ABSTRACT
Infection with Helicobacter pylori is the strongest modifiable risk factor for stomach cancer. Nine out of 10 people who develop stomach cancer in the distal part of the stomach have been infected with H. pylori. Infection of the gastric mucosa with H. pylori most commonly occurs during childhood and if not treated can cause chronic inflammation and gastric carcinogenesis. Stomach cancer is a major contributor to the ethnic gap in cancer incidence and mortality in New Zealand, with Māori and Pacific peoples having three to six times higher rates of stomach cancer than New Zealand Europeans. Infection with H. pylori is the main driver of this inequity. Testing asymptomatic individuals with non-invasive tests (serology, stool or C-urea breath tests), and treating those who are infected with H. pylori using antibiotics and proton pump inhibitors has been found to be effective in reducing stomach cancer incidence, with an estimated one-third reduction in risk. A programme based on this approach is likely to be cost-effective in New Zealand, and pro-equity particularly if targeted towards Māori and Pacific peoples. However, there are substantial gaps in our knowledge base that must be filled before such a programme can be considered.
Introduction
The last three decades have seen a substantial increase in our knowledge of Helicobacter pylori and its impacts (Fraser Citation2004; Lee, Chiang, Liou, et al. Citation2016). H. pylori – a bacterium that lives in the lining of the stomach (McDonald et al. Citation2015) – is now well-established internationally as the strongest modifiable risk factor for stomach cancer (International Agency for Research on Cancer Citation1994). Stomach cancer is one of the most common malignancies worldwide, with H. pylori infection implicated in 9 out of 10 people with cancer in the distal (lower) stomach (International Agency for Research on Cancer Citation1994; Plummer et al. Citation2015). Significantly, the high global burden of stomach cancer has prompted the International Agency for Research on Cancer (IARC) to announce that all countries should have a strategy to address stomach cancer, which includes exploring the possibility of introducing population-based H. pylori testing and treatment programmes (Herrero et al. Citation2014).
Māori, and Pacific peoples living in New Zealand, have substantially higher rates of stomach cancer than New Zealand Europeans (Blakely et al. Citation2010; Ministry of Health Citation2016; Teng, Atkinson, et al. Citation2016). Some of this excess stomach cancer incidence, particularly among younger Māori, is caused by an increased propensity toward mutation of the CDH1 gene amongst Māori (Hakkaart et al. Citation2019). However, as in other countries around the world, the primary driver of inequities in stomach cancer incidence within New Zealand, remains the differential rates of H. pylori infection by ethnicity (Teng, Blakely, et al. Citation2016).
The Treaty of Waitangi and the United Nations Declaration on the Rights of Indigenous Peoples outline responsibilities of the New Zealand Government to the indigenous Māori population, which include achieving equitable health outcomes with non-Māori (Ministry of Health Citation2002; United Nations Citation2007). Large and sustained inequities in stomach cancer rates between Maori and Pacific peoples compared to New Zealand Europeans (Blakely et al. Citation2011) also provide a moral imperative to act. Yet despite stomach cancer being a disease that is amenable to both primary and secondary prevention, there has been little focus to date in New Zealand on the prevention of stomach cancer through measures to address the environmental risk factors and high rates of H. pylori infection.
Here, we provide background information about stomach cancer and stomach cancer inequities, primarily within the New Zealand context. We then discuss the natural history and unequal impact of H. pylori infection, followed by the evidence – including cost-effectiveness – for testing-and-treating H. pylori. We finish with research implications and policy considerations for New Zealand.
Stomach cancer
In 2018, stomach cancer was the fifth most commonly diagnosed cancer globally, behind lung, breast, prostate and colorectal cancer (Bray et al. Citation2018; Rawla and Barsouk Citation2019). It is also one of the most common causes of cancer mortality worldwide, with a death rate second only to lung cancer (Ferlay et al. Citation2010; Bray et al. Citation2018). Globally, there is wide geographical variation in stomach cancer incidence, with rates varying up to 10-fold between high- and low-risk populations (Forman and Burley Citation2006; Guggenheim and Shah Citation2013). Internationally, indigenous populations such as indigenous Siberians, Mapuche in South America and Inuit in Arctic regions of Greenland, Canada and Alaska all have higher incidence rates of stomach cancer than their non-indigenous counterparts (Arnold et al. Citation2014; Rawla and Barsouk Citation2019), most probably driven by higher rates of H. pylori infection (Arnold et al. Citation2014). Similarly to incidence, mortality rates are mostly poorer for indigenous peoples when compared to their majority non-indigenous populations (Arnold et al. Citation2014). For example, from 2012 to 2016 stomach cancer mortality rates among American Indians and Alaskan Natives were reported as 11.2 in men and 6.1 in women. The corresponding rates for Non-Hispanic White Americans were 7.8 and 3.5, respectively (Siegel et al. Citation2019). Mortality for Indigenous Australian men has been shown to be greater than two-fold increased than that of the total New South Wales population (Supramaniam et al. Citation2006; Morrell et al. Citation2012). While mortality for Indigenous Australian women showed a near two-fold increase when compared to non-Indigenous women (1.98; 95% CI, 1.15–3.16) (Morrell et al. Citation2012).
Compared to other international contexts, New Zealand does not have a high overall incidence of stomach cancer. The world age-standardised incidence rate for stomach cancer is 15.7 in males and 7.0 in females (per 100,000) (Bray et al. Citation2018). By comparison, in New Zealand the rate is 7.2 for males and 3.9 for females (Ministry of Health Citation2016). However, these rates disguise differential rates by ethnicity: the most recent data available from 2013 show that the age-standardised incidence rate of stomach cancer for Māori males and females was 17.0 and 12.9 per 100,000 respectively (Ministry of Health Citation2016), and the age-standardised mortality rates were 15.9 and 8.0 for Māori males and females respectively (Ministry of Health Citation2016). These rates place stomach cancer among the 10 most common cancers for Māori males and the fifth most common for Māori females (Ministry of Health Citation2016).
Although stomach cancer incidence declined over the time period 1981–2004 for all ethnic groups in New Zealand, substantial inequities have persisted (Blakely et al. Citation2010). Rates for Māori and Pacific peoples living in New Zealand have been consistently three to six times higher than those for New Zealand Europeans (Teng, Atkinson, et al. Citation2016). Additionally, between 1981 and 2004 a widening of relative stomach cancer rates between Māori and European/Other females was observed (Blakely et al. Citation2010). Stomach cancer is among the top contributors to the ethnic gap in cancer incidence between Māori, Pacific and European/Other peoples, and ranks second only to lung cancer as a contributor to ethnic inequities in mortality (Teng, Atkinson, et al. Citation2016). Infection with H. pylori is shown to be the major contributor to ethnic inequities in stomach cancer incidence, accounting for 50% and 82% of the excess distal stomach cancer incidence among Māori and New Zealand Pacific men respectively compared to New Zealand European males (Teng, Blakely, et al. Citation2016).
In addition to infection with H. pylori, stomach cancer has a number of other potentially modifiable environmental risk factors. These factors include tobacco and alcohol use, obesity, and diets with large amounts of smoked, salted or pickled food, while diets high in fresh fruit and vegetables appear to have a protective effect (Forman and Burley Citation2006; Guggenheim and Shah Citation2013). Additionally, there is a strong deprivation gradient in both incidence and mortality for stomach cancer (Blakely et al. Citation2010; Soeberg et al. Citation2012) with consistent evidence of increased risk within more deprived groups within any given population (Kelley and Duggan Citation2003; Forman and Burley Citation2006). This excess incidence of stomach cancer reflects similar increased levels of H. pylori infection amongst these groups (Kelley and Duggan Citation2003; MacDonald Citation2013). In New Zealand, both Māori and non-Māori with stomach cancer are more likely to live in areas of higher deprivation (Signal Citation2016). In addition, Māori are more likely to live in more highly deprived areas than non-Māori and this has been shown to account for up to 14% of the stomach cancer incidence and mortality differences between Māori and non-Māori New Zealanders (Robson et al. Citation2010).
