ABSTRACT
The use of tracking tunnels to record footprints is becoming an important tool for monitoring fauna. This study demonstrated that Duvaucel’s gecko (Hoplodactylus duvaucelii) footprints (toe dimensions, foot length and foot area) can be used to predict snout-to-vent length (SVL). Back feet were larger than front feet and, as long as this was taken into account, all anatomical features of footprints predicted SVL with R2 values > 0.73. Measurements of foot area were easy to make and provided R2 values of 0.94–0.97. Footprint analysis of a population of Duvaucel’s geckos on Motuora Island in the Hauraki Gulf, confirmed the presence of island-born geckos and improved estimates of the number of individuals. We propose that image analysis of footprints can be used to increase knowledge of populations of geckos that occur at low density, are cryptic and/or hard to capture, and are being monitored by volunteers.
Introduction
The examination of footprints is a well-established technique that is widely used as a tool in ecological research for establishing the presence, distribution, age structure and habitat preferences of large mammalian herbivores (Western et al. Citation1983) and carnivores (Sharma et al. Citation2005), small mammals under conservation threat (Palma and Gurgel-Goncalvais Citation2007; Mills et al. Citation2016) or that are introduced pests that represent threats to native fauna and flora (King and Edgar Citation1977; Brown et al. Citation1996; Jones et al. Citation2004; Morgan et al. Citation2009; Russel et al. Citation2009) and more recently for monitoring at-risk populations of reptiles (van Winkel Citation2008; Jarvie and Monks Citation2014) and invertebrates (Carpenter et al. Citation2016). For larger animals it is often possible to identify individuals from pugmarks left on the ground (Sharma et al. Citation2005). For smaller animals, tracking tunnels (Yarnell et al. Citation2014) or tracking tubes (Stolen et al. Citation2014) have been used to collect footprints, which are left directly in soft clay (Harrington et al. Citation2008) or recorded on cardboard or paper after the animal has walked across ink, charcoal or chalk (Jones et al. Citation2004). A number of studies have successfully discriminated between relatively similar species (Harrington et al. Citation2008; Russel et al. Citation2009; Angeli et al. Citation2014) and others have discriminated between individuals from the same species (Sharma et al. Citation2005; Herzog et al. Citation2007; Ellison and Swanson Citation2016). Studies on rodent pests have found good relationships between the density of animals estimated by trapping and the activity in tracking tunnels (Brown et al. Citation1996; Blackwell et al. Citation2002) and positive correlations have also been found between density of lizard populations and footprints left in tracking tunnels (Oligosoma aeneum and Oligosoma moco; Siyam Citation2006).
Intentional translocation is an important approach that has been used in the conservation of New Zealand reptiles (Romijn and Hartley Citation2016). Generally, there are four recognisable stages that define progress of a translocated reptile population: (1) survival and growth of founders, (2) evidence of reproduction indicated by detection of new individuals excluding those born by females that were gravid at release, (3) population growth as indicated by the capture of more animals than were originally released and (4) viable population indicated by consistently high numbers of animals including immature animals and a relatively small proportion of founders (Miller et al. Citation2014). Assessment of such progress relies on established monitoring procedures such as systematic searches, live trapping and use of artificial retreats (Lettink and Monks Citation2016). Tracking tunnels are sometimes used to supplement standard approaches; they offer advantages including: minimal disturbance of the environment, no need to handle the animals, minimal need for training of personnel, relatively inexpensive to implement, and they can record the presence of cryptic animals that are hard to observe or capture and/or occur at low frequencies. As such, the use of tracking tunnels is an approach that lends itself to projects relying on volunteers, including those monitoring reptiles (Baling et al. Citation2013).
The subjects of this study were Duvaucel’s geckos (Hoplodactylus duvaucelii). They are New Zealand’s largest extant gecko, growing up to 161 mm in snout-to-vent length (SVL), are nocturnal, take up to seven years to reach maturity (Gill and Whitaker Citation1996; Jewell Citation2008) and are classified as ‘relicts’ with the only remaining populations being found on offshore islands (Hitchmough et al. Citation2013) except for one mainland record for which the existence of the population needs further confirmation (Morgan-Richards et al. Citation2016). The security of these populations is being improved through translocation onto islands that are free from mammalian predators (Romijn and Hartley Citation2016), but survey approaches undertaken within the first decade after the release can struggle to accurately inform conservationists on the success of H. duvaucelii translocations (Bell and Herbert Citation2017). Indeed, Bell and Herbert (Citation2017) were only able to establish the success of a translocation of 40 Duvaucel’s geckos onto Mana Island using survey and hand-capture data aggregated across years 11 to 15 post-release.
