ABSTRACT
In the blast furnace, nut coke is utilized in a mixture with the ferrous burden to improve the gas permeability. Although applied in a broad range (10–40 mm, 2–23 wt-%), limited information is available on changed burden behaviour in its presence. In the present study, the detailed characterization was performed on the iron ore pellets quenched during sintering, softening and before complete melting. The quantified information of the phase distribution across the pellets is compared for the samples mixed with and without nut coke. The principal role played by the nut coke is on bringing higher reduction and lower sintering among the pellets. For the pellet mixed with nut coke, at the core, ∼25 vol.-% of the material is observed in a network arrangement. The core structure consists of a wüstite matrix (10–20 vol.-%) reinforced with the iron nuclei (5–15 vol.-%). On the contrary, in the absence of nut coke, the pellet core is observed being hollow.
Introduction
In a blast furnace, the reduction of the ferrous burden (iron ore, sinter and pellet) is accompanied by the burden shrinkage, softening and melting in the cohesive zone. The cohesive zone starts with the softening of the ferrous burden and ends with the melting and dripping. Thus higher resistance to the gas flow is experienced in this zone [Citation1]. This restricts the gas intake capacity of the blast furnace and hence limits the productivity [Citation2]. In order to improve the permeability in the cohesive zone, a method like the addition of nut coke (10–40 mm, 2–23 wt-% replacement of regular coke) to ferrous burden has gained researchers and industrial attention [Citation3]. The nut coke addition has the ability to improve the shaft permeability [Citation4], reduction kinetics [Citation5], enhance burden softening-melting properties [Citation6], and extend the thermal reserve zone temperature [Citation3,Citation7].
The presence of nut coke brings fundamental changes on the ferrous burden, which transforms the burden properties. These on a macroscale affect the bed properties. This brings the need of thorough comparative understanding on the interior of the ferrous burden during the shrinkage, softening and melting steps in the presence and absence of the nut coke.
Materials and methods
Iron ore pellets are a commonly used raw material for iron production from the blast furnace route. Hence, commercially supplied iron ore pellets (olivine fluxed) were used in the present work. The chemical analysis with XRF (X-ray fluorescence, Pananalytical, AxiosMax) of the raw pellets is given in . Pellets and nut coke used were in the size range of 10–13 mm and 10–15 mm, respectively.
Table 1. Chemical analysis of the iron ore pellets (wt-%).
In order to understand the effect of nut coke addition with the ferrous burden, Song [Citation6,Citation8] has performed a series of experiments both at room temperature and at high temperatures. His studies were aimed to investigate the bed permeability, reduction behaviour and softening-melting properties of the mixed charged ferrous burden. Nut coke addition was found to improve these properties. To further investigate the principal reasons for enhanced burden properties, Song [Citation8] has performed a series of quenching experiments under simulated blast furnace conditions. The thermal and gas profile followed during the experiments is given in . For the sample bed quenching from 1300oC and 1400oC, segments up to 9 and 10, respectively, were followed and then samples were quenched in nitrogen gas atmosphere at the rate of 5oC/min. In the present study, this work is carried forward. These quenched pellets were thoroughly investigated to understand the microscopic development occurred during the shrinkage, softening and before complete melting.
Table 2. Thermal and gas profile followed during the experiments [Citation8].
Similar to the blast furnace layered burden structure, iron ore pellets were sandwiched between two regular coke layers inside the crucible. In the case of pellet mixed with nut coke, 20 wt-% nut coke (replacement of the regular coke) was mixed with the pellets before sandwiching in between the regular coke. All experiments were conducted with equal amounts of the pellets (500 g) and total coke (100 g). More information about the experimental set-up, procedure and condition can be found in the refs [Citation6,Citation8].
Sample selection
Compared to burden mass and volume in the blast furnace, the sample mass used in the study is very small. However, in the microscale, this burden sees similar surroundings and atmosphere as in the blast furnace. Therefore, by the analysis of one or two representative pellets, the burden bed behaviour can be explained effectively.