Stomach cancer site
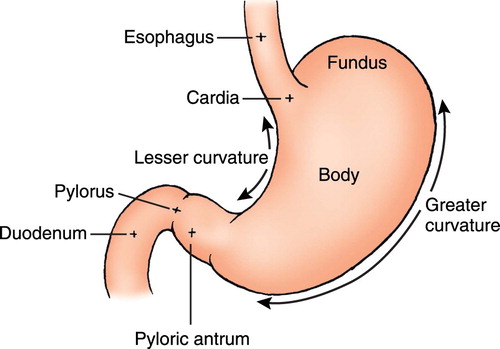
shows the divisions or sub-sites of the stomach. Collectively tumours arising in the cardia, fundus or body of the stomach can be referred to as proximal tumours while those arising in the antrum or pyloric region can be referred to as distal tumours (American Cancer Society Citation2013). In addition to the distinction between proximal and distal tumours, another important distinction is made between tumours located in the cardia (adjacent to the oesophagus) versus those located in other areas of the stomach (non-cardia), as they have different epidemiologic patterns and causes (Guggenheim and Shah Citation2013). Ethnicity, in particular, is important to stomach cancer site with marked variation in tumour location observed in different ethnic groups (Guggenheim and Shah Citation2013). For example in the United States, incidence of non-cardia stomach cancer is high among African-Americans (Crew and Neugut Citation2006; Forman and Burley Citation2006), American Indians and Alaskan Natives (Arnold et al. Citation2014), while white Americans are twice as likely as these ethnic groups to have tumours located in the cardia region of the stomach (Crew and Neugut Citation2006; Forman and Burley Citation2006; Arnold et al. Citation2014). Comparably, in New Zealand, Māori (Biggar et al. Citation2011; Arnold et al. Citation2014; Signal et al. Citation2015) and Pacific peoples (Biggar et al. Citation2011) have much higher proportions of non-cardia based tumours when compared to New Zealand Europeans, who have higher proportions of tumours located within the cardia region of the stomach (Biggar et al. Citation2011; Arnold et al. Citation2014; Signal et al. Citation2015).
Figure 1. Divisions of the stomach (Wilson and Stevenson Citation2007).
Hereditary stomach cancer
Approximately 1%–3% of confirmed stomach cancers arise from known inherited cancer predisposition disorders (Lynch et al. Citation2005; Hu et al. Citation2012), including syndromes such as Lynch, Peutz-Jeghers, Cowden and Li-Fraumeni syndromes, familial adenomatous polyposis, and hereditary diffuse gastric cancer (HDGC) (Lynch et al. Citation2005; Hu et al. Citation2012; Rawla and Barsouk Citation2019). The latter of these, HDGC, is the best-described form of hereditary stomach cancer and comprises approximately 1% of all stomach cancers (Blair et al. Citation2006). It is well known in New Zealand (Abrams and Wang Citation2010; Blair et al. Citation2013) due to a large Māori family, who in collaboration with researchers, published seminal work describing a molecular basis for familial stomach cancer (Guilford et al. Citation1998). For the individuals and families affected, the impact of the genetic mutation associated with HDGC is substantial, and the genetic screening and interventions now available are life-saving (Guilford et al. Citation2010; Hakkaart et al. Citation2019) Approximately 6% of advanced cancers among Māori have been identified as having a CDH1 mutation – the mutation predominantly responsible for HDGC (Hakkaart et al. Citation2019). This is a substantial burden of disease attributable to a known hereditary factor. However, the vast majority of stomach cancers among Māori (and non-Māori) cannot be explained by known hereditary risk factors – with H. pylori and other environmental risk factors likely to be the most important contributors to the New Zealand population burden of stomach cancer (Blair et al. Citation2013; Teng, Blakely, et al. Citation2016; Ellison-Loschmann et al. Citation2017).
Stomach cancer prognosis
Stomach cancer has a poor prognosis, with many patients diagnosed at an advanced stage (Dicken et al. Citation2005; Abrams and Wang Citation2010; Allum et al. Citation2011) and most countries reporting five-year survival rates between 10% and 30% (Dicken et al. Citation2005; Crew and Neugut Citation2006; Forman and Burley Citation2006; Mercer and Robinson Citation2008). In New Zealand, nearly half of all patients are diagnosed with metastatic disease and the survival rate from stomach cancer overall is 20% (Signal Citation2016). However during 1991–2004, Māori with stomach cancer had excess mortality of 25% above that of non-Māori (Soeberg et al. Citation2012), and more recently (2006–2008) 30% poorer stomach cancer survival when compared with non-Māori (Signal Citation2016). Stage at diagnosis does not appear to be a contributor to this differential survival: no difference in stage was observed between Māori and non-Māori in two New Zealand studies that used clinical note review to provide accurate data about stomach cancer stage (Biggar et al. Citation2011; Signal et al. Citation2015).
Notwithstanding the social costs associated with a stomach cancer diagnosis and premature death, each person diagnosed with stomach cancer in New Zealand is estimated to cost the health system $46,000, with costs of stomach cancer treatment totalling approximately $17 million annually (Blakely et al. Citation2015). These social and fiscal costs, in addition to the inequitable burden of disease borne by Māori, and Pacific peoples living in New Zealand, emphasise the strong need for investment in stomach cancer prevention.
H. pylori
H. pylori and stomach cancer pathogenesis
H. pylori is classed as a group one carcinogen by IARC, with the lifetime risk of stomach cancer amongst those infected with H. pylori estimated at 1%–3% (International Agency for Research on Cancer Citation1994). Infection of the gastric mucosa with H. pylori most commonly occurs in childhood, and can result in chronic long-lasting inflammation or gastritis. Chronic inflammation can promote gastric carcinogenesis, typically via the Correa cascade of atrophic gastritis, intestinal metaplasia, and dysplasia () (Correa Citation1996; Moss Citation2017). H. pylori expresses an array of proteins that interact with receptors in stomach epithelial cells, and signal cellular pathways that change the expression of genes involved in inflammation, cellular proliferation, invasion and metastasis (International Agency for Research on Cancer and World Health Organization Citation2014). Decades of H. pylori-related inflammation can lead to gene methylation (epigenetic changes), and chronic exposure to reactive oxygen and nitrogen species that cause DNA damage and gene mutations leading to the development of cancer (International Agency for Research on Cancer and World Health Organization Citation2014). H. pylori virulence factors such as cytotoxin-associated gene A (CagA), vacuolating cytotoxin (VacA), or lipopolysaccharide (LPS) also play a role in carcinogenesis by modulating cellular signalling pathways (International Agency for Research on Cancer and World Health Organization Citation2014). For example, it is known that CagA positive H. pylori increases the risk of stomach cancer more than the Cag-A negative H. pylori strain (Huang et al. Citation2003). Additionally, different CagA subtypes carry differing risks of cancer. The Eastern strains prevalent in Asia, and in Māori (Fraser Citation2004) are more pathogenic than Western strains (Yuan et al. Citation2017). Ethnic differences in the virulence strains of H. pylori may contribute to the Māori/non-Māori stomach cancer incidence gap, although the current pattern of virulence factors in New Zealand is unknown.
Figure 2. Multifactorial pathway leading to gastric carcinoma (Wroblewski et al. Citation2010).
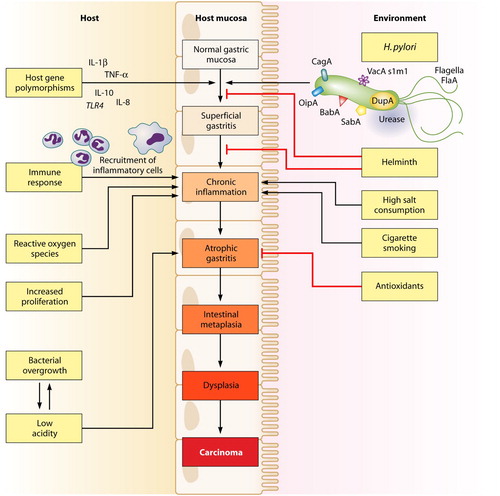
A subject of debate is whether H. pylori infection alone can be responsible for the development of stomach cancer (Forman and Burley Citation2006; World Health Organization Citation2008), or whether other cofactors need also be present (Brenner et al. Citation2004; Crew and Neugut Citation2006). The vast majority of people infected with H. pylori do not go on to develop stomach cancer (Crew and Neugut Citation2006), and globally, there are regions with high prevalence of H. pylori but paradoxically low prevalence of stomach cancer, e.g. south Asia and many African countries (Yamaoka Citation2010; Fock Citation2014). Thus, it is presumed that infection with the bacterium creates an internal environment that when combined with one or more other factors, creates a much higher risk of stomach cancer than that seen by any one risk factor alone (Brenner Hermann et al. Citation2002; Crew and Neugut Citation2006; World Health Organization Citation2008; Gonzalez and Lopez-Carrillo Citation2010; Blair et al. Citation2013). Better understanding of these co-factors – such as age at acquisition of infection, the prevalent strains of H. pylori, and environmental factors such as diets high in nitrates, salted or pickled foods and tobacco use (Ishaq and Nunn Citation2015) – will contribute to further understanding of stomach cancer pathogenesis and prevention. Importantly to the New Zealand context, there appears to be an interaction between infection with H. pylori and tobacco use on the subsequent development of stomach cancer (Gonzalez and Lopez-Carrillo Citation2010), especially for distal tumours (Brenner Hermann et al. Citation2002; Gonzalez and Lopez-Carrillo Citation2010). Māori have among the highest rates of tobacco use in the world and tend to begin smoking at an early age (Ministry of Health Citation2011), and so tobacco use is proposed as a potentially important driver of stomach cancer for Māori (Ellison-Loschmann et al. Citation2017). shows that many host, bacterial, and environmental factors interact in combination to contribute to the Correa cascade leading to the development of stomach cancer.
