ABSTRACT
Human infants are born with sparse microflora in their gastrointestinal tracts. Acquisition of pioneer bacteria and their initial colonisation are affected by a number of factors, including mode of birth, feeding practices, exposure to antibiotics, and environmental conditions. Subsequent diversification and development of the microbiota continues dynamically until it reaches maturity when the infant is around 3 years old. An important window of opportunity to affect the structure of developing microflora is likely to coincide with the weaning period when a gradual transition in diet from milk-based infant feeding to first family-based solid foods occurs. Although the ability of many dietary factors to modulate the composition of gastrointestinal microflora is well established, it is not so well understood in children. Moreover, there are cultural, economic, varying feeding practices, and geographical factors that play roles in the choice of complementary foods in different regions of the world. We provide a review of recent literature on development of infant microflora and how complementary feeding practices from different cultures may affect the infant gut microbiota during early childhood.
Introduction
Often regarded as a ‘living organ’ on its own right, the human gut microbiome consists of microbial entities connected by a highly complex network of microbial competition and cooperation. The intimate relationship between the structure and function of the human gut microbiome and health is now well recognised. Impaired acquisition of pioneer bacteria, followed by a reduction in microbial diversity and the ensuing gut dysbiosis, have been shown to be associated with many childhood and later life disorders (Hooper et al. Citation2012; Tremaroli and Backhed Citation2012; Jain and Walker Citation2015; Tang and Hazen Citation2017; Yoshida et al. Citation2018; Pulikkan et al. Citation2019). Our understanding of the role of human gut microbiota in human physiology and health has made rapid advances in the recent years as a result of the emergence of molecular ‘tool box’ and multiple integrative ‘omics’ technologies, including metagenomics, metabolomics and transcriptomics. The crucial role that microbiota play in shaping the immune system and the digestive functions of developing infants is now well recognised (Macpherson and Harris Citation2004; Purchiaroni et al. Citation2013; Gomez de Aguero et al. Citation2016; Macpherson et al. Citation2017; Robertson et al. Citation2019). In addition to other factors, including mode of birth (Dominguez-Bello et al. Citation2010; Perez-Munoz et al. Citation2017) and host genetics (Spor et al. Citation2011; Bonder et al. Citation2016), diet is one of the strongest environmental factors that determines the diversity of infant gut microbiota. Differences in early feeding practices such as breastfeeding versus formula-feeding have an impact on determining the initial bacterial composition and the alpha diversity of the infant gut microbiome (Penders et al. Citation2006; Chong et al. Citation2018,). Profound and sustained changes occur to the community structure of the infant gut microbiome with the introduction of solid foods (Fallani et al. Citation2011; Koenig et al. Citation2011; Stewart et al. Citation2018). During this period of dietary transition, the microbial composition of the infant gut continuously and flexibly adapts to the introduced dietary components, acquiring new bacterial strains and establishing dominant species that have the ability to utilise these new substrates for growth (Backhed et al. Citation2015). This period represents a critical window of opportunity to influence the community structure of a developing infant’s microbiome via dietary interventions. Parental choices for first foods thus may have a significant impact on shaping the structure and diversity of infants’ developing gut microbiota, leading to better health outcomes later in life (Movassagh et al. Citation2017). In this review we summarise current knowledge on the developmental trajectory of the infant microbiome and the role that early nutrition plays in its healthy development and maturation.
Initial acquisition of gut bacteria
There has been a long-standing belief that the anatomical, physiological, and immunological features of placenta ensure that prenatal developments of a foetus occur in a sterile environment, and the first microbes are acquired from the mother during the birthing process. However, this view may not be accurate, because the presence of microorganisms in meconium, placenta, and amniotic fluid has been reported in several studies (Jimenez et al. Citation2008; Aagaard et al. Citation2014; Blaser and Dominguez-Bello Citation2016; Collado et al. Citation2016). While these observations suggest that microbial colonisation may begin in utero preceding birth, such claims still lack direct and unequivocal evidence (Perez-Munoz et al. Citation2017; Robertson et al. Citation2019). A vertical transfer of microbiota from mother to the new-born is a much more likely way of initial acquisition of microbes during birth. The establishment of dominant bacterial types often depends on the mode of birth and other environmental factors. During a vaginal birth, the baby acquires its bacteria from its mother, and the mother’s gut microbiome appears to be the biggest influencer of the infant’s gut microbiome (Ferretti et al. Citation2018). In case of a caesarean delivery, however, the mother-to-baby transmission pattern is disrupted. The more common bacteria that are found in babies born by caesarean section are reported to be bacteria associated with a hospital setting (Shao et al. Citation2019). The long-term differences in the composition of gut microbiota of babies born via caesarean section in comparison to vaginal birth have been documented in many studies, where the microbiome of the former has been generally found to be less diverse than that of the latter (Jakobsson et al. Citation2014; Backhed et al. Citation2015). Interestingly in another recent study, Chu et al. (Citation2017) assessed the impact of mode of delivery on the maturation of infant microbiome community structure and function across multiple body sites. Their study concluded that within the first 6 weeks of life, the infant microbiota undergoes significant reorganisation that is primarily driven by body site and not by mode of delivery.
