ABSTRACT
Consistent differences among individuals in the boldness-shyness continuum have been described in a variety of species. Environments with higher levels of predation are likely to select for shyer behavioural responses, due to the increased susceptibility of being ‘fearless’ in a high-risk environment. In this study, we compared the behavioural responses in two populations of South Island robin (Petroica australis), one of which is sympatric with a range of introduced predators (Kaikoura mainland) and one with no introduced predators (Motuara Island). We found robins on Motuara Island were significantly bolder than mainland robins. This was evidenced by robins in this low-risk environment being more likely to approach mealworms placed closer to a researcher. Robins in Kaikoura were also significantly slower than Motuara Island robins in latency (time to approach mealworms) but faster to remove five mealworms placed nearest to a researcher (handling time). These differences may be driven by bolder individuals having a disadvantage on the mainland as it exposes them to a higher risk from introduced predators. Although the extent to which these differences have a genetic basis is unknown, our results suggest that sympatry with introduced predators may favour more risk-averse behaviours in robins and other native species.
Introduction
Personality refers to consistent differences in behaviour between individuals (Brodin et al. Citation2013; Réale et al. Citation2007; Sih et al. Citation2004). Although personality is often considered on an individual level, it can also be applied when comparing two or more populations (Réale et al. Citation2007). One of the commonly studied personality types is ‘shyness-boldness’, defined as an individual’s reaction to a risky but not novel situation. Bold individuals tend to be less risk aversive than shy individuals, and spend more time engaged in activities that increase their vulnerability to predation, such as basking or foraging in risky habitats (Beckmann et al. Citation2013; Carter et al. Citation2012a, Citation2012b).
The degree of shyness-boldness in an individual’s personality can have an effect on its behaviour in a variety of different ecological contexts, such as foraging (Bergvall et al. Citation2011), nest defence (Burtka and Grindstaff Citation2013), aggression (Bell and Sih Citation2007) and tameness (Barnett et al. Citation2013). Nevertheless, individual differences in behaviour may be able to persist in the same population due to trade-offs which favour one personality type in one situation but the other type in another situation. For example, in great tits (Parus major), low population densities favour fast explorers (which take more risks) while high population densities favour slow explorers (Nicolaus et al. Citation2016). As population densities change from year to year due to changing environmental conditions, the fluctuating selection pressures can maintain personality variation within a population (Nicolaus et al. Citation2016). However, if environmental conditions show directional change, selection can favour one personality tendency, causing it to become more prevalent over time.
Predation risk is likely to select for particular behavioural responses, especially if different responses alter the risks of being depredated (Quinn and Cresswell Citation2005). For example, bold bighorn ewes (Ovis canadensis) were less likely to experience predation by cougars (Puma concolor) (Réale and Festa-Bianchet Citation2003). Similarly, the pups of active and aggressive female red squirrels (Tamiasciurus hudsonicus) grew faster and survived better (Boon et al. Citation2007). Similar patterns are seen in domestic animals (Hemsworth et al. Citation1999; Korhonen and Niemelä Citation1996; Madden and Whiteside Citation2014). If predation risk has the potential to select for certain personality traits, the most advantageous traits are likely to differ across species and situations. The difference in predation rates on individuals with different personalities may be due to different levels of activity (thus attracting a predator’s attention), differences in length of time spent in riskier habitats, or greater willingness to explore or approach novel stimuli (Sih et al. Citation2004).
If personality traits provide benefits in relation to avoidance of predation, it is likely that two populations experiencing different levels of predation risk would diverge in their average behavioural responses. Indeed, it has been found that personality traits often exhibit geographical variation (Foster Citation1999). For example, tadpoles of the common frog (Rana temporaria) on islands were bolder and faster explorers compared to their counterparts on the mainland where they were subjected to greater rates of predation (Brodin et al. Citation2013). The same pattern was found in a comparative study of tadpoles across 13 species that experienced different rates of predation (Richardson Citation2001). Sticklebacks (Gasterosteus aculeatus) similarly had differing activity and aggressiveness levels depending on whether they were from a predator-naive or predator-sympatric population (Bell Citation2005; Dingemanse et al. Citation2007). Despite well-documented differences in personality types, the underlying mechanisms responsible are seldom known. Personality differences among populations could be explained by environmental factors, genetic differences or a combination of both (Sih et al. Citation2004).
Until humans arrived, the only predators on terrestrial birds in New Zealand were birds of prey such as falcons (Falco novaeseelandiae) and owls (Ninox novaeseelandiae and Sceloglaux albifacies). As a result, endemic New Zealand birds have been isolated from mammalian predators for most of their evolutionary history and they lack many of the anti-predator adaptations seen in continental species. Many endemic birds could not cope with these novel predators and went extinct, while most of the surviving species have undergone widespread decline (Blackburn et al. Citation2004; Diamond and Veitch Citation1981; Remeš et al. Citation2012; Worthy and Holdaway Citation2002). Nonetheless, a number of species have managed to survive on the New Zealand mainland, perhaps by changes in their behaviour that are advantageous against introduced predators (Massaro et al. Citation2008).