The unequal impact of H. pylori
H. pylori is exchanged between people via the oral or gastro/faecal-oral route, with poverty and household overcrowding implicated in its transmission (McCallion et al. Citation1996; Broutet et al. Citation2001; Baker et al. Citation2013; Herrero et al. Citation2014). Globally, there are an estimated 4.4 billion individuals infected with H. pylori (Hooi et al. Citation2017), however, there is wide variation in the prevalence of H. pylori between and within countries. Generally, Indigenous – and minority – populations have much higher H. pylori prevalence than their non-Indigenous counterparts, most probably reflecting differences in socioeconomic position within these populations. For example, Alaskan Natives in the United States have H. pylori prevalence of 75% and non-whites a prevalence between 35% and 62%, while prevalence among non-Hispanic white Americans ranges from 18% to 26% (Hooi et al. Citation2017).
Higher rates of H. pylori are also linked to poverty and household crowding in New Zealand. From studies internationally, people living in conditions with the greatest household crowding have an average 80% higher infection risk with H. pylori when compared to those experiencing the least crowding (RR 1.82; 95% CI, 1.55–2.14), independent of age and socioeconomic status (Baker et al. Citation2013). Māori and Pacific peoples are substantially more likely to live in the overcrowded housing conditions that are a key risk factor in the transmission of H. pylori (Baker et al. Citation2012, Citation2013); and as a likely consequence of this, the prevalence of the infection is consistently higher for Māori and Pacific peoples than New Zealand Europeans (Fraser et al. Citation1996, Citation2010). For example, from 1983 to 1999 (the most recent data available) Māori had an H. pylori seroprevalence of 35%, substantially higher than the 18% seroprevalence seen among New Zealand Europeans (McDonald et al. Citation2015). Seroprevalence for Pacific peoples was even higher at 62%, more than 3 times greater than New Zealand European seroprevalence (McDonald et al. Citation2015).
Stomach cancer prevention
As previously discussed, high-risk individuals and families may undergo genetic screening and interventions – such as prophylactic gastrectomy – to prevent stomach cancer (Guilford et al. Citation2010; Hakkaart et al. Citation2019; Rawla and Barsouk Citation2019). However, at a population level, preventing new H. pylori infections is likely to be the most effective long-term primary prevention strategy for reducing stomach cancer rates (Fock Citation2014). Addressing household overcrowding is fundamental to such a strategy and could also offer the advantage of reducing other infections associated with close-contact and poverty (Baker et al. Citation2013; McDonald et al. Citation2015). Other primary prevention measures of particular importance and relevance to stomach cancer consist of dietary measures, such as reducing salt intake, reducing levels of obesity and tobacco control (Talley et al. Citation2008; Allum et al. Citation2011; Pasechnikov et al. Citation2014). All of these measures would have broad population health benefits, although evidence is limited regarding their impact on future rates of stomach cancer (Talley et al. Citation2008; Pasechnikov et al. Citation2014). In contrast, reducing the prevalence of H. pylori infection using measures to detect and treat H. pylori could contribute to reductions in future incidence of stomach cancer by up to a third, with completed randomised controlled trials showing a 33% reduction in risk of stomach cancer from testing for and treating high risk asymptomatic populations (Ford et al. Citation2015). Current New Zealand primary care guidelines only recommend testing for and treating H. pylori in the presence of indigestion in combination with other major risk factors, such as symptoms that are severe or persistent, family history of stomach cancer, and if of Māori, Pacific or Asian descent and aged ≥40 years (New Zealand Guidelines Group Citation2009; Bpac NZ Ltd Citation2014).
H. pylori testing-and-treating in asymptomatic populations as a strategy for stomach cancer prevention
Internationally there is increasing interest in investigating and implementing measures to prevent stomach cancer, primarily focused on the testing and treatment of H. pylori (Herrero et al. Citation2014). Randomised trials have been conducted in China (Wong et al. Citation2004; Ma et al. Citation2012; Wong et al. Citation2012; Zhou et al. Citation2014), the United Kingdom (Wald Citation2014) and central Europe (Austria, Czech Republic, and Germany) (Miehlke et al. Citation2001). These studies determined initial H. pylori status through endoscopy, C-urea breath testing and/or serology. All studies compared placebo treatment to either double (Ma et al. Citation2012; Wong et al. Citation2012; Zhou et al. Citation2014) or triple (Miehlke et al. Citation2001; Wong et al. Citation2004; Wald Citation2014) antibiotic therapy with concomitant proton pump inhibitor (PPI) for participants positive for H. pylori. They achieved H. pylori eradication rates (re-assessed in the intervention group after treatment) between 71% (Wong et al. Citation2012) and 89% (Miehlke et al. Citation2001). Treatment compliance was reported in one study with 99% of participants completing all medication (Ma et al. Citation2012), although this was achieved with intensive monitoring of medication administration.
Additionally, large-scale community-based randomised trials focusing on eradicating H. pylori in asymptomatic individuals have recently been initiated in Linqu County, China (Pan et al. Citation2015) and in Changhua County, Taiwan (Sugano Citation2015; Lee, Chiang, Liou, et al. Citation2016). In China (Pan et al. Citation2015), initial testing for H. pylori was undertaken with a C-urea breath test, while treatment for the intervention group consisted of double antibiotic therapy with adjuvant PPI for 10 days. The China study reported a treatment compliance rate of 89% and a 73% eradication rate. The Taiwanese (Lee, Chiang, Liou, et al. Citation2016) study is coupled to colorectal cancer screening by using faecal antigen test for H. pylori infection alongside immunochemical faecal occult blood testing (for colorectal cancer), with an eligible age range of 50–69 years. This study used a retest-and retreat practice with second-line treatment where required, with an overall eradication rate of 98%.
Although outcome results from the above programmes will not be available for a number of years, testing for and treating H. pylori in asymptomatic individuals has been found to be effective in reducing stomach cancer in previous clinical trials. A Cochrane review and meta-analysis in 2014 reported an estimated relative risk of stomach cancer of 0.66 (95% CI, 0.46–0.95) for treated compared with untreated groups (Ford et al. Citation2014). More recent meta-analyses of randomised trials and observational studies reinforced this finding (RRs 0.62, 0.67, 0.46, 0.56) (Lagergren Citation2016; Lee, Chiang, Chou, et al. Citation2016; Rokkas et al. Citation2017; Seta et al. Citation2017).
Potential harms of testing for and treating H. pylori
While testing for and treating H. pylori can offer benefits in addition to reductions in stomach cancer incidence – such as reductions in dyspepsia and peptic ulcer disease, and associated decreases in health care use (Hung and Wong Citation2009; Herrero et al. Citation2014) – there is some evidence of potential harms. The presence of H. pylori has been linked to decreased prevalence of gastro-oesophageal reflux disease, Barrett’s oesophagus, and oesophageal adenocarcinoma, although evidence is conflicting (McColl Citation2007; Hung and Wong Citation2009; IARC Helicobacter pylori Working Group Citation2014). There is also some indication that H. pylori may protect infected individuals from immunologically mediated diseases, such as asthma, eczema and allergic rhinitis, albeit the causal relationship for these diseases is not well established (IARC Helicobacter pylori Working Group Citation2014).
