?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Ernest Rutherford carried out his post-graduate research in the Cavendish Laboratory as one of the first generation of graduate students from outside Cambridge to study for a higher degree by research. His first experiments in radioactivity were carried out in the period 1896–1898. He returned to Cambridge as Cavendish Professor in 1919, following a remarkable period of discovery in nuclear physics at McGill University in Canada and at Manchester University. He was appointed Cavendish Professor in succession to J.J. Thomson and Director of the Cavendish Laboratory during these 'golden years' of nuclear physics until his sudden death in 1937. His achievements and those of his numerous colleagues, students and collaborators during these tumultuous years are described, much of their work under Rutherford's personal direction. These included the transmutation of nuclei by α-particle impact with Chadwick, the discovery of the neutron by Chadwick and the splitting of the atom by Cockcroft and Walton. At the same time, others were sowing the seeds for the remarkable expansion of physics research in the post-War era.
Introduction
Ernest Rutherford's connections with the Cavendish Laboratory fall into two distinct phases. The first relates to the years 1895–1898 when he was one of the first non-Cambridge students to study for a BA degree by research. In the second phase, from 1919 to 1937, he held the Cavendish Professorship of Experimental Physics. By then, he had made many of his most important experimental discoveries while holding the Macdonald Research Professorship at McGill University in Canada (1898–1907) and the Langworthy Professorship of Physics at the Victoria University of Manchester in England (1907–1919).
Ernest Rutherford had been an outstanding undergraduate and graduate student at Canterbury College at Christchurch, now the University of Canterbury, where he studied from 1891 to 1895. In his final year, he applied for a prestigious 1851 Scholarship, funded from the remarkable profits of the Great Exhibition of 1851 to provide fellowships for British and Empire graduate students. One of these was awarded by the Commissioners to an applicant from New Zealand every second year. Exceptionally, Rutherford was awarded one of two in 1895 ‘for long and valuable research in Electricity and Magnetism’. On 1 August 1895, the 23-year-old Rutherford set sail for the UK and Cambridge.Footnote1
Graduate students from outside Cambridge join the laboratory
In 1895, Cambridge University made an important change to its regulations – graduates from other Universities were allowed to be admitted as ‘research students’. After two years of residence, they could submit a dissertation on their research work and be awarded the degree of Bachelor of Arts (BA) by research – this would become a Doctor of Philosophy, PhD, in 1921. The first physics graduate students from abroad included Ernest Rutherford from New Zealand and the Irish physicists John Townsend from Dublin and John McClelland from Galway (). Through the period 1895 to 1898, more than half the active research workers in the Laboratory were from overseas. These students added enormously to the research strength of the Laboratory. C.T.R. Wilson remarked on the sudden change of atmosphere within the Laboratory as a result of this influx of bright young research students.
Rutherford, radioactivity and β-particles
At the end of 1893 while still at Canterbury College, Rutherford began his physics research career with an investigation into the magnetisation of iron by high frequency discharges. He continued work in this area when he arrived in Cambridge and developed a magnetic detector for electromagnetic waves (). In December 1895, he demonstrated the operation of the detector at a distance of 200 yards from a Hertzian spark transmitter. The first outdoor use of the detector occurred on 22 February 1896 when he set up a spark transmitter on Jesus Green and detected the radiated pulses at a distance of 350 yards in a house on Park Parade. On the following day, a successful transmission over a distance of nearly three quarters of a mile was achieved. Rutherford held the world record for the reception distance at the time. In the same year, however, Marconi came to England and developed a system for the transmission of Morse code signals by means of electromagnetic waves, the beginning of wireless telegraphy.
As soon as the discovery of radioactivity was announced by Henri Becquerel in 1896, Rutherford took up the study of the rays released in radioactive decays, a topic which was to dominate his subsequent research. In his first publication on the subject, he established that there are at least two different types of radiation emitted by radioactive substances (Rutherford Citation1899). He called the component which is most easily absorbed α-radiation (or α-rays) and the much more penetrating component β-radiation (or β-rays).
McGill and Manchester universities
Rutherford's great years from 1898 to 1919 saw a number of key experiments and results which established his reputation as an experimenter of genius. They included:
The elucidation of radioactive decay chains with the chemist Frederick Soddy. For this work, he was awarded the Nobel Prize in Chemistry in 1908.
An estimate of the age of the Earth from long-lived radioactive species.
The demonstration that α-particles are the same as the nuclei of helium atoms.
The discovery of the nucleus of the atom from the scattering of α-particles through the process now known as Rutherford scattering.
The measurement of e/m for α-particles.
the first demonstration of the artificial destruction of nitrogen nuclei by energetic α-particles.
The aftermath of war
Rutherford was 47 years old when he returned to Cambridge. The War had interrupted his work and that of his students. In 1919, Rutherford wrote to Geiger, who had survived the War in Berlin:
We are all feeling very rusty scientifically after the War, and it will be some years before we can get going properly, for apparatus is very dear and difficult to get … (Crowther Citation1974)
Besides the problems of regenerating the research programme, there was a backlog of four years of student intake who wished to study physics. In 1919 teaching was required for about 600 undergraduates and 50 naval officers, about twice as many as could be accommodated in the Laboratory. Rutherford wrote a Memorandum to the University explaining the urgent need for investment in further buildings and infrastructure. His requirements were:
Increased laboratory and lecture space for the teaching of Physics.
Provision of new, well-equipped laboratories for Applied Physics, Optics and Properties of Matter.
Provision of three additional lecturers of high standing, competent to direct advanced study in research in the new departments mentioned above.
The endowment of a second Chair of Physics.