Duvaucel’s geckos have been translocated onto islands (van Winkel Citation2008; Bell and Herbert Citation2017) including a number within the inner Hauraki Gulf of which seven are publically owned and four are the subject of community-led ecological restoration (Towns et al. Citation2012). Motuora Island is one of these and is fortunate that despite being farmed in the past it has remained free of mammalian predators, except for an incursion by a single rat (Rattus norvegicus) in 2008. The island is being reforested by volunteers from the Motuora Island Restoration Trust and has a formal ecosystem-based restoration plan (Towns et al. Citation2012). Twenty adult Duvaucel’s geckos (10 females and 10 males) obtained from Korapuki Island in the Mercury Island Group were released at a site on the southern tip of Motuora Island in 2006 (van Winkel Citation2008).
The overall aim of the study was to use Duvaucel’s geckos to determine if lizard footprints collected from tracking tunnels could be used to provide more detailed information on the state of populations undergoing conservation monitoring by volunteer groups. Footprints might contribute information on: the size of individual geckos, the frequency with which geckos of different sizes occur in the population and, recognising that a single gecko might leave multiple sets of footprints, the number of individuals that had contributed footprints. There were a number of steps towards achieving this aim: (1) establish a simple method for measuring the various dimensions and characteristics of the footprints, which could be used in this study and future studies; (2) identify what characteristics of the footprints are best at predicting the SVL of individual geckos; and (3) use these predictors of size to assess population SVL distribution and identify individuals during monitoring of a population of geckos on an offshore island.
Materials and methods
Geckos from the captive breeding programme
Some of the Duvaucel’s geckos collected from Korapuki Island for translocation onto islands in the Hauraki Gulf were retained as part of a captive breeding programme being undertaken by Massey University. Five geckos from each of the six cohorts currently part of the breeding programme were used in this study. The original geckos captured from the wild were included along with the progeny from each successive breeding season (age cohorts = 1, 2, 3, 4 and 5 years old). For each gecko the SVL was measured, the weight was determined and footprints were collected using commercial tracking cards (Blacktraka™; Gotcha Traps Ltd., Warkworth), each of which included an ink pad and two adjacent zones on which footprints could be collected. One tracking card was used per gecko, which allowed recording of footprints on both of the adjacent zones. Each gecko was placed on the ink pad before allowing them to walk across the card.
The plain cards with the footprints were separated from the ink pad and allowed to dry for a few days before scanning to provide an electronic record of the prints. Cards were scanned using Photoshop® 6.0 version 6.0.1 (settings: Reflective, Photo, eight-bit grey-scale, 300 DPI) and saved as .jpeg files (quality setting 7). Measurements of footprints in the scanned images were undertaken using public domain software, Image J (Schneider et al. Citation2012). The calibration function was used to ensure that measurements, in mm, reflected the actual size of footprints. Then it was possible to magnify the images to a size that allowed more precise measurements of the dimensions of the footprints.
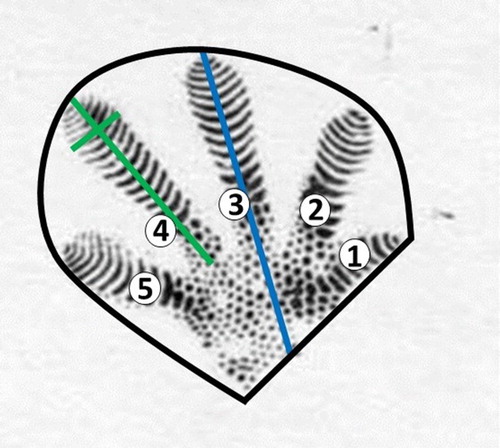
For each gecko, two footprints representing a full print from a front foot and back foot, were selected and the locations were identified by X and Y coordinates (mm) on the image. Preliminary visual examination of the geckos and their footprints had clearly shown that the front foot was smaller than the back foot. For each selected footprint the length from the heel to the tip of the longest toe (fourth toe on a hind foot) was measured, and for each toe, the length and width was measured using the cursor tool. The area of each footprint was determined using a drawing tool to trace a shape defining the area from the heel to the tip of each toe (mm2; ). All statistical analysis was carried out in R 3.2.2 (R Core Team Citation2013). The anatomical measurements were used independently as the explanatory variable in a linear regression model to predict SVL (). The front and back foot were modelled separately. For the anatomical measurement with the highest R2 statistic, 95% confidence and prediction intervals were estimated for each regression line, the confidence intervals provided information on the true population mean and the prediction interval provides information on the distribution of the data. Model assumptions and fit (linear relationship and residuals independently and normally distributed with constant variance) were checked using standard residual plots.