However, perhaps the most important consideration in regards to the potential harm of treating H. pylori is the risk of antibiotic resistance. Treatment resistance of H. pylori is a developing problem worldwide (De Francesco et al. Citation2010) that also affects New Zealand, where there is evidence of increasing antibiotic resistance (Fraser et al. Citation1999; Hsiang et al. Citation2013). Studies conducted in Auckland in 1999 and 2013 showed a doubling in clarithromycin resistance of H. pylori isolates from 7% to 16%, and an apparent increase in metronidazole resistance from 32% to almost 50% (Fraser et al. Citation1999; Hsiang et al. Citation2013). Additionally, there is concern about the effects of antibiotic use on the gut microbiome, although the full impact of these effects on human health are not yet known (Herrero et al. Citation2014). Finally, while antibiotic resistance in H. pylori is likely a consequence of antibiotic overconsumption, the widespread use of antibiotics in a test-and-treat programme has the potential to further contribute to increasing resistance in other bacterial species and the principles of antibiotic stewardship must be carefully applied in any such programme (Fallone et al. Citation2019). These potential harms must be weighed against the likely benefit of H. pylori eradication, which include a reduction in the future burden of stomach cancer.
Cost-effectiveness of H. pylori test-and-treat programmes
Researchers in the United States, Singapore, Canada and the United Kingdom have modelled the cost-effectiveness of H. pylori test-and-treat programmes, based on various testing strategies (serology, faecal or C-urea breath) and information on in-country disease profiles. While these studies generally did not include potential harms, all of the studies found that H. pylori testing and treatment was cost-effective given country-specific willingness to pay (Parsonnet et al. Citation1996; Fendrick et al. Citation1999; Roderick et al. Citation2003; Ford et al. Citation2005; Xie et al. Citation2009).
A recent New Zealand-based cost-utility analysis found that H. pylori testing-and-treating was likely to be cost-effective, particularly for Māori and probably for Pacific peoples (Teng et al. Citation2017). This study assumed healthy individuals would undergo faecal antigen testing to identify infection and establish eradication for those requiring antibiotics. First-line therapy was assumed to be triple therapy (omeprazole, amoxicillin and clarithromycin for seven days), with second-line eradication using quadruple therapy. The cost of potential harm from Clostridium difficile was included in the model. The total population cost was estimated to be $24,600 (NZD) per quality-adjusted life-year (QALY) gained, but was considerably lower at $11,985 per QALY gained for Māori. A programme based on serology testing was estimated to be slightly more cost-effective than one based on faecal testing, but resulted in less overall health gain. While modelling was not carried out for Pacific peoples specifically, it is likely that any test-and-treat programme would also be highly cost-effective for Pacific peoples living in New Zealand given their high rates of both H. pylori (McDonald et al. Citation2015) and stomach cancer (Blakely et al. Citation2011).
Implications and considerations for New Zealand
IARC has stated that all countries should have a strategy to address the burden of stomach cancer, which includes exploring the possibility of introducing population-based H. pylori testing and treatment programmes, tailored to each country’s local context (Herrero et al. Citation2014; International Agency for Research on Cancer and World Health Organization Citation2014). However, before a New Zealand-based test-and-treat programme can be considered, there are substantial gaps in the New Zealand knowledge base that must be addressed. It is critical to determine the current prevalence and distribution of H. pylori in New Zealand in order to estimate the scope of a potential programme. There are also a number of practical questions that would require consideration and addressing if a test-and-treat programme was to be implemented in New Zealand (National Health Committee Citation2003). These include the question of which test would be most appropriate, what the target population would be in terms of age, sex and ethnicity, what treatment regimens would be appropriate and feasible, and fundamentally, whether such a programme would be acceptable within New Zealand. Each of these factors will now be discussed.
Current New Zealand H. pylori prevalence
Accurate knowledge of H. pylori prevalence by age and ethnicity is critical to determine the scope, impact and resource requirements of any potential programme (International Agency for Research on Cancer Citation2014; International Agency for Research on Cancer and World Health Organization Citation2014) and comprises part of New Zealand (and international) criteria to inform decisions about prospective new screening programmes (National Health Committee Citation2003). The most recent New Zealand-based prevalence data was determined in 1999 (McDonald et al. Citation2015) and it is highly likely that prevalence rates have since changed. It is anticipated that current age-related differences in prevalence will be large with past evidence suggesting a 2–5-fold difference across age groups (McDonald et al. Citation2015). Updating this information – stratified by ethnicity – is fundamental to identifying the sub-populations most likely to benefit from any organised test-and-treat programme.
Determining the current H. pylori prevalence in the New Zealand population will also contribute to international efforts to estimate the future burden of stomach cancer and other H. pylori-related diseases (International Agency for Research on Cancer Citation2014). Furthermore, additional considerations could be usefully investigated in conjunction with determining current H. pylori prevalence rates. For example, there is a need to better understand the New Zealand distribution of the risk factors for H. pylori infection, the pattern of H. pylori virulence and antibiotic resistance factors and the prevalence of the co-factors that are important in the development of stomach cancer.
Potential tests for population-based H. pylori testing
Endoscopic biopsy (gastroscopy) is considered the clinical diagnostic gold standard test for H. pylori in patients with gastritis, with very high sensitivity (Patel et al. Citation2014). However, the invasive nature and high costs (largely related to performing the endoscopic procedure) make endoscopic biopsy a poor choice for a test-and-treat programme (Ansari and Yamaoka Citation2018). Less invasive tests for H. pylori are commonly used in clinical practice (Best et al. Citation2018) and also have sensitivity and specificity sufficient for population-based prevention programmes, although each has advantages and disadvantages. These less invasive tests are each discussed below.
An inexpensive faecal antigen test can determine who may be infected, with a sensitivity of 94.4% and specificity of 92% which is sufficient for a test-and-treat programme. Faecal antigen tests are the currently recommended test in clinical practice for reasons of cost, sensitivity, and specificity (Elwyn et al. Citation2007; Atkinson and Braden Citation2016; Best et al. Citation2018). Additionally, faecal antigen is the currently funded test in New Zealand laboratories (DHB Shared Services Citation2013) for reasons of cost, resource use (lab time) and accuracy (Bpac NZ Ltd Citation2014). Faecal antigen has other advantages; it detects currently active infection and so can be used to assess the need to treatment and to test for eradication after treatment (Bpac NZ Ltd Citation2014). Faecal collection also allows for PCR-based testing of genetic attributes of H. pylori, including specific strains, pathogenicity factors and antibiotic resistance genes (Beckman et al. Citation2017). The wide uptake of PCR-based methods means these tests are now more closely integrated to common clinical practice and are becoming increasingly affordable (Langley et al. Citation2015). The main disadvantages of the faecal antigen test are the potential reluctance of some patients to provide a faecal sample (an important factor discussed later in this manuscript) and as with most tests which detect active colonisation by H. pylori, it is negatively affected by the recent use of antibiotics or PPIs (Bpac NZ Ltd Citation2014).
The C-Urea breath test is also used in test-and-treat programmes internationally, due to its high sensitivity (>90%) and specificity (90%) (Elwyn et al. Citation2007; Atkinson and Braden Citation2016; Best et al. Citation2018). Similarly to the faecal antigen test, the C-Urea breath test detects active colonisation by H. pylori and is negatively affected by the recent use of antibiotics or PPIs, thus these medications need to be discontinued two or more weeks before administration of the test (Bpac NZ Ltd Citation2014; Malfertheiner et al. Citation2017). The inability to gather information on antibiotic resistance and further genetic attributes of H. pylori is a limiting factor of this testing method. However, perhaps the most limiting factor of the C-Urea breath test in the New Zealand context is that it is not widely used as C-Urea breath testing is non-funded (Bpac NZ Ltd Citation2014), expensive, requires on-site testing at a location with the necessary equipment and takes some time to conduct (up to 30 min) (Bpac NZ Ltd Citation2014; Ansari and Yamaoka Citation2018).
