Abstract
Increasing our knowledge of the costs and benefits associated with commensal interactions is necessary both for a better understanding of how ecological communities are structured and for determining conservation actions. We investigated the concomitant use of a burrow by a nesting seabird, the fairy prion (Pachyptila turtur), and a medium-sized predatory reptile, the tuatara (Sphenodon punctatus). Arrival time of fairy prions at the burrow each night was found to be delayed by the presence of a tuatara at the burrow. Use of the burrow by tuatara almost halved the time fairy prions spent at the burrow with their chick. The effect of this cohabitation on each species can be expected to change over both space and time, i.e. with changes in population densities and season. Hence it is possible that in newly established colonies of birds with low numbers in the presence of tuatara, tuatara predation may have a deleterious impact.
Introduction
Commensal and mutualistic interactions occur frequently among species of terrestrial vertebrates, and have important but largely unmeasured effects on fitness (Dickman Citation1992). This is particularly the case when an individual has to share living space, for example a burrow or retreat site, with another species, and when one species has a disproportionate effect on that living space, for example by altering the structure or the microclimate, or restricting the use of that retreat site. Shelters, nests and burrows constructed by many species of vertebrates are often co-utilised by heterospecifics, and this permits exploitation of environments that would otherwise be inaccessible. For example, associations involving use of rodent runways by shrews and weasels (e.g. King Citation1989), or use of owl burrows by small mammals are well known (Machicote et al. Citation2004). However, benefits to the commensal species and possible costs or advantages to hosts in these situations have been poorly studied (Dickman Citation1992).
The tuatara (Sphenodon punctatus), a medium-sized reptile, is often found cohabiting in burrows with various petrel species. Despite a long awareness of their coexistence, the exact nature of the symbiotic relationship between tuatara and seabirds such as fairy prions (Pachyptila turtur) is still unclear. However, there is sufficient evidence to suggest that tuatara benefit greatly from living within the same burrow alongside such seabirds. The birds excavate the burrows in which many tuatara live (though the reptiles can dig their own), and by incorporating their mineral-rich guano they create conditions that may encourage ground-dwelling invertebrates, which form the bulk of the diet of the tuatara (Dawbin Citation1962, 1982; Crook Citation1974; Walls Citation1981). Hence, higher tuatara densities are found in areas with more prions (CitationMarkwell 1998). The bird's eggs and chicks may provide an easy meal for larger males and important nutrients at an energy-demanding time of year as they approach the mating season (Crook Citation1975; Walls Citation1978, Citation1981). Studies on Stephens Island revealed that despite predation on the eggs, chicks and even adults, the prion population as a whole is not significantly decreased by tuatara (Markwell Citation1998). To our knowledge there has been no documentation of the behavioural response of fairy prions to tuatara in the burrow, apart from reports of prions expelling tuatara from their burrows (Newman Citation1987; Gaston & Scofield Citation1995). Both species are subject to conservation measures to repopulate depauperate island faunas resulting from the effects of invasive rodents. Understanding the inter-specific interactions will inform the likely future success of management actions for both species.
In contrast to previous studies, which have targeted the association between fairy prions and tuatara from the tuatara's perspective (Newman Citation1987), in terms of predation levels (Walls Citation1978) or burrow occupation (Newman Citation1978; Gaston & Scofield Citation1995; Markwell Citation1998), we investigated the interaction in terms of potential effects on the fairy prions’ use of the burrow. Our goal was to determine whether tuatara influenced fairy prion activity levels during the burrow prospecting stage (September), egg incubation (October/November), chick feeding (January), or general use of their burrow.
Methods
Study area and species
This study was conducted on Stephens Island (also known by its Māori name, Takapourewa), a 150-ha island located in Cook Strait, New Zealand (40°40′S, 174°00′E), where approximately 1.83 million pairs of fairy prions breed (Craig Citation2010). Fairy prions are active at their colonies during their breeding season from spring (October) to late summer (January). This study was conducted over two successive breeding seasons (October 2009 to January 2011) in an area called Keepers Bush (c.3 ha), which consists of regenerating coastal forest with canopy height approximately 3–5 m (Newman Citation1987).