Although there is little doubt about the occurrence of vertical transmission of bacterial strains from mother to infant (Backhed et al. Citation2015; Asnicar et al. Citation2017; Sakwinska et al. Citation2017), there is still uncertainty about which maternal body site serves as the primary source of the initial bacterial inoculum that colonises and subsequently dominates the early infant gut. Some studies found little similarity between the bacterial taxa present in infant faecal samples and those present in maternal vaginal samples (Sakwinska et al. Citation2017). This suggests that vaginal inoculum may not be the source of bacteria that succeed in colonising and becoming the initial dominant species in the infant gut. A study by Nayfach et al. (Citation2016) used molecular techniques and sophisticated bioinformatics to track the transmission of specific bacterial taxa from mother to infant in a precise manner. They analysed the faecal metagenomes of 96 Swedish mothers and their infants collected over more than a year, and showed that the early colonising bacterial species transmitted to the infant originated from the maternal gastrointestinal tract, whereas late colonisers were often transmitted from other sources in the external environmental.
Typically, faecal samples of a new-born are enriched with facultative anaerobic bacteria belonging to genera such as Enterococcus, Escherichia/Shigella, Streptococcus and Staphylococcus (Iozzo and Sanguinetti Citation2018). These facultative anaerobes consume oxygen to slowly create habitable anaerobic environments (Arrieta et al. Citation2014; Backhed et al. Citation2015), priming the gut for the subsequent colonisation by more strict anaerobes including those of the genera Bifidobacterium and Clostridium.
The composition of breast milk also plays an important role in facilitating the selection of early colonisers, particularly the human milk oligosaccharides (HMOs). More than 200 structurally diverse unconjugated 3–15 carbon unit long oligosaccharides consisting of the monosaccharides glucose, galactose, N-acetylglucosamine (GlcNAc), fucose, and N-acetylneuramic acid (sialic acid) are present in human breast milk (Ayechu-Muruzabal et al. Citation2018). Bifidobacterial genomes, especially Bifidobacterium longum subsp. infantis, show a high degree of specialisation for utilising HMOs (Underwood et al. Citation2015), often encoding gene clusters consisting of numerous Carbohydrate-Active enZymes (CAZymes), ABC transporters, and solute-binding proteins that specifically target oligosaccharides derived from HMOs (Thomson et al. Citation2018), thus enabling B. longum subsp. infantis to competitively colonise the gut of breast-fed infants. One of the reasons that many commercial infant formulae are supplemented with non-digestible oligosaccharides such as galacto-oligosaccharides and fructo-oligosaccharides is to partially mimic the prebiotic properties of human breast milk. Although the dominance of bifidobacteria in the infant gut is often considered an indication of normal gut maturation, not all infants seem to reach this developmental milestone. In some studies, high abundances of Proteobacteria and Firmicutes, and very low numbers of Actinobacteria – regardless of the birth modes – have been reported in healthy neonates and infants from China and Brazil (Kuang et al. Citation2016). Similarly in other studies, Bacteroides rather than bifidobacteria were reported to be the dominant group of bacteria in breast-fed babies (Tannock et al. Citation2016; de Muinck and Trosvik Citation2018). It is known that certain Bacteroides can degrade HMOs via mucin utilisation pathways (Marcobal et al. Citation2011), which may explain this observation.
In a recent review Korpela and de Vos (Citation2018) provided a global view of the temporal progression of microbiota development in the infant gut during early life. They looked at cumulative data from more than 34 studies conducted in different geographic regions of the world and analysed age-associated changes in the relative abundance of five of the most abundant classes of bacteria in the infant gut. illustrates an age-adjusted evaluation of the average relative abundances in the infant gut from birth to 24 months of age using a subset of the microbiome metadata compiled by Korpela and de Vos. Metadata from 29 studies were used in (data points from infants who had been given antibiotics or who were over the age of 24 months were excluded). Temporal changes in the infant gut microbial populations of key bacterial groups can be clearly observed from these data. Initial colonising bacteria are bacilli (aerobes and facultative aerobes), which give way to colonisation by strict anaerobes such as bifidobacteria and Bacteroides. Bacterial taxa such as Proteobacteria that initially colonise the new-born gut gradually make way for the colonisation of bifidobacteria and Bacteroides and as the complexity of diet increases, there is an increase in relative abundance of Clostridia. While variables such as sample origin (country), birth mode and feeding practices are acknowledged as influencers of the microbial landscape, the age and maturation of the infant gut also influence the microbial colonisation patterns.
Figure 1. Average relative abundance of the five most abundant bacterial classes from the early life microbiota studies (N = 29), as a subset from the data compiled by Korpela and de Vos (Citation2018). Studies with infants who had received antibiotics or those who were over 24 months of age were excluded. The trend lines were calculated using a loess smoother on the square-root of age, with each study weighted by its sample size. (Adapted from the original figure published in Current Opinion in Microbiology 44: 70–78, under Elsevier Copyright license# 4647300186567, obtained on 13 August 2019.)