Serological testing (IgG-antibody-based tests) is another inexpensive method, with lower sensitivity and specificity than faecal antigen tests (at a specificity of 90%, sensitivity of 84%) (Best et al. Citation2018). The main disadvantages of serological testing are that it is not possible to conduct detailed genetic work (strain, pathogenicity, antibiotic resistance) in serum samples, and serum-based tests cannot always differentiate current and historic infection, because the test can detect antibodies that can remain for years following eradication of H. pylori. In a population-based test-and-treat programme this latter point would likely necessitate confirmation of current infection through faecal or breath testing in those with a positive serological result. Serological testing also has a disadvantage for needle-phobic individuals. Nevertheless, the negative predictive value of a serological test is high, and results are not affected by recent medications (antibiotics and PPI) that may temporarily alter H. pylori colonisation (Atkinson and Braden Citation2016).
The recommended test in the context of a potential New Zealand test-and-treat programme is yet to be evaluated. In any organised test-and-treat strategy, the predictive value of the test is dependent on both the performance of the test and the prevalence rate of the disease (H. pylori) in the population of interest (Lee et al. Citation2016). Balancing such programme considerations and the technical aspects of each test with available resource and test acceptability will be vitally important to any evaluation and resultant recommendations.
Treatment regimens
As with any organised screening programme, access to effective treatment is critical (National Health Committee Citation2003; International Agency for Research on Cancer and World Health Organization Citation2014). Historically, low-cost treatment regimens using 2 or 3 generic antibiotics plus a PPI (e.g. omeprazole) for 7–14 days, could eradicate H. pylori infection in more than 80% of cases (Gatta et al. Citation2013). However, optimal H. pylori treatment requires careful attention to local antibiotic resistance and eradication patterns in demographic subgroups (Herrero et al. Citation2014; Ansari and Yamaoka Citation2018; Choi JH et al. Citation2018). Determining these patterns has previously required gastric biopsy and H. pylori culture, an invasive and technically challenging process. More recently, novel methods have been developed that allow the detection of resistance mutations in the H. pylori gene using faecal samples (Beckman et al. Citation2017). This provides opportunities to implement H. pylori antibiotic resistance surveillance strategies and locally appropriate treatment regimens (Ansari and Yamaoka Citation2018). While there are ongoing RCTs being conducted internationally to address the question of optimal treatment regimes (Leja et al. Citation2017), and New Zealand treatment guidelines (Bpac NZ Ltd Citation2014), the optimal strategy for the treatment of H. pylori infection in New Zealand is currently unknown.
Any treatment strategy in the context of an organised H. pylori test-and-treat programme should be comprehensive, focusing not only on first-line regimens but also including plans for treatment failures and evaluation of the overall rate of eradication (Lee, Chiang, Liou, et al. Citation2016; Choi JH et al. Citation2018). International RCTs have achieved eradication rates of up to 98% using retest-and retreat strategies (Ford et al. Citation2014; Lee, Chiang, Liou, et al. Citation2016; Seta et al. Citation2017) and it is likely a similar strategy would be needed in New Zealand. However importantly, reinfection with H. pylori is uncommon once eradicated in adults with the reinfection rate reported as less than 1% in developed countries (Zhang et al. Citation2009). Thus it is likely that a one-off programme would be needed, with each individual or family treated until confirmed negative for H. pylori infection, then discharged from the programme.
No vaccines are currently available for H. pylori. Vaccine prototypes have been trialled but there is not yet any evidence of additional protection in adults (Malfertheiner et al. Citation2018). At some point in the future, a vaccine may replace test-and-treat strategies as a method for primary prevention of stomach cancer (Maleki Kakelar et al. Citation2019).
Target population
Defining a target population group(s) is also fundamental to the development of any organised programme (National Health Committee Citation2003). Given their much higher prevalence of both infection with H. pylori and stomach cancer, it is likely that Māori and Pacific peoples in New Zealand would benefit the most from a test-and-treat programme. Cost-effectiveness modelling has also shown that targeting these populations would be the most cost-effective strategy in the New Zealand context (Teng et al. Citation2017). There is precedent set in New Zealand for an ethnically targeted screening programme with the recent pilot of an abdominal aortic aneurysm (AAA) screening programme for Māori only (Waitemata District Health Board Citation2019). However, there are other population groups that may also benefit from organised H. pylori testing-and-treating such as those living in more highly deprived areas and recent migrants from high prevalence areas or countries globally.
Currently, the best age to provide an H. pylori test-and-treat programme remains unclear. Most international RCTs have been focused on middle-aged adults (mean ages 42–53 years old, ranging from 20 to 75 years old) (Ford et al. Citation2014; Rokkas et al. Citation2017). However, given the natural history of stomach cancer, primary prevention is recommended before precancerous conditions such as atrophy or intestinal metaplasia take place (Fock Citation2014; Moss Citation2017). This is thought to be 10–20 years before the incidence of stomach cancer starts to increase in the population of interest (Fock Citation2014). In the total New Zealand population, stomach cancer appears to increase in the 45–64 age group (Ministry of Health Citation2016). Importantly, the average age of Māori and Pacific peoples when diagnosed with stomach cancer is 10 years younger than non-Māori/non-Pacific (Biggar et al. Citation2011; Signal et al. Citation2015), and so the age in which stomach cancer increases in these populations is also likely to be 10 years earlier. Thus, while further work needs to be undertaken to determine the New Zealand-specific target age group, testing-and-treating H. pylori is likely to be most effective in a younger age groups. However, there is some evidence that eradication of H. pylori reverses the premalignant changes of gastric atrophy and intestinal metaplasia (Lee, Chiang, Liou, et al. Citation2016; Ansari and Yamaoka Citation2018) and may prevent metachronous tumours – tumours developing more than six months after the detection of a primary tumour – after curative endoscopic resection of early stomach cancer (Choi IJ et al. Citation2018; Zhao et al. Citation2019). Because of this, testing-and-treating older age groups may still be useful. An additional consideration is whether to treat children and families of infected individuals identified through an H. pylori test-and-treat programme to prevent re-infection and accelerate reductions in prevalence (Lee, Chiang, Liou, et al. Citation2016; Ansari and Yamaoka Citation2018). As previously discussed, determining current H. pylori prevalence across age and ethnicity, is also vital to informing the population groups best targeted in a New Zealand test-and-treat programme.
In the future, better knowledge of genetic attributes of H. pylori and of the co-factors in the development of stomach cancer may help to stratify H. pylori-infected individuals into cohorts at high or low risk of stomach cancer (Moss Citation2017). New approaches investigating biomarkers and their potential role in the identification of people at greater risk of stomach cancer are also being pursued in the United States (Herrero et al. Citation2014). Thus, highly targeted approaches to eradicating H. pylori at the individual level may be possible at a future date.
The acceptability of an H. pylori test-and-treat programme in New Zealand
Any screening programme can fail without adequate participation. Acceptability of the programme to the target population is important not only for uptake but also for on-going participation through the programme, such as adherence to the antibiotic treatment schedule (Lee, Chiang, Liou, et al. Citation2016). Any New Zealand strategy to test-and-treat H. pylori will require careful attention to the delivery of the programme, including the method of testing and its acceptability within the target population. Aspects such as test accuracy and cost may need to be traded off against collection method/s and storage if uptake is to be optimised. For example, the low cost and ease of analysis of faecal tests may need to be carefully weighed against the acceptability of the relatively high cost and more complicated option of breath testing.
Our research groups have recently completed foundational qualitative research into the acceptability of a targeted New Zealand test-and-treat programme generally, and into the acceptability of faecal testing more specifically. While these studies are currently unpublished, early findings from this research suggest that a targeted H. pylori test-and-treat programme for Māori and Pacific peoples would likely be acceptable – at least to key stakeholders and participants of two focus group meetings held with Māori and Pacific community members – although a significant level of community engagement, consultation and ongoing work would be required prior to any implementation efforts. Additionally, the Māori cultural concepts of tapū and noa (Sachdev Citation1989) were found to be relevant in the context of faecal sampling acceptability in interviews and focus groups with Māori, Pacific and European participants. Mixing tapū and noa – such as items connected with food or cooking (noa) with items connected with the body (tapū) (Sachdev Citation1989) – was not acceptable to the majority of participants. Considerations such as this may impact on the acceptability of testing methods and thus likely affect the uptake of any organised programme.