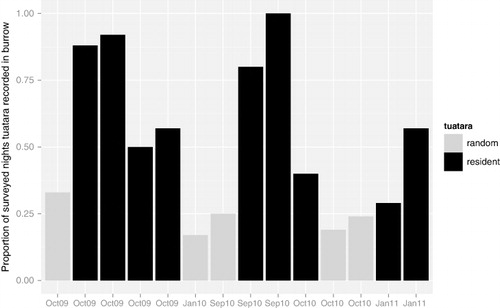
Fairy prions (length 25 cm, weight 90–175 g) display high levels of natal philopatry and site fidelity (Craig Citation2010). The tuatara, endemic to New Zealand, is a medium-sized reptile; females are up to 450 mm in length and 500 g in weight, and males up to 600 mm and 1 kg (Dawbin Citation1982). Stephens Island is home to the largest population of tuatara, with estimated densities of up to 2700/ha in Keepers Bush (Moore et al. Citation2007) and total numbers estimated to be between 30,000 and 50,000 individuals (Newman Citation1982). Tuatara inhabiting forested areas are active throughout the 24-h period (Gillingham & Miller Citation1991). The burrows range from 0.2 m to 0.7 m in depth (Markwell Citation1997), and can be complex with many connected chambers and openings, or simple with only one or two entrances (Newman Citation1982). Most burrows are probably constructed by the fairy prions (Newman Citation1982). It is likely that all burrows within the study area were visited at least once by a tuatara, as tuatara tend to use more than one burrow at any given time (Newman Citation1987) and will opportunistically use any nearby burrow as a retreat when startled (pers. obs.). Hence, burrows were divided into two binomial categories: (1) contains a resident tuatara or not, and (2) a more inclusive category of used regularly or occasionally by one or more tuatara, or not used by any tuatara ().
Recording movements of animals at burrows
A Radio Frequency Identification (RFID) scanner was placed around the entrance to each burrow (n = 10 each trip) and connected to a battery and logger, which were set to default 1-minute re-read settings to prevent excessive readings of the same individual if it was sitting at the entrance to the burrow. Data were downloaded in the field directly into a laptop computer most afternoons, weather permitting. Between October 2009 and January 2011, 44 individual fairy prions at 22 burrows were tagged and monitored. Prion pairs in these burrows were banded on the left leg with a single stainless steel D-band and had a PIT tag (Passive Integrated Transponder, ‘Allflex’ 11.5 mm × 2 mm tags) injected at the base of the neck (Carter et al. Citation2009). Handling time of each fairy prion never exceeded 10 minutes, and upon release each bird either resumed incubating the egg if caught in October (day capture) or went back into its burrow and resumed feeding their chick if caught in January (night capture). Burrows occupied by PIT-tagged birds were monitored for 2–3 weeks during pre-breeding (September, only in year 2), egg incubation (October/November) and chick rearing (January) periods spanning two breeding seasons. Not all birds were present at all burrows for each complete 2- to 3-week period. Within the study area almost all tuatara had been previously identified individually with a PIT tag (‘Allflex’ 11.5 mm × 2 mm tags), toe clip and/or unique sequence of coloured beads in their caudal crest (Moore et al. Citation2009). Ambient temperature was recorded hourly using a data logger (hobo TidbiT®, Onset, Massachusettes) tied to a tree 1 m above ground in the forest shade.
The first record of an individual bird passing through a scanner on any given night was assumed to represent arrival at a burrow. Activity at the burrow during the prospecting stage (September) was defined as the total length of time that an individual bird spent at the burrow in a night. It was measured as the difference in time between the first and last passes through the scanner at the entrance of the burrow on each night. In January, time spent inside the burrow feeding the chick was measured in the same manner as prospecting activity. Visits of less than 5 minutes were discounted (three records), as this time was deemed too short for a feeding event to have occurred. Although it was possible to detect how much time each bird spent passing through the entrance to the burrow each night, the time spent incubating an egg could not be investigated. Because of the volume of passes through each scanner and the default 1-minute re-read settings, the individual bird left behind each night to sit on the egg during the day could not be identified with certainty. Burrows could have been checked manually during the day but it was decided that this would create too much disturbance and compromise the non-invasive monitoring with the scanners. Therefore, because we could not identify with certainty which bird remained behind, we could not detect the amount of time each bird spent incubating (October).