In the study performed by Song [Citation8] it was found that significant bed shrinkage occurred around 1300oC, and sample melting was about to occur after 1400oC. Therefore, in the current study detailed investigations were performed on the pellets quenched at 1300oC and 1400oC for both cases, with (20 wt-%) and without mixed nut coke. Photographs of the quenched sample beds are shown in . The pellets located away from the graphite crucible wall and approximately cut through their centre are marked as representative pellets. In the case of pellets mixed with nut coke, to understand the effect brought by the nut coke addition, pellets located close to the nut coke were selected for the detailed analysis. Encircled representative pellets in are selected for detailed investigations. For the sample bed quenched at 1300oC, representative pellets look similar by brief observation under the microscope. Thus, one representative pellet was selected for the detailed analysis from the quenched sample bed. In the case of sample quenched from 1400oC, variation in the present phases appeared among the representative pellets. Hence, two representative pellets were selected for the closer investigation.
Figure 1. Photographs of the sample bed quenched from high temperatures [Citation8]. Encircled pellets are selected for detail analysis.
![Figure 1. Photographs of the sample bed quenched from high temperatures [Citation8]. Encircled pellets are selected for detail analysis.](/cms/asset/628f9d6c-89e0-4d8e-9fcf-a3818a4fcb3f/yirs_a_1510873_f0001_oc.jpg)
Phase identification
Phases, mineralogy (iron, wüstite and slag), and pore distribution patterns were investigated in the sample pellets. Different phases present in the samples were determined by X-ray diffraction (XRD, Bruker D8 discover). XRD analyses were carried out with CoKα radiation with an accelerating voltage of 45 kV and a filament current of 25 mA. Parallel beam geometry was applied for the analysis. The scanning rate was 1.2 degree/min and the diffraction angle (2θ) was between 30o and 80o. Phases were analysed both at pellet shells and core. Phase details and features were examined under field emission scanning electron microscope (FSEM, Joel 6500F). The composition of the phases were semi-quantitatively analysed using Energy Dispersive Spectroscopy (EDS). An acceleration voltage of 15 kV and 8 nA probe current were applied during the analysis.
Phase quantification
Quantification of the phases was performed via a series of investigations. To bring out the clear effects of nut coke addition, characterized phases were closely compared with the case when nut coke was absent in the pellet bed.
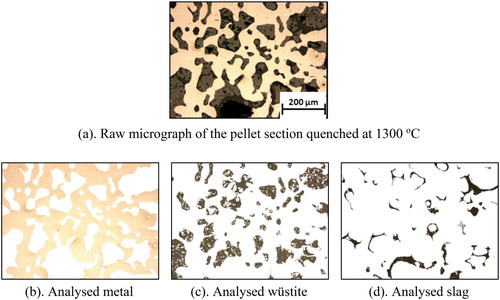
For the phase quantification in ferrous burden, advance reflected light microscopes are generally used and their accuracy is stated to be high [Citation9,Citation10]. Thus in present studies, phases distribution in the pellet samples were logged by reflected light microcopy (Olympus BX60M) and quantified by the image analysis techniques (). Two randomly selected diameters in the pellet cross-section were photographed from one end to the other, approximately 530 μm in each frame at 100× magnification. These micrographs were analysed for phase concentration and distribution with the help of ‘GIMP-2’ open source image processing software [Citation11]. The shape and contrast difference among the phases were exploited for the analysis. The number of pixels per phase was utilized to estimate the volume percentage of different phases present in a micrograph. To further enhance the accuracy in the examination of the features, SEM-EDS analysis were cross-referred. The pores appeared black in the optical micrographs. Their pixels were estimated by subtracting metal, wüstite and slag pixels from the total micrograph pixels. This exercise was repeated for all the captured micrographs of the pellet. To view a complete cross-section of the selected pellet a Keyence optical microscope (VHX-5000) was operated in the stitching mode.