Gecko population on Motuora Island
A project using volunteers to monitor lizards on Motuora Island was initiated in late 2010. Monitoring was carried out over five nights in 2011 (10–15 February), 2012 (2–7 January) and 2013 (19–24 January). Thirty-seven tracking tunnels had been set up according to a grid design (c.10 × c.10 m spacing) established for the original release in 2006 (van Winkel Citation2008) and remain in place for monitoring purposes. On the first day, the tracking cards were placed in the tunnels along with a slice of banana as bait. Tunnels were monitored daily, and if footprints were observed, the card was removed and replaced with a fresh card. The banana bait was replaced as needed. Cards with gecko footprints were processed as described above for the captive population. Then using the predictions developed using the captive population, the SVL of wild geckos was estimated.
Results
Geckos from the captive breeding programme
Sampling from across all age cohorts provided geckos with SVL that ranged from 58 to 124 mm and weights that ranged from 5.0 to 73.2 g (). All anatomical features of footprints demonstrated a strong relationship with SVL (). The strongest relationships according to linear regression models were associated with the area for both the front (R2 = 0.94) and back footprint (R2 = 0.97; ), both of which were relatively fast and easy to collect from images. Residual plots showed that the assumptions were met and the model fitted well. The front foot and back foot were modelled separately, providing an estimation of SVL (mm) using foot area (mm2): SVL = 49.06 + 0.29 × area (Adjusted R2 = 0.94) and SVL = 46.40 + 0.19 × area (Adjusted R2 = 0.97) for the front and back feet, respectively (, ).
Figure 2. Relationships between footprint area and snout-to-vent length (SVL) for Duvaucel’s geckos from a captive breeding programme. Scatterplot of footprint area and SVL showing linear regression model (solid lines), 95% confidence intervals (dotted line) and 95% prediction intervals (dashed line) for the front and back feet.
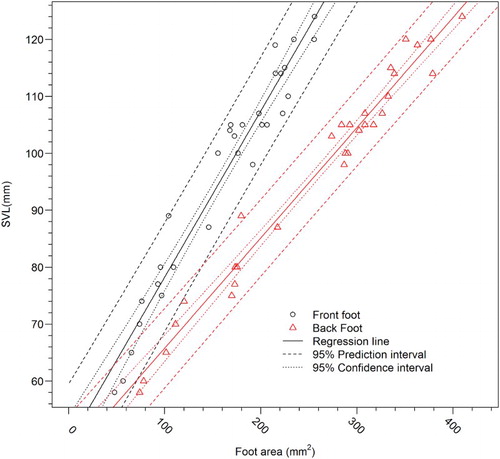
Table 1. Summary of snout-to-vent length (SVL) and weight of the individuals (n = 5) from each cohort of the captive population; values represent the mean with the range presented in parentheses.
Table 2. Linear regression models for predicting snout-to-vent length (SVL) of Duvaucel’s geckos using various footprint characteristics.
Gecko population on Motuora Island
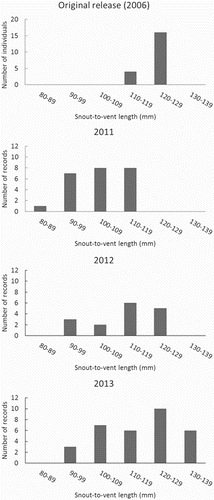
The percentage of tunnels in which footprints were recorded on one or more nights was 30% in 2011 (24 cards with footprints), 19% in 2012 (16 cards with footprints) and 43% in 2013 (32 cards with footprints) (). Anatomical analysis relied on prints left by front feet, because identifiable and un-smudged prints from back feet were rarely if ever left on the cards. There are a number of possible reasons for this: (1) back footprints were obscured by tail smudges or other footprints and/or (2) geckos only got ink on their front feet. The latter occurred when the geckos stepped onto the ink pad with their front feet and then, after eating the banana, backed away before exiting the tunnels. Such an explanation is supported by frequent observations that all the inked footprints pointed towards the ink-pad and were darkest adjacent to the ink-pad and became fainter as the gecko backed away from the ink-pad. In other patterns of footprints it could be seen that geckos backed away and then turned on the card before leaving the tracking tunnel. The 92.5 mm distance to the middle of the ink pad meant that the large geckos in the current study were able to reach the banana bait without their back feet touching the ink. There were few smaller geckos, which are more likely to get ink on all feet as they feed on the bait.