The South Island robin (Petroica australis) is an ideal species to study the relationship between behaviour responses and predation risk. On the main islands of New Zealand, robins have been in sympatry with kiore (Rattus exulans) for c. 700 years, and with a range of other mammalian predators that followed European colonisation c. 140 years ago (King Citation1984; Worthy and Holdaway Citation2002). In contrast, robins on a number of offshore islands are currently allopatric with most or all introduced mammalian predators, providing an opportunity to study the behavioural response of birds in a situation similar to their ancestral state, prior to introduction of novel predators. If exposure to a high risk of predation favours changes in personality one would expect individuals in mammalian-free islands to be bolder on average. In the absence of high predation risk, shyness and hesitation may lead to missing foraging opportunities or losing higher quality territories (Mittelbach et al. Citation2014). Alternatively, on the mainland, where predation by introduced mammals is currently the greatest cause of mortality (Armstrong et al. Citation2006; Boulton et al. Citation2008; Brown Citation1997), shyness should be advantageous if this causes birds to hesitate, avoid novel objects and thus avoid predation (Mittelbach et al. Citation2014). In this study, we compared the behavioural responses of robins in two populations that differed in exposure to mammalian predators by timing an individual’s willingness to approach a researcher to collect items of food in the form of mealworm larvae (Tenebrio molitor).
Methods
Study sites
This study was conducted at two sites, one on mainland New Zealand near the town of Kaikoura, and one on predator-free Motuara Island in the Marlborough Sounds. Robins were studied in two forest patches in the Kaikoura area, one at Kowhai Bush (−42.376, 173.616) and another at Waimangarara Bush (−42.341, 173.659). Kowhai Bush is an ∼240 ha native forest dominated by kanuka (Kunzea ericoides). The understory is composed of various native and introduced shrubs (Hunt and Gill Citation1979). Waimangarara Bush is an ∼65 ha native forest located 5 km north of Kowhai Bush. It is composed of similar vegetation and holds a similar diversity of bird species. The two sites are joined through a continuous band of montane forest at their western edges but are otherwise separated by farmland. Kowhai Bush receives little predator control, and this primarily targets possums (Trichosurus vulpecula) around the perimeter adjacent to farmland. Parts of Waimangarara Bush had been subject to more extensive predator control prior to this study, however, pest control was similar to that of Kowhai Bush during this study (B. Dunnet, pers. comm.). Introduced mammalian predators that are present at the Kaikoura sites include feral cats (Felis catus), stoats, (Mustela erminea), ferrets (M. putorius), weasels (M. nivalis), rats (Rattus rattus and R. norvegicus), mice (Mus musculus) and possums.
Motuara is a 59 ha island located ∼1.8 km offshore in the Marlborough Sounds (−41.093, 174.271). Forest structure is similar to that at Kaikoura (Maloney and McLean Citation1995). Robins were introduced to Motuara Island in 1973 from nearby Nukuwaiata Island (Taylor et al. Citation2005). In contrast to the introduced mammalian predators present on the mainland study site, neither the source population on Nukuwaiata, nor the current descendent population on Motuara Island has ever been exposed to any mammalian predators other than the kiore for any of their evolutionary history. Furthermore, kiore were eradicated from Motuara Island in 1990 (Maloney and McLean Citation1995). The difference in predation risk is evident from rates of nest predation: in Kowhai Bush on the mainland 37.5% of nests fail from predation (unpubl., data), while on Motuara Island, only 5.1% of nests are subject to predation (MacFarlane Citation2019).
Study species
South Island robins (Petroica australis) (hereafter robins) are passerine forest birds that are endemic to New Zealand. They are insectivorous and largely feed on the ground. Breeding pairs are territorial and the male feeds the nesting female while both parents feed their nestlings and fledglings. Breeding occurs between September and January and 2–3 clutches are produced per season (Powesland Citation1983). Like many island birds, robins are relatively tame around humans. Most of the adult birds at both Kaikoura and Motuara Island had prior experience with researchers and many had already been fitted with unique colour band combinations so that individuals could be identified. Robins at both sites were visited by researchers at similar levels and it is unlikely that one population was more habituated to people than the other. Alternatively, if banding had made them more fearful of humans, this was also consistent between populations. Robins on Motuara Island are isolated from mainland populations as they do not cross large expanses of open space or water. There are no robin populations on the adjacent mainland to Motuara Island, minimising gene flow. In contrast, it is possible for robins from one mainland population to disperse into the Kaikoura area, although it is not known how often this occurs. Any robins dispersing from other mainland populations would be similarly exposed to the full range of mammalian predators present at Kaikoura.