Despite this setback, there was no lack of remarkable talent wishing to join the Laboratory. The most prominent among the new intake were the following:
James Chadwick, a former student of Rutherford's, followed him to Cambridge supported by a Clerk Maxwell studentship. After completing his PhD, the first in the Cavendish, he was appointed an Assistant Director of Research to support Rutherford's research.
In 1914 Charles Ellis was sent to Germany for military studies and was interned with Chadwick on the outbreak of the War. He became Rutherford's research assistant and then a University Lecturer in 1926.,
Patrick Blackett had commanded a destroyer during the War and embarked upon a six-month course at Cambridge designed for returning naval officers. He became Rutherford's research student and was appointed a University Lecturer in 1930.
In 1919, Arthur Compton was awarded one of the first two one-year US National Research Council Fellowships to enable students to study abroad. He chose to work with George Thomson on the scattering and absorption of γ-rays.
Pyotr Kapitsa joined the Laboratory in 1921. He had been a member of a delegation from the Soviet Union seeking to renew scientific relations after the Communist Revolution and the Russian Civil War. On reaching Cambridge, Kapitsa asked if he could join the Laboratory and, despite the severe overcrowding, he was admitted by Rutherford who recognised a kindred spirit.
George Thomson, the son of J.J., had studied physics in the Cavendish as an undergraduate. Returning to Cambridge after the War, he worked on positive rays before taking up the Professorship of Physics at Aberdeen in 1922 where he carried out his pioneering experiments on electron diffraction.
Edward Appleton joined the army in 1914 and was assigned to signal duties. This introduced him to thermionic values and radio propagation, both topics which he espoused on his return to Cambridge in 1919 as a graduate student. He was appointed an assistant demonstrator in 1920.
Support for the expanding UK research programme was provided by the Government's Department of Scientific and Industrial Research (DSIR) founded in 1915 – its budget was £1M. Rutherford and his colleagues took full advantage of these opportunities.
During Rutherford's tenure of the Cavendish Chair, the nature of physics research was to change dramatically.
There were now numerous centres of excellence in experimental physics in Europe and the USA and competition and the potential for controversy increased considerably.
From 1925, the discovery of quantum mechanics provided a new challenge for theoretical physics, which could not be ignored by the experimentalists.
The electronic revolution was well underway with the development of electronics, as well as radio communications.
The move towards ‘big-science’ was gathering momentum and this would require investment on scales previously undreamt of.
Nuclear transmutations
In 1914, Ernest Marsden investigated the ranges of hydrogen nuclei produced when α-particles were projected through a volume filled with hydrogen. As predicted by Darwin and Bohr, the ranges of the hydrogen nuclei were expected to be four times that of the α-particles, about 28 cm in air, and far greater than any of the α-particles produced by the known radioactive elements (Marsden Citation1914). This experiment was repeated by Rutherford in his last years at Manchester, using energetic α-particles produced in the radioactive decays of radium-C (bismuth) but now colliding them with oxygen, nitrogen or carbon dioxide gas.
In a series of four papers of 1919, Rutherford gave a detailed account of the apparent anomalies observed in these experiments. He confirmed Marsden's result that energetic protons bombarding hydrogen gas had ranges four times greater than the most energetic α-particles (Rutherford Citation1919a). Long-range particles were observed in collision experiments with air and nitrogen, but not with oxygen and carbon dioxide. Rutherford concluded that the long-range particles only appeared in collision with nitrogen gas (Rutherford Citation1919b). They had to be hydrogen nuclei, what he was to refer to as protons, liberated from the nucleus in collisions between the α-particle and the nuclei of nitrogen atoms.
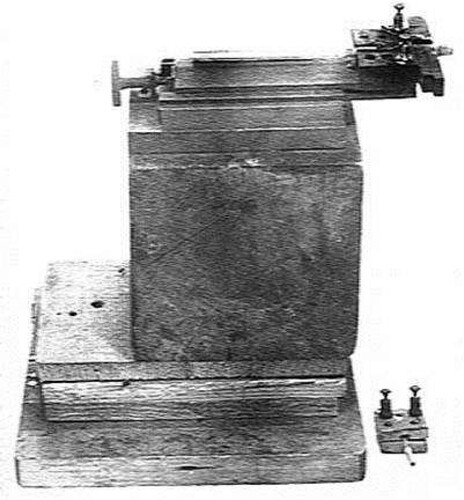
Rutherford and Chadwick began a programme of training in experimental technique for the graduate students, including the protocols devised for scintillation counting. Accurate counting required considerable concentration by a trained experimenter who had to be properly dark adapted. They began a refined set of experiments published in 1921. With the famous apparatus shown in , they found that, with dry air in the brass tube, particles with ranges up to 40 cm were observed. When the dry air was replaced by carbon dioxide or hydrogen, no ranges greater than 29 cm were observed. They found that long-range tracks, greater than 29 cm, were found in the disintegrations of nitrogen, aluminium, boron, fluorine, sodium and phosphorus.
Figure 3. The apparatus with which Rutherford and Chadwick refined measurements of the nuclear disintegration of different atomic nuclei (Rutherford and Chadwick Citation1921).
These results were not without controversy. Two young researchers at the Radium Institute in Vienna, Hans Pettersson and Gerhard Kirsch, found that, contrary to the experiments of Rutherford and his colleagues, most of the light elements, including beryllium, magnesium, lithium and silicon, showed evidence of disintegration with ranges between 10 and 18 cm. They also found that nuclear disintegrations were much more frequent than in the Cavendish experiments. In 1926, Kirsch and Pettersson published their book Atomzertrümmerung (Atomic Fragmentation) in which they set out their very different perspective on the results of the disintegration experiments. This dispute cast doubt upon the Cavendish results. Rutherford made every effort to contain the dispute and avoid public disagreements.