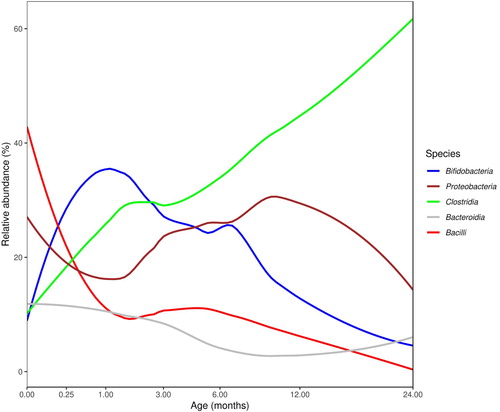
Given that the postnatal development and assembly of the gut microbial community is associated with long-term health benefits, it would be helpful if some microbial markers of normal and healthy progression of gut microbiota could be identified. One approach that has been suggested and used in the literature is description of age-discriminatory bacterial taxa. The changes in relative abundance of these taxa provide a microbial signature that describes a programme of normal gut microbiota development shared by biologically unrelated healthy infants/children from birth to 3 years of age (Subramanian et al. Citation2014).
Current infant complementary feeding practices
The World Health Organization (WHO) guideline recommends ‘exclusive breastfeeding for the first 6-months of age and continuation for up to 2-years of age and beyond’ (https://www.who.int/nutrition/topics/exclusive_breastfeeding/en/). Breastmilk provides sufficient energy and nutrients up until 6 months of age, beyond which breastfeeding alone becomes increasingly insufficient to provide appropriate energy density and essential nutrients for a growing child. Complementary foods therefore must accompany and gradually replace breast milk not only to provide essential micro- and macronutrients for growth but also to promote long-term health outcomes. There is no consensus on the timing of introduction of complementary foods; timing and nature of foods to be given vary between countries depending on cultural influences and food habits. Practical challenges of having accessibility to fresh foods and affordability of fortified commercial food products can also affect food choices. In developing African and South Asian countries, fewer than 15% of children have access to the supply of energy-dense meats (Ayana et al. Citation2017) to replace breast milk as the source of protein and fat after breast feeding ceases. Animal-sourced foods are high in many macronutrients (protein) and micronutrients (vitamin A, vitamin B12, riboflavin, calcium, iron, and zinc) that have high bioavailability (Murphy and Allen Citation2003). Many children (especially in lower socio-economic groups) in developing countries where meat consumption is low are often deficient in vitamin B12 (Murphy and Allen Citation2003). Monotonous cereal- and starch-based diets are often low in essential micronutrients such as calcium, iron and zinc and fall short of complementing breast milk (Krebs et al. Citation2013; Osendarp et al. Citation2016). Although consumption of animal-sourced foods is often associated with a high quality diet and lean tissue mass gain (Robinson et al. Citation2009), many children growing up in developing countries lack regular intakes of animal-sourced foods, and hence must rely on plant-based diets, especially fruits and vegetables rich in vitamin A and vitamin C, for essential nutrition. However, statistics reported that children growing up both in developing and developed countries lack regular intakes of fruits and vegetables. Globally, only 29.4% of children meet the minimum dietary diversity (White et al. Citation2017). In Ethiopia, only 23.7% of children have acceptable dietary diversity, with the major dietary sources being cereals and legumes, while intakes of vegetable-, fruit-, and animal-sourced foods are very low (Baye et al. Citation2012). In India, minimum dietary diversity among children aged 6–23 months was reported as 15% (Senarath et al. Citation2012), with only 31.5% of 6- to 23-month-old children consuming vitamin A-rich fruits and vegetables (White et al. Citation2017). In China, cereal rice, porridge, dumplings, whole eggs, meat, vegetables, and fruits were dominant choices of complementary foods (Li et al. Citation2003). Porridge, which was reported to be the major complementary food for infants in China and South Asia, is low in nutrient density and needs to be consumed in a large volume to fulfil the high energy requirements of growing infants (Dewey Citation2016). In socio-economically challenged settings, commercially available fortified foods are often unaffordable, and fortified foods may have limited benefits since infants and children often do not consume enough food for fortification to have an impact (Dutta et al. Citation2006). In the USA, up to 27% of infants aged 6 to18 months consume no vegetables, and up to 23% consume no fruits on any given day (Siega-Riz et al. Citation2010). Reduced vegetable/fruit intake is partly associated with consumption of commercially prepared infant foods that are often high in salt and sugar (Campoy et al. Citation2018). Specific individual complimentary foods used in most European countries generally meet or exceed dietary requirements for energy, macronutrients and micronutrients, although in some subgroups, the intake of specific nutrients such as ω-3 polyunsaturated fatty acids (PUFAs), vitamin D, or iodine is borderline (EFSA NDA Panel Citation2013). There are several opinions regarding the right age for introduction of complementary foods and if attention should also be paid to taste and texture. It has been suggested that a prolonged use of pureed foods should be avoided and by 8 months of age finger foods such as soft cooked vegetable slices should be introduced (Marduel and Vernet Citation2018). The European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition (CoN) suggests that 9–10 months of age is the critical window for introducing lumpy solid foods, and its introduction in later age is related to a high risk of feeding difficulties and unhealthy eating habits (Agostoni et al. Citation2008; Mennella and Trabulsi Citation2012; Fewtrell et al. Citation2017).