Results
Phase identification
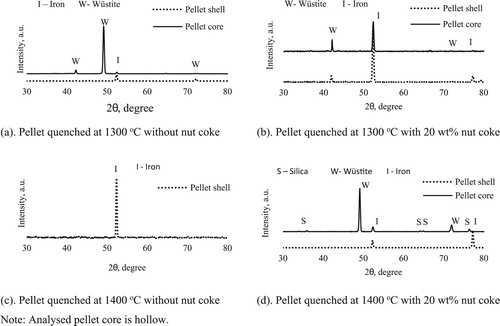
To identify the phases present across the pellet cross-section, XRD investigations were performed on the selected pellets. The diffraction patterns of the quenched pellets are given in . A strong peak of iron at the pellet periphery (shell) for all the selected samples shows that the reduction reactions progressed topo-chemically (surface to centre).
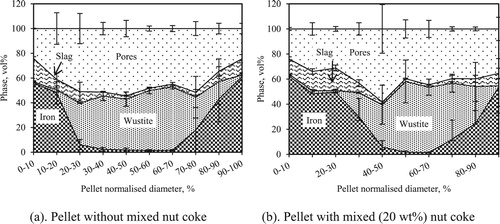
In the sample quenched at 1300oC, differences in the phases present between the pellet shell and core was detected ((a,b)). In the pellet without mixed nut coke, iron and wüstite were observed as the primary phase present at shell and core, respectively ((a)). In the pellet mixed with 20 wt-% nut coke, iron was observed as the principal phase at the shell. And at the core, a sharp peak of iron along with wüstite indicates its presence in significant amount ((b)).
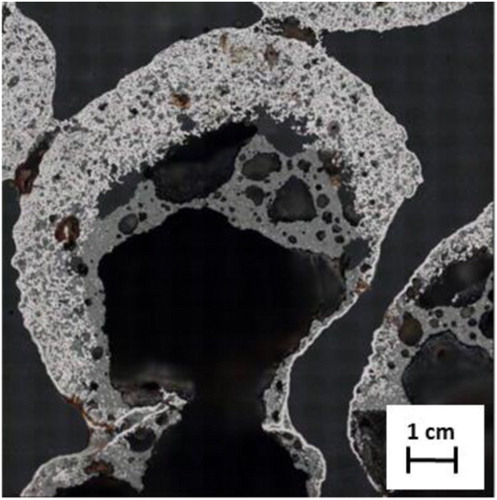
In the sample quenched at 1400oC, iron was again recognized at the pellet shell as the major phase for both the cases ((c,d)). In the pellet mixed with nut coke, along with iron and silica, wüstite was observed as the leading phase in the core. On the other hand, in the pellet without nut coke, the core was observed to be hollow ((d)). Hence on hollow cores, XRD investigation was not performed.
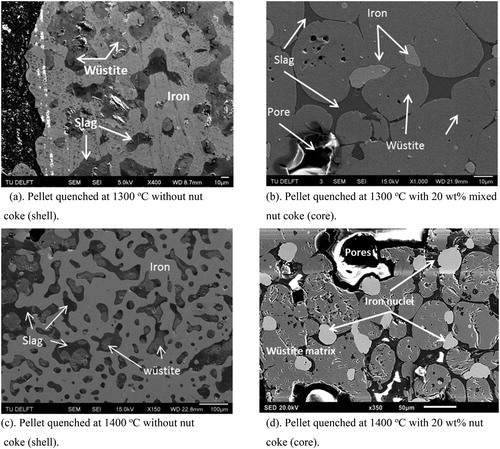
In line with the XRD investigations, the identified phases were reconfirmed by the SEM-EDS analysis. For both cases and quenching temperatures (1300oC and 1400oC), the pellet shell consists of an iron matrix with entrapped partially reduced iron oxide (wüstite) and slag ((a,c)). In the pellet with nut coke quenched at 1300oC, along with wüstite and slag, the presence of iron nuclei at the pellet core was noted ((b)). In the same pellet, at the core dispersed pores in the size range of 10–100μm were also observed.