Data analysis
Data were analysed using the statistical software SPSS, version 18.0 (SPSS Inc., Chicago, IL, USA) and R, version 2.11.1 (R Development Core Team Citation2011). Generalised linear mixed models (Lindstrom & Bates Citation1990) in the package ‘lme4’ with procedure ‘glmer’ in program R were used to account for individual random effects within a linear regression framework. These models were used to investigate the predictors of first arrival of fairy prions at the burrow each night (measured as time to the nearest minute) and activity at the burrow entrance (measured as time to the nearest minute between first and last pass through the burrow entrance in a night). In addition to an individual bird random effect, the effects of year, month, night, temperature measured at midnight in degrees Celsius, phase of moon (eight standard moon phases: 1, full moon; 2, waxing gibbous; 3, first quarter; 4, waxing crescent; 5, new moon; 6, waning crescent; 7, first quarter; and 8, waning gibbous), burrows with a resident tuatara, and burrows subject to infrequent visits by tuatara were initially tested. A burrow was identified as having a ‘resident tuatara’ if the same tuatara passed through the scanner every day of the sampling period, and categorised as ‘subject to infrequent visits’ where tuatara (often a number of different individuals) passed through the scanner on at least 25% of the dates during the sampling period. Potentially important interaction terms in these mixed-effects models were also examined, including interactions of month*year, resident.tuatara*year and tuatara*year, as annual variation was possible, particularly with respect to weather and individual behaviour.
Akaike's information criterion corrected for small sample size (AICc; Akaike Citation1978; Anderson et al. Citation1998) defined as AICc = –2 × log-likelihood + 2 × d.f., where d.f. is the number of degrees of freedom in the model, was used to evaluate the model(s). Akaike model weights (wi) were calculated to compare models and determine which model(s) fit the data: wi = exp (−0.5 × ∆AICc /∑ exp (−0.5 × ∆AICc), where ∆AICc is the difference in AICc values between the best performing model and the model of interest. If several models share some weight in explaining the variability within the data set (i.e. wi > 0), or if ∆AICc was <3 points, each model and the effect of all the covariates involved in those models was briefly discussed.
Results
Data available across 2 years for individual fairy prions varied from one to five fieldtrips (). A total of 6864 fairy prion tag readings were obtained. Two tuatara without PIT tags occasionally used two marked burrows, so these observations were added to the data set of tag readings from the scanners. Most burrows were visited at least once by a tuatara. However, considerable variation was observed among burrows: one burrow during one time period was regularly used by three tuatara, whereas another went through a full 2 weeks without any visits from a tuatara.
Table 1 List of burrows monitored over five fieldtrips (only nine burrows in January 2011 due to faulty logger).
Arrival time at the burrow
Two models shared some weight in predicting first arrival time at the burrow (). The top model, with 62% of the overall AICc weight, retained a fixed effect of month and individual heterogeneity (an individual random effect). The second model, with AICc weight of 17%, included the effect of month and infrequent visits from tuatara. Birds at burrows frequented by tuatara arrive later (mean first arrival 0010 h ± 7 min) than when they are the sole occupants of a burrow (mean first arrival 2344 h ± 9 min).
Table 2 Selection results for models testing for the effects of year, month, night, temperature measured at midnight in °C, moon phase, resident tuatara at burrow (tuat.res) and infrequent visits by tuatara (tuatara) on first arrival at burrow (measured as time to the nearest minute).
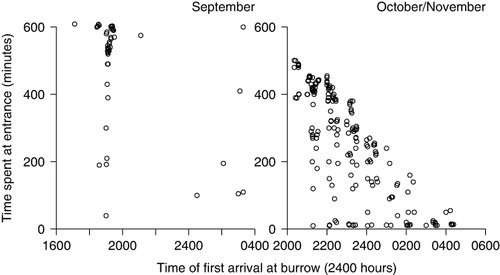
The timing of first arrival at the burrow grew significantly later as the season progressed (F2 = 131.835, P = 0.001), but there was no significant difference between years one and two (F1 = 6.474, P = 0.011). In September, dusk fell between 1805 and 1814 h, and the prions arrived at their burrows 2.7 ± 0.4 h later, with a mean arrival time of 2244 h ± 12 min. In October/November when the fairy prions were incubating their eggs, dusk fell between 1951 and 2014 h, and the prions arrived 3.3 ± 0.1 h after dusk with the mean first arrival at the burrow 2331 h ± 9 min. January had the latest arrival times, with dusk falling between 2057 and 2049 h, and the prions arrived 4.2 ± 0.1 h later, at 0106 h ± 7 min. Hence, birds arrived later each month even when dusk was accounted for. Individual arrival time was found to be highly correlated among all months (r = 0.167, P < 0.01 for all correlations). Thus, birds with early arrival times in September had early arrival times in October and January, and birds with early arrival times in year one had early arrival times in year two.