At the heart of the dispute were the protocols used to estimate the ranges and frequency of nuclear disintegrations. In 1925, Chadwick carried out a very thorough investigation of the techniques and protocols to ensure the reliability of the results, but even then the source of the disagreements could not be unravelled. Eventually, in 1927, Hans Thirring, Professor of Physics at Vienna University, initiated exchange visits between Cambridge and Vienna so that their procedures could be compared. Petterrson visited Cambridge in 1927 but the differences were not resolved. On Chadwick's return visit in December 1927, he was able to repeat the Vienna experiments using the Cambridge protocols. The experiments confirmed the Cambridge results, to the annoyance of the Viennese physicists. Stefan Meyer, Director of the Radium Institute, offered to retract the Viennese results, but Chadwick refused, preferring the outcome to remain private. He expected that eventually the Vienna results would fade into obscurity without public humiliation of his colleagues. All parties recognised that a less subjective means of detecting the products of nuclear decay was needed. The cloud chamber pictures produced by C.T.R. Wilson, the Geiger counter and the opportunities offered by the development of radio valves were soon to replace the scintillation technique.
Shimizu, Blackett and the cloud chamber
Rutherford immediately turned his thoughts to how the disintegration of nitrogen nuclei by fast α-particles could be photographed using the cloud chamber technique perfected by C.T.R. Wilson in 1912. He gave the problem to a new graduate student Takeo Shimizu, recognising that only about one in 100,000 of the α-particles would undergo a close collision with a nitrogen nucleus. Shimizu's devised a reciprocating mechanism which would allow 50 to 200 expansions of the chamber per minute and then recorded the images of the particle tracks using cinematographic film. Shimizu was able to take 1000 images or more per hour. On a 200-foot reel of film, 3000 α-particle tracks were observed.
Shimizu had to return to Japan in 1921 and Blackett was asked by Rutherford to continue the development of the reciprocating cloud chamber. Blackett made a number of important improvements to Shimizu's apparatus with the results described in his Nobel Prize lecture of 1948,
This preliminary work done, production was started in earnest in 1924 and 23,000 photographs were taken within a few months. With an average of 18 tracks a photograph these gave over 400,000 tracks, each of which had to be scrutinised for anomalous behaviour. … Eight forked tracks were found which had a quite different appearance from those showing normal elastic collisions, and these were readily identified as the sought for transmutation of nitrogen. (Blackett Citation1964)
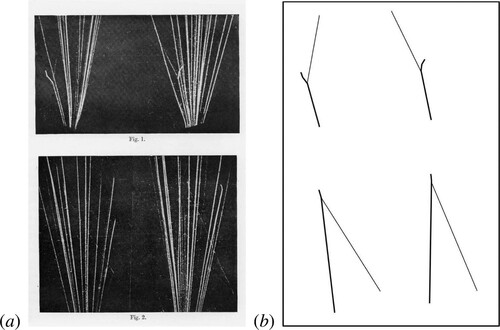
The key features of the 8 images were interactions in which a long-range proton was ejected as well as a heavy nucleus, but no α-particle was released. Stereographic images of two of the eight events are shown in A with diagrams showing the anomalous inelastic collisions (Figure B) (Blackett Citation1925). These famous images were unambiguous evidence for nuclear transmutations – the α-particle was absorbed into the nucleus and then, rather than causing the disintegration of the nucleus as a whole, as Rutherford had conjectured, the nucleus was transformed into a nucleus of and a fast proton ejected through the interaction
(1)
(1) The rare
isotope was only discovered several years later in mass spectrograph experiments.
Figure 4. (left) The ejection of protons from nitrogen nuclei by α-particles. In the top stereographic pair, the proton is ejected in the forward direction. In the lower image it travels in the backward direction (Blackett Citation1925). (right) A schematic diagram showing the tracks of the incoming α-particles and the paths of the nuclei in heavy black lines and the paths of the protons in thin lines in Figures – in the left-hand panel.
Blackett and Ochiallini – cosmic rays and the discovery of the positron
In 1929, Dmitri Skobeltsyn, working in his father's laboratory in Leningrad, constructed a cloud chamber to study the properties of the β-rays emitted in radioactive decays. Among the tracks, he noted some which were hardly deflected at all. He identified these with secondary electrons produced by cosmic rays, the first pictures of their tracks.
With Geiger and Müller's improvements of the Geiger counter, individual cosmic rays could be detected and their arrival times determined very precisely. In 1929, Bothe and Kolhörster introduced the technique of coincidence counting for studying cosmic rays. They placed slabs of lead and gold up to 4 cm thick between the counters and measured the decrease in the number of coincidences when the absorber was introduced. The mass absorption coefficient agreed very closely with that of the atmospheric attenuation of the cosmic radiation. The experiment strongly suggested that the cosmic radiation had to consist of charged particles rather than γ-rays.
Blackett developed a strong interest in the new quantum mechanics, among the fruits being Nevill Mott's prediction of the angular distribution of α-particles scattered by helium nuclei. Mott, then a junior research fellow at Gonville and Caius College, realised that, because helium nuclei and α-particles are identical bosons, they had to obey Bose-Einstein statistics in their scattering properties. At 45 to the direction of incidence, slow α-particles should display twice the probability as compared with the Rutherford scattering formula (Mott Citation1928). The experiments of Blackett and Frank Champion were in excellent agreement with Mott's predictions (Blackett and Champion Citation1931). Rutherford was impressed by Mott's theoretical prediction, remarking, ‘If you think of anything like this again, come and tell me’, a considerable encouragement for the 23-year old Mott (Mott Citation1984).