As discussed earlier, in pellet without mixed nut coke at 1400oC, the core was hollow. However, strikingly in the core of pellet mixed with nut coke, the iron nuclei were observed to be embedded on the wüstite matrix. In the same pellet, the presence of bigger size (100–400 μm) pores was also noted at the core ((d)).
Phase distribution
Pellet quenched at 1300oC
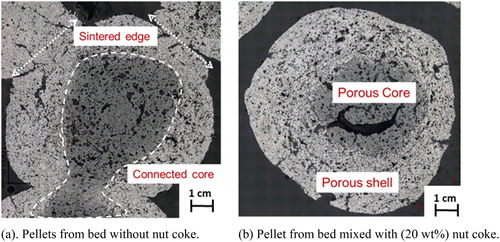
Photographs of the pellet cross sections quenched from 1300oC are shown in . In the pellet without mixed nut coke, close contact among surrounding pellets was seen ((a)). Reduced iron shells of the pellet were sintered with the neighbour pellets. In some instances, due to poor availability of the pellet surface for reduction and sintering at low temperatures (1050oC) [Citation12], pellets were seen connected even before the formation of an iron shell. This brings the situation of a common shell and connected core among two or more pellets ((a)). In the case of quenched pellets with 20 wt-% nut coke, a uniform porous and broader iron shell was witnessed. Furthermore, limited contact (sintering) among the pellets were observed. Quantified phase information for the pellets quenched at 1300oC with and without nut coke is given in . At the outer layer of both pellets, iron was present as the dominant phase. The iron-rich region was thicker for the pellet mixed with nut coke compared to the pellet without nut coke. The total volume of unreduced iron oxide (wüstite) was observed to be large and concentrated at the core for pellet without mixed nut coke.
Phase distribution in pellet quenched at 1400oC
Pellets without mixed nut coke
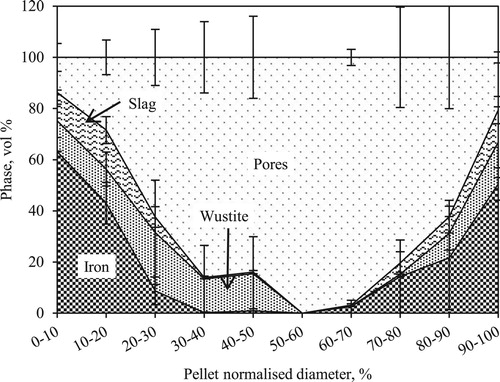
Detailed observation under the microscope for pellets quenched at 1400oC revealed significant variation in the features. Thus, for the pellets quenched at 1400oC the investigations were repeated with two representative pellets. shows the phase distribution within the pellet cross-section. Analysed results are plotted against the normalized pellet diameter. It is clear from that the central part of the pellet is hollow. The material concentration steeply decreases from about 90 vol.-% to 0 vol.-% by reaching nearly 40% of the diameter from both ends of the pellet. Iron is present as the dominant phase in the pellet shell. Within the shell, the presence of both wüstite and solidified slag was observed in a similar range of 10–15 vol.-%, and ∼15 vol.-% of pores were present at the shell. The metal concentration decreases from the shell to the core. It decreases sharply to 0 vol.-% at 40% of its normalized diameter from both ends. Contrary to the metal phase, wüstite and slag concentrations (volume percentage) decreased in a gradual manner from pellet shell to the core. The pore volume was observed to increase continuously from the shell to core.