Prospecting
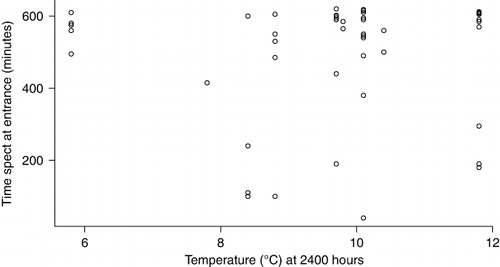
The best model was the null model, including individual heterogeneity (44% AICc weight; ). Two other models could not be discounted. The second model included the presence of a mate at the burrow and temperature at midnight. When both birds of a pair were present at the burrow on any night, they were more active, with 24.91 ± 5.2 passes per individual through the burrow entrance versus 17.9 ± 6.2 when only one of a pair was present (F1 = 8.937, P = 0.003), although there was substantial variation, with three birds recorded over 100 times at the entrance in one night (one lone bird and two in a pair). On warmer nights, there was a trend for birds to stay longer at the burrow (), but this was not significant (r2 = 0.0006, P = 0.583). The third model included first arrival time at the burrow. Birds that arrived later spent less time at the burrow (). Neither a resident tuatara nor occasional visits by tuatara had any effect on activity time spent at the entrance in September.
Table 3 Results for models testing for the effects of year, month, night, temperature measured at midnight in °C, first arrival time, resident tuatara at burrow (tuat.res), infrequent visits by tuatara (tuatara) and mate on activity at the burrow (measured as time to the nearest minute) in September, October/November and January.
Feeding chicks
Two models explained 100% of the overall AICc weight for the length of time at the burrow (). A model retaining a fixed effect of a resident tuatara interacting with year and individual heterogeneity via an individual random effect, and a model that retained a fixed effect of random visits by tuatara*year and individual heterogeneity via an individual random effect each accounted for 50% of the AICc weight. The effect that a resident tuatara had on time spent with chick varied with year. In 2010, prions occupying a burrow with a resident tuatara spent 20.0 ± 2.3 min a night with their chick compared with a mean time of 23.1 ± 2.9 min for those without a resident tuatara. In 2011 this difference was more pronounced: prions occupying a burrow with a resident tuatara spent 15.4 ± 1.4 min a night with their chick whereas those without a resident tuatara spent a mean time of 33.7 ± 8.4 min. There was a similar effect with those burrows subject to visits from non-resident tuatara: in 2010 those in burrows never used by tuatara spent a mean total of 30.0 ± 1.9 min with their chick, and those occasionally used by tuatara spent 22.8 ± 4.5 min. In 2011 these differences were again magnified. Those in burrows never used by tuatara spent a mean total of 35.7 ± 9.2 min with their chick, in contrast with 15.4 ± 1.4 min for those occasionally used by tuatara.
Success at individual burrows
Birds at five out of ten burrows in the first year successfully incubated eggs to the hatchling stage (). Of these, two were used by tuatara, and three of the five failed burrows were used by tuatara. Only four of the ten eggs in the second year resulted in live chicks in January 2011 (one with a resident tuatara) and of the six failed nests, three were occupied by a resident tuatara. All chicks, in all burrows, in both years survived until at least the end of January, and at the end of the sampling period, none had yet fledged in 2010 but two had in 2011.
Discussion
Some key relationships in the life of an organism are the interactions with individuals of other species within the community. The use of PIT tags and dataloggers as a means of documenting activity at a seabird colony enabled us to investigate the concomitant occupation of a burrow by two different taxa with very limited disturbance to behaviour. As a result, we revealed that tuatara are interfering with fairy prions’ use of their breeding burrow.
