ABSTRACT
Kiore (Pacific rat; Rattus exulans) is both a target for eradication and a taonga or highly valued species in New Zealand, and its abundance and distribution vary considerably throughout the country. We investigated reports of an abundant kiore population on Slipper Island (Whakahau), off the east coast of New Zealand’s North Island, in March 2017. We trapped kiore to examine their distribution across a range of habitats with varying degrees of human activity. Kiore were captured in all habitats, with particularly high abundance at a campground with a fruiting fig tree (50 kiore per 100 trap nights corrected for sprung traps). We found no evidence of other rat species; Slipper Island appears to remain one of few New Zealand islands with kiore but without ship rats (Rattus rattus) and Norway rats (R. norvegicus), the two other rat species present in New Zealand. Slipper Island potentially provides opportunities to research kiore behaviour and population dynamics in a New Zealand commensal environment, and genetics of an isolated island population.
TUHINGA-WHAKARĀPOPOTO
Ki ētahi ko te kiore muritai (Rattus exulans) he kīrearea hei haepapa. Ki ētahi atu anō, he taonga kāmehameha ki Aotearoa. Ko tōna huhua me te marara hoki o tana noho ka tāhapa ki tēnā ki tēnā tauwāhi o te motu. I mātaihia e mātou ētahi pūrongo (mai i te tau 2017) e kiia nei he taupori huhua e noho ana ki runga o te moutere Whakahau, ki waho atu o te takutai rāwhiti o te Ika a Māui. I tārorehia ētahi kiore muritai e mātou hei mātakitaki i te whānui o tā rātou noho marara puta noa i ētahi nohoanga whakaehu i runga anō i te tokomaha, te tokoiti rānei o te tangata. I hopukina ngā kiore muritai nei ki ngā momo nohoanga katoa (ahakoa te tokomaha te tokoiti rānei o te tangata). E hia kē ngā kiore i hopukina ki tētahi whenua-puni i reira he rākau piki (Ficus carica) e tipu ana i taua wāhi, 50 ngā kiore i hopukina i ngā pō tārore 100. Ka mutu, i whakatikahia tēnei nama mō ngā tārore turupana. Kāore mātou i kite taunakitanga mō ētahi atu momo kiore. Te āhua nei ko te moutere Whakahau tētahi o ngā moutere whakamutunga o Aotearoa kei reira te kiore muritai e noho tonu ana engari kāore hoki i reira te kiore kaipuke (Rattus rattus) te kiore nōwei rānei (R. norvegicus). Ko ēnei momo kiore e rua e kitea whānuitia ana ki Aotearoa. Kei runga o Whakahau pea te āheinga kia rangahauhia ngā whanonga me ngā taitaupori o te kiore muritai ki tētahi nohoanga tauutuutu ki Aotearoa. Kei reira hoki te āheinga kia rangahauhia ngā mātauranga momo whakaheke o te kiore nei ki te taupori pūreirei ā moutere nei.
TINO-KUPU:
Introduction
Globally, invasive species are recognised as a key conservation challenge and are the focus of considerable research and targeted eradication efforts at significant economic cost. Invasive mammalian predators have driven species declines and extinctions worldwide and threaten further losses (Doherty et al. Citation2016). In New Zealand, controlling introduced mammalian predators such as rats, possums and mustelids, and creating predator free sanctuaries are primary conservation tools. The kiore (or Pacific rat; Rattus exulans) was the first mammalian predator to arrive in New Zealand, on the seafaring vessels of Polynesian voyagers in c. 1280 AD (Matisoo-Smith et al. Citation1998; Wilmshurst et al. Citation2008). At that time, New Zealand’s native fauna was dominated by a rich suite of birds (many flightless), diverse geckos and skinks, and numerous large invertebrates, with bats the only extant terrestrial mammals. Seabirds were abundant, nesting on offshore islands and the mainland (Holdaway et al. Citation2001), and fruits and seeds were plentiful in the lush temperate and sub-tropical forests (McGlone Citation1989; Campbell and Atkinson Citation1999). Māori oral tradition (Mead and Grove Citation2003; Wehi, Cox, Whaanga, Roa, unpubl. data) indicates that kiore were managed and harvested in forested areas (Haami Citation1994), but were also associated with human settlements in New Zealand, consistent with their commensal status throughout much of their international range (Williams Citation1973; Atkinson and Towns Citation2005; Hingston Citation2015).