Pellets with mixed nut coke
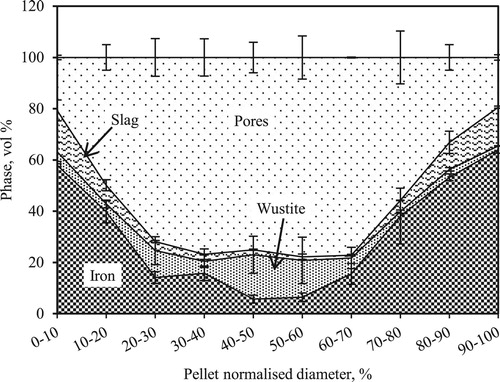
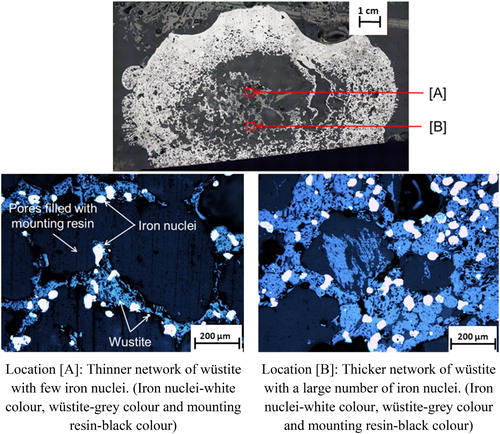
Similar to the case when nut coke was absent, in the presence of (20 wt-%) mixed nut coke (), a high material concentration was observed around the pellet periphery. It decreased from about 90 vol.-% at the pellet edge to approximately 25 vol.-% around the core. The notable difference between the pellet with and without mixed nut coke at 1400oC is the presence of approximately 25 vol.-% of materials at the core in the pellets mixed with nut coke. Material at the core consists of about 5–15 vol.-% metal and 15–20 vol.-% wüstite along with a minimal quantity of the solidified slag. Zooming in the section around the pellet core revealed that these material were present in a network structure (). The matrix consists of wüstite with iron nuclei embedded in it. The iron nuclei are irregular in shape, present in a size range of 10–20μm. The wüstite matrix thickness was inconsistent around the pellet core. The pore sizes were observed in a wide range of 100–400μm.
Discussion
Pellet behaviour in absence of nut coke
Under reducing atmosphere, the topo-chemical reduction of iron ore pellets results in the formation of a product layer of iron metal at the periphery (shell). Because of the load on the burden and sintering phenomenon at high temperature, this iron layer becomes dense and often turns out to be impermeable for the reducing gases. These phenomena hamper the reduction kinetics, leading to the formation of a partially reduced pellet core. As the pellet reaches the high-temperature zone inside the blast furnace, this partially reduced core together with fluxes and gangue minerals in and around the core melts to form the initial slag. This melt pool of slag tries to percolate or break the shell to pass on to its neighbour pellet or to the next available voids [Citation13]. This phenomenon leads to the formation of ‘hollow icicles’ in the pellet bed (). Thereafter with further increase in iron carburization and temperature, the pellet shell ultimately collapses. This results in filling of intrinsic pores and inter pellet voids in the bed. Owing to the liquid filled in the pores and voids, a high-pressure drop is experienced in this temperature zone inside the blast furnace. These series of events were explained by Gudenau et al. [Citation13] after systematic experimentation. Corroborates the observation of Gudenau et al. [Citation13] in the present study, the hollow core was observed in the pellet quenched at 1400oC without nut coke (). Observed pellets were connected to their neighbours and it appears that the liquid from the core has already transferred to the pellet located on the lower side.
Figure 10. Hollow icicles formation in the cohesive zone, adapted from Gudenau et al. [Citation13].
![Figure 10. Hollow icicles formation in the cohesive zone, adapted from Gudenau et al. [Citation13].](/cms/asset/73898d96-c669-4dc4-be23-77f53b9b40d2/yirs_a_1510873_f0010_oc.jpg)
As a result of close contact among the pellets, the probability of sintering among the pellets is high in the case of pellets without nut coke. The connection was observed to start even before core melting, and it is evident from the pellets quenched at 1300oC without nut coke ((a)). Beacuse of close contact and sintering among the pellets, the shell and core seem to be connected. In the sintered pellet cluster, reducing gases find it hard to penetrate inside the core, resulting in a large quantity of the left over partially reduced iron oxide (wüstite) concentrated at the pellet core. Once melting initiates at a high temperature, this situation further accelerates the melting to cause the ‘physical core collapse’ in the pellet, ultimately leading to the collapse and melting of the complete pellet bed.