Fairy prions tended to enter burrows frequented by tuatara later in the evening compared to burrows not used by tuatara. Tuatara may sit at the entrance to their burrow for several hours (Walls Citation1983) and may temporarily block access by fairy prions. The timing of the prions’ arrival at the colony and the subsequent frenetic use of the burrow coincides with nightfall, when tuatara tend to emerge from their burrows (Saint Girons et al. Citation1980), which could mean that fairy prions are delayed from entering their burrow if tuatara are at or near the entrance. One limitation of the PIT tags and RFID was that we could not confirm when the prions arrived at the colony, only when they entered the burrow.
In September, neither a resident tuatara nor visits by other tuatara seemed to have any effect on the level of activity at a burrow. It is possible that when the birds are socialising while prospecting for burrows and reacquainting with mates there is too much disturbance for the tuatara so it vacates the burrow. When both individuals of a pair are present on any one night, they enter and exit the burrow more often than when alone at the burrow. This may also be an opportunity for birds to strengthen pair bonds. Breeding success among seabirds is strongly associated with continued partnership with the same mate (Bradley et al. Citation1990). September is also the only period in which our only environmental factor, temperature, affects activity levels at the burrow. Nightly variability of activity at the colony was affected by weather conditions in ancient murrelets (Synthliboramphus antiquus) (Jones et al. Citation1990) and wind speed influenced arrival times of grey-faced petrels (Pterodroma macroptera gouldi) at a colony (Ross & Brunton Citation2002). It is possible that weather conditions play a greater role during this period in the absence of any parental duties.
Tuatara affect the amount of time that an adult fairy prion will spend in a burrow with their chick. Both a resident tuatara and visits from random tuatara almost halved the time that an adult prion spent in the burrow. However, impacts on chick survival remain unclear. If conditions at a burrow present an above average challenge for a successful reproductive event, then birds may reduce the amount of effort put into the rearing of their offspring. The successful incubation of the egg to the hatchling stage was not correlated with the presence of a tuatara and none of the chicks in our study burrows suffered predation by tuatara. However, limited numbers and no way of ascertaining the cause of nest failures meant we were unable to determine whether they were a result of tuatara predation/interference or some other factor such as inexperienced breeding adults.
The coexistence with tuatara is perhaps the most striking feature of the natural history of petrels such as the fairy prion in New Zealand. For example, both Māori (Ramstad et al. Citation2007) and European naturalists who lived on or visited the islands off New Zealand on which the fairy prion occurs described or noted the behaviour as something extraordinary. However, categorising the relationship between tuatara and petrels has always been difficult.
Understanding the mechanisms and conditions underlying species interactions is a key challenge for ecosystem conservation (Stachowicz Citation2001). The classification of interactions has also become useful in terms of conservation efforts, such as in the identification of obligate mutualisms necessary for the functioning of a particular species or community. Since we now have evidence that tuatara are interfering with fairy prions’ use of their burrow, we cannot call this interaction a commensalism. Neither can we call it a mutualism, as there is no evidence to support the fact that fairy prions are gaining any fitness benefits from the association. There is a potential benefit to prions of tuatara presence, if this discourages prospecting prions from entering the burrow and competing for use of the burrow (this effect is hinted at as tuatara presence reduces or delays burrow use/visits by resident prions). However, assessing this would require a longer-term study looking at rates of burrow turnover and mate fidelity with tuatara presence. In certain instances, it may be that this interaction is best classed as a parasitism with the tuatara benefitting from burrow use, an enhanced microclimate (Corkery et al. Citation2014) and easy predation opportunities (Walls Citation1978; Markwell Citation1998) in certain cases (although none were documented in the present study) to the detriment of the lifetime reproductive success of the fairy prions. In other instances it may simply be a case of competition for a limited resource (a burrow) with the outcome varying depending on the individuals and the circumstances involved. It is now recognised that commensal and mutualistic associations are dynamic; they form and dissolve under different conditions of predator risk, resource levels, competition and many other factors (Dickman Citation1992). Further study into exactly how this dynamic relationship shifts in response to burrow availability and population densities of tuatara and fairy prions would possibly reveal more about this intriguing cohabitation.