As the sole exotic rodent for about 500 years until European colonisation in the early nineteenth century (Holdaway Citation1989), kiore spread quickly across mainland New Zealand (Holdaway Citation1989; Towns et al. Citation2006; Gibbs Citation2009), reaching offshore islands as opportunity arose (Bellingham et al. Citation2010). However, subsequent introductions of Norway rats (Rattus norvegicus) and ship rats (R. rattus) associated with European colonisation of New Zealand in the late 18th and 19th centuries (Haami Citation1994; Atkinson and Towns Citation2005) altered this distribution. These rodents competed with kiore and have now largely replaced them on mainland New Zealand (Russell and Clout Citation2004). Hence, kiore now have a restricted distribution, in parts of Fiordland on the mainland, and on offshore islands (Atkinson and Towns Citation2005; Russell and Clout Citation2004; Towns Citation2011).
Over the last 50 years, rodent damage to native ecosystems has been increasingly well documented and has led to targeted rodent eradication efforts in New Zealand and elsewhere (Russell and Broome Citation2016), particularly on offshore islands in order to protect threatened species (Bellingham et al. Citation2010). However, complete eradication of kiore is not universally desired by New Zealanders, as kiore are highly valued by some Māori as ‘taonga tuku iho’ passed down through generations to the present day (Kapa Citation2003; McClelland Citation2002; Clive Stone, Ngātiwai, pers. comm. 2016). Therefore, a small kiore sanctuary has been established on Mauitaha and Araara Islands off the coast of Northland (Department of Conservation Citation2010), with the goal of maintaining a harvestable kiore population at this location (Wehi et al. in prep). Despite different perspectives on kiore management, understanding kiore ecology and behaviour contributes to both control and management goals.
Slipper Island (Whakahau) is one of only a few remaining New Zealand offshore islands where kiore appear to be abundant (Russell and Russell Citation2018). However, this has not always been the case. In a 1973 survey, Norway rats but not kiore were reported on Slipper Island; kiore were recorded only on the nearby, smaller Rabbit and Penguin Islands (Hayward and Moore Citation1974). In contrast, in a recent (December 2016) survey of fauna on Slipper Island, Russell and Russell (Citation2018) observed only kiore but not Norway rats. They hypothesised that kiore were abundant and Norway rats no longer present. Nonetheless, they were unable to capture kiore to quantify kiore abundance on the basis of trapping indices, and suggested neophobia in the kiore population as an explanation. In this study, we investigated the abundance and distribution of kiore on Slipper Island by trapping them in different habitats, including some closely associated with human activity. We also recorded body mass and length, sex and reproductive maturity of captured kiore.
Materials and methods
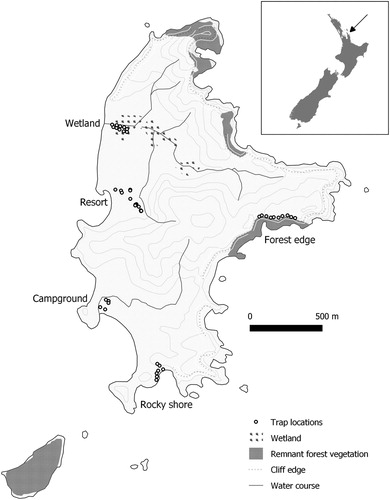
Slipper Island (37.07° S, 175.92° E; ) is a privately-owned 242 ha island that has been largely cleared of native vegetation. The island is 4 km from the Coromandel Peninsula on the east coast of New Zealand’s North Island (). It is predominantly grazed farmland, with a small resort, an old orchard, a campground, several holiday homes, and small remnants of native forest across the eastern cliffs. Two wetlands are fenced to exclude livestock, with restoration of vegetation underway. A ground-based rat control programme in 2002–2005 reduced kiore numbers but did not eliminate them (Russell and Russell Citation2018). Apart from kiore and possibly Norway rats (see above), there are no other introduced predatory mammals on Slipper Island.
During 20–24 March 2017, we set Victor Professional snap traps for 1–4 nights on Slipper Island in five locations >450 m apart, each in a different potential kiore habitat, with varying degrees of human activity (, ). Adjacent traps within each group or line were 26 m apart on average (median 24 m, range 1–65 m). The primary objective was to collect kiore carcasses for a dietary study, so traps were placed somewhat irregularly, with additional traps laid on the final day. We placed traps at the resort and wetland locations on the first night and added the remaining three locations on the second night. At the resort, ten traps were set on the first night, and seven traps were added on the fourth night near fruiting trees. At the forest edge site, five snap traps and five Elliott live traps were set. All trapping was undertaken under an animal ethics permit from Manaaki Whenua – Landcare Research (17-02-13).