IARC recommends that any potential test-and-treat programme be undertaken with multi-sectorial – including community – partnerships (IARC Helicobacter pylori Working Group Citation2014). Developing any such programme in partnership with Māori and Pacific peoples was also seen as critical in our stakeholder engagement work. While this work is preliminary, the findings suggest that key stakeholders including Māori and Pacific peoples themselves see stomach cancer as an important health issue that is worthy of a higher profile and further investigation into an H. pylori test-and-treat programme is warranted.
Conclusion
Infection with H. pylori has a known strong association with the development of stomach cancer (Crew and Neugut Citation2006; Kato and Asaka Citation2010; Blair et al. Citation2013). Importantly, it is shown to be the primary driver of the excess stomach cancer incidence seen among Māori and Pacific peoples living in New Zealand over that of New Zealand Europeans (Teng, Blakely, et al. Citation2016). Testing and treatment for H. pylori is generally acceptable and affordable and is currently being trialled in a number of countries (Herrero et al. Citation2014). Such a programme is likely to be effective in preventing stomach cancer (Ford et al. Citation2014) and if implemented for Māori and Pacific peoples in New Zealand, particularly cost-effective and pro-equity (Teng et al. Citation2017). The recent progress globally in knowledge about H. pylori itself, the availability of accurate non-invasive testing methods, better treatment regimens and evidence from high-quality studies that eradication of H. pylori reduces risk of stomach cancer in later life, collectively presents an opportunity for stomach cancer prevention and addressing inequities that was previously unavailable. More needs to be done to understand and explore this approach within the New Zealand context.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abrams JA, Wang TC. 2010. Adenocarcinoma and other tumors of the stomach. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran’s gastrointestinal and liver disease. Philadelphia (PA): Saunders.
- Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R, the British Association of Surgical Oncology. 2011. Guidelines for the management of oesophageal and gastric cancer. Gut. 60(11):1449–1472.
- American Cancer Society. 2013. Stomach cancer. Atlanta (GA): American Cancer Society.
- Ansari S, Yamaoka Y. 2018. Current understanding and management of Helicobacter pylori infection: an updated appraisal. F1000Res. 7:F1000 Faculty Rev-1721.
- Arnold M, Moore SP, Hassler S, Ellison-Loschmann L, Forman D, Bray F. 2014. The burden of stomach cancer in indigenous populations: a systematic review and global assessment. Gut. 63(1):64–71.
- Atkinson NS, Braden B. 2016. Helicobacter pylori infection: diagnostic strategies in primary diagnosis and after therapy. Digestive Diseases and Sciences. 61(1):19–24.
- Baker M, Goodyear R, Telfar Barnard L, Howden-Chapman P. 2012. The distribution of household crowding in New Zealand: an analysis based on 1991 to 2006 census data. Wellington: University of Otago.
- Baker MG, McDonald AM, Zhang J, Howden-Chapman P. 2013. Infectious diseases attributable to household crowding in New Zealand: A systematic review and burden of disease estimate. Wellington: University of Otago/Ministry of Health.
- Beckman E, Saracino I, Fiorini G, Clark C, Slepnev V, Patel D, Gomez C, Ponaka R, Elagin V, Vaira D, et al. 2017. A novel stool PCR test for Helicobacter pylori may predict clarithromycin resistance and eradication of infection at a high rate. Journal of Clinical Microbiology. 55(8):2400–2405.
- Best LM, Takwoingi Y, Siddique S, Selladurai A, Gandhi A, Low B, Yaghoobi M, Gurusamy KS. 2018. Non-invasive diagnostic tests for Helicobacter pylori infection. The Cochrane Database of Systematic Reviews. 3:Cd012080.
- Biggar M, Srinivasa S, Wickramarachchi S, Babor R, Poole GH, Hill A. 2011. Gastric cancer location and histological subtype in Pacific people and Māori defies international trends. New Zealand Medical Journal. 124:39–44.
- Blair V, Kahokehr A, Sammour T. 2013. Cancer in Māori: lessons from prostate, colorectal and gastric cancer and progress in hereditary stomach cancer in New Zealand. Australia New Zealand Journal of Surgery. 83:42–48.
- Blair V, Martin I, Shaw D, Winship I, Kerr D, Arnold J, Harawira P, McLeod M, Parry S, Charlton A, et al. 2006. Hereditary diffuse gastric cancer: diagnosis and management. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association. 4(3):262–275.
- Blakely T, Atkinson J, Kvizhinadze G, Wilson N, Davies A, Clarke P. 2015. Patterns of cancer care costs in a country with detailed individual data. Medical Care. 53(4):302–309.
- Blakely T, Shaw C, Atkinson J. 2010. Cancer trends: trends in incidence by ethnic and socioeconomic group, New Zealand 1981–2004. Wellington: Ministry of Health.
- Blakely T, Shaw C, Atkinson J, Cunningham R, Sarfati D. 2011. Social inequalities or inequities in cancer incidence? Repeated census-cancer cohort studies, New Zealand 1981–1986 to 2001–2004. Cancer Causes & Control: CCC. 22(9):1307–1318.
- Bpac NZ Ltd. 2014. The changing face of Helicobacter pylori testing. Best Tests. 23.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 68(6):394–424.
- Brenner H, Arndt V, Bode G, Stegmaier C, Ziegler H, Stümer T. 2002. Risk of gastric cancer among smokers infected with Helicobacter pylori. International Journal of Cancer. 98(3):446–449.
- Brenner H, Arndt V, Stegmaier C, Ziegler H, Rothenbacher D. 2004. Is Helicobacter pylori infection a necessary condition for noncardia gastric cancer? American Journal of Epidemiology. 159(3):252–258.
- Broutet N, Sarasqueta AM, Sakarovitch C, Cantet F, Lethuaire D, Mégraud F. 2001. Helicobacter pylori infection in patients consulting gastroenterologists in France: prevalence is linked to gender and region of residence. European Journal of Gastroenterology and Hepatology. 13(6):677–684.
- Choi IJ, Kook M-C, Kim Y-I, Cho S-J, Lee JY, Kim CG, Park B, Nam B-H. 2018. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. New England Journal of Medicine. 378(12):1085–1095.
- Choi JH, Yang YJ, Bang CS, Lee JJ, Baik GH. 2018. Current status of the third-line Helicobacter pylori eradication. Gastroenterology Research and Practice. 2018:7.
- Correa P. 1996. Helicobacter pylori and gastric cancer: state of the art. Cancer Epidemiology and Prevention Biomarkers. 5(6):477–481.
- Crew KD, Neugut AI. 2006. Epidemiology of gastric cancer. World Journal of Gastroenterology. 12(3):354–362.
- De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Ierardi E, Zullo A. 2010. Worldwide H. pylori antibiotic resistance: a systematic review. Journal of Gastrointestinal and Liver Diseases: JGLD. 19(4):409–414.
- DHB Shared Services. 2013. Laboratory test schedule.
- Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. 2005. Gastric adenocarcinoma: review and considerations for future directions [review]. Annals of Surgery. 241(1):27–39.
- Ellison-Loschmann L, Sporle A, Corbin M, Cheng S, Harawira P, Gray M, Whaanga T, Guilford P, Koea J, Pearce N, et al. 2017. Risk of stomach cancer in Aotearoa/New Zealand: a Māori population based case-control study. PloS One. 12(7):e0181581.
- Elwyn G, Taubert M, Davies S, Brown G, Allison M, Phillips C. 2007. Which test is best for Helicobacter pylori? A cost-effectiveness model using decision analysis. British Journal of General Practice. 57(538):401–403.
- Fallone CA, Moss SF, Malfertheiner P. 2019. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology. 157(1):44–53.
- Fendrick AM, Chernew ME, Hirth RA, Bloom BS, Bandekar RR, Scheiman JM. 1999. Clinical and economic effects of population-based Helicobacter pylori screening to prevent gastric cancer. Archives of Internal Medicine. 159:142–148.
- Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. 2010. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 127(12):2893–2917.
- Fock KM. 2014. Review article: the epidemiology and prevention of gastric cancer. Alimentary Pharmacology & Therapeutics. 40(3):250–260.
- Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. 2005. A community screening program for Helicobacter pylori saves money: 10-year follow-up of a randomized controlled trial. Gastroenterology. 129(6):1910–1917.
- Ford AC, Forman D, Hunt R, Yuan Y, Moayyedi P. 2015. Helicobacter pylori eradication for the prevention of gastric neoplasia. The Cochrane Library. 7:1–54.
- Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. 2014. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 348:g3174.
- Forman D, Burley VJ. 2006. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Practice & Research Clinical Gastroenterology. 20(4):633–649.
- Fraser A. 2004. Helicobacter pylori: a historical perspective 1983–2003. New Zealand Medical Journal. 117(1194):U896.
- Fraser AG, Moore L, Hackett M, Hollis B. 1999. Helicobacter pylori treatment and antibiotic susceptibility: results of a five-year audit. Australian and New Zealand Journal of Medicine. 29(4):512–516.
- Fraser AG, Scragg R, Metcalf P, McCullough S, Yeates NJ. 1996. Prevalence of helicobacter pylori infection in different ethnic groups in New Zealand children and adults. Australian and New Zealand Journal of Medicine. 26(5):646–651.
- Fraser AG, Scragg R, Schaaf D, Metcalf P, Grant CC. 2010. Helicobacter pylori infection and iron deficiency in teenage females in New Zealand. The New Zealand Medical Journal. 123(1313):38–45.
- Gatta L, Vakil N, Vaira D, Scarpignato C. 2013. Global eradication rates for Helicobacter pylori infection: systematic review and meta-analysis of sequential therapy. BMJ. 347:f4587. doi:10.1136/bmj.f4587.
- Gonzalez CA, Lopez-Carrillo L. 2010. Helicobacter pylori, nutrition and smoking interactions: their impact in gastric carcinogenesis. Scandinavian Journal of Gastroenterology. 45(1):6–14.
- Guggenheim DE, Shah MA. 2013. Gastric cancer epidemiology and risk factors. Journal of Surgical Oncology. 107(3):230–236.
- Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. 1998. E-cadherin germline mutations in familial gastric cancer. Nature. 392(6674):402–405.
- Guilford P, Humar B, Blair V. 2010. Hereditary diffuse gastric cancer: translation of CDH1 germline mutations into clinical practice. Gastric Cancer. 13(1):1–10.
- Hakkaart C, Ellison-Loschmann L, Day R, Sporle A, Koea J, Harawira P, Cheng S, Gray M, Whaanga T, Pearce N, et al. 2019. Germline CDH1 mutations are a significant contributor to the high frequency of early-onset diffuse gastric cancer cases in New Zealand Māori. Familial Cancer. 18(1):83–90.
- Herrero R, Park JY, Forman D. 2014. The fight against gastric cancer – the IARC working group report. Best Practice & Research Clinical Gastroenterology. 28(6):1107–1114.
- Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, et al. 2017. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 153(2):420–429.
- Hsiang J, Selvaratnam S, Taylor S, Yeoh J, Tan YM, Huang J, Patrick A. 2013. Increasing primary antibiotic resistance and ethnic differences in eradication rates of Helicobacter pylori infection in New Zealand – a new look at an old enemy. New Zealand Medical Journal. 126(1384):64–76.
- Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. 2012. Gastric cancer: classification, histology and application of molecular pathology. Journal Gastrointestinal Oncology. 3(3):251–261.
- Huang JQ, Zheng GF, Sumanac K, Irvine EJ, Hunt RH. 2003. Meta-analysis of the relationship between CagA seropositivity and gastric cancer. Gastroenterology. 125(6):1636–1644.
- Hung IFN, Wong BCY. 2009. Assessing the risks and benefits of treating Helicobacter pylori infection. Therapeutic Advances in Gastroenterology. 2(3):141–147.
- IARC Helicobacter pylori Working Group. 2014. Helicobacter pylori eradication as a strategy for preventing gastric cancer. Lyon: International Agency for Research on Cancer.
- International Agency for Research on Cancer. 1994. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: World Health Organization.
- International Agency for Research on Cancer. 2014. Protocol: international prevalence surveys of Helicobacter pylori in high and low risk areas for stomach cancer (epidemiological investigation of gastric malignancies – ENIGMA). Lyon: International Agency for Research on Cancer.
- International Agency for Research on Cancer, World Health Organization. 2014. Helicobacter pylori eradication as a strategy for preventing gastric cancer. Lyon: International Agency for Research on Cancer.
- Ishaq S, Nunn L. 2015. Helicobacter pylori and gastric cancer: a state of the art review. Gastroenterology and Hepatology From Bed to Bench. 8(Suppl1):S6–S14.
- Kato M, Asaka M. 2010. Recent knowledge of the relationship between Helicobacter pylori and gastric cancer and recent progress of gastroendoscopic diagnosis and treatment for gastric cancer. Japanese Journal of Clinical Oncology. 40(9):828–837.
- Kelley JR, Duggan JM. 2003. Gastric cancer epidemiology and risk factors. Journal of Clinical Epidemiology. 56(1):1–9.
- Lagergren JEL. 2016. Eradication of Helicobacter pylori and gastric and oesophageal cancer: a systematic review and metaanalysis of cohort studies. Journal of the National Cancer Institute. 108(9):1582–1588.
- Langley G, Besser J, Iwamoto M, Lessa FC, Cronquist A, Skoff TH, Chaves S, Boxrud D, Pinner RW, Harrison LH. 2015. Effect of culture-independent diagnostic tests on future emerging infections program surveillance. Emerg Infect Dis. 21(9):1582–1588.
- Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. 2016. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 150(5):1113–1124.e5.
- Lee YC, Chiang TH, Liou JM, Chen HH, Wu MS, Graham DY. 2016. Mass eradication of Helicobacter pylori to prevent gastric cancer: theoretical and practical considerations. Gut and Liver. 10(1):12–26.
- Leja M, Park JY, Murillo R, Liepniece-Karele I, Isajevs S, Kikuste I, Rudzite D, Krike P, Parshutin S, Polaka I, et al. 2017. Multicentric randomised study of Helicobacter pylori eradication and pepsinogen testing for prevention of gastric cancer mortality: the GISTAR study. BMJ Open. 7(8):e016999.
- Lynch HT, Grady W, Suriano G, Huntsman D. 2005. Gastric cancer: new genetic developments. Journal of Surgical Oncology. 90(3):114–133.
- Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, Liu WD, Hu Y, Han ZX, Crystal-Mansour S, et al. 2012. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. Journal of the National Cancer Institute. 104(6):488–492.
- MacDonald A. 2013. Casting a long shadow: the role of household crowding on Helicobacter pylori infection, and excess stomach cancer incidence among Māori and Pacific people. Wellington: Otago.
- Maleki Kakelar H, Barzegari A, Dehghani J, Hanifian S, Saeedi N, Barar J, Omidi Y. 2019. Pathogenicity of Helicobacter pylori in cancer development and impacts of vaccination. Gastric Cancer. 22(1):23–36.
- Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, et al. 2017. Management of Helicobacter pylori infection—the Maastricht V/florence consensus report. Gut. 66(1):6–30.
- Malfertheiner P, Selgrad M, Wex T, Romi B, Borgogni E, Spensieri F, Zedda L, Ruggiero P, Pancotto L, Censini S, et al. 2018. Efficacy, immunogenicity, and safety of a parenteral vaccine against Helicobacter pylori in healthy volunteers challenged with a Cag-positive strain: a randomised, placebo-controlled phase 1/2 study. The Lancet Gastroenterology & Hepatology. 3(10):698–707.
- McCallion WA, Murray LJ, Bailie AG, Dalzell AM, O'Reilly DPJ, Bamford KB. 1996. Helicobacter pylori infection in children: relation with current household living conditions. Gut. 39(1):18–21.
- McColl KEL. 2007. Helicobacter pylori and oesophageal cancer – not always protective. Gut. 56(4):457–459.
- McDonald AM, Sarfati D, Baker MG, Blakely T. 2015. Trends in Helicobacter pylori infection among Māori, Pacific, and European birth cohorts in New Zealand. Helicobacter. 20(2):139–145.
- Mercer D, Robinson E. 2008. Stomach. In: Townsend CM, Beauchamp RD, Evers BM, Mattox KL, editors. Sabiston textbook of surgery: the biological basis of modern surgical practice. 18th ed. Philadelphia (PA): Saunders-Elsevier; p. 1223–1277.
- Miehlke S, Kirsch C, Dragosics B, Gschwantler M, Oberhuber G, Antos D, Dite P, Lauter J, Labenz J, Leodolter A, et al. 2001. Helicobacter pylori and gastric cancer: current status of the Austrain Czech German gastric cancer prevention trial (PRISMA-study). World Journal of Gastroenterology. 7(2):243–247.