Table 3. Individual Duvaucel’s geckos as identified by estimates of snout-to-vent length (SVL) and their distribution within the grid pattern of tracking tunnels.
The conversion of footprints into estimates of SVL enabled the generation of frequencies of geckos in the various size categories (). However, these observations were subject to the risk that a highly active gecko might be over-represented in the data. An attempt to further understand the population was pursued through use of estimated SVL as the signature of individual geckos; relying also on the spatial and temporal distribution of records of the footprints alongside knowledge of the habitat and how it changes across the study area. Raw data on footprint area could be used to do this, but a decision was made to continue to use estimated SVL as this measure provides a context that enables herpetologists to use their prior knowledge and experience with these and other reptiles. provides an example of how this type of assessment might be undertaken. Geckos within 5 mm estimated SVL that visited neighbouring tunnels or the same tunnel over successive nights were considered to be the same individual. Although there was some ambiguity regarding the association of some footprints with individual geckos, the reallocation of these footprints to different geckos did not alter estimates of the overall number of geckos detected. Using this approach, we speculate that the minimum number of geckos responsible for the prints was as follows: (1) in 2011 the 24 sets of footprints may have been made by nine geckos, (2) in 2012 the 16 sets of footprints may have been made by eight geckos and (3) in 2013 the 32 sets of footprints may have been made by nine geckos.
Discussion
Using tracking tunnels to monitor this low-density population of translocated geckos represents another valuable input of volunteers to species conservation. Through analyses of gecko footprints, the data collected were expanded beyond records of distribution and presence/absence to provide information on the overall health and demography of the Duvaucel’s gecko population on Motuora Island.
Prediction of size of geckos using footprints
The dimensions of all characteristics of the footprints were good predictors of SVL. The best predictors of SVL were those that took into account the entire foot through whole footprint measures such as area or length, although individual toe measurements were also highly correlated with SVL. In almost all cases the geckos did not leave an impression from the first toe. Footprint area represents the area within a geometric boundary that encloses the entire foot and which is defined at the outermost edge by the tips of the toes and the heel of the foot; this approach is based on one of many well-established morphometric approaches (Bookstein Citation1982).
Assessing utility of SVL estimates in field research
Identification that footprints were from Duvaucel’s geckos was straightforward because the only other gecko species present was Raukawa geckos (Woodworthia maculata). These geckos were introduced during 2010 elsewhere on the island, and were not expected to be encountered at the Duvaucel’s gecko release site during our study. Further confirmation was obtained through examination of footprints and particularly the number of lamellae on the fourth toe of the hind foot (15–20 for Duvaucel’s gecko and 12–15 for Raukawa gecko; Jewell Citation2008). In locations where species have overlapping distributions some care and experience would be needed to identify the species based on footprint morphology—however, there are substantial differences in foot shape in terrestrial, partially arboreal and fully arboreal gecko species as well as differences in the counts of lamellae on the toes of terrestrial geckos (Siyam Citation2006; Jewell Citation2008; Jarvie and Monks Citation2014).
The study confirmed that tracking tunnels are an efficient way of monitoring Duvaucel’s geckos in the wild. Estimates of SVL added to the value obtained from the tracking tunnels by providing insights into the distribution of SVL in the population. Duvaucel’s gecko are a long-lived species and the larger footprints (i.e. estimated SVL > 110 mm) may have been left by founders. However, many of the estimated SVLs were < 110 mm, suggesting that they had been made by island-born geckos.