Measurement of behavioural responses
The relative boldness of robins was determined by their willingness and time taken to approach a human researcher. As the robins at both sites have experience with humans, the situation was not novel (i.e. all had seen humans previously) but given their hesitancy to approach researchers, we assumed that robins viewed close proximity as a risk. At various intervals between 17 November 2012 and 17 December 2013, researchers walked around the study sites both on and off marked trails. Robins were attracted by making noise, including stomping, clapping, whistling and calling. When a bird appeared, it was fed a single mealworm larvae to keep its interest during the experimental setup. This consisted of clearing the leaf litter from three patches of ground in a straight line. The patches were 30, 100 and 300 cm from where the researcher stood motionless (referred to as the observer position). Each patch was the same size (approximately 10 cm2). When the robin approached within 5 m and faced the researcher, five mealworms were placed at one of the patches. The researcher then retreated to the observer position and the approach time (latency) and total time to remove all five mealworms by the robin were noted. Once all mealworms were consumed or removed, the researcher waited 1 min before placing another five mealworms in a second patch. Once the second 5 mealworms were removed, the trial was repeated a third time in the last remaining patch. The order in which each patch was tested was chosen randomly. In each trial, the researcher returned to the observer position after placing the mealworms. If a robin failed to approach a patch within 5 min (i.e. it was present but too shy to approach the patch), the worms were removed and the total time noted as 300 s. Latency was defined as the time (in seconds) taken to remove the first mealworm. Similarly, if a bird failed to take a mealworm, it was assigned a latency of 300 s. Total time (in seconds) was defined as the period taken by the robin to remove all five mealworms. Handling time was defined as the difference between latency and total time (i.e. time from removal of first mealworm to removal of last mealworm). Robins that failed to take any mealworms were excluded from analyses of handling time. In all trials, only adult birds were tested. We also only used trials that were not interrupted by other birds. If a neighbouring robin disrupted the trial, the researcher repeated the trial further from the presumed territory border. If the robin had a mate or fledglings present, the trial was only carried out if the other birds did not take worms directly, although they may have been fed by or begged at the focal bird. Banding records were later checked to confirm the sex of each bird although some birds were of unknown sex.
Eight birds (4 males, 4 females) were tested in Kowhai Bush and 21 birds (16 males, 4 females, 1 sex unknown) were tested in Waimangarara Bush, for a total of 29 birds tested in the mainland sites at Kaikoura (20 males, 8 females, 1 unknown). Eighty-five birds were tested on Motuara Island (56 males, 26 females, 3 sex unknown). In analyses examining differences in response between the sexes, individuals of unknown sex were excluded. A total of 25 robins (6 in Kaikoura and 10 on Motuara Island) were retested up to 3 times with a period of at least 24 h between tests.
Statistical analysis
We compared the behavioural responses between robins from the mainland (Kaikoura) and a predator-free island (Motuara) by running generalised linear mixed models (GLMM) with the ‘glmmTMB’ package (Brooks et al. Citation2017) on R software version 4.1.2 (R Development Core Team Citation2018). A binomial distribution was applied to the models predicting whether or not a bird approached the mealworms (likelihood of approach), whereas models predicting latency, total time and handling time were log transformed to follow a normal distribution. Multiple models were run for each response variable including the distance from the researcher (30, 100 and 300 cm) and the location (mainland, island) as explanatory variables and a combination of covariables (i.e. sex, breeding or non-breeding season, and order of tested distances). To account for the unbalanced experimental design and pseudo-replication (same birds tested multiple times), the date of the experiment and the identity of the birds were included as random factors in all models.
For each response variable, the model with the lowest corrected Akaike Information Criterion (AICc) (Sakamoto et al. Citation1986) calculated using the ‘MuMIN’ package (Barton Citation2022) was considered as the best model. Model diagnostics were run using the ‘DHARMa’ package (Hartig Citation2022) and R-squared was calculated using the ‘MuMIN’ package. The ‘predict’ function associated with the ‘glmmTMB’ package (Brooks et al. Citation2017) was used to calculate fitted means and their standard errors. To obtain the general effect of each explanatory variable included in the best models, an analysis of variance (ANOVA) applying a Wald chi-square test was run using the ‘car’ package (Fox and Weisberg Citation2019).
Results
Probability of approach in relation to distance
Overall, the model including distance to researcher, location (island vs. mainland) and their interaction showed the best fit (AICc = 265.27, .a) with a significant influence on the likelihood of the robin approaching the mealworms (χ2 = 14.48, P = .001; χ2 = 13.84, P = .0002; χ2 = 9.44, P = .009, respectively). Robins from Motuara Island were more likely to approach for mealworms than robins from the mainland (Kaikoura), but only at 30 cm distance from the researcher (, .a). On the mainland, fewer robins approached when mealworms were at 30 cm than when 100 and 300 cm, whereas no difference was observed with robins from Motuara Island (, .a).
Table 1. Likelihood of South Island robins at Kaikoura (predators present) and on Motuara Island (no introduced predators) approaching a researcher.