Complementary foods and their interaction with microbiome
With the introduction of complementary foods, the primary source of dietary carbohydrates changes from lactose and oligosaccharides present in breast milk or infant formulae to plant-derived polysaccharides. As discussed earlier, the microbiome of breast-fed babies is often dominated by HMO-utilising Bifidobacterium spp. (e.g. B. longum subsp. infantis, Bifidobacterium bifidum, and Bifidobacterium breve), (Tannock et al. Citation2013; Bergstrom et al. Citation2014). Compared with HMOs, the composition and chemical structures of plant-derived carbohydrates are highly diverse, resulting in selection of microbes that can utilise these complex substrates for growth. Among the members of the genus Bifidobacterium, B. breve, B. longum subsp. longum, B. adolescentis, and B. catenulatum are known to ferment mucin as well as a wide range of plant-derived oligosaccharides and short-chain sugars including malto-, fructo-, xylo, and galacto-oligosaccharides (Pokusaeva et al. Citation2011), and therefore they can readily adapt to the changing diet, and persist. Some members of Lachnospiraceae, Ruminococcaceae, Bacteroidaceae, and Prevotellaceae families are particularly effective in fermenting both insoluble and soluble dietary fibres and crystalline starch granules (Flint and Bayer Citation2008). These ‘primary degrading’ taxa of infant gut bacteria essentially initiate the process of carbohydrate utilisation and orchestrate the expansion of the microbial diversity by releasing a variety of oligomeric degradation products for neighbouring gut bacteria that are less well adapted for degradation of complex carbohydrates (Flint et al. Citation2012 ). Among dietary fibres, pectin, hemicellulose, and resistant starch are particularly relevant to infant nutrition, as these are typically abundant in first foods based on cereals, fruits and vegetables, including baby cereals produced with oatmeal, rice and barley, mashed or pureed fruits and vegetables, cooked and mashed starchy vegetables, beans, and legumes. In a recent study Gopalsamy et al. (Citation2019) examined whether high amylose maize starch (a resistant starch and a known prebiotic for adults) could be fermented by the developing microbiota of infants. They collected faecal samples from 17 infants at two time points, pre-weaning and within 10 weeks of the introduction of first solids. Fermentation of resistant starch and the associated changes in the composition of microflora were assessed using in vitro batch fermentation at 24 h. This study showed that independently of weaning stage, infant faecal inocula were capable of fermenting resistant starch. Furthermore, the introduction of solids not only resulted in increasing the fermentation capacity of inocula but also increased the Shannon diversity index with increased abundance of Bifidobacterium and Bacteroides. Many other known bacteria including Faecalibacterium prausnitzii, Eubacterium rectale, and Ruminococcus bromii, and many unassigned members of Clostridiales are known to degrade complex carbohydrates and produce short chain fatty acids (SCFAs) such as butyrate and propionate (Maier et al. Citation2017). In an infant gut, F. prausnitzii, Roseburia spp., and Eubacterium hallii (Bergstrom et al. Citation2014) start appearing at around 12 months of age, which often coincides with the introduction of more diverse dietary carbohydrates. Members of the families Ruminococcaceae and Lachnospiraceae, and Bacteroides spp. may (Yassour et al. Citation2018) or may not be present before introduction of solid foods (Koenig et al. Citation2011), but they eventually increase in abundance with the addition of solid foods into the infant diet (Laursen et al. Citation2017). The degradation by-products of complex carbohydrates allow a rapid expansion of secondary bacterial populations that perform essential functions such as vitamin synthesis (Yatsunenko et al. Citation2012; Backhed et al. Citation2015) and SCFA synthesis (Roy et al. Citation2006). It is important to consider that pectic polysaccharides constitute one of the major complex carbohydrate components of plant cell walls, and have been shown to stimulate the colonic production of butyrate, possibly through utilisation of pectic oligosaccharides by butyrate-producing bacteria such as F. prausnitizii (Lopez-Siles et al. Citation2012; Chung et al. Citation2016). F. prausnitizii is one of the most abundant gut bacteria and plays an important role in providing butyric energy to the colonocytes. Together with hemicellulose-rich whole grains and bran xyloglucan and arabinoxylan, the most abundantly present forms of hemicellulosic carbohydrates have been shown to promote acetate, propionate, and butyrate production (Rumpagaporn et al. Citation2015; Brahma et al. Citation2017; Huang et al. Citation2019). In addition, butyrogenic species that belong to the family Lachnospiraceae, whose members include Roseburia intestinalis and E. rectale, also use arabinoxylan for growth (Sheridan et al. Citation2016). Thus early complementary food choices have significant potential to affect the community structure of the developing infant microbiome.
