity purposes and health protection, as this is typically based upon the collection of larvae, with preserved (or otherwise non-viable) specimens provided for laboratory identification, rather than through the rearing of live specimens. For species that lack distinguishing larval characters, accurate identification can be difficult. For instance, Culex quinquefasciatus, a mosquito species belonging to the Culex pipiens complex (Miller et al. Citation1996), can be difficult to identify because members of this complex are morphologically alike (Weitzel et al. Citation2009; Nelms et al. Citation2013). Identification problems can be further compounded due to the fact that Cx. quinquefasciatus has been known to hybridise with other species, in particular with Cx. pipiens (e.g. Barr Citation1957; Sanogo et al. Citation2008), where their ranges overlap (Farajollahi et al. Citation2011). This hybridisation has been detected in many areas of North America (Barr Citation1957; Sanogo et al. Citation2008; Kothera et al. Citation2009), and this phenomenon has also contributed to the difficulty in unequivocally distinguishing this species worldwide (Farajollahi et al. Citation2011). If live specimens are available, link-rearing can also be helpful, although it may also be difficult to examine certain features of live larvae.
New Zealand is a temperate landmass with a depauperate mosquito fauna consisting of 15 species (NZBEL Citation2017): 12 endemic, including three Culex species, and three exotics. The endemic Culex pervigilans Bergroth is New Zealand’s most widespread mosquito (Belkin Citation1968), whereas the endemic Culex asteliae Belkin and Culex rotoruae Belkin are comparatively more ecologically specialised and thereby limited in distribution (Belkin Citation1962, Citation1968). Of the three exotic species, Cx. quinquefasciatus was the first recorded in New Zealand, in 1848 (Laird et al. Citation1994), and was thought to have arrived in water barrels on sailing ships (Weinstein et al. Citation1997). Although Cx. quinquefasciatus is generally found throughout tropical regions (Farajollahi et al. Citation2011), this species had become greatly abundant within coastal areas of the North Island of New Zealand by 1990 (Laird Citation1990). Its presence is a concern for wildlife and human health as this species is a known vector of important mosquito-borne diseases such as the West Nile virus, avian malaria and avian pox (van Riper et al. Citation1986; Tompkins & Gleeson Citation2006; Savage et al. Citation2007), and is a potential vector for future introduced arthropod-borne viruses.
There are difficulties in identifying Cx. quinquefasciatus larvae in New Zealand due to its morphological similarity to the endemic Cx. pervigilans. Since Cx. pervigilans is a geographically isolated endemic mosquito, this similarity may well be due to convergence, nevertheless it has led to the speculation of possible hybridisation between the two species (Belkin Citation1968). The only molecular comparison of these species in New Zealand to date found no evidence of hybridisation (Smith & Fonseca Citation2004); however, those authors also acknowledged that further study is necessary because their sample size was extremely small, being limited to only nine Cx. pervigilans and three Cx. quinquefasciatus adult specimens from a single population in Auckland.
We conducted an investigation into the mosquito fauna of an urban Zoological Park in Auckland, New Zealand, 2016, and collected 651 Cx. quinquefasciatus larvae. During a detailed examination of all specimens, it was found that Cx. quinquefasciatus larvae displayed noticeable variability in morphology, and it was clear that a number of diagnostic characters deviated from the descriptions in the most recent key to the Culicidae of New Zealand (Snell Citation2005). Here we present information about the variability exhibited by this urban population of Cx. quinquefasciatus. It is intended that these data be a resource for improving the accuracy of identification of this species in the future.
Methods
The Auckland Zoo is situated in an urban environment, adjacent to a park and recreational area. It covers approximately 17 hectares, and is home to over 875 animals belonging to 135 different species. The environs were surveyed for mosquito larvae over three days in January and February, 2016. Every accessible pool of standing water outside animal enclosures was inspected. Larvae were collected by dipping or with plastic pipettes, including a large domestic version known as a ‘turkey baster’.