Latency and total times
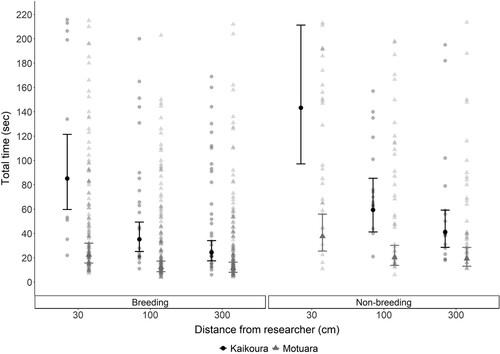
For both latency and total time, the models including distance to researcher, location (island vs. mainland), their interaction and breeding period showed the best fit (AICc = 1556.14, .b-c). When predicting latency, the distance from the researcher had the strongest effect (χ2 = 202.57, P < .001) followed by the interaction (χ2 = 54.69, P < .001), location (χ2 = 38.26, P < .001) and breeding period (χ2 = 15.25, P < .001). When predicting total time, distance from the researcher had the strongest effect (χ2 = 89.54, P < .001) followed by location (χ2 = 30.42, P < .001), breeding period (χ2 = 15.25, P = .002) and the interaction (χ2 = 6.16, P = .046) (, .c). Overall, latency and total time both increased with decreasing distance to researcher, with robins quicker to approach during the breeding season regardless of location ( and , .b, c). The only exception to this pattern was on Motuara Island, where there was no difference in latency between 100 and 300 cm (, .b).
Figure 1. Fitted mean latency times (±SE) for South Island robins to approach and remove the first mealworm at three distances (30, 100, 300 cm) from researcher at Kaikoura (mainland site, black dots) and on Motuara Island (grey triangles) depending on the season (breeding, non-breeding season). Fitted means were back transformed from a log scale to exponential for the purpose of this figure. Raw values are also given, with darker shades indicating more overlapping values.
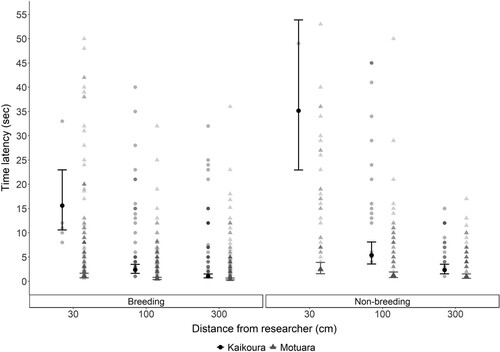
Figure 2. Fitted mean total time (±SE) for South Island robins to remove five mealworms, from first to last mealworm, at three distances (30, 100, 300 cm) from researcher at Kaikoura (mainland site, black dots) and on Motuara Island (grey triangles) depending on the season (breeding, non-breeding season). Fitted means were back transformed from a log scale to exponential for the purpose of this figure. Raw values are also given, with darker shades indicating more overlapping values.
Handling time
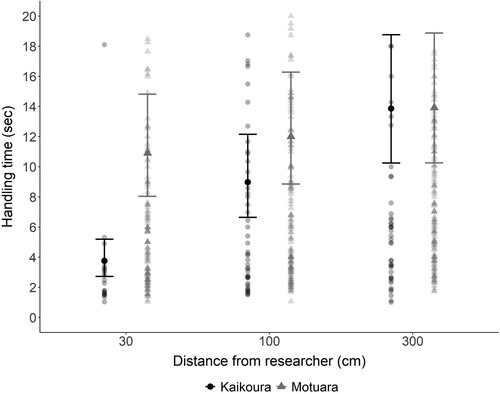
Handling time was significantly influenced by distance from the researcher (χ2 = 19.15, P < .001) followed by the interaction (χ2 = 16.64, P < .001) and location (χ2 = 4.34, P = .037) (Tables A1.d). The handling time of robins from the mainland decreased with decreasing distance to researcher whereas distance did not significantly affect handling time for robins from Motuara Island (, Tables A2.d). Although there was no significant difference overall between robins from Motuara Island and the mainland, handling time was higher for robins from Motuara Island at 30 cm (, Tables A2.d).
Figure 3. Fitted mean handling time (±SE) for South Island robins to remove five mealworms, from first to last mealworm at three distances (30, 100, 300 cm) from researcher at Kaikoura (mainland, black dots) and on Motuara Island (grey triangles). Fitted means were back transformed from a log scale to exponential for the purpose of this figure. Raw values are also given, with darker shades indicating more overlapping values.
Discussion
Robins on Motuara Island (low predation risk) were found to differ in their behavioural responses to the presence of the researcher than robins at Kaikoura (high predation risk). This was shown by the increased willingness of robins on Motuara Island to approach closer and by a reduced latency time to approach a researcher to retrieve mealworms. Although robins may not necessarily view humans as predators, approaching a larger animal should still be considered risky, as suggested by the difference in approach distances and latency times we observed. This difference in approach times is likely due to differences in predation risk, as Motuara Island currently has no introduced mammalian predators, while for mainland robins, predation is the highest cause of mortality on both adults and their nests (Powesland Citation1983).
On Motuara Island, robin densities are around ten times greater than on the mainland (Mackintosh and Briskie Citation2005) and it is possible that the bolder responses shown by robins when faced with the presence of a researcher could allow those individuals to be more successful competitors in gaining territories, mates and food. Alternatively, at the Kaikoura sites, where density is lower and interspecific competition is presumed to be less intense, shyer responses may be more advantageous as they may make them better at avoiding mortality through predation from a wide range of introduced mammalian predators that are present.