The influence of dietary carbohydrates on the composition of the developing microbiome of children has been demonstrated in several studies. By comparing cohorts of children, de Filippo et al. (Citation2010) showed that the microbiome of children living in Burkina Faso who consumed diets rich in complex carbohydrates from cereal and legume sources was more enriched in Bacteroidetes than that of children living in Italy (57% vs 22%). In addition, diets enriched with complex carbohydrates also resulted in increased abundance of several specialised starch-degrading bacterial taxa such as Prevotella and Xylanibacter in Burkina Faso children, which were not found in the Italian cohort. Instead, Italian children had microbiomes enriched in Firmicutes (63% vs 27%), which was probably driven by their fibre-poor, but calorie-rich diet. These differences in the glycan-degrading capacities of Burkina Faso and Italian cohorts’ microbiota showed a strong correlation to high dietary glycan consumption. It is worth pointing here that the two childhood cohorts used in this study also differed in their environment therefore environmental factors also played a role in the observed differences in the gut microbiota.
In another study, Malawian, Amerindian, and rural African children whose main diets consisted of maize, corn, cassava, and locally produced cereals, grains, and vegetables showed higher microbial diversity and similarity than their US and European counterparts who consumed a modern Western diet (Yatsunenko et al. Citation2012). Despite the large inter-individual and chronological fluctuations observed in the human gut microbial composition, studies showed that the diet-driven functional microbial core probably remains resilient to dietary alterations for an extended period of time, possibly as an adaptation mechanism to provide a safety buffer against unexpected dietary events such as starvation or overeating (Thaiss et al. Citation2016). An example of microbiome stabilisation was observed among samples obtained from acutely malnourished children living in sub-Saharan Africa and South Asia, who suffered from micronutrient deficiencies, compromised immune systems, increased susceptibility to infection and diarrhoea, were underweight, stunted, and wasted, which together heavily contributed to mortality and long-lasting disabilities observed in these children (Black et al. Citation2008). Studies showed that the faecal microbiome of Bangladeshi and Malawian children (<3 years of age) diagnosed with severe acute malnutrition were immature, and deviated from the age-discriminatory regression models derived from the microbiome of healthy children (Smith et al. Citation2013; Subramanian et al. Citation2014). Short-term (1 month) administration of commercial therapeutic foods supplemented with micronutrients did not promote sustainable changes in the microbiome configuration before, during and after treatment, with the microbiota composition remaining persistently stagnated compared with that of healthy groups, and the children mostly remained chronically underweight >4 months after treatment (Smith et al. Citation2013; Subramanian et al. Citation2014). There is growing evidence that the gut microbiome and host genetics are largely independent, and hereditary transmission of microbiota is minimal except during the neonatal period (Yatsunenko et al. Citation2012; Rothschild et al. Citation2018). Lifestyle-changing long-term intervention measures encompassing diet, geography (He et al. Citation2018), and sanitation have emerged as important adjustable parameters that may determine microbiome stability and dysbiosis leading to disease susceptibility, and young children are not exceptions to these rules.
Concluding remarks
There are many factors that influence this gradual temporal maturation of the infant gut microbiome. The major factors include age, birth mode, early feeding practices, geography, immediate microbial environment, exposure to antibiotics, and complementary foods at the time of weaning. While many of these factors are immutable, the choice of complementary foods offers an attractive opportunity to modulate and influence the developing infant gut microbiome, although the use of these foods depends on cultural and geographical influences, palatability, consumer acceptance and availability.
Acknowledgements
We acknowledge Dr Duncan Hedderley for his expert advice with Figure 1.
Disclosure statement
No potential conflict of interest was reported by the author(s).
ORCID
Caroline C. Kim http://orcid.org/0000-0003-3393-8364
Shanthi G. Parkar http://orcid.org/0000-0002-5737-7867
Pramod K. Gopal http://orcid.org/0000-0002-3434-5555
References
- Aagaard KJ, Antony KM, Ganu R, Petrosino J, Versalovic J. 2014. The placenta harbors a unique microbiome. Sci Transl Med. 6(237):237ra65. doi:10.1126/scitranslmed.3008599.
- Agostoni C, Decsi T, Fewtrell M, Goulet O, Kolacek S, Koletzko B. 2008. Complementary feeding: a commentary by the ESPGHAN Committee on nutrition. J Pediatr Gastroenterol Nutr. 46:99–110.
- Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. 2014. The intestinal microbiome in early life: health and disease. Front Immunol. 5:427. doi:10.3389/fimmu.2014.00427.
- Asnicar F, Manara S, Zolfo S, Tin Truong D, Scholz M, Armanini F, Ferretti P, Gorfer V, Pedrotti A, Tett A, Segata N. 2017. Studying vertical microbiome transmission from mothers to infants by strain-level metagenomic profiling. mSystems 2: e00164-16.