Larvae were killed in hot water (Upton Citation1991), placed in 70% ethanol, and kept in the dark to avoid bleaching (Walker & Crosby Citation1988). Specimens were examined microscopically on a Leica Z16 or Leica M205C microscope, over a range of × 0.57 to × 160 magnification. Species identification was based on Snell’s (Citation2005) key, plus a mantle plate character from Belkin (Citation1962, Citation1968) (), as routinely adopted by entomologists from the Mosquito Consulting Services. Snell (Citation2005) recommended that her key be applied to 3rd and 4th instars, which were analysed separately from < 3rd instars. Additional confirmation was provided by Mosquito Consulting Services based on the examination of a representative subset of samples.
Table 1. The nine diagnostic characters used to identify Culex quinquefasciatus larvae, based on Snell (Citation2005) and Belkin (Citation1962, Citation1968). In addition, a Culex pervigilans mantle plate character is also given for comparative purposes.
The proportion (%) of larvae that consistently conformed with Snell’s (Citation2005) key characters and Belkin’s (Citation1962, Citation1968) mantle plate character, was calculated for both < 3rd instar and 3rd/4th instar larvae. In addition, the co-occurrence of pairs of characters was determined for 3rd/4th instars, where these characters were assessable (visible and/or present in undamaged larvae).
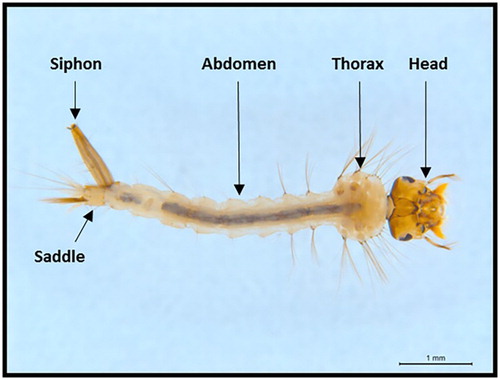
The main body parts of a Cx. quinquefasciatus larva are presented (). Examples of benchmark images of the distinguishing characters used for identifying Cx. quinquefasciatus larvae are provided () and some examples of exceptions to these traits are illustrated (, ).
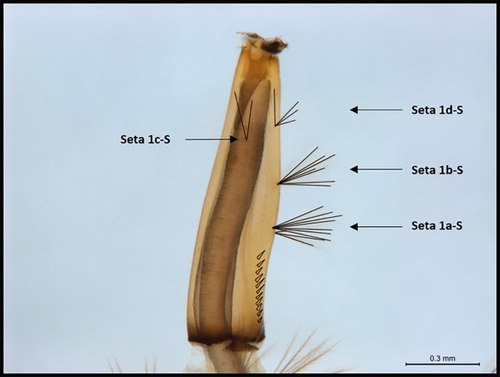
Figure 2. Standard morphological characters used to identify Culex quinquefasciatus larvae. A, Four pairs of ventro-lateral hair tufts, and seta 1d-S not aligned (this image is of a < 3rd instar larvae); B, the pecten teeth, and seta 1a-S positioned above the pecten teeth; C, distinct siphon bulge; D, lateral comb hairs; E, anal papillae about length of the saddle. and dorsal anal papillae longer than ventral anal papillae; F, mantle plate; G, mantle plate drawing modified from Belkin (Citation1962); H, Culex pervigilans mantle plate drawing modified from Belkin (Citation1962).
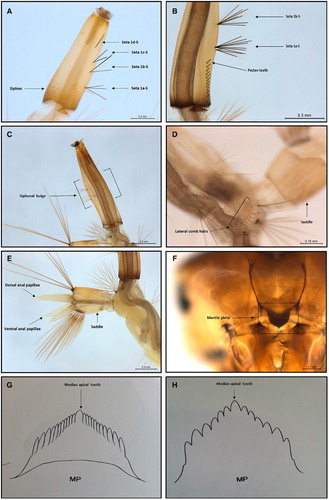
Figure 3. Some morphological variations observed in Culex quinquefasciatus larvae. A, Seta 1a-S in line with pecten teeth rather than above; B, seta 1a-S positioned below the level of pecten teeth rather than above; C, slight bulge of siphon, rather than distinct bulge; and D, anal papillae longer than saddle rather than equal.