From the 1930s to the early 1950s, the cosmic rays provided a natural source of very high energy particles which were energetic enough to penetrate into the nuclei of atoms. Blackett realised that the coincidence technique developed by Bothe and Kolhörster could be combined with the cloud chamber so that the chamber was only triggered when a cosmic ray particle passed through.
At the instigation of Bruno Rossi, Guiseppe (Beppe) Occhialini was sent to Cambridge in 1931 to work with Blackett in order to master cloud chamber techniques. By the summer of 1932, they were obtaining cloud chamber images at a rate of one every two minutes with an 80% chance of these being associated with cosmic rays. They obtained many excellent photographs of the positive electrons, on many occasions observing showers containing equal numbers of positive and negative electrons (Blackett and Occhialini Citation1933). The discovery of the positive electron or positron coincided almost exactly with Dirac's theory of electrons and positrons, which Blackett had no hesitation in adopting.
Wynn-Williams, thyratrons and the scale of two counter
Despite Rutherford's attachment to the scintillation counting technique, more objective and reliable means of counting charged particles were required. The breakthrough came in 1926 when Heinrich Greinacher and his colleagues at the University of Bern constructed high gain linear amplifiers to detect the ionisation currents of individual α-particles and protons. A number of the Cavendish graduate students had a particular aptitude and enthusiasm for the burgeoning field of electronic circuitry, among them Eryl Wynn-Williams being interested in applying these techniques to the counting of ionising particles. Initially, he and his colleagues took the output signal from an ionisation chamber and fed it directly into the grid of the first stage of the amplifier. The amplified signal could then be used to drive an Einthoven string galvanometer which recorded the pulses on a cylindrical chart. This was the counting device used by Chadwick in his discovery of the neutron.
Mechanical counters were inadequate to cope with the large counting rates required. Thyratrons, which became commercially available in 1928, behaved like a two-state system rather than a linear amplifier. The BTH company generously donated six thyratrons to the Laboratory. In 1930, Wynn-Williams devised an electronic circuit in which several thyratrons were connected in a ring such that only one thyratron at a time could pass a current. A ring of five thyratrons connected to a mechanical counter could handle five times the pulse rate of the mechanical counter (Wynn-Williams Citation1931).
He then realised that the circuit could be considerably simplified if the ring were reduced to just a pair of thyratrons, which resulted in much improved performance and stability. He optimised the use of valves for counting by connecting the pairs of thyratrons in series so that each pair counted only every second pulse received by the preceding pair (). He termed this invention the ‘scale-of-two’ counter (Wynn-Williams Citation1932). By this means, particles could be counted at a rate up to 1250 events per second. This innovation marked the beginning of the use of the binary numbers in electronic computation. The detector and power supplies were mounted on a trolley which could be moved from lab to lab ().
Figure 5. A ‘next vintage’ version of the Wynn-Williams' scale of two counters. In the 1980s, he tried to find the original, but it was long gone. This second generation counter survived (Wynn-Williams Citation1984).

Figure 6. This famous photograph of Rutherford and Ratcliffe of 1932 was taken by Wynn-Williams who switched on the illuminated sign ‘Talk Softly Please’ just before Rutherford's arrival. The trolley contains the rebuilt Wynn-Williams and Ward amplifier as well as the three power supply batteries, enabling them to be moved from one laboratory to another (Lewis Citation1984).

Chadwick and the discovery of the neutron
Rutherford's Bakerian lecture to the Royal Society of London in 1920 provides a vivid picture of the state of knowledge of the properties of the atomic nucleus at that time (Rutherford Citation1920). The commonly held explanation was that the nucleus was composed of electrons and protons, these ‘inner’ electrons neutralising the ‘extra’ protons. The fact that certain nuclei ejected electrons in radioactive β-decays supported this point of view. Rutherford speculated in his 1920 review that the neutral mass in the nucleus might be associated with a new type of particle, similar to the proton but with no electric charge. He also proposed the existence of deuterium as an isotope of hydrogen, consisting of one proton and one neutron
Chadwick was quickly converted to the concept of what was named the neutron but little attention was paid to the proposal outside Cambridge. Nonetheless, Chadwick, Rutherford and their colleagues made a number of attempts to find evidence for them. As Chadwick himself wrote much later:
From time to time in the course of the following years, sometimes together, sometimes myself alone, we made experiments to find evidence of the neutron, both its formation and its emission from atomic nuclei. I shall mention some of the more respectable of these attempts; there were others which were so desperate, so far-fetched as to belong to the days of alchemy. (Chadwick Citation1984)
In 1930, Walther Bothe and Herbert Becker published their discovery of a very penetrating form of radiation emitted when light elements such as beryllium are bombarded by α-particles. They postulated that the neutral particles were high energy γ-rays. In 1931, Irène Joliot-Curie found that the penetrating particles were much more penetrating than had been previously thought. She assumed that the rays must be similar to the cosmic rays, which were interpreted as very high energy γ-rays.
Three weeks later, Irène Joliot-Curie and her husband Frédéric Joliot published the results of a further series of experiments in which the penetrating neutral radiation encountered a block of paraffin wax, a material rich in hydrogen atoms. They found that energetic protons were emitted with energies up to 4.5 MeV. If these were produced by Compton scattering by high energy γ-rays, their energies would have to be MeV.