Pellet behaviour in presence of nut coke
Reduction
Similar to the without nut coke scenario, the reduction reactions in the case of pellets mixed with nut coke progresses topo-chemically. The notable difference is the presence of a thicker iron product layer for the pellets mixed with nut coke for both temperatures (1300oC and 1400oC). Being a source of carbon, nut coke enhances both direct and indirect reduction reactions resulting in a higher reduction degree [Citation14]. Consequently, a comparatively smaller quantity of the wüstite is present at the pellet core ((b)). The wüstite (FeO) phase is known to form a slag with gangue and additives at a relatively low temperature (∼1200oC). That means in the pellet mixed with nut coke, less raw materials (FeO) are available for the liquid (slag) formation. In the presence of more solid material at the core, a lower order of pellet deformation will occur. This delays the deformation and melts formation in the pellets to improve its high-temperature properties.
Furthermore, the presence of iron nuclei even at the core supports the argument of a higher reduction degree in the presence of nut coke, and on the contrary, no material was observed at the pellet core when nut coke was absent ( and ). At the pellet core, iron nuclei are observed embedded in the matrix of wüstite and act as anchor points for the core structure.
Because of the higher surface area (smaller size), gasification reaction is faster for the nut coke compared to the regular coke. This will result in bigger sized regular coke going to the hearth to maintain a healthy (permeable) blast furnace hearth operation.
Sintering
In the pellet bed mixed with nut coke, limited sintering among the pellets are witnessed ((c,d)). On the other hand, close contact and sintering among the pellets are noticed in the pellet bed when nut coke is absent ((a,b), (a), ). In the representative pellets from the bed mixed with nut coke quenched from 1300oC and 1400oC, no sintering with the neighbour is noted ( and (b)). Nut coke brings the ‘spacer effect’ to physically hinder the contact among the pellets. By physical hindering, nut coke prevents the sintering of two or more pellets. Thus at high temperatures, mixed nut coke particles limit the pellet cluster formation. As a result, more surface area and micropores on the pellet surface are available for reduction reactions. Therefore, at high temperatures nut coke enhances the reduction reactions to avoid reduction retardation phenomenon [Citation15], which is known to occur in the pellet bed.
Bed structure
In the pellet bed mixed with nut coke comparatively less bed displacement was experienced at high temperatures [Citation8]. In pellets with mixed nut coke, due to the presence of material (approximately 25 vol.-%) at the cores, it offers higher resistance against the deformation. The reinforcement given to the core wüstite network structure with iron nuclei is clearly marked in the present study ().
Furthermore, to support the bed structure, nut coke acts as the skeleton for the pellet bed. Pellets undergo less sintering due to the support offered by the nut coke structure against the applied load on the top ((b)). This brings more reducing gases to the interior of the pellet to enhance the reduction process. Together with the network structure filled core, a wider iron product layer enhances the high-temperature properties of the pellet. In the case of a pellet mixed with nut coke, a broader iron layer offers higher resistance against the deformation to increase the softening temperature. During melting of the pellets, the nut coke mixed in the bed is expected to support the structure and maintain gas permeability within the bed, hence to offer comparatively less resistance to the gas flow in the bed [Citation8]. Additionally, this will prevent the sudden collapse of the pellet bed.
Conclusions
Based on detailed characterization on the quenched iron ore (olivine fluxed) pellets mixed with (20 wt-%) and without nut coke, the following conclusions are drawn.
In pellet bed quenched from high temperature (1400oC) without nut coke, the presence of a hollow pellet core was reconfirmed with the common understanding of pellet behaviour in the cohesive zone of the blast furnace.