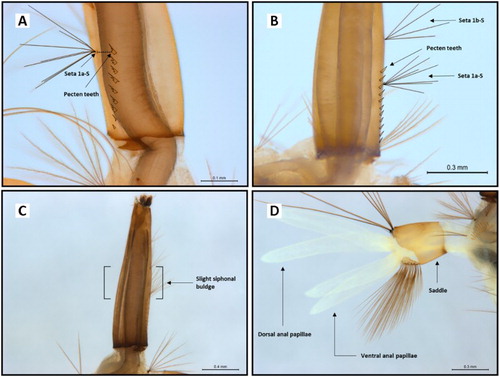
Figure 4. Culex quinquefasciatus larvae displaying seta 1c-S out of alignment, instead of seta 1d-S.
Voucher specimens of Cx. quinquefasciatus larvae were deposited with the Entomology Collection at the Auckland War Memorial Museum (accession numbers: AMNZ96464–96472).
Results
Of the 651 Cx. quinquefasciatus larvae collected, 149 were < 3rd instar and 414 were either 3rd or 4th instars. Instar level could not be determined for 88 larvae, and these individuals were excluded from the analysis. (See Tables S1, S2, for summary data for all inspected 3rd/4th instar and < 3rd instar larvae.)
The Cx. quinquefasciatus larvae commonly displayed morphological variation, but some characters were more consistent (less variable) than others. The three most consistent characters (observed in > 90% of larvae, where this character was visible) were:
However, even within these characters some variation was observed; e.g. the number of pecten and mantle plate teeth ranged from 6 to 15 (B, A,B) and 8–13, respectively. (Comparative reference drawings of the mantle plate teeth from Cx. quinquefasciatus and Cx. pervigilans from Belkin (Citation1962, Citation1968) are provided in G,H). The least consistent trait was the seta 1a-S position in relation to the pecten teeth (seta 1a-S usually above pecten teeth) (B, A,B). This was present in only 7.4% of < 3rd instar and 9.4% of 3rd/4th instar larvae (). The second least consistent trait was the siphon hair tuft alignment (1d-S not aligned) (, ), which was observed in 35.6% of < 3rd instar and 38.6% of 3rd/4th instar larvae ().
Figure 5. The nine diagnostic characters used for identification of Culex quinquefasciatus and their observed frequency (presence) in < 3rd instar (n = 149) and 3rd/4th instar larvae (n = 414) collected at the Auckland Zoo.
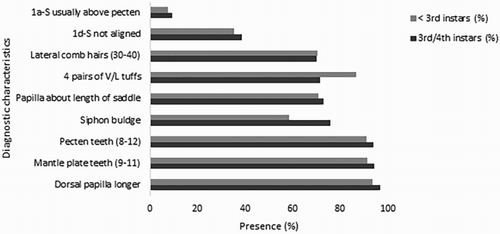
The consistency of the remaining four characters for < 3rd instar and 3rd/4th instar larvae was as follows: siphon shape (58.4% and 75.8%, respectively) (C, ), length of anal papillae in relation to the saddle (70.7% and 72.9%) (E, ), number of siphonal ventrolateral hair tufts (86.6% and 71.7%) (A), and lateral comb patch (70.5% and 70.0%) (D). (The percentages for observed character occurrences are summarised in ).
It is important to note that only six out of the nine diagnostic features (characters 1–6) were visible in 100% of specimens. Three traits (characters 7–9) were sometimes not always assessable being either missing or not visible. The percentages of how assessable these three characters were, are as follows:
Notably, the only two diagnostic features that differed greatly between the younger and older larvae were the presence of four pairs of ventro-lateral hair tufts and the siphon bulge (). Surprisingly, not one of the diagnostic characters was 100% consistent across all larvae with visible characters. Additional information about how the characters varied are provided in Tables S1 and S2.