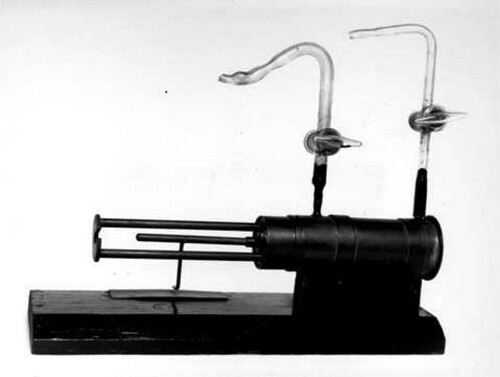
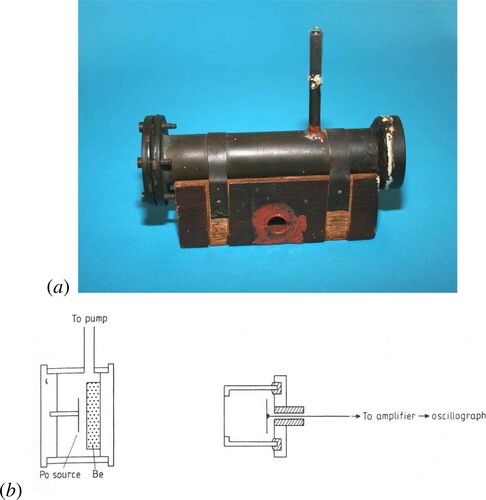
When news of this result reached Cambridge, Rutherford's uncharacteristic response was ‘I don't believe it’. Chadwick was convinced that the beryllium radiation was a flux of the long sought-for neutrons. In his apparatus shown in , the beryllium radiation was produced in collisions between the α-particle and the beryllium screen in what Chadwick referred to as the ‘source vessel’. The ionisation chamber was directly connected to the amplifier and oscillograph. Feather described what Chadwick, working at fever pitch, achieved in a virtuoso set of experiments:
Figure 7. A, The ‘source vessel’ with which Chadwick discovered the neutron (Chadwick Citation1932). B, The layout of Chadwick's neutron experiment. The source vessel is on the left and the ionisation chamber on the right (Hendry Citation1984).
So within ten days Chadwick had measured the range of the protons under various conditions, had detected the recoil of atoms of helium, lithium, beryllium, carbon, nitrogen, oxygen, and argon, and had determined the maximum ionisation produced when these recoil atoms were liberated in the gas in the ionisation chamber. It was obvious at once that the whole picture made sense numerically if the penetrating radiation from beryllium consisted of neutrons of mass roughly equal to the proton mass … (Feather Citation1984)
By the time of his Bakerian Lecture in 1933, Chadwick had adopted the point of view that the neutron was indeed a new elementary particle (Chadwick Citation1933). The final nail in the coffin of the proton plus electron model was provided in 1934 by the experiment suggested to Chadwick by Maurice Goldhaber, a recently arrived graduate student from Germany (Chadwick and Goldhaber Citation1934). Goldhaber pointed out that the mass of the neutron could be determined in collisions between γ-rays and deuterium nuclei,
(2)
(2) The mass of the neutron was found to be greater than 1.0077 and less than 1.0086 in atomic mass units. Thus, the neutron was more massive than the hydrogen atom which had an accurately determined mass of 1.0078 amu. By then, even the cautious Chadwick was thoroughly convinced he had discovered a new particle.
Cockcroft, Gamow and Walton – ‘splitting the atom’
Throughout the 1920s, the projectiles used to probe the nucleus were the products of natural radioactivity, α-particles and β-rays, but the α-particles provided only a limited range of particle energies. A second problem was that the fluxes of the projectiles were low. The obvious solution was to develop techniques to produce beams of accelerated energetic particles. The fluxes of particles provided by positive ray tubes were many thousands of times greater than those from naturally occurring radioactive substances. In his Presidential Address to the Royal Society of 1927, Rutherford made an impassioned plea:
It has long been my ambition to have available for study a copious supply of atoms and electrons which have an individual energy far transcending that of the α and β-particles from radioactive bodies. I am hopeful that I may yet have my wish fulfilled, but it is obvious that many experimental difficulties will have to be surmounted before this can be realised, even on a laboratory scale (Rutherford Citation1928).
Even before Rutherford's address to the Royal Society, Thomas Allibone had arrived at the Laboratory sponsored by the engineering firm, Metropolitan-Vickers. He was already familiar with the equipment necessary to create voltages of half a million volts and so his project was to build an accelerator which could produce a beam of 0.5 MeV electrons. Rutherford knew that atomic nuclei had potential barriers of 8 MeV or more, but these experiments might be interesting for studies with lower energy electrons. Allibone succeeded in producing beams of 300 keV electrons.
John Cockcroft, who worked in the same room, also had a background in electrical engineering, again with the support of Metropolitan-Vickers. Ernest Walton arrived in 1928 from Dublin, supported by an 1851 studentship, specifically to work on the acceleration of particles to high energies. The perspective changed suddenly with the arrival in Cambridge of a memorandum by the young George Gamow.
Gamow had read Rutherford's Presidential Address of 1927 on the problem of understanding the α-decay in thorium C, or polonium-212 (
Pu). Geiger's α-scattering experiments had shown that the height of the electrostatic potential barrier of the nucleus was at least 8.57 MeV, and yet the α-particles observed in the decay of thorium C
had energies less than half this value, 4.2 MeV. Gamow realised that this was an example of barrier penetration in quantum mechanics. He, and independently Ronald Gurney and Edward Condon almost simultaneously, solved Schrödinger's equation for a deep, rectangular nuclear potential and derived a relationship between the decay constant λ of the nucleus for α-particle decay and the energy of the α-particle. The theory naturally accounted for the very narrow range of energies of the α-particle and the enormous range of their decay constants, as found by Geiger and Nuttall in 1911.