- Ministry of Health. 2002. He Korowai Oranga: Maori health strategy. Wellington: Ministry of Health.
- Ministry of Health. 2011. Māori smoking and tobacco use 2011. Wellington.
- Ministry of Health. 2016. Cancer: new registrations and deaths 2013. Wellington: Ministry of Health.
- Morrell S, You H, Baker D. 2012. Estimates of cancer incidence, mortality and survival in aboriginal people from NSW, Australia. BMC Cancer. 12(1):168.
- Moss SF. 2017. The clinical evidence linking Helicobacter pylori to gastric cancer. Cellular and Molecular Gastroenterology and Hepatology. 3(2):183–191.
- National Health Committee. 2003. National Health Committee: screening to improve health in New Zealand: criteria to assess screening programmes. Wellington: Ministry of Health. https://www.nsu.govt.nz/publications/screening-improve-health-new-zealand-criteria-assess-screening-programmes.
- New Zealand Guidelines Group. 2009. Suspected cancer in primary care: guidelines for investigation, referral and reducing ethnic disparities. Wellington: Ministry of Health.
- Pan KF, Zhang L, Gerhard M, Ma JL, Liu WD, Ulm K, Wang JX, Zhang L, Zhang Y, Bajbouj M, et al. 2015. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: baseline results and factors affecting the eradication. Gut. 65:9–18.
- Parsonnet J, Harris RA, Hack HM, Owens DK. 1996. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. The Lancet. 348(9021):150–154.
- Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. 2014. Gastric cancer: prevention, screening and early diagnosis. World Journal of Gastroenterology. 20(38):13842–13862.
- Patel SK, Pratap CB, Jain AK, Gulati AK, Nath G. 2014. Diagnosis of Helicobacter pylori: what should be the gold standard? World Journal of Gastroenterology. 20(36):12847–12859.
- Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. 2015. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 136(2):487–490.
- Rawla P, Barsouk A. 2019. Epidemiology of gastric cancer: global trends, risk factors and prevention. Przeglad Gastroenterologiczny. 14(1):26–38.
- Robson B, Purdie G, Cormack D. 2010. Unequal impact II: Māori and non-Māori cancer statistics by deprivation and rural-urban status, 2002–2006. Wellington: Ministry of Health.
- Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, Patel P. 2003. The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model. Health Technology Assessment. 7(6):1–86.
- Rokkas T, Rokka A, Portincasa P. 2017. A systematic review and meta-analysis of the role of Helicobacter pylori eradication in preventing gastric cancer. Ann Gastroenterol. 30(4):414–423.
- Sachdev PS. 1989. Mana, Tapu, Noa: Maori cultural constructs with medical and psycho-social relevance. Psychological Medicine. 19(4):959–969.
- Seta T, Takahashi Y, Noguchi Y, Shikata S, Sakai T, Sakai K, Yamashita Y, Nakayama T, Katoh M. 2017. Effectiveness of Helicobacter pylori eradication in the prevention of primary gastric cancer in healthy asymptomatic people: A systematic review and meta-analysis comparing risk ratio with risk difference. PLoS One. 12(8):e0183321.
- Siegel RL, Miller KD, Jemal A. 2019. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. 69(1):7–34.
- Signal V. 2016. Making sense of stomach cancer inequities in Aotearoa New Zealand. Wellington: University of Otago.
- Signal V, Sarfati D, Cunningham R, Gurney J, Koea J, Ellison-Loschmann L. 2015. Indigenous inequities in the presentation and management of stomach cancer in New Zealand: a country with universal health care coverage [research support, non-U.S. Gov’t]. Gastric Cancer. 18(3):571–579.
- Soeberg M, Blakely T, Sarfati D, Tobias M, Costilla R, Carter K, Atkinson J. 2012. Cancer trends: rends in cancer survival by ethnic and socioeconomic group, New Zealand 1991–2004. Wellington: University of Otago and Ministry of Health.
- Sugano K. 2015. Screening of gastric cancer in Asia. Best Practice & Research: Clinical Gastroenterology. 29(6):895–905.
- Supramaniam R, Grindley H, Pulver LJ. 2006. Cancer mortality in aboriginal people in New South Wales, Australia, 1994–2002. Australian and New Zealand Journal of Public Health. 30(5):453–456.
- Talley NJ, Fock KM, Moayyedi P. 2008. Gastric cancer consensus conference recommends Helicobacter pylori screening and treatment in asymptomatic persons from high-risk populations to prevent gastric cancer. The American Journal of Gastroenterology. 103(3):510–514.
- Teng AM, Atkinson J, Disney G, Wilson N, Sarfati D, McLeod M, Blakely T. 2016. Ethnic inequalities in cancer incidence and mortality: census-linked cohort studies with 87 million years of person-time follow-up. BMC Cancer. 16(1):755.
- Teng AM, Blakely T, Baker MG, Sarfati D. 2016. The contribution of Helicobacter pylori to excess gastric cancer in indigenous and Pacific men: a birth cohort estimate. Gastric Cancer. 20:752–755.
- Teng AM, Kvizhinadze G, Nair N, McLeod M, Wilson N, Blakely T. 2017. A screening program to test and treat for Helicobacter pylori infection: cost-utility analysis by age, sex and ethnicity. BMC Infectious Diseases. 17(1):156.
- United Nations. 2007. United Nations declaration on the rights of indigenous peoples. Geneva: United Nations.
- Waitemata District Health Board. 2019. Abdominal aortic aneurysm (AAA) screening pilot for Māori. Auckland.
- Wald NJ. 2014. The treatment of Helicobacter pylori infection of the stomach in relation to the possible prevention of gastric cancer. Lyon, France: International Agency for Research on Cancer.
- Wilson RL, Stevenson CE. 2007. Anatomy and physiology of the stomach. In: Yeo CL, editor. Shackelford’s surgery of the alimentary tract. 8th ed. Philadelphia: Saunders; p. 636.
- Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, et al. 2004. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. Jama. 291(2):187–194.
- Wong BC, Zhang L, Ma JL, Pan KF, Li JY, Shen L, Liu WD, Feng GS, Zhang XD, Li J, et al. 2012. Effects of selective COX-2 inhibitor and Helicobacter pylori eradication on precancerous gastric lesions. Gut. 61(6):812–818.
- World Health Organization. 2008. World cancer report 2008. Lyon: International Agency for Research on Cancer.
- Wroblewski LE, Peek RM, Jr., Wilson KT. 2010. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clinical Microbiology Reviews. 23(4):713–739.
- Xie F, O'Reilly D, Ferrusi IL, Blackhouse G, Bowen JM, Tarride JE, Goeree R. 2009. Illustrating economic evaluation of diagnostic technologies: comparing Helicobacter pylori screening strategies in prevention of gastric cancer in Canada. Journal of the American College of Radiology. 6(5):317–323.
- Yamaoka Y. 2010. Mechanisms of disease: Helicobacter pylori virulence factors. Nature Reviews Gastroenterology & Hepatology. 7(11):629–641.
- Yuan XY, Yan JJ, Yang YC, Wu CM, Hu Y, Geng JL. 2017. Helicobacter pylori with east Asian-type cag PAI genes is more virulent than strains with western-type in some cag PAI genes. Brazilian Journal of Microbiology. 48(2):218–224.
- Zhang YY, Xia HH, Zhuang ZH, Zhong J. 2009. Review article: ‘true’ re-infection of Helicobacter pylori after successful eradication – worldwide annual rates, risk factors and clinical implications. Alimentary Pharmacology & Therapeutics. 29(2):145–160.
- Zhao B, Zhang J, Mei D, Luo R, Lu H, Xu H, Huang B. 2019. Does Helicobacter pylori eradication reduce the incidence of metachronous gastric cancer after curative endoscopic resection of early gastric cancer: a systematic review and meta-analysis. Journal of Clinical Gastroenterology. doi:10.1097/mcg.0000000000001195.
- Zhou L, Lin S, Ding S, Huang X, Jin Z, Cui R, Meng L, Li Y, Zhang L, Guo C, et al. 2014. Relationship of Helicobacter pylori eradication with gastric cancer and gastric mucosal histological changes: a 10-year follow-up study. Chinese Medical Journal. 127(8):1454–1458.