No two characters co-occurred in all larvae (). The most consistent pair of characters was the length of dorsal anal papillae (in relation to ventral anal papillae) with the number of pecten teeth (). The least consistent character pairs were the siphon hair tuft alignment with the position of seta 1a-S (in relation to the pecten teeth) (). The co-occurrence of siphon hair tuft alignment with any other character did not exceed 40% (). The co-occurrence of seta 1a-S position (in relation the pecten teeth) with any other character did not exceed 10% (). In contrast, the higher co-occurrences of characters with the three most consistent traits highlight that the dorsal papillae, mantle plate and pecten teeth are reliable diagnostic features.
Table 2. Co-occurrence of the nine main diagnostic features used in the identification of Culex quinquefasciatus 3rd and 4th instar larvae.
Discussion
None of the nine characters examined consistently conformed in every individual Cx. quinquefasciatus larva collected from the Auckland Zoo. Conformity of individual characters ranged from 9.4% to 96.7% for 3rd/4th instar larvae and from 7.4% to 93.6% for < 3rd instar larvae. The three most consistent characters were the length of dorsal anal papillae in relation to ventral anal papillae, the number of mantle plate teeth and number of pecten teeth. Therefore, we can confirm that these are the most reliable traits for identifying Cx. quinquefasciatus larvae in Auckland, when these are visible.
The ability to see the mantle plate character does pose a challenge when encountering larvae with dark-coloured heads (as observed in 3rd/4th instar larvae). A possible solution to this may be to allow bleaching of these specimens in natural sunlight, if loss of colouration would not compromise the research value of the specimen. Alternatively, specimens could be slide-mounted using a Lactophenol-PVA solution to clear the larva, making this feature on the head more visible (Rochelle Knox, unpublished data). However, neither of these options was attempted in this study.
The least consistent characters were the seta 1a-S position in relation to the pecten teeth (seta 1a-S usually above pecten teeth), and the siphon hair tuft alignment (1d-s not aligned). The lack of consistency suggests that these features are not reliable for discriminating Cx. quinquefasciatus larvae.
The seta 1a-S was most frequently observed below the level of pecten teeth (b), as seen in 68.4% of < 3rd instar and 62.1% of 3rd/4th instar larvae. This positioning (seta 1a-S overlapping with the pecten teeth) is not a diagnostic character for Cx. pervigilans or any other New Zealand mosquito species (Graham Citation1929; Belkin Citation1962, Citation1968; Dumbleton Citation1968; Snell Citation2005). Therefore, it is unlikely that this trait would be indicative of another Culicidae species within New Zealand. In fact, in overseas literature there does appear to be some contradiction in the positioning of seta 1a-S in relation to the pecten teeth for Cx. quinquefasciatus larvae. For example, Huang (Citation1977) and Russell (Citation1993) have depicted seta 1a-S overlapping (rather than clearly above) the pecten teeth, whereas Sirivanakarn and White (Citation1978) characterised seta 1a-S above the pecten teeth. Whether this is the result of worldwide geographic variation exhibited by Cx. quinquefasciatus is unclear.
Like the seta 1a-S position in relation to the pecten teeth, the siphon hair tuft alignment was not generally useful for Cx. quinquefasciatus identification, as the most common tuft alignment was seta 1c-S not aligned (), seen in 50.3% of < 3rd instar and 43.0% of 3rd/4th instar larvae. Seta 1c-S not aligned is a trait generally indicative of Snell’s (Citation2005) character for Cx. pervigilans. However, we are confident that these larvae were not Cx. pervigilans, as this variable trait (siphon hair tuft alignment) was observed in larvae that carried multiple characters strongly consistent with Cx. quinquefasciatus, particularly with the mantle plate and dorsal papillae. The only exceptions to this were a single 3rd/4th instar larva, and two < 3rd instar larvae. Although the mantle plate was not visible in these three larvae, they were identifiable by their otherwise distinct Cx. quinquefasciatus characters, including the siphon bulge and pecten teeth.