The unique characteristics of footprints such as missing toes might easily identify individual geckos, but the possibility that estimates of SVL might also allow us to identify individual geckos is less likely. Even so, success would offer the opportunity to translate the frequencies with which footprints are recorded into estimates of the number of individual geckos contributing to these records and, hence inform assessments of population growth and viability. The estimates of the number of geckos contributing to footprint records will vary depending on the ‘rules’ used to allocate footprints to an individual. For example, if one was more stringent on the requirements for estimated SVL to be identical, then the proposed number of geckos in the population would increase. Alternatively, if the stipulation that geckos should only have visited the same or neighbouring tunnels was relaxed, then the number of geckos in the population would decrease. However, the approach will only be relevant for small and low-density populations with high levels of variability in size and age. Such populations may occur when individuals that have been translocated to a new site start to breed, but as these populations become established and densities increase the likelihood that two neighbouring geckos cannot be discriminated on the basis of SVL may increase. The assumption that spatial distribution can help to distinguish between geckos of similar estimated SVL that forage in different parts of the study area is reasonable according to optimal foraging theory (Molles Citation2010). For example, it seems unlikely that in 2013 Gecko 1 should regularly be found in tunnels in the southeast (tunnels = A1, A3, B1, B2) and then travel to forage in the northwest (tunnel = F5) without visiting any tunnels between these localities (). The presumption that Gecko 1 was foraging for banana within an approximately 200 m2 area (defined at the corners by visits to grid tunnels: A1, A3, B1 and B3) seems reasonable given that some Motuora Island geckos have been logged travelling an average of 28 m per night in the period after translocation (van Winkel Citation2008). The main risk in the analysis is the level of error associated with estimating SVL because many of the wild prints were of poor quality or were obscured by multiple overlaying prints and tail smears. Validation of the appropriateness of using estimated SVL as a signature for individual geckos will require experimental testing, for example by assessing tracking tunnel data following temporary experimental capture and removal of targeted individuals.
The low frequency of observations or captures of Duvaucel’s geckos during the study has meant that evaluation of the condition of the population on Motuora Island needed to rely on tracking tunnel records. The population seems to be doing well: estimates of SVL show that footprints were from geckos that were born on the island as well as individuals released in 2006. Some geckos seem to leave records in neighbouring tunnels across a number of nights, suggesting that they are foraging and exploiting food sources within a localised and temporary territory, whereas other geckos leave a single record, suggesting that they were moving through the study area. Geckos were mainly recorded in the southeastern or northwestern parts of the study area, although in 2013 some geckos moved into the northeastern quadrant. The middle of the study area has a large patch of thick grass, within which no records of footprints were collected. In an earlier study using radio-tracking to follow geckos (van Winkel Citation2008), it was noted that the geckos were active in this patch of grassland. This suggests that researchers need to be cautious when inferring that a lack of tracking tunnel records reflects an absence of individuals in the habitat. It may be that: (1) the behaviour of geckos (e.g. transit versus feeding) may have not been conducive to visiting the tunnels, (2) the density and complexity of the habitat may have impeded movement of geckos to the tracking tunnels or (3) the density of the habitat limited the diffusion of aroma compounds and hence the ability of the bait to attract animals into the tunnels.
Two further cautions are: first, there have been observations suggesting that some smaller gecko species such as W. maculata (55–82 mm SVL; Jewell Citation2008) have entered tunnels to consume bait, but left no footprints on the card having exited along the wall of the tunnels (NF & TD Harker, personal communication). Second, some geckos may avoid using the tunnels. Indeed, in a subsequent study on Motuora Island it was observed that geckos were recaptured in funnel traps located adjacent to tracking tunnels without ever visiting the tunnels, although the same bait was used (NF & TD Harker, personal communication). These more recent studies have also found that progeny from the 2006 release have spread beyond the original release site and it is therefore likely that monitoring in this original area does not accurately reflect the growth of the population on the island.
In 2013 there was a further release of geckos onto the island with the potential for the new population to move into the current study area. This supplementation of geckos brought the current phase of monitoring to an end.
Conclusion
Image analysis proved to be a simple and effective approach for rapidly analysing the dimensions of footprints collected during tracking tunnel-based monitoring of Duvaucel’s geckos. The dimensions of these footprints, particularly footprint area, were good predictors of SVL and provided valuable additional insights into the status of the population being monitored. The study demonstrated progress of the translocated gecko population in terms of both survival of founders and detection of new individuals (i.e. Stages 1 and 2; Miller et al. Citation2014). The analysis allowed more informed speculation regarding the number of individuals and, based on these estimates, the population in the survey area has not yet fulfilled Miller et al.’s criteria for Stage 3: population growth as indicated by the capture of more animals than were originally released (Miller et al. Citation2014). Overall, the project demonstrated the successful implementation of a translocation framework (Miller et al. Citation2014), in circumstances where volunteers were involved in post-release monitoring at a site that could not be easily or frequently visited by experts to collect appropriate intensive data using more intrusive capture techniques. Our footprint analysis methodology is especially useful for determining initial outcomes in the first few years, where it is difficult to find island-born animals due to their cryptic behaviour in a large and complex environment.