If an anti-predator behaviour (i.e. being shy) is costly, it should be lost or diminished in a population in the absence of predators (Blumstein Citation2002). Other studies have shown similar trade-offs between foraging and shyness. For example, bolder females in fallow deer (Dama dama) raise heavier calves but suffer from higher predation than shyer deer (Bergvall et al. Citation2011). In contrast, the shyer females may be subject to less predation but their calves are more likely to be smaller or starve. Bolder Namibian rock agamas (Agama planiceps) were found to spend more time basking and feeding and had larger territories but were depredated more than their shyer counterparts (Carter et al. Citation2012a). If robins experience a similar trade-off, it is likely that being bold would be favoured in populations subject to low predation risk. Robins on other mammalian predator-free islands we have visited were also noted, albeit anecdotally, to be tamer and bolder than mainland robins.
Although our results are consistent with differences in predation risk explaining the differences observed in the degree of boldness, there are alternative explanations that could affect the outcome of our experiments. For example, it is possible that our sample of birds included a biased number of bold individuals at either or both sites. This would be the case if the shyest birds were too timid to even approach the experimental setup. However, if this was the case, we would not expect to still see differences in how birds responded at the experimental setup if only the bold individuals approached. Furthermore, assuming only the boldest birds of Kaikoura approached, we would expect to have less difference between the sites. Despite this, there was still a difference in behavioural responses between the two sites.
The differences in behavioural responses could also be due to differences in food availability between the two study sites, as population densities are higher on Motuara Island than the mainland (primarily because of the high number of introduced predators on the mainland). Risk taking is likely to be higher when an animal is in poor energetic state (David et al. Citation2012; Verdolin Citation2006). However, after eating a few mealworms, robins cached the remaining mealworms we presented. The birds that did not approach to collect mealworms were typically observed to hop back and forth looking directly at the mealworms, suggesting they were motivated to approach, but too shy to come any closer. It has also been found that food supplementation did not affect nest and fledgling survival on Motuara Island (Mackintosh and Briskie Citation2005), suggesting that food limitation was not widespread and unlikely to explain the behavioural differences we observed.
Season (breeding or non-breeding) was included in the best-fitting models for both latency and total time, with robins appearing to be less hesitant to approach the researcher to retrieve the mealworms when tested during the breeding season (summer), than when tested in the non-breeding season. This pattern could arise for a number of reasons. First, robins during the breeding season may be willing to take more risks when having nestlings to feed, or in the immediate post-breeding season when they are moulting and have higher energetic costs. Alternatively, robins may be more reluctant to approach the researcher in the non-breeding season as both our study sites tend to be wetter in the winter, and this may make invertebrates in the leaf litter more accessible. During the summer breeding season, the drier conditions may make invertebrates less accessible and force robins to take more risks. Further experiments are needed to assess the causes of the differences we observed, but our results confirm the need for researchers to consider seasonal effects when measuring behavioural differences between populations.
It was not possible in our study to determine whether behavioural differences between Motuara Island and the mainland were due to genetic or learned influences or a combination of both. Some studies have found that consistent behavioural traits are genetically determined (Dingemanse et al. Citation2004; Drent et al. Citation2003) and heritable (Bize et al. Citation2012; Brent et al. Citation2014), but others show that environmental factors have a stronger influence than heritability (Chervet et al. Citation2011; Fox and Millam Citation2004; Groothuis and Carere Citation2005; Nicolaus et al. Citation2012). The ability of robins to adapt to novel predators suggests they have a degree of plasticity in how they behave and that predator recognition may be experience dependent (Maloney and McLean Citation1995; McLean et al. Citation1999). Different species likely have differing levels of plasticity, on individual and evolutionary levels, and some studies have shown that plasticity itself varies between populations (Dingemanse et al. Citation2012; Richardson Citation2001), especially if the population experiences changes within lifetimes. It appears that individual robins can learn and plastically change their behaviour in response to altered predation risk (McLean et al. Citation1999), but that environment conditions, such as altered predation risk, can guide selection towards certain behavioural traits as we found in our study.
There is potential to carry out a range of further studies relating to behavioural differences between island and mainland populations of birds. Robins are an ideal study species for this work as they are tame and do not need to be forced into unnatural situations to be tested. This is a potential problem with personality studies involving animals in a laboratory setting as behaviour can change from the wild as animals adapt to the captive environment (e.g. Butler et al. Citation2006), and if individuals vary in their ability to adapt to captivity, this can lead to differences in personality from that observed in the wild (e.g. Wilson et al. Citation1993). Nevertheless, it should be possible to adapt many lab studies to wild robins to determine how robins compare in other behavioural measures other than the bold-shy continuum and if these differ consistently between island and mainland populations. It would be of particular interest to find out if the behavioural differences we found have a genetic basis, as has been found in great tits (Parus major) (Dingemanse et al. Citation2004; Drent et al. Citation2003) and blue tits (Cyanistes caeruleus) (Kluen et al. Citation2012). This could be achieved via translocation experiments, to see whether or not bolder island birds or their offspring stay bold or become shyer when moved to the mainland or vice versa. Repeated testing of robins across several seasons and years would also be useful to estimate the repeatabilities of individual personality, and to determine if the population level differences we detected are driven by changes in the frequency of robins with bold or shy personalities in each population. Finally, the differences in behaviour that we found could have implications for the translocation of threatened species in New Zealand, where birds from island populations are sometimes used to reintroduce or supplement populations on the mainland. If such introductions occur in areas with invasive mammalian predators, it may be beneficial to select shyer individuals for translocations, especially if bolder individuals are subject to an increased risk of predation.