The remaining four characters (siphon shape, length of anal papillae in relation to the saddle, number of siphonal ventro-lateral hair tufts and lateral comb patch) generally conformed to Snell’s (Citation2005) key, but they were less consistently observed across specimens than the three primary characters. As a result, we recommend that these features are used only as secondary traits to back up identification initially based on the three most consistent distinguishing characters.
Identification keys for mosquito larvae are generally based on larger instar stages (e.g. Snell Citation2005; Farajollahi & Price Citation2013). However, the current study indicates that the three most consistent characters used to identify Cx. quinquefasciatus 3rd/4th instar larvae can also be used to identify < 3rd instar stages. Overall, the diagnostic features displayed by < 3rd instar and 3rd/4th instar larvae were relatively similar. The number of siphonal ventro-lateral hair tufts and the siphon shape were the only two characters that differed frequently between the two age groups.
The number of siphonal ventro-lateral hair tufts was found to be a more reliable identifying feature in < 3rd instar larvae, whereas the siphon shape was more reliable for identifying 3rd/4th instar larvae. This appears to indicate that the siphon bulge becomes more pronounced in 3rd/4th instars, as larvae develop. In contrast, the larger proportion of mature individuals that were found to have fewer than four pairs of ventro-lateral hair tufts indicates that older larvae may be prone to losing siphonal hair tufts as they grow. Nonetheless, our finding suggests that features of < 3rd instar larvae may also be useful in distinguishing mosquito species.
Conspecific mosquito larvae from geographically discrete populations have been shown to display morphological variation (e.g. Morais et al. Citation2010; Dehghan et al. Citation2016). Therefore, in a system where two mosquito species may potentially hybridise, it can be challenging to determine if regional variation is due to environmental factors, local genetic characteristics of individual species, or hybridisation. This is a problem faced in New Zealand with Cx. pervigilans and the exotic Cx. quinquefasciatus because, anecdotally, regional differences have been reported in Cx. pervigilans morphology, in parts of the country where Cx. quinquefasciatus has not yet established (Rochelle Knox, unpublished data). This is particularly interesting in the light of Belkin’s (Citation1968) speculation regarding Cx. pervigilans and Cx. quinquefasciatus hybridisation. However, since Smith and Fonseca’s (Citation2004) limited sample did not detect hybridisation, there is currently no molecular evidence of introgression between these two species in New Zealand. Therefore, further molecular characterisation is essential, particularly as the level of overlap in the ranges of these two species has progressively increased since the arrival of Cx. quinquefasciatus in the nineteenth century (Laird Citation1990, Citation1995).
The high degree of variability exhibited by Cx. quinquefasciatus collected during this survey was remarkable. Whether or not the morphology of this species varies in this same way across the entirety of its range is yet to be determined. Regional differences in Cx. quinquefasciatus within New Zealand would not be entirely surprising, as in other parts of the world regional morphological divergence of Cx. quinquefasciatus has been observed. For example, Dehghan et al. (Citation2016) reported 100% of Cx. quinquefasciatus from a central Iranian city had a double branch of seta 1 in the abdominal segments III–IV, whereas only 50% of specimens from a southeastern city had a double branch, the remainder displaying a single branch. Future research will be able to determine if the reliable characters identified in this study also prove to display regional variation in New Zealand. Likewise, further investigation into the extent of regional morphological variability in Cx. pervigilans would be useful.
This study contributes to understanding the morphological variability exhibited by mosquito larvae, and should hopefully assist with the identification of Cx. quinquefasciatus in Auckland and potentially elsewhere in New Zealand. Accurately identifying this invasive species is important not only due to its role in disease transmission, but also because this will enable us to identify future novel mosquito incursions by related species such as the temperate-adapted Cx. pipiens.
Table S1. Morphological characters of the 414 Culex quinquefasciatus larvae (3rd/4th instars).
Download MS Word (52.5 KB)Table S2. Morphological characters of the 149 Culex quinquefasciatus larvae (<3rd instars).