- Ayana D, Tariku A, Feleke A, Woldie H. 2017. Complementary feeding practices among children in Benishangul Gumuz Region, Ethiopia. BMC Res Notes. 10:335. doi:10.1186/s13104-017-2663-0.
- Ayechu-Muruzabal V, van Stigt AH, Mank M, Willemsen LEM, Stahl B, Garssen J, Van’t Land B. 2018. Diversity of human milk oligosaccharides and effects on early life immune development. Front Pediatr. 6:239. doi:10.3389/fped.2018.00239.
- Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al. 2015. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host & Microbe. 17(5):690–703.
- Baye K, Guyot J, Icard-Verniere C, Mouquet-Rivier C. 2012. Nutrient intakes from complementary foods consumed by young children (aged 12-23 months) from North Wollo, northern Ethiopia: the need for agro-ecologically adapted interventions. Public Health Nutr. 16(10):1741–1750.
- Bergstrom A, Skov TH, Bahl MI, Roager HM, Christensen LB, Ejlerskov KT, Mølgaard C, Michaelsen KF, Licht TR. 2014. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl Environ Microbiol. 80(9):2889–2900.
- Black R, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. 2008. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 371(9608):243–260.
- Blaser MJ, Dominguez-Bello MG. 2016. The human microbiome before birth. Cell Host & Microbe. 20(5):558–560.
- Bonder MJ, Kurilshikov A, Tigchelaar EF, Mujagic Z, Imhann F, Vila AV, Deelen P, Vatanen T, Schirmer M, Smeekens SP, et al. 2016. The effect of host genetics on the gut microbiome. Nat Genet. 48:1407–1412.
- Brahma S, Martinez I, Walter J, Clarke J, Gonzalez T, Memon R, Rose DJ. 2017. Impact of dietary pattern of the fecal donor on in vitro fermentation properties of whole grains and brans. J Funct Foods. 29:281–289.
- Campoy C, Campos D, Cerdó T, Diéguez E, García-Santos J. 2018. Complementary feeding in developed countries: the 3 Ws (when, what, and why?). Ann Nutr Metab. 73(Suppl 1):27–36.
- Chong CYL, Bloomfield FH, O'Sullivan JM. 2018. Factors affecting gastrointestinal microbiome development in neonates. Nutrients. 10:3. pii E274/nu10030274.
- Chu DM, Ma J, Prince AL, Anthony KM, Seferovic MD, Aagaard KM. 2017. Maturation of infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 23(3):314–326. Doi:10.1038/nm.4272.
- Chung W, Walker AW, Louis P, Parkhill J, Vermeiren J, Bosscher D, Duncan SH, Flint HJ. 2016. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol. 14:3. doi:10.1186/s12915-015-0224-3.
- Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. 2016. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep. 6:23129. doi:10.1038/srep23129.
- de Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U.S.A. 107(33):14691–14696.
- de Muinck EJ, Trosvik P. 2018. Individuality and convergence of the infant gut microbiota during the first year of life. Nat Commun. 9(1):2233. doi:10.1038/s41467-018-04641-7.
- Dewey KG. 2016. Reducing stunting by improving maternal, infant and young child nutrition in regions such as South Asia: evidence, challenges and opportunities. Matern Child Nutr. 12:27–38.
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. 2010. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 107(26):11971–11975.
- Dutta T, Sywulka SM, Frongillo EA, Lutter CK. 2006. Characteristics attributed to complementary foods by caregivers in four countries of Latin America and the Caribbean. Food Nutr Bull. 27(4):316–326.
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). 2013. Scientific opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA J. 11: 3408.
- Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, Gil A, Vieites JM, Norin E, Young D, et al. 2011. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology. 157:1385–1392.
- Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, Armanini F, Truong DT, Manara S, Zolfo M, et al. 2018. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. 24(1):133–145. e135.
- Fewtrell M, Bronsky J, Campoy C, Domellof M, Embleton N, Fidler MN. 2017. Complementary feeding: a position paper by the European society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on nutrition. J Pediatr Gastroenterol Nutr. 64:119–132.
- Flint HJ, Bayer EA. 2008. Plant cell wall breakdown by anaerobic microorganisms from the mammalian digestive tract. Ann NY Acad Sci. 1125:280–288.
- Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. 2012. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 3(4):289–306.
- Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, et al. 2016. The maternal microbiota drives early postnatal innate immune development. Science. 351(6279):1296–1301.
- Gopalsamy G, Mortimer E, Greenfield P, Bird AR, Young GF, Christophersen C. 2019. Resistant starch is actively fermented by infant faecal microbiota and increases microbial diversity. Nutrient. 11:1345–1361. doi:10.1390/nu11061345.
- He Y, Wu W, Zheng HM, Li P, McDonald D, Sheng HF, Chen MX, Chen ZH, Ji GY, Zheng ZD, et al. 2018. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat Med. 24(10):1532–1535.
- Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science. 336(6086):1268–1273.
- Huang JQ, Wang Q, Xu Q, Zhang Y, Lin B, Guan X, Qian L, Zheng Y. 2019. In vitro fermentation of O-acetyl-arabinoxylan from bamboo shavings by human colonic microbiota. Int J Biol Macromol. 125:27–34.