Understanding the effects that management actions have on interspecific associations is a priority for individual species recovery. The Recovery Plan for Tuatara (Gaze Citation2001) considers small nesting seabirds to be a habitat feature that is favourable for the long-term survival of tuatara. Conversely, interference and predation by tuatara can have a negative effect on the breeding success of fairy prions, but it is thought that tuatara are unlikely to be a major cause of population regulation of the birds (Walls Citation1978). However, it is possible that in newly established colonies with low numbers of birds, e.g. as the result of translocations (see Miskelly et al. Citation2009), tuatara predation may have a deleterious impact. Hence, understanding the mechanisms and conditions underlying species interactions is fundamental to conservation and failure to consider the ecological network within which species are embedded may lead to counterproductive management measures, particularly where species are threatened or at low numbers in restored populations.
Acknowledgements
The following organisations provided financial support: Victoria University Doctoral Research Scholarship, Faculty of Science at Victoria University, Centre for Biology and Restoration Ecology (CBRE), Allan Wilson Centre for Molecular Ecology and Evolution, and Forest and Bird. This work was also supported by Ngāti Kōata iwi with permission from Department of Conservation (permit NM-23620-RES). Thank you also to the many field assistants who helped out on this project.
References
- Akaike H 1978. A Bayesian analysis of the minimum AIC procedure. Annals of the Institute of Statistical Mathematics 30: 9–14. 10.1007/BF02480194
- Anderson D, Burnham K, White G 1998. Comparison of Akaike information criterion and consistent Akaike information criterion for model selection and statistical inference from capture-recapture studies. Journal of Applied Statistics 25: 263–282. 10.1080/02664769823250
- Bradley J, Wooller R, Skira I, Serventy D 1990. The influence of mate retention and divorce upon reproductive success in short-tailed shearwaters Puffinus tenuirostris. Journal of Animal Ecology 59: 487–496. 10.2307/4876
- Carter GM, Barnes GRG, Castro I 2009. The use of radio frequency identification tags (passive integrated transponders) in a field study of ship rats, Rattus rattus. Kokako 16: 1–21.
- Corkery I, Bell B, Nelson N 2014. Investigating kleptothermy: a reptile-seabird association with thermal benefits. Physiological and Biochemical Zoology 87: 216–221. 10.1086/674566
- Crook IG 1974. Are tuataras dependent on petrels? Wildlife: A Review 5: 43–46.
- Crook IG 1975. The tuatara. In: Kuschel G ed. Biogeography and ecology in New Zealand. Department of Internal Affairs Wildlife Publication No. 167. The Hague, The Netherlands, Dr W Junk, BV Publishers. Pp. 331–352.
- Craig, ED 2010. Takapourewa titiwainui (fairy prion; Pachyptila turtur): how nest site selection affects breeding success, with applications for translocation. Unpublished MSc thesis. Dunedin, University of Otago.
- Dawbin WH 1962. The tuatara in its natural habitat. Endeavour 21: 16–24. 10.1016/0160-9327(62)90129-1
- Dawbin WH 1982. The Tuatara, Sphenodon punctatus (Reptilia: Rhynchocephalia): a review. In: Newman DG ed. New Zealand herpetology. Wellington, New Zealand Wildlife Service. Pp. 149–181.
- Dickman C 1992. Commensal and mutualistic interactions among terrestrial vertebrates. Trends in Ecology & Evolution 7: 194–197. 10.1016/0169-5347(92)90072-J
- Gaston AJ, Scofield P 1995. Birds and tuatara on North Brother Island, Cook Strait, New Zealand. Notornis 42: 27–41.
- Gaze P 2001. Tuatara recovery plan, 2001–2011. Threatened species recovery plan 47. Wellington, Department of Conservation Te Papa Atawhai. http://www.doc.govt.nz/documents/science-and-technical/TSRP47.pdf (accessed 11 February 2015).
- Gillingham JC, Miller TJ 1991. Reproductive ethology of the tuatara Sphenodon punctatus: applications in captive breeding. International Zoo Yearbook 30: 157–164. 10.1111/j.1748-1090.1991.tb03479.x
- Jones IL, Gaston AJ, Falls JB 1990. Factors affecting colony attendance by ancient murrelets (Synthliboramphus antiquus). Canadian Journal of Zoology 68: 433–441. 10.1139/z90-064
- King CM 1989. The advantages and disadvantages of small size to weasels, Mustela species. In: Gittleman JL ed. Carnivore behavior, ecology, and evolution. New York, Cornell University Press. Pp. 302–334.