Download MS Word (52.5 KB)Acknowledgements
We would like to thank the staff at the Auckland Zoo, in particular Dr Richard Jakob-Hoff and Sian Buley for enabling this study to happen, Mark Disbury (Mosquito Consulting Services) for helping with the identification of specimens, and John Early (Auckland Museum’s Curator of Entomology) for his input and for providing access to his microscope and camera. We would also like to thank Dr Allen Heath for his feedback and helpful suggestions.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
José G. B. Derraik http://orcid.org/0000-0003-1226-1956
References
- Barr AR. 1957. The distribution of Culex p. pipiens and C. p. quinquefasciatus in North America. The American Journal of Tropical Medicine and Hygiene. 6(1):153–165. doi: 10.4269/ajtmh.1957.6.153
- Belkin JN. 1962. The mosquitoes of the South Pacific (Diptera, Culicidae). Vol. 2. Berkeley: University of California Press.
- Belkin JN. 1968. Mosquito studies VII, the Culicidae of New Zealand. Contributions of the American Entomological Institute. 3(1):1–182.
- Dehghan H, Sadraei J, Moosa-Kazemi SH, Abolghasemi E, Solimani H, Nodoshan AJ, Najafi MH. 2016. A pictorial key for Culex pipiens complex (Diptera: Culicidae) in Iran. Journal of Arthropod-Borne Diseases. 10(3):291–302.
- Dumbleton LJ. 1968. A synopsis of the New Zealand mosquitoes (Diptera Culicidae) and a key to the larvae. Tuatara. 16(3):167–179.
- Emlen DJ. 1997. Alternative reproductive tactics and male-dimorphism in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Behavioral Ecology and Sociobiology. 41(5):335–341. doi: 10.1007/s002650050393
- Farajollahi A, Fonseca DM, Kramer LD, Kilpatrick AM. 2011. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infection, Genetics and Evolution. 11(7):1577–1585. doi: 10.1016/j.meegid.2011.08.013
- Farajollahi A, Price DC. 2013. A rapid identification guide for larvae of the most common North American container-inhabiting Aedes species of medical importance. Journal of the American Mosquito Control Association. 29(3):203–221. doi: 10.2987/11-6198R.1
- Graham DH. 1929. Mosquitoes of the Auckland district. Auckland Institute. 205–244.
- Huang YM. 1977. The mosquitoes of Polynesia with a pictorial key to some species associated with filariasis and/or dengue fever. Mosquito Systematics. 9(3):289–322.
- Kothera L, Zimmerman EM, Richards CM, Savage HM. 2009. Microsatellite characterization of subspecies and their hybrids in Culex pipiens complex (Diptera: Culicidae) mosquitoes along a north-south transect in the central United States. Journal of Medical Entomology. 46(2):236–248. doi: 10.1603/033.046.0208
- Laird M. 1990. New Zealand's northern mosquito survey, 1988–89. Journal of the American Mosquito Control Association. 6(2):287–299.
- Laird M. 1995. Background and findings of the 1993–94 New Zealand mosquito survey. New Zealand Entomologist. 18(1):77–90. doi: 10.1080/00779962.1995.9722010
- Laird M, Calder L, Thornton RC, Syme R, Holder PW, Mogi M. 1994. Japanese Aedes albopictus among four mosquito species reaching New Zealand in used tires. Journal of the American Mosquito Control Association. 10(1):14–23.
- Miller BR, Crabtree MB, Savage HM. 1996. Phylogeny of fourteen Culex mosquito species, including the Culex pipiens complex, inferred from the internal transcribed spacers of ribosomal DNA. Insect Molecular Biology. 5(2):93–107. doi: 10.1111/j.1365-2583.1996.tb00044.x
- Morais SAD, Moratore C, Suesdek L, Marrelli MT. 2010. Genetic-morphometric variation in Culex quinquefasciatus from Brazil and La Plata, Argentina. Memorias do Instituto Oswaldo Cruz. 105(5):672–676. doi: 10.1590/S0074-02762010000500012
- Nelms BM, Kothera L, Thiemann T, Macedo PA, Savage HM, Reisen WK. 2013. Phenotypic variation among Culex pipiens complex (Diptera: Culicidae) populations from the Sacramento Valley, California: horizontal and vertical transmission of West Nile virus, diapause potential, autogeny, and host selection. The American Journal of Tropical Medicine and Hygiene. 89(6):1168–1178. doi: 10.4269/ajtmh.13-0219
- NZBEL. New Zealand Biosecure webpage, viewed 28/4/2017, http://www.smsl.co.nz/NZBEL.html.