Acknowledgements
We thank University of Canterbury for the use of the Edward Percival Field Station, with thanks to staff involved in administration and maintenance of this facility. We thank Christina Wu, David Lloyd-Jones and Archie MacFarlane for field assistance. We also thank the Department of Conservation and staff for supporting and facilitating this research through visits to Motuara Island. This project was approved by the University of Canterbury Animal Ethics Committee (permit 2012/29R) and the Department of Conservation (permit NM-34960-RES).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Armstrong DP, Raeburn EH, Lewis RM, Ravine D. 2006. Modeling vital rates of a reintroduced New Zealand robin population as a function of predator control. Journal of Wildlife Management. 70:1028–1036. doi:10.2193/0022-541X(2006)70[1028:MVROAR]2.0.CO;2.
- Barnett C, Salter M, Chevallier C, Robertson N, Berard O, Burns KC. 2013. The ability of North Island robins to discriminate between humans is related to their behavioural type. PLoS One 8:e64487. doi:10.1371/journal.pone.0064487.
- Barton K. 2022. MuMIn: Multi-model inference. R package version 1.46.0.
- Beckmann C, Biro PA, Wright J. 2013. On the validity of a single (boldness) assay in personality research. Ethology. 119:937–947. doi:10.1111/eth.12137.
- Bell AM. 2005. Behavioural differences between individuals and two populations of stickleback (Gasterosteus aculeatus). Journal of Evolutionary Biology. 18:464–473. doi:10.1111/j.1420-9101.2004.00817.x.
- Bell AM, Sih A. 2007. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecology Letters. 10:828–834. doi:10.1111/j.1461-0248.2007.01081.x.
- Bergvall UA, Schäpers A, Kjellander P, Weiss A. 2011. Personality and foraging decisions in fallow deer, Dama dama. Animal Behaviour. 81:101–112. doi:10.1016/j.anbehav.2010.09.018.
- Bize P, Diaz C, Lindstrom J. 2012. Experimental evidence that adult antipredator behaviour is heritable and not influenced by behavioural copying in a wild bird. Proceedings of the Royal Society of London B. 279:1380–1388. doi:10.1098/rspb.2011.1789.
- Blackburn TM, Duncan CP, Evans RP, Gaston KL, J K. 2004. Avian extinction and mammalian introductions on oceanic islands. Science. 305:1955–1958. doi:10.1126/science.1101617.
- Blumstein DT. 2002. Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. Journal of Biogeography. 29:685–692. doi:10.1046/j.1365-2699.2002.00717.x.
- Boon AK, Reale D, Boutin S. 2007. The interaction between personality, offspring fitness and food abundance in North American red squirrels. Ecology Letters. 10:1094–1104. doi:10.1111/j.1461-0248.2007.01106.x.
- Boulton RL, Richard Y, Armstrong DP. 2008. Influence of food availability, predator density and forest fragmentation on nest survival of New Zealand robins. Biological Conservation. 141:580–589. doi:10.1016/j.biocon.2007.12.007.
- Brent LJN, Semple S, MacLarnon A, Ruiz-Lambides A, Gonzalez-Martinez J, Platt ML. 2014. Personality traits in rhesus macaques (Macaca mulatta) are heritable but do not predict reproductive output. International Journal of Primatology. 35:188–209. doi:10.1007/s10764-013-9724-6.
- Brodin T, Lind MI, Wiberg MK, Johansson F. 2013. Personality trait differences between mainland and island populations in the common frog (Rana temporaria). Behavioral Ecology and Sociobiology. 67:135–143. doi:10.1007/s00265-012-1433-1.
- Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Machler M, Bolker BM. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal. 9:378–400. doi:10.32614/RJ-2017-066.
- Brown KP. 1997. Predation at nests of two New Zealand endemic passerines; implications for bird community restoration. Pacific Conservation Biology. 3:91–98. doi:10.1071/PC970091.
- Burtka JL, Grindstaff JL. 2013. Repeatable nest defense behavior in a wild population of Eastern bluebirds (sialia sialis) as evidence of personality. Acta Ethologica. 16:135–146. doi:10.1007/s10211-013-0143-7.
- Butler SJ, Whittingham MJ, Quinn JL, Cresswell W. 2006. Time in captivity, individual differences and foraging behaviour in wild-caught chaffinches. Behaviour. 143:535–548. doi:10.1163/156853906776240632.