Acknowledgements
We thank the Motuora Island Restoration Society for their support; Melinda Rixon, Dylan van Winkel and Marleen Baling for the training they provided and for their foresight in establishing reptile monitoring for the island; Motuora Island Rangers, Sian Potier and Toby Shanley for advice and hospitality during our time on the island, Liz Maire for her encouragement and help in collecting data, Dr Ian Hallett for advice on image analysis and Kate Richards for her support in statistical analysis. Jo Monks and Chrissie Painting for advice and helpful critiques during revision of the manuscript and Trent Bell for his advice and for the framework to interpret these data. Tim Harker undertook research using the captive geckos as part of a scholarship awarded under the Liggins Education Network for Science Student–Scientist Mentor Programme and would like to thank his mentors Helen Mora (Senior Educator, Liggins Education Network for Science) and Elizabeth Bouchard (Onehunga High School). Research was undertaken under DOC National Permit Number AK-29843-FAU.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Angeli T, de Oliveira ML, Duarte JMB. 2014. Differentiation of deer species of the genus Mazama by track morphometry. Studies on Neotropical Fauna and Environment. 49:199–203. doi: 10.1080/01650521.2014.958898
- Baling M, van Winkel D, Rixon M, Ruffell J, Ji W, Ussher G. 2013. A review of reptile research and conservation management on Tiritiri Matangi Island, New Zealand. New Zealand Journal of Ecology. 37:272–281.
- Bell TP, Herbert SM. 2017. Establishment of a self-sustaining population of a long-lived, slow-breeding gecko species (Diplodactylidae: Hoplodactylus duvaucelii) evident 15 years after translocation. Journal of Herpetology. 51:37–46. doi: 10.1670/15-106
- Blackwell GL, Potter MA, McLennan JA. 2002. Rodent density indices from tracking tunnels, snap-traps and Fenn traps: do they tell the same story? New Zealand Journal of Ecology. 26:43–51.
- Bookstein FL. 1982. Foundations of morphometrics. Annual Review of Ecology and Systematics. 13:451–470. doi: 10.1146/annurev.es.13.110182.002315
- Brown KP, Moller H, Innes J, Alterio N. 1996. Calibration of tunnel tracking rates to estimate relative abundance of ships rats (Rattus rattus) and mice (Mus musculus) in a New Zealand forest. New Zealand Journal of Ecology. 20:271–275.
- Carpenter JK, Monks JM, O’Donnell CFJ. 2016. Developing indices of relative abundance for monitoring cave and ground wētā (Orthoptera) in southern beech forest, New Zealand. New Zealand Journal of Zoology. 43:149–162. doi: 10.1080/03014223.2015.1117500
- Ellison SA, Swanson BJ. 2016. Individual identification of raccoons (Procyon lotor) using track plate foot printing. The American Midland Naturalist. 176:306–312. doi: 10.1674/0003-0031-176.2.306
- Gill B, Whitaker T. 1996. New Zealand frogs and reptiles. Auckland: Bateman Fieldguides.
- Harrington LA, Harrington AL, Macdonald DW. 2008. Distinguishing tracks of mink Mustela vision and polecat M. putorius. European Journal of Wildlife Research. 54:367–371. doi: 10.1007/s10344-007-0145-8
- Herzog CJ, Kays RW, Ray JC, Gompper ME, Zielinski WJ, Higgins R, Tymeson M. 2007. Using patterns in track-plate footprints to identify individual fishers. Journal of Wildlife Management. 71:955–963. doi: 10.2193/2006-408
- Hitchmough R, Anderson P, Barr B, Monks J, Lettink M, Reardon J, Tocher M, Whitaker T. 2013. Conservation status of New Zealand reptiles, 2012. Wellington: New Zealand Department of Conservation.
- Jarvie S, Monks JM. 2014. Step on it: can footprints from tracking tunnels be used to identify lizard species? New Zealand Journal of Zoology. 41:210–217. doi: 10.1080/03014223.2014.911753
- Jewell T. 2008. A photographic guide to reptiles and amphibians of New Zealand. Auckland: New Holland Publishers (NZ) Ltd.