Table 1. Kiore captures and capture indices at five locations in different habitats on Slipper Island, New Zealand, in March 2017. Traps were Victor Professional snap traps, with five Elliott live-traps at the forest edge location. Capture indices are expressed as kiore caught per 100 corrected trap-nights (kiore per 100CTN). Captures resulting from additional evening trap-checks, which did not affect the capture index, are not included.
Traps were baited with peanut butter mixed with rolled oats and cleared and reset daily. Traps remained set all day, and at the resort some traps were again cleared and reset in the evening. Captured kiore were sexed, weighed, and their head and body length measured (from nose tip to pelvic girdle; Cunningham and Moors Citation1996); live captures were first euthanased with cervical dislocation. All carcasses were retained and frozen for later dissection to assess reproductive status. Males with epididymis visible to the naked eye and females with moderately developed to enlarged uterus were considered reproductive (Moller and Craig Citation1987).
Because previous records had indicated that Norway rats were present on Slipper Island (Hayward and Moore Citation1974), we noted potential evidence of their presence. We collected one sample of unusually large faecal pellets, which were sent to EcoGene (Auckland, New Zealand) for DNA-based diagnostic testing for species identification.
To index kiore abundance, we calculated a kiore capture index for each group of traps and for all traps combined, based on the number caught per 100 trap nights corrected for sprung traps (captures per 100CTN; Nelson and Clark Citation1973; Cunningham and Moors Citation1996). When additional evening trap-checks resulted in multiple daily captures at some traps, these additional captures were excluded from index calculations. We used Welch’s t-tests for two samples with unequal variances to compare kiore abundance indices (captures per 100CTN) between trap-lines where we had and had not observed fruiting exotic trees (post-hoc test), and to compare body mass between male and female kiore. Capture indices were square-root transformed prior to statistical comparison, to stabilise the variance as recommended for count-based data (Zar Citation1996). Means are expressed with standard errors throughout. Statistical analyses were completed in R version 3.6.2 (R Core Team Citation2019).
Results
We trapped 30 kiore in daily trap-checks on all traplines combined (). Kiore were readily observed by day and night in exotic fruit trees (feijoa Acca sellowiana and fig Ficus carica) and grassy places, and we caught four additional kiore, not included in capture indices, as the result of extra evening trap-checks at the resort. Kiore capture indices ranged from 50.0 kiore/100CTN at the campground and 34.2/100CTN in the resort, to 2.5/100CTN in the wetland. However, capture indices in the campground and resort, where kiore were readily seen feeding in fruit trees, did not significantly exceed capture indices in the other three locations (post-hoc comparison; t2.1 = 2.9, P = 0.13). Only one kiore was captured in an Elliott live trap, which were set at the forest edge location only (five Elliott and five snap traps; ); five other kiore were caught in snap traps at this location. The combined capture index across all five habitats was 22.9/100CTN.
Weights of captured kiore (n = 32) ranged between 49–111 g, and head and body lengths ranged between 109–144 mm (; ). Two scavenged carcasses could not be measured. Despite a higher percentage of immature males than females (below), the mean body mass of males (84.6 g ± 3.9; range 59–111 g) was higher than that of females (73.1 g ± 3.7; 49–108 g; t27.9 = 2.2, P = 0.03). More than half of all kiore (69%) captured were reproductive, 100% of females (n = 18) and 29% of males (n = 14). We caught a single lactating female and no obviously pregnant females (i.e. with visible embryos). The sex ratio of captured kiore was approximately even (14 males:18 females).
Figure 2. Body mass and length of 14 male and 18 female kiore trapped on Slipper Island, New Zealand, in March 2017. Head and body length were measured from nose to pelvic girdle.