- Carter A, Goldizen A, Heinsohn R. 2012a. Personality and plasticity: temporal behavioural reaction norms in a lizard, the Namibian rock agama. Animal Behaviour. 84:471–477. doi:10.1016/j.anbehav.2012.06.001.
- Carter AJ, Marshall HH, Heinsohn R, Cowlishaw G. 2012b. How not to measure boldness: novel object and antipredator responses are not the same in wild baboons. Animal Behaviour. 84:603–609. doi:10.1016/j.anbehav.2012.06.015.
- Chervet N, Zottl M, Schurch R, Taborsky M, Heg D. 2011. Repeatability and heritability of behavioural types in a social cichlid. International Journal of Evolutionary Biology. 2011:1–15. Article ID 321729. doi:10.4061/2011/321729.
- David M, Auclair Y, Cézilly F, Wright J. 2012. Assessing short- and long-term repeatability and stability of personality in captive zebra finches using longitudinal data. Ethology. 118:932–942. doi:10.1111/j.1439-0310.2012.02085.x.
- Diamond JM, Veitch CR. 1981. Extinctions and introductions in the New Zealand avifauna: cause and effect? Science. 211:499–501. doi:10.1126/science.211.4481.499.
- Dingemanse NJ, Both C, Drent PJ, Tinbergen JM. 2004. Fitness consequences of avian personalities in a fluctuating environment. Proceedings of the Royal Society of London. Series B: Biological Sciences. 271:847–852. doi:10.1098/rspb.2004.2680.
- Dingemanse NJ, Bouwman KM, van de Pol M, van Overveld T, Patrick SC, Matthysen E, Quinn JL. 2012. Variation in personality and behavioural plasticity across four populations of the great tit Parus major. Journal of Animal Ecology. 81:116–126. doi:10.1111/j.1365-2656.2011.01877.x.
- Dingemanse NJ, Wright J, Kazem AJ, Thomas DK, Hickling R, Dawnay N. 2007. Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. Journal of Animal Ecology. 76:1128–1138. doi:10.1111/j.1365-2656.2007.01284.x.
- Drent PJ, van Oers K, van Noordwijk AJ. 2003. Realized heritability of personalities in the great tit (Parus major). Proceedings of the Royal Society of London. Series B: Biological Sciences. 270:45–51. doi:10.1098/rspb.2002.2168.
- Foster SA. 1999. The geography of behaviour: An evolutionary perspective. Trends in Ecology & Evolution. 14:190–195. doi:10.1016/S0169-5347(98)01577-8.
- Fox J, Weisberg S. 2019. An R Companion to applied regression (Third). Sage.
- Fox RA, Millam JR. 2004. The effect of early environment on neophobia in orange-winged Amazon parrots (Amazona amazonica). Applied Animal Behaviour Science. 89:117–129. doi:10.1016/j.applanim.2004.05.002.
- Groothuis TG, Carere C. 2005. Avian personalities: characterization and epigenesis. Neuroscience & Biobehavioral Reviews. 29:137–150. doi:10.1016/j.neubiorev.2004.06.010.
- Hartig F. 2022. DHARMa: residual diagnostics for hierarchical (multi-level/mixed). Regression models. R package version 0.4.5.
- Hemsworth PH, Pedersen V, Cox M, Cronin GM, Coleman GJ. 1999. A note on the relationship between the behavioural response of lactating sows to humans and the survival of their piglets. Applied Animal Behaviour Science. 65:43–52. doi:10.1016/S0168-1591(99)00047-7.
- Hunt DM, Gill BJ. 1979. Ecology of kowhai bush, kaikoura. Mauri Ora Special Publication 2.
- King CM. 1984. Immigrant killers: introduced predators and the conservation of birds in. Auckland: Oxford University Press.
- Kluen E, Kuhn S, Kempenaers B, Brommer JE. 2012. A simple cage test captures intrinsic differences in aspects of personality across individuals in a passerine bird. Animal Behaviour. 84:279–287. doi:10.1016/j.anbehav.2012.04.022.
- Korhonen H, Niemelä P. 1996. Temperament and reproductive success in farmbred silver foxes housed with and without platforms. Journal of Animal Breeding and Genetics. 113:209–218. doi:10.1111/j.1439-0388.1996.tb00606.x.
- MacFarlane AET. 2019. Long-term consequences of genetic rescue on island populations of South Island robins [dissertation]. Christchurch: University of Canterbury.
- Mackintosh M, Briskie JV. 2005. High levels of hatching failure in an insular population of the South Island robin: a consequence of food limitation? Biological Conservation. 122:409–416. doi:10.1016/j.biocon.2004.09.002.
- Madden JR, Whiteside MA. 2014. Selection on behavioural traits during ‘unselective’ harvesting means that shy pheasants better survive a hunting season. Animal Behaviour. 87:129–135. doi:10.1016/j.anbehav.2013.10.021.