- Iozzo P, Sanguinetti E. 2018. Early dietary patterns and microbiota development: still a way to go from descriptive interactions to health-relevant solutions. Front Nutr. 5:5. doi:10.3389/fnut.2018.00005.
- Jain N, Walker WA. 2015. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nat Rev Gastroenterol Hepatol. 12(1):14–25.
- Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, Björkstén B, Engstrand L, Andersson AF. 2014. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 63(4):559–566.
- Jimenez JE, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, Fernández L, Rodríguez JM. 2008. Is meconium from healthy newborns actually sterile? Res Microbiol. 159(3):187–193.
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. 2011. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 108(Suppl 1):4578–4585. doi:10.1073/pnas.1000081107.
- Korpela K, de Vos WM. 2018. Early life colonization of the human gut: microbes matter everywhere. Curr Opin Microbiol. 44:70–78.
- Krebs NF, Sherlock LG, Westcott J, Culbertson D, Hambidge KM, Feazel LM, Robertson CE, Frank DN. 2013. Effects of different complementary feeding regimens on iron status and enteric microbiota in breastfed infants. J Pediatr. 163(2):416–423.
- Kuang YS, Li SH, Guo Y, Lu JH, He JR, Luo BJ, Jiang FJ, Shen H, Papasian CJ, Pang H, et al. 2016. Composition of gut microbiota in infants in China and global comparison. Sci Rep. 6:36666. doi:10.1038/srep36666.
- Laursen MF, Bahl MI, Michaelsen KF, Licht TR. 2017. First foods and gut microbes. Front Microbiol. 8:356.
- Li L, Li S, Ali M, Ushijima H. 2003. Feeding practice of infants and their correlates in urban areas of Beijing, China. Pediatr Int. 45:400–406.
- Lopez-Siles M, Khan TM, Duncan SH, Harmsen HJ, Garcia-Gil LJ, Flint HJ. 2012. Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. Appl Environ Microbiol. 78(2):420–428.
- Macpherson AJ, Gomes de Aguero M, Ganal-Vonarburg SC. 2017. How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol. 17(8):508–517.
- Macpherson AJ, Harris NL. 2004. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 4:478–485.
- Maier TV, Lucio M, Lee LH, VerBerkmoes NC, Brislawn CJ, Bernhardt J, Lamendella R, McDermott JE, Bergeron N, Heinzmann SS, et al. 2017. Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. mBio. 8(5):e01343–17.
- Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, Lebrilla CB, Weimer BC, Mills DA, German JB, Sonnenburg JL. 2011. Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host & Microbe. 10(5):507–514.
- Marduel BA, Vernet M. 2018. Introduction of new food textures during complementary feeding: observations in France. Arch Pediatr. 25:6–12.
- Mennella JA, Trabulsi JC. 2012. Complementary foods and flavor experiences: setting the foundation. Ann Nutr Metab. 60:40–50.
- Movassagh EZ, Baxter-Jones ADG, Kontulainen S, Whiting SJ, Vatanparast H. 2017. Tracking dietary patterns over 20 years from childhood through adolescence into young adulthood: the Saskatchewan pediatric bone mineral accrual study. Nutrients. 9:9. pii: E990. doi: 10.3390/nu9090990.
- Murphy SP, Allen LH. 2003. Nutritional importance of animal source foods. J Nutr. 133(11 Suppl 2):3932S–3935S.
- Nayfach S, Rodriguez-Mueller B, Garud N, Pollard KS. 2016. An integrated metagenomics pipeline for strain profiling reveals novel patterns of bacterial transmission and biogeography. Genome Res. 26:1612–1625.
- Osendarp SJ, Broersen B, van Liere MJ, De-Regil LM, Bahirathan L, Klassen E, Neufeld LM. 2016. Complementary feeding diets made of local foods can be optimized, but additional interventions will be needed to meet iron and zinc requirements in 6- to 23-month-old children in low- and middle-income countries. Food Nutr Bull. 37(4):544–570.
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. 2006. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 118(2):511–521.
- Perez-Munoz ME, Arrieta M, Ramer-Tait AE, Walter J. 2017. A critical assessment of the ‘sterile womb’ and ‘in utero colonization’ hypothesis: implications for research on the pioneer infant microbiome. Microbiome. 5:48.
- Pokusaeva K, Fitzgerald GF, van Sinderen D. 2011. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 6(3):285–306.
- Pulikkan J, Mazumder A, Grace T. 2019. Chapter 13: role of the gut microbiome in autism spectrum disorders – in reviews on biomarker studies in psychiatric and neurodegerative disorders. Adv Exp Med Biol. 1118:253–269.
- Purchiaroni F, Tortora A, Gabrielli M, Bertucci F, Gigante G, Ianiro G. 2013. The role of intestinal microbiota and the immune system. Eur Rev Med Pharmacol Sci. 17:323e33.
- Robertson RC, Manges AR, Finlay BB, Prendergast AJ. 2019. The human microbiome and child growth – first 1000 days and beyond. Trends Microbiol. 27(2):131–147.