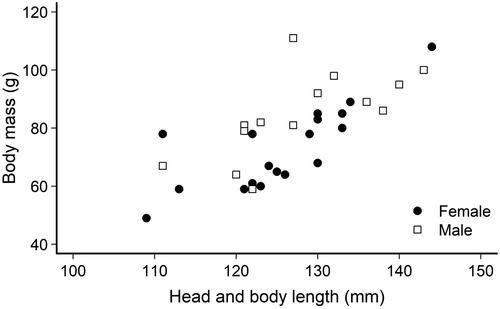
Table 2. Measurements and reproductive condition of kiore captured on Slipper Island, New Zealand, in March 2017. Data are sorted by sex and body mass. Head and body length were measured from nose to pelvic girdle. Uterus size was scored as Undeveloped, Moderately developed or Enlarged. Scrotal/Abdominal indicates one testis in each position. Reproductive condition was assessed on the basis of moderate or enlarged uterus (females), and visible tubules on the epididymis (males).
We did not observe any rat species other than kiore on Slipper Island. Two trapped rats were scavenged (with heads intact) in traps at the campground. DNA testing of a large, fresh faecal pellet (14–17 mm long, within the size range for Norway rat and larger than typical kiore pellets; Atkinson and Towns Citation2005) from beside one of these traps detected kiore DNA but not Norway rat DNA.
Discussion
Kiore appeared to be flourishing on Slipper Island in the absence of other rodent species and introduced mammalian predators and were widespread across the island. Our trapping data indicate that kiore can be numerous in human modified habitats in New Zealand, in line with observations of kiore commensality described elsewhere in the ecological literature (Williams Citation1973; references in Atkinson and Towns Citation2005) and narratives of kiore eating Māori crops and plantations, and around settlements (see for example Mead and Grove Citation2003; Haami Citation1994; Roberts et al. Citation2004).
The population size and dynamics of kiore are influenced by inter-specific competition, presence of mammalian predators and competitors, season, and geography (Atkinson and Towns Citation2005). Kiore capture indices in past studies at various times of year on northern New Zealand islands of similar size to Slipper Island (242 ha), which also lacked other rodent species and mammalian predators, were similar to the overall capture index in this study (22.1 kiore per 100CTN) (Figure 13 and appendix 2 in Moller and Craig Citation1987). On smaller Mauitaha/West Chicken Island (22 ha) in autumn 2016 (the same season as the present study), the combined kiore capture index in live-traps and snap-traps was 7.6 kiore per 100CTN (Wehi et al. in prep.).
Kiore numbers fluctuate annually as the result of seasonal breeding (Atkinson and Towns Citation2005). In addition, seasonal resources such as fruit trees may act as feeding ‘hot spots’ that draw in kiore from surrounding areas, perhaps especially young animals that are dispersing. Hence, the high capture rates that we recorded at the resort and campground may be temporary. However, because our traplines were more than 450 m apart (much greater than kiore home range lengths, which are usually <100 m; Bramley Citation2014), any attraction of kiore to the fruiting trees would be unlikely to directly affect kiore abundance at our trap-lines in other parts of the island. Examining habitat utilisation and population fluctuations over a longer period would help reveal some of these dynamics.
Kiore capture indices in some habitats on Slipper Island () may not fully reflect the potential abundance of kiore there. The forest edge capture index could have been affected by possibly lower capture success in Elliott traps, which were set only at that location. Ongoing poison efforts have been focused around the resort, where the carcasses of poisoned rats were readily observed during this study. In addition, when traps near resort fruit trees were cleared, some were re-sprung within minutes; such additional captures within a single day were not included in capture indices (see Methods). The overall high capture index despite poison efforts suggests that conditions for kiore were very favourable on Slipper Island during this study.
Kiore on Slipper Island were readily observed feeding on abundant cultivated fruit at the resort and campground. Food in the form of seeds is provided by the introduced pasture grasses dominating the island. On Tiritiri Matangi Island, grass seed was a significant seasonal resource that affected kiore breeding and population dynamics (Moller and Craig Citation1987). On islands with extensive exotic grass cover, kiore may breed only in spring and early summer when grass seed is present (Moller and Craig Citation1987; Atkinson and Towns Citation2005). In contrast, on forested islands, the kiore breeding season tends to extend into autumn (Moller and Craig Citation1987; Atkinson and Towns Citation2005). Our captures of few reproductive males, no females that were obviously pregnant and only one that was lactating in early autumn on Slipper Island, which is dominated by exotic grasses, are consistent with these findings. Further, we caught no newly independent juveniles; our smallest captures were a 49 g female and a 59 g male, and it is not unusual to trap much smaller individuals (18–35 g; Moller and Tilley Citation1986; Wehi et al. in prep.).