Cockcroft repeated Gamow's calculations and, in a memorandum to Rutherford, showed that, because of barrier penetration, protons accelerated to only 300 keV could penetrate a boron nucleus with about 0.6% probability and so this accelerating electric potential would be sufficient to penetrate the boron nucleus.
Cockcroft and Walton joined forces in a determined effort to produce powerful fluxes of high energy protons. Cockcroft persuaded Rutherford to obtain £1000 from the University to buy a 300 kV transformer. They also benefitted from the purchase of prototype Apiezon pumps, invented by C.R. Burch at Metropolitan-Vickers. With the copious use of the recently introduced plasticine, also produced by Burch, by 1930 they had constructed an accelerator with beam energy 280 keV, but no nuclear interactions were observed (Cockcroft and Walton Citation1930).
At this point, Cockcroft and Walton had to vacate their laboratory and move to much larger premises in the former Balfour Library Building. They decided to build a new accelerator with the objective of reaching 800 MeV. is the classic image of the Cockcroft-Walton experiment with Walton inside the tent observing the fluorescent screen with Cockcroft on the left. On the morning of 14 April 1932, Walton succeeded in observing the first artificial nuclear disintegrations by bombarding lithium with high energy protons (Cockcroft and Walton Citation1932). Cockcroft immediately confirmed the observation and then Rutherford was invited to observe the zinc sulphide screen aglow with scintillations. Rutherford exclaimed:
Figure 8. The apparatus with which Cockcroft and Walton artificially disintegrated lithium nuclei (Cockcroft and Walton Citation1932). Walton is sitting inside the little tent, observing the decay products on a luminescent screen. Cockcroft is on the left.

Those scintillations look mighty like alpha particle ones to me. … I should know an alpha particle scintillation when I see one, for I was in at the birth of the alpha particle and have been observing them ever since.
The process involved was
(3)
(3) The energies of the accelerated protons were precisely known, as were the rest masses of the lithium and helium atoms. The decrease in mass in the above interaction corresponded to the liberation of
MeV of kinetic energy, shared equally between the two emitted α-particles. From the observed ranges of the α-particles, the total liberated energy was 17.2 MeV. This excellent agreement between theory and experiment provided the first direct experimental test of Einstein's mass-energy relation
.
Cockcroft and Walton went on to bombard many more light elements with high energy protons – Be, B, C, O, F, Na, Al, K, Ca, Fe, Co, Ni, Cu, Ag, Pb and U – scintillations were observed from all these elements. The strongest fluxes of α-particles resulted from the disintegrations of lithium Li, boron
B and fluorine
F. As they pointed out, these are all elements with atomic mass numbers of the 4n + 3 type, where n is the number of α-particles. The addition of a proton to each of these would result in the formation of a ‘new α-particle’ inside the nucleus. This experiment marked the beginning of experimental high energy physics with artificially accelerated charged particles.
Mark Oliphant completed his PhD on the bombardment of metal surfaces with positive ions in December 1929. It was recognised that he had great technical skill and was invited by Rutherford to design and build a simplified version of the Cockcroft-Walton machine. It operated up to voltages of 200 kV and produced fluxes of protons one hundred times greater than the earlier machine.
At the end of 1933, the chemist Gilbert Lewis of the University of California at Berkeley donated two tiny phials to the Laboratory containing a total of 0.5 cc of heavy water, . Oliphant was given the responsibility of looking after these precious drops and carried out a brilliant series of experiments involving deuteron collisions (Oliphant et al. Citation1933). The apparatus was modified so that the particle collisions could be photographed in a Wilson cloud chamber and Philip Dee and Walton succeeded in photographing both proton and deuteron collisions with lithium and boron targets (Dee and Walton Citation1933).
The most brilliant of Oliphant's discoveries were those of tritium and helium-3
. The voltages available in Oliphant's new machine were doubled and beams of high energy deuterons were collided with compounds containing deuterium. Large numbers of protons and neutrons were liberated in these collisions as well as particles with mass number 3 and atomic numbers one and two. These were identified with the species tritium
and helium-3
created in the interactions:
(4)
(4) There is a delightful reminiscence by Oliphant of Rutherford's continuing enthusiasm for nuclear physics at the age of 63.
We found a group of particles which clearly carried a double charge and appeared to be α-particles, in numbers equal to the protons and tritons. Rutherford produced hypothesis after hypothesis … . I went to bed tired out. At 3.00 am the telephone rang. … . I heard an apologetic voice express sorrow for waking me up, then excitedly say: ‘I've got it. Those short-range particles are helium of mass three.’ Shocked into attention, I asked on what possible grounds could he conclude that this was so, as no possible combination of twice two could give two particles of mass three and one of unity. Rutherford roared: ‘Reasons, reasons! I feel it in my water!’ (Oliphant Citation1984).
Ellis, pauli, fermi and β-decay
In contrast to the α-rays, the β-decay process resulted in a broad continuum spectrum of electron energies as well as line spectra. Rutherford had carried out important β-ray experiments in Manchester with Robinson and W. F. Rawlinson in 1912–1913 following the discovery by Otto Baeyer, Otto Hahn and Lise Meitner that groups of electrons with characteristic speeds are ejected by most β-emitting nuclei.
After the War, at Rutherford's suggestion, Charles Ellis took up these complex challenges and showed great experimental skill in establishing the line spectra of the β-rays emitted in what was called the inner photoeffect. The basic equation employed was the standard photoelectric rule, but now applied to nuclear γ-rays:
(5)
(5) where
is the kinetic energy of the emitted electron,
is the energy of the γ-ray emitted in the radioactive decay and
is the binding energy of the electron. By analogy with the origin of optical spectral lines, there had to be quantised energy levels within the nucleus itself.