- Lindstrom MJ, Bates DM 1990. Nonlinear mixed effects models. Biometrics 46: 673–687. 10.2307/2532087
- Machicote M, Branch LC, Villarreal D 2004. Burrowing owls and burrowing mammals: are ecosystem engineers interchangeable as facilitators? Oikos 106: 527–535. 10.1111/j.0030-1299.2004.13139.x
- Markwell TJ 1997. Video camera count of burrow-dwelling fairy prions, sooty shearwaters, and tuatara on Takapourewa (Stephens Island), New Zealand. New Zealand Journal of Zoology 24: 231–237. 10.1080/03014223.1997.9518118
- Markwell TJ 1998. Relationship between tuatara Sphenodon punctatus and fairy prion Pachyptila turtur densities in different habitats on Takapourewa (Stephens Island), Cook Strait, New Zealand. Marine Ornithology 26: 81–83.
- Miskelly CM, Taylor GA, Gummer H, Williams R 2009. Translocations of eight species of burrow-nesting seabirds (genera Pterodroma, Pelecanoides, Pachyptila and Puffinus: Family Procellariidae). Biological Conservation 142: 1965–1980. 10.1016/j.biocon.2009.03.027
- Moore J, Daugherty C, Godfrey S, Nelson N 2009. Seasonal monogamy and multiple paternity in a wild population of a territorial reptile (tuatara). Botanical Journal of the Linnean Society 98: 161–170. 10.1111/j.1095-8312.2009.01271.x
- Moore JA, Hoare JM, Daugherty CH, Nelson NJ 2007. Waiting reveals waning weight: monitoring over 54 years shows a decline in body condition of a long-lived reptile (tuatara, Sphenodon punctatus). Biological Conservation 135: 181–188. 10.1016/j.biocon.2006.10.029
- Newman DG 1978. Tuataras and petrels. Wildlife: A Review 9: 16–23.
- Newman DG 1982. Tuatara, Sphenodon punctatus, and burrows, Stephens Island. In: Newman DG ed. New Zealand herpetology. Wellington, New Zealand Wildlife Service. Pp. 213–223.
- Newman DG 1987. Burrow use and population densities of tuatara (Sphenodon punctatus) and how they are influenced by fairy prions (Pachyptila turtur) on Stephens Island, New Zealand. Herpetologica 43: 336–344.
- Ramstad KM, Nelson NJ, Paine G, Beech D, Paul A, Paul P et al. 2007. Maori traditional ecological knowledge complements science in conservation of tuatara (Sphenodon). New Zealand Journal of Zoology 34: 269–270.
- R Core Team 2011. R: A language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing. ISBN 3-900051-07-0. http://www.R-project.org/ (accessed 11 February 2015).
- Ross EL, Brunton DH 2002. Seasonal trends and nightly variation in colony attendance of grey-faced petrels (Pterodroma macroptera gouldi). Notornis 49: 153–157.
- Saint Girons H, Bell BD, Newman DG 1980. Observations on the activity and thermoregulation of the tuatara, Sphenodon punctatus (Reptilia: Rhynchocephalia), on Stephens Island. New Zealand Journal of Zoology 7: 551–556.
- Stachowicz JJ 2001. Mutualisms, positive interactions, and the structure of ecological communities. Bioscience 51: 235–246. 10.1641/0006-3568(2001)051[0235:MFATSO]2.0.CO;2
- Walls GY 1978. The influence of the tuatara on fairy prion breeding on Stephens Island, Cook Strait. New Zealand Journal of Ecology 1: 91–98.
- Walls GY 1981. Feeding ecology of the tuatara (Sphenodon punctatus) on Stephens Island, Cook Strait. New Zealand Journal of Ecology 4: 89–97.
- Walls GY 1983. Activity of the tuatara and its relationships to weather conditions on Stephens Island, Cook Strait, with observations on geckos and invertebrates. New Zealand Journal of Zoology 10: 309–318. 10.1080/03014223.1983.10423920