Successful population eradication as well as management to achieve population maintenance or growth (as might be desired for taonga species) require understanding of habitat use and behaviour, including neophobia. On Slipper Island, kiore were active day and night and showed no sign of neophobia. Some traps set at the base of fruit trees were sprung within minutes of being set, and kiore observed feeding in fig trees were easy to approach closely. These observations contrast with a suggestion of possible neophobia in the same population in springtime (Russell and Russell Citation2018). At the time of our autumn study, kiore may have been particularly abundant following summer breeding. They therefore may have had to compete for food, hence becoming less neophobic.
In summary, we detected abundant kiore and no other rodent species on Slipper Island. The scavenged kiore we recorded were most likely eaten by other kiore, or possibly by predatory birds (list in Russell and Russell Citation2018), given that there are no other known introduced predatory mammals such as cats. The island may therefore present an opportunity for future behavioural studies (for example, of neophobia) of kiore at high density in the absence of other rodents. In addition, thought could be given to the translocation or maintenance of some individuals from the Slipper Island kiore population as insurance for the protected population on Mauitaha Island, given the high cultural value placed on kiore, and in light of current rodent eradication plans throughout New Zealand as part of the Predator Free 2050 initiative (Russell et al. Citation2015). Maintaining Slipper Island kiore as an insurance population for this taonga species would require a detailed management plan that considers both kiore genetics and potential impacts on human habitation (for example, the need to incorporate targeted rat control). Establishing the genetic diversity of this population, and whether any genetic bottlenecks have taken place, would be a useful first step.
Acknowledgements
We warmly thank Markus Gronwald for field assistance on Slipper Is. We thank Slipper Island Resort Ltd and Wendy Wu for permission to survey the island and for transport and accommodation, and caretakers Brian and Fiona Brakenridge and Nick for helping plan our visit. James Russell facilitated access to Slipper Island, Grace Yee assisted with identification of reproductive maturity, and Gretchen Brownstein helped create the map. We are grateful to Isabel Castro and James Russell for their helpful comments on the manuscript. HR, PMW and DJW were supported by Rutherford Discovery Fellowship 14-LCR-001, and the Strategic Science Investment Fund for Crown Research Institutes from the Ministry of Business, Innovation and Employment provided additional funding for HR and DJW. Nā Holden Hohaia te whakarāpopoto i whakamāori.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Atkinson IAE, Towns DR. 2005. Kiore. In: King CM, editor. The handbook of New Zealand mammals. 2nd ed. Melbourne: Oxford University Press; p. 159–174.
- Bellingham PJ, Towns DR, Cameron EK, Davis JJ, Wardle DA, Wilmshurst JM, Mulder CPH. 2010. New Zealand island restoration: seabirds, predators, and the importance of history. New Zealand Journal of Ecology. 34(1):115–136.
- Bramley GN. 2014. Home ranges and interactions of kiore (Rattus exulans) and Norway rats (R. norvegicus) on Kapiti Island, New Zealand. New Zealand Journal of Ecology. 38(2):328–334.
- Campbell DJ, Atkinson IAE. 1999. Effects of kiore (Rattus exulans Peale) on recruitment of indigenous coastal trees on northern offshore islands of New Zealand. Journal of the Royal Society of New Zealand. 29(4):265–290.
- Cunningham DH, Moors PJ. 1996. Guide to the identification and collection of New Zealand rodents. Wellington: Department of Conservation. http://www.doc.govt.nz/documents/science-and-technical/rodent-identification.pdf.
- Department of Conservation. 2010. DOC and Ngatiwai Trust Board sign management agreement for two of the Marotere (Chicken) Islands http://www.doc.govt.nz/news/media-releases/2010/doc-and-ngatiwai-trust-board-sign-management-agreement-for-two-of-the-marotere-chicken-islands.
- Doherty TS, Glen AS, Nimmo DG, Ritchie EG, Dickman CR. 2016. Invasive predators and global biodiversity loss. Proceedings of the National Academy of Sciences. 113:11261–11265.
- Gibbs GW. 2009. The end of an 80-million year experiment: a review of evidence describing the impact of introduced rodents on New Zealand’s ‘mammal-free’ invertebrate fauna. Biological Invasions. 11:1587–1593.