During the 1920s, there was an ongoing debate about whether the broad continuum electron energy spectrum could be attributed to what was termed ‘ordinary’ processes, meaning that the electrons were created with a single energy which was then redistributed by processes such a Compton scattering, or whether the continuous energy spectrum was intrinsic to the radiative decay process itself. An example of the continuous energy spectrum of electrons found in the decay of radium-E (Bi) is shown in . In that example, there is an upper limit to the energies of the emitted electrons of just over 1 MeV, but the spread of energies extends to less than 4% of this value, the maximum occurring at just less than 300 keV with an average energy of 390 keV.
Figure 9. A example of the energy spectrum of electrons emitted in the β-decay process. This spectrum shows the continuous electron energy spectrum found in the radioactive decay of radium-E (Bi) (Neary Citation1940).
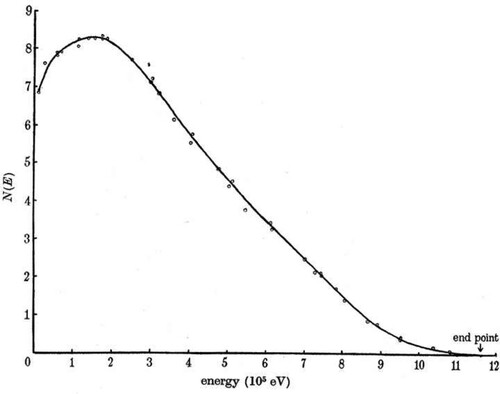
After two years of challenging experiments, Ellis and William Alfred Wooster completed calorimetric experiments in which they showed that the average energy deposited in their calorimeter was about 350 keV per disintegration, rather than about 1 MeV as might be expected if all the energy was injected with maximum energy of 1 MeV and then dissipated by ‘ordinary’ processes (Ellis and Wooster Citation1927). In these experiments, the precision of the calorimetry was such that temperature differences of K could be reliably measured. The experiment indicated that the observed continuum electron energy spectrum was indeed the intrinsic energy spectrum of the β-decay process.
In 1930, Pauli suggested that the problem might be solved by invoking the existence of a neutral particle which he called a ‘neutron’. Pauli's radical proposal was contained in an impassioned letter to his expert colleagues at their meeting in Tübingen.
I have come to a desperate way out regarding the ‘wrong’ statistics of the N- and Li nuclei, as well as the continuous β-spectrum, in order save the ‘alternation law’ of statistics and the energy law. To wit, the possibility that there could exist in the nucleus electrically neutral particles, which I will call neutrons, which have spin 1/2 and satisfy the exclusion principle and which are further distinct from light-quanta in that they do not move with light velocity. The mass of the neutrons should be of the same order of magnitude as the electron mass and in any case not larger than 0.01 times the proton mass. … . The continuous β-spectrum would then become understandable from the assumption that in β-decay a neutron is emitted along with the electron in such a way that the sum of the energies of the neutron and the electron is constant. … ’
Chadwick's discovery of the neutron, the neutral partner of the proton, changed the picture. In 1933, Fermi suggested that Pauli's ‘neutron’ might be better called a neutrino and that usage was established from then on.
In fact, Ellis and Mott were very close to discovering the neutrino. Not long after Pauli's proposal, they found that the maximum energy of the emitted electron in β-decay is equal to the differences between the initial and final states of the nuclei concerned. This was an important result since it meant that Pauli's neutrino had to have an extremely small rest mass (Ellis and Mott Citation1933). Mott remarked philosophically in his memoir of the period,
We really had in Ellis's work much of the evidence for the existence of the neutrino, and, with hindsight, it is a pity we didn't say so. (Mott Citation1984)
Nuclear fission
Unlike the electron or the α-particle, the neutron could readily penetrate the Coulomb barrier of the nucleus. The discipline of nuclear physics was transformed, since heavy nuclei such as uranium could be bombarded with neutrons, resulting in the formation of new isotopes. Furthermore, the Cockcroft and Walton experiment had demonstrated the validity of Einstein's relation and the production of energy in nuclear reactions. Rutherford was not optimistic about the use of these discoveries for energy generation. In his address to the British Association in 1933, he is reported as stating:
We might in these processes obtain very much more energy than the proton supplied, but on the average we could not expect to obtain energy in this way. It was a very poor and inefficient way of producing energy, and anyone who looked for a source of power in the transformation of the atoms was talking moonshine.
Leo Szilard was well aware, however, of the significance of these experiments for nuclear energy generation. In 1933, Szilard realised that a self-sustaining nuclear reaction chain would be possible if the liberated neutrons could be used to initiate further nuclear interactions.
Hahn, Meitner and Fritz Strassmann carried out experiments involving the bombardment of uranium with neutrons but in 1938, following the Anschluss, Meitner lost her citizenship, fled to Sweden and continued her collaboration with Hahn by mail. Hahn informed Meitner of his discovery of traces of barium when uranium was bombarded with neutrons. This came as a complete surprise since barium has only 40% the atomic weight of uranium. Meitner soon convinced herself and Hahn that the barium resulted from the nuclear fission of the uranium nuclei. Meitner and her nephew Otto Frisch, who was also working in Sweden and would become Jacksonian Professor in the Laboratory after the War, published the results of their calculations that a new type of nuclear reaction had been observed in Hahn and Strassmann's experiments.
Szilard and others immediately realised that this provided a route to a nuclear chain reaction, both for the generation of nuclear power and for the creation of nuclear weapons. Rutherford died suddenly in 1937 and did not live to see the realisation of practical means of generating nuclear energy by applying the great discoveries which he and his colleagues had made during this ‘golden era’ of nuclear physics.