- Jones C, Moller H, Hamilton W. 2004. A review of potential techniques for identifying individual stoats (Mustela erminea) visiting control or monitoring stations. New Zealand Journal of Zoology. 31:193–203. doi: 10.1080/03014223.2004.9518372
- King CM, Edgar RL. 1977. Techniques for trapping and tracking stoats (Mustela erminea); a review, and a new system. New Zealand Journal of Zoology. 4:193–212. doi: 10.1080/03014223.1977.9517953
- Lettink M, Monks JM. 2016. Survey and monitoring methods for New Zealand lizards. Journal of the Royal Society of New Zealand. 46:16–28. doi: 10.1080/03036758.2015.1108343
- Miller KA, Bell TP, Germano JM. 2014. Understanding publication bias in reintroduction biology by assessing translocations of New Zealand’s herpetofauna. Conservation Biology. 28:1045–1056. doi: 10.1111/cobi.12254
- Mills CA, Godley BJ, Hodgson DJ, Cameron EZ. 2016. Take only photographs, leave only footprints: novel applications of non-invasive survey methods for rapid detection of small, arboreal animals. PLoS ONE. 11:e0146142. doi: 10.1371/journal.pone.0146142
- Molles CM Jr. 2010. Ecology: concepts and applications, 5th ed. New York: McGraw-Hill.
- Morgan DKJ, Waas JR, Innes J. 2009. An inventory of mammalian pests in a New Zealand city. New Zealand Journal of Zoology. 36:23–33. doi: 10.1080/03014220909510136
- Morgan-Richards M, Hinlo AR, Smuts-Kennedy C, Innes J, Ji W, Barry M, Brunton D, Hitchmough RA. 2016. Identification of a rare Gecko from North Island New Zealand, and genetic assessment of its probable origin: a novel mainland conservation priority? Journal of Herpetology. 50:77–86. doi: 10.1670/13-128
- Palma ART, Gurgel-Goncalvais R. 2007. Morphometric identification of small mammal footprints from ink tracking tunnels in the Brazilian Cerrado. Revista Brasileira de Zoologia. 24:333–343. doi: 10.1590/S0101-81752007000200011
- R Core Team. 2013. R: a language and environment for statistical computing. https://www.R-project.org/.
- Romijn RL, Hartley S. 2016. Trends in lizard translocations in New Zealand between 1988 and 2013. New Zealand Journal of Zoology. 43:191–210. doi: 10.1080/03014223.2016.1146311
- Russel JC, Hasler N, Klette R, Rosenhahn B. 2009. Automatic track recognition of footprints for identifying cryptic species. Ecology. 90:2007–2013. doi: 10.1890/08-1069.1
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 9:671–675. doi: 10.1038/nmeth.2089
- Sharma S, Jhala Y, Sawarkar VB. 2005. Identification of individual tigers (Panthera tigris) from their pugmarks. Journal of Zoology. 267:9–18. doi: 10.1017/S0952836905007119
- Siyam SM. 2006. Reptile monitoring: development of an effective, passive monitoring technique [M.Sc. thesis], Auckland, New Zealand: University of Auckland.
- Stolen ED, Oddy DM, Breininger DR, Gann SL, Legare SA, Weiss SK, Holloway-Adkins KG, Schaub R, Legare ML. 2014. Preventing tracking-tube false detections in occupancy modeling of southeastern beach mouse. Journal of Fish and Wildlife Management. 5:270–281. doi: 10.3996/032014-JFWM-025
- Towns DR, Bellingham PJ, Mulder CPH, Lyver POB. 2012. A research strategy for biodiversity conservation on New Zealand’s offshore islands. New Zealand Journal of Ecology. 36:1–20.
- van Winkel D. 2008. Efficiency of techniques for post-translocation monitoring of the Duvaucel’s gecko (Hoplodactylus duvaucelii) and evidence of avian predation on lizards [M.Sc. thesis], Auckland, New Zealand: Massey University.
- Western D, Moss C, Georgiadis N. 1983. Age estimation and population age structure of elephants from footprint dimensions. Journal of Wildlife Management. 47:1192–1197. doi: 10.2307/3808191
- Yarnell RW, Pacheco M, Williams B, Neumann JL, Rymer DJ, Baker PJ. 2014. Using occupancy analysis to validate the use of footprint tunnels as a method for monitoring the hedgehog Erinaceus europaeus. Mammal Review. 44:234–238. doi: 10.1111/mam.12026