Whereas, in a pellet bed mixed with 20 wt-% nut coke (1400oC), the core was observed to be filled with about 25 vol.-% of the material, which was unevenly distributed in a network structure. In the matrix of wüstite, iron nuclei reinforced the structure to bring additional strength to support the pellets against the deformation.
In the case of pellets mixed with nut coke, for both quenching temperatures (1300oC and 1400oC), a broader outer iron layer (shell) and iron nuclei at the core is a clear result of enhanced reduction kinetics offered by the presence of nut coke.
It is experimentally confirmed that nut coke not only acts as a reducing agent when placed in close proximity of the iron ore pellets but also acts as a ‘spacer’ to physically hinder the contact and sintering among the pellets.
Nut coke addition in mixture with the iron ore pellets has promised higher reduction degree, lower sintering among the pellets and stable bed structure.
Acknowledgements
This research was carried out under project T41.5.13490 in the framework of the research program of the Materials innovation institute (M2i), in the department of Materials Science and Engineering (MSE) of the Delft University of Technology in the Netherlands.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Dharm Jeet Gavel http://orcid.org/0000-0003-0058-7190
Rob Boom http://orcid.org/0000-0002-0519-0208
Yongxiang Yang http://orcid.org/0000-0003-4584-6918
Additional information
Funding
References
- Geerdes M, Toxopeus H, van der Vliet C, et al. Modern blast furnace ironmaking : an introduction. 2nd ed Amsterdam: IOS Press BV; 2009.
- Biswas AK. Principles of blast furnace iron making - theory and practice. Brisbane: Cootha Publishing House; 1981.
- Gavel DJ. A review on nut coke utilisation in the ironmaking blast furnaces. Mater Sci Technol. 2017;33:381–387. doi: 10.1080/02670836.2016.1183073
- Babich A, Senk D, Yaroshevskiy S. Effect of nut coke on blast furnace shaft permeability. Proceeding of 3rd Scanmet; 2008; Lulea. 227–236.
- Mousa EA, Senk D, Babich A, et al. Influence of nut coke on iron ore sinter reducibility under simulated blast furnace conditions. Ironmak Steelmak. 2010;37:219–228. doi: 10.1179/030192309X12506804200906
- Song Q, Yang Y, Boom R. Effect of nut coke on the reduction behavior in iron- making blast furnace. Baosteel Tech Res. 2015;9:8–16.
- Sato A, Aritsuka M, Yamagata Y, et al. The operation with larger amount of nut coke under high pulverised coal rate. CAMP-ISIJ. 1995;8:1064.
- Song Q. Effect of nut coke on the performance of the ironmaking blast furnace [dissertation]. Delft: Delft University of Technology; 2013.
- Kamesh TKS, Simonsson M, Viswanathan NN, et al. Establishing a novel methodology to correlate the macroscopic and microscopic degree of sintering in magnetite pellets during induration. Steel Res. Int. 2018;89:1–8.
- Nellros F. Quantitative image analysis - A focus on automated characterization of structures in optical microscopy of iron ore pellets[dissertation]. Lulea: Lulea University of Technology; 2013.
- Gimp.org[Internet]: [cited 2017 Oct 1]. Available from: https://www.gimp.org/.
- DeAlencar JPS, DeResende VG, DeCastro LFA. Effect of temperature on morphology of metallic iron and formation of clusters of iron ore pellets. Metall Mater Trans B. 2016;47B:85–88. doi: 10.1007/s11663-015-0471-2
- Gudenau HW, Senk D, Wang S, et al. Research in the reduction of iron ore agglomerates including coal and C-containing dust. ISIJ Int. 2005;45:603–608. doi: 10.2355/isijinternational.45.603
- Babich A, Senk D, Gudenau HW. Effect of coke reactivity and nut coke on blast furnace operation. Ironmak Steelmak. 2009;36:222–229. doi: 10.1179/174328108X378242
- Mousa E, Senk D, Babich A. Reduction of pellets-nut coke mixture under simulating blast furnace conditions. Steel Res Int. 2010;81:706–715. doi: 10.1002/srin.201000047