- Oliveira VM, Santos CSG, Lana PC, Camargo MG. 2010. Morphological variations caused by fixation techniques may lead to taxonomic confusion in Laeonereis (Polychaeta: Nereididae). Zoologia. 27(1):146–150. doi: 10.1590/S1984-46702010000100022
- Russell RC. 1993. Mosquitoes and mosquito-borne disease in Southeastern Australia. A guide to the biology, relation to disease, surveillance, control and the identification of mosquitoes in Southeastern Australia. Sydney: University of Sydney.
- Sanogo YO, Kim C-H, Lampman R, Halvorsen JG, Gad AM, Novak RJ. 2008. Identification of male specimens of the Culex pipiens complex (Diptera: Culicidae) in the hybrid zone using morphology and molecular techniques. Journal of Medical Entomology. 45(2):203–209. doi: 10.1093/jmedent/45.2.203
- Šašić L, Ačanski J, Vujić A, Ståhls G, Radenković S, Milić D, Vidaković DO, Đan M. 2016. Molecular and morphological inference of three cryptic species within the Merodon aureus species group (Diptera: Syrphidae). PloS one. 11(8):1–27. doi: 10.1371/journal.pone.0160001
- Savage HM, Aggarwal D, Apperson CS, Katholi CR, Gordon E, Hassan HK, Anderson M, Charnetzky D, Millen LMC, Unnasch EA, et al., 2007. Host choice and West Nile virus infection rates in blood-fed mosquitoes, including members of the Culex pipiens complex, from Memphis and Shelby County, Tennessee, 2002–2003. Vector-Borne and Zoonotic Diseases. 7(3):365–386. doi: 10.1089/vbz.2006.0602
- Sirivanakarn S, White GB. 1978. Neotype designation of Culex quinquefasciatus Say (Diptera: Culicidae). Proceedings of the Entomological Society of Washington. 80(3):360–372.
- Smith JL, Fonseca DM. 2004. Rapid assays for identification of members of the Culex (Culex) pipiens complex, their hybrids, and other sibling species (Diptera: Culicidae). The American Journal of Tropical Medicine and Hygiene. 70(4):339–345.
- Snell AE. 2005. Identification keys to larval and adult female mosquitoes (Diptera: Culicidae) of New Zealand. New Zealand Journal of Zoology. 32(2):99–110. doi: 10.1080/03014223.2005.9518401
- Tompkins DM, Gleeson DM. 2006. Relationship between avian malaria distribution and an exotic invasive mosquito in New Zealand. Journal of the Royal Society of New Zealand. 36:51–62. doi: 10.1080/03014223.2006.9517799
- Upton MS. 1991. Methods for collecting, preserving, and studying insects and allied forms. Australian Entomological Society. 3(3):1–86.
- van Riper III C, van Riper SG, Goff ML, Laird M. 1986. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecological Monographs. 56(4):327–344. doi: 10.2307/1942550
- Walker AK, Crosby TK. 1988. The preparation and curation of insects. Wellington: Science Information Publishing Centre (DSIR).
- Weinstein P, Laird M, Browne GN. 1997. Exotic and endemic mosquitoes in New Zealand as potential arbovirus vectors. Wellington Ministry of Health, 1–16.
- Weitzel T, Collado A, Jöst A, Pietsch K, Storch V, Becker N. 2009. Genetic differentiation of populations within the Culex pipiens complex and phylogeny of related species. Journal of the American Mosquito Control Association. 25(1):6–17. doi: 10.2987/08-5699.1