- Robinson S. M., Marriott L. D., Crozier S. R., Harvey N. C., Gale C. R., Inskip H. M., Baird J., Law C. M., Godfrey K. M., Cooper C., et al. 2009. Variations in infant feeding practice are associated with body composition in childhood: a prospective cohort study. J Clin Endocrinol Metab. 94(8):2799–2805.
- Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, et al. 2018. Environment dominates over host genetics in shaping human gut microbiota. Nature. 555(7695):210–2015.
- Roy CC, Kien CL, Bouthillier L, Levy E. 2006. Short-chain fatty acids: ready for prime time? Nutr Clin Pract. 21(4):351–366.
- Rumpagaporn P, Reuhs BL, Kaur A, Patterson JA, Keshavarzian A, Hamaker BR. 2015. Structural features of soluble cereal arabinoxylan fibers associated with a slow rate of in vitro fermentation by human fecal microbiota. Carbohydr Polym. 130:191–197.
- Sakwinska O, Foata F, Berger B, Brussow H, Combremont S, Mercenier A, Dogra S, Soh S, Yen J, Heong G. 2017. Does the maternal vaginal microbiota play a role in seeding the microbiota of neonatal gut and nose? Benef Microbes. 8:763–778.
- Senarath U, Agho KE, Akram D, Godakandage SSP, Hazir T, Jayawickrama H, Joshi N, Kabir I, Khanam M, Patel A, Pusdekar Y. 2012. Comparisons of complementary feeding indicators and associated factors in children aged 6-23 months across five South Asian countries. Matern Child Nutr. 8:89–106.
- Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, Kumar N, Stares MD, Rodger A, Brocklehurst P, et al. 2019. Stunted microbiota and opportunistic pathogen colonization in Caesarean-section birth. Nature. 574:117–121.
- Sheridan P, Martin JC, Lawley TD, Browne HP, Harris HMB, Bernalier-Donadille A, Duncan SH, O'Toole PW, Scott K P, Flint H J. 2016. Polysaccharide utilization loci and nutritional specialization in a dominant group of butyrate-producing human colonic Firmicutes. Microb Genom. 2(2):e000043. doi:10.1099/mgen.
- Siega-Riz AM, Deming DM, Reidy KC, Fox MK, Condon E, Briefel RR. 2010. Food consumption patterns of infants and toddlers: where are we now? J Am Diet Assoc. 110(12):S38–S51.
- Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC, et al. 2013. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 339(6119):548–555.
- Spor A, Koren O, Ley R. 2011. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Microbiol. 9:279–290.
- Stewart CJ, Ajami NJ, O'Brien JL, Hutchinson DS, Smith DP, Wong MC, Ross MC, Lloyd RE, Doddapaneni H, Metcalf GA, et al. 2018. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 562(7728):583–588.
- Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, et al. 2014. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 510(7505):417–421.
- Tang WHW, Hazen SLH. 2017. The gut microbiome and its role in cardiovascular diseases. Circulation. 135(11):1008–1010.
- Tannock GW, Lawley B, Munro K, Gowri Pathmanathan S, Zhou SJ, Makrides M, Gibson RA, Sullivan T, Prosser CG, Lowry D, Hodgkinson AJ. 2013. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl Environ Microbiol. 79(9):3040–3048.
- Tannock GW, Lee PS, Wong KH, Lawley B. 2016. Why don’t all infants have bifidobacteria in their stool? Front Microbiol. 7:834. Doi 10.3389/fmicb.2016.00834.
- Thaiss CA, Itav S, Rothschild D, Meijer MT, Levy M, Moresi C, Dohnalová L, Braverman S, Rozin S, Malitsky S, et al. 2016. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. 540(7634):544–551.
- Thomson P, Medina DA, Garrido D. 2018. Human milk oligosaccharides and infant gut bifidobacteria: molecular strategies for their utilization. Food Microbiol. 75:37–46.
- Tremaroli V, Backhed F. 2012. Functional interactions between the gut microbiota and host metabolism. Nature. 489(7415):242–249.
- Underwood MA, German JB, Lebrilla CB, Mills DA. 2015. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatr Res. 7:229–235. doi:10.1038/pr.
- White JM, Begin F, Kumapley R, Murray C, Krasevec J. 2017. Complementary feeding practices: current global and regional estimates. Matern Child Nutr. 13(S2):12505.
- Yassour M, Jason E, Hogstrom LJ, Arthur TD, Tripathi S, Siljander H, Selvenius J, Oikarinen S, Hyöty H, Virtanen SM, et al. 2018. Strain-level analysis of mother-to-child bacterial transmission during the first few months of life. Cell Host & Microbe. 24(1):146–154.
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. 2012. Human gut microbiome viewed across age and geography. Nature. 486(7402):222–227.
- Yoshida N, Yamashita T, Hirata KI. 2018. Gut microbiome and cardiovascular diseases. Diseases. 6:3. pii: E56. doi: 10.3390/diseases6030056.