- Haami BJTM. 1994. Chapter 5, The kiore rat in Aotearoa: A Maori perspective. In: Morrison RJ, Geraghty PA, Crowl L, editor. Science of Pacific Island Peoples. Vol. 3, Fauna, flora and medicine. Suva, Fiji: Institute of Pacific Studies; p. 65–75.
- Hayward BW, Moore PR. 1974. Auckland University field club scientific camp to Shoe Island and the Slipper Island Group, August 1973. Tane. 20:1–3.
- Hingston M. 2015. Phylogeography of the commensal Rattus exulans with implications for its use a bioproxy for human migrations [dissertation]. Auckland: University of Auckland.
- Holdaway RN. 1989. New Zealand's pre-human avifauna and its vulnerability. New Zealand Journal of Ecology. 12(Suppl):11–25.
- Holdaway RN, Worthy TH, Tennyson AJD. 2001. A working list of breeding bird species of the New Zealand region at first human contact. New Zealand Journal of Zoology. 28(2):119–187.
- Kapa D. 2003. The eradication of kiore and the fulfilment of kaitiakitanga obligations. Auckland University Law Review. 9:1326–1352.
- Matisoo-Smith E, Roberts RM, Irwin GJ, Allen JS, Penny D, Lambert DM. 1998. Patterns of prehistoric human mobility in Polynesia indicated by mtDNA from the Pacific rat. Proceedings of the National Academy of Sciences USA. 95:15145–15150.
- McClelland PJ. 2002. Eradication of Pacific rats (Rattus exulans) from Whenua Hou Nature Reserve (Codfish Island), Putauhinu and Rarotoka Islands, New Zealand. In: Veitch CR, Clout MN, editor. Turning the Tide: the eradication of invasive species. Gland, Switzerland: IUCN; p. 173–181.
- McGlone MS. 1989. The Polynesian settlement of New Zealand in relation to environmental and biotic changes. New Zealand Journal of Ecology. 12(Suppl):115–129.
- Mead HM, Grove N. 2003. Ngā Pēpeha a ngā Tīpuna. Wellington: Victoria University Press.
- Moller H, Craig JL. 1987. The population ecology of Rattus exulans on Tiritiri Matangi Island, and a model of comparative population dynamics in New Zealand. New Zealand Journal of Zoology. 14:305–328.
- Moller H, Tilley JAV. 1986. Rodents and their predators in the eastern Bay of Islands. New Zealand Journal of Zoology. 13:563–572.
- Nelson Jr L, Clark FW. 1973. Correction for sprung traps in catch/effort calculations of trapping results. Journal of Mammalogy. 54:295–298.
- R Core Team. 2019. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. URL https://www.R-project.org/.
- Roberts M, Haami B, Benton R, Satterfield T, Finucane ML, Henare M, Henare M. 2004. Whakapapa as a Māori mental construct: Some implications for the debate over genetic modification of organisms. The Contemporary Pacific. 1–28.
- Russell JC, Broome KG. 2016. Fifty years of rodent eradications in New Zealand: another decade of advances. New Zealand Journal of Ecology. 40(2):197–302.
- Russell JC, Clout MN. 2004. Modelling the distribution and interaction of introduced rodents on New Zealand offshore islands. Global Ecology and Biogeography. 13:497–507.
- Russell JC, Innes JG, Brown PH, Byrom AE. 2015. Predator-Free New Zealand: Conservation Country. Bioscience. 65:520–525.
- Russell JC, Russell KJ. 2018. Terrestrial fauna survey of Slipper Island (Whakahau). New Zealand Journal of Zoology. 45(1):73–82.
- Towns DR. 2011. Eradications of vertebrate pests from islands around New Zealand: what have we delivered and what have we learned? In: Veitch CR, Clout MN, Towns DR, editors. Island invasives: eradication and management. Gland: IUCN; p. 364–371.
- Towns DR, Atkinson IAE, Daugherty CH. 2006. Have the harmful effects of introduced rats on islands been exaggerated? Biological Invasions. 8:863–891.
- Williams JM. 1973. Ecology of Rattus exulans (Peale) reviewed. Pacific Science. 27:120–127.
- Wilmshurst JM, Anderson AJ, Higham TFG, Worthy TH. 2008. Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proceedings of the National Academy of Sciences USA. 105(22):7676–7680.
- Zar JH. 1996. Biostatistical analysis, 3rd ed. Upper Saddle River, NJ: Prentice Hall.