- Maloney RF, McLean IG. 1995. Historical and experimental learned predator recognition in free-living New-Zealand robins. Animal Behaviour. 50:1193–1201. doi:10.1016/0003-3472(95)80036-0.
- Massaro M, Starling-Windhof A, Briskie JV, Martin TE. 2008. Introduced mammalian predators induce behavioural changes in parental care in an endemic New Zealand bird. PLoS ONE. 3(6):e2331. doi:10.1371/journal.pone.0002331.
- McLean IG, Hölzer C, Studholme BJS. 1999. Teaching predator-recognition to a naive bird: implications for management. Biological Conservation. 87:123–130. doi:10.1016/S0006-3207(98)00024-X.
- Mittelbach GG, Ballew NG, Kjelvik MK. 2014. Fish behavioral types and their ecological consequences. Canadian Journal of Fisheries and Aquatic Sciences. 71:927–944. doi:10.1139/cjfas-2013-0558.
- Nicolaus M, Tinbergen JM, Bouwman KM, Michler SP, Ubels R, Both C, Kempenaers B, Dingemanse NJ. 2012. Experimental evidence for adaptive personalities in a wild passerine bird. Proceedings of the Royal Society B: Biological Sciences. 279:4885–4892. doi:10.1098/rspb.2012.1936.
- Nicolaus M, Tinbergen JM, Ubels R, Both C, Dingemanse NJ. 2016. Density fluctuations represent a key process maintaining personality variation in a wild passerine bird. Ecology Letters. 19:478–486. doi:10.1111/ele.12584.
- Powesland R. 1983. Breeding and mortality of the South Island robin in Kowhai Bush, Kaikoura. Notornis. 30:265–282.
- Quinn JL, Cresswell W. 2005. Personality, anti-predation behaviour and behavioural plasticity in the chaffinch Fringilla coelebs. Behaviour. 142:1377–1402. doi:10.1163/156853905774539391.
- R Development Core Team. 2018. R: A language and environment for statistical computing.
- Réale D, Festa-Bianchet M. 2003. Predator-induced natural selection on temperament in bighorn ewes. Animal Behaviour. 65:463–470. doi:10.1006/anbe.2003.2100.
- Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biological Reviews. 82:291–318. doi:10.1111/j.1469-185X.2007.00010.x.
- Remeš V, Matysioková B, Cockburn A. 2012. Nest predation in New Zealand songbirds: exotic predators, introduced prey and long-term changes in predation risk. Biological Conservation. 148:54–60. doi:10.1016/j.biocon.2012.01.063.
- Richardson JML. 2001. A comparative study of activity levels in larval anurans and response to the presence of different predators. Behavioral Ecology. 12:51–58. doi:10.1093/oxfordjournals.beheco.a000378.
- Sakamoto Y, Ishiguro M, Kitagawa G. 1986. Akaike information criterion statistics. Dordrecht: D Reidel Publishing Company.
- Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology & Evolution. 19:372–378. doi:10.1016/j.tree.2004.04.009.
- Taylor SS, Jamieson IG, Armstrong DP. 2005. Successful island reintroductions of New Zealand robins and saddlebacks with small numbers of founders. Animal Conservation. 8:415–420. doi:10.1017/S1367943005002337.
- Verdolin JL. 2006. Meta-analysis of foraging and predation risk trade-offs in terrestrial systems. Behavioral Ecology and Sociobiology. 60:457–464. doi:10.1007/s00265-006-0172-6.
- Wilson DS, Coleman K, Clark AB, Biederman L. 1993. Shy-bold continuum in pumpkinseed sunfish (Lepomis gibbosus): an ecological study of a psychological trait. Journal of Comparative Psychology. 107:250–260. doi:10.1037/0735-7036.107.3.250.
- Worthy TH, Holdaway RN. 2002. The lost world of the moa: prehistoric life of New Zealand. Bloomington: Indiana University Press.
Appendix 1
Table A1. List of generalised linear mixed models run predicting the likelihood of approach to retrieve mealworms (a), latency time to approach and remove the first mealworm (b), total time to remove five mealworms (c) and handling time from first to last mealworm (d) with number of birds included (n), degree of freedom (df), corrected AIC (AICc), delta AICc obtained using ‘MuMIn’ package.
Notes: Approach models were fitted with binomial error distribution whereas latency, total time and handling time were log transformed with a normal (Gaussian) error distribution. In bold, best fitted model. Distance refers to distance between researcher and placements of mealworms (30, 100 or 300 cm), location refers to either mainland (Kaikoura) or island (Motuara) study site, season as either breeding or non-breeding season, sex as either male or female, order as sequence in which the three distances were tested, and ID as identity of the robin.
Table A2. Detailed outputs of the best model predicting the likelihood of approach to retrieve mealworms (a), latency time to approach and remove the first mealworm (b), total time to remove five mealworms (c) and handling time from first to last mealworm (d) with either the mainland (Kaikoura) or Motuara Island as a reference (intercept), number of tested birds (n), AIC and R-square (R2) calculated using ‘MuMIn’ package. See for definitions of the factors.