The exodus of the ‘radioactivists’
The Laboratory annual photograph of 1932 shows many of the protagonists of these dramatic years () – it includes nine Nobel Prize winners. The key role of Rutherford in leading the research programme through this dramatic period of discovery is unambiguous. His achievements up to 1919 when he took up the Cavendish Chair were extraordinary enough, but to repeat the feat through the subsequent 18 years has scarcely been paralleled in any area of the physical sciences.
The one negative aspect of Rutherford's dominant role in the Laboratory was his reluctance to seek the resources needed to continue the development of research in nuclear physics, even when they were offered. It is estimated that the annual budget for research and teaching apparatus was about $2000, quite inadequate for a laboratory which had to cater for 400–500 students per year. The reason for the success of the research programme was undoubtedly Rutherford's ability to invent, and inspire, simple experiments which enabled him to draw profound conclusions by careful attention to detail.
In 1933, Chadwick recommended the construction of a high tension laboratory, but Rutherford was against it – Chadwick decided that there was no future for nuclear physics at Cambridge. Cockcroft tried to persuade Rutherford to allow him and his colleagues to go ahead with the construction of a cyclotron which had been successfully developed by Lawrence and his colleagues at Berkeley. Rutherford was disinclined to go ahead with this quite different approach to the acceleration of high energy particles. It was to be three years before the construction of a cyclotron was begun in the Cavendish.
Rutherford's reluctance to invest in larger machines for nuclear physics undoubtedly led to the decision of most of his distinguished colleagues to set up their own laboratories at other UK Universities.
Blackett took up the Professorship of Physics at Birkbeck College in 1933 and then the Langworthy Professorship at the Victoria University of Manchester in 1937.
Chadwick took up the Chair of Experimental Physics at Liverpool University in 1935.
Ellis was appointed to the Wheatstone chair of physics at King's College London, in succession to Appleton in 1936.
At the outbreak of the Second World War, Cockcroft was appointed Assistant Director of Scientific Research in the Ministry of Supply.
Walton became a Fellow of Trinity College Dublin in 1934, and in 1946 was appointed Erasmus Smith's Professor of Natural and Experimental Philosophy at the College.
Wynn-Williams was appointed assistant lecturer in physics at Imperial College, London in 1935.
Oliphant became one of Rutherford's Assistant Directors of Research but he too left to became Poynting Professor of Physics at the University of Birmingham in 1937, working with Chadwick who had secured the funds to build a cyclotron.
Kaptisa was detained in Moscow in 1934 and C.T.R. Wilson retired from the Jacksonian Chair in 1934, to be replaced by Appleton. But a new generation of gifted physicists was beginning to flourish who would take the Laboratory in new directions.
The seeds of the future research programme
Rutherford was eventually persuaded that larger, more expensive accelerators were needed but he did not live to see them. Already, however, the seeds of the future physics research programme had been sown by those researchers not involved in the nuclear physics programme.
Edward Appleton began his research career in 1914 under Lawrence Bragg. The work for which he won the Nobel Prize in Physics was his elucidation of the properties of radio wave propagation in the ionosphere and the equations which describe these phenomena, the Appleton–Hartree equations. After 1939, leadership of the radio group fell to Ratcliffe, whose expertise was to prove of immense value to the subsequent flourishing of radio astronomy under the leadership of Martin Ryle.
Kapitsa's technical expertise and bold approach to experiment led to his production of extremely large magnetic fields and the construction of the Kaptisa helium liquefier. In 1930, he persuaded Rutherford to seek funds for a laboratory dedicated to housing his high magnetic field and cryogenic facilities. Rutherford was enthusiastically supportive and obtained £15,000 from the Royal Society Mond fund. These developments led to the development of the Low Temperature Physics group in the Mond Laboratory which blossomed after the War.
Geoffrey (G.I.) Taylor had his office next to Rutherford's where he and his research assistant worked on some of the most challenging problems in experimental continuum and fluid mechanics. Taylor's interest in the plasticity of solid materials dated from his studies of the failure of aircraft propeller shafts while working at the Royal Aircraft Factory at Farnborough in 1914. In 1934, dislocations were postulated by Taylor, and independently by Orowan and Polanyi, to explain the plastic behaviour of metals under stress and the phenomenon of work hardening (Taylor Citation1934). The roles of dislocations was demonstrated after the War through the development of electron microscopy under Ellis Cosslett and the formation of the Metal Physics Group.
Theoretical support was provided by Ralph Fowler – he married Rutherford's daughter Eileen in 1921. He was allocated an office next but one to Rutherford's in the Laboratory. Fowler was the key figure in fostering theoretical physics in Cambridge – he was Dirac's PhD supervisor. Fowler was appointed to the newly-founded Plummer Professorship of Theoretical Physics in January 1932 and Dirac to the Lucasian Professorship of Mathematics in succession to Larmor later that same year.
The end of an era
Rutherford died unexpectedly in 1937 after a brief illness. His passing marked the end of an extraordinary era of discovery in experimental physics in the Laboratory. He bequeathed to his successor Lawrence Bragg a laboratory in the process of change. Many of the new areas of research were to become major themes of the post-war years. But the War was to overshadow everything and led to quite new approaches to physics research.
Further Reading
This essay is primarily an account of the scientific content of Rutherford's achievements while he was a postdoc and Cavendish Professor in the Laboratory. From the point of view of a professional historian of science, mine is a ‘technical (internalist/positivist) account of the work’. For more details of the background to this story from the perspective of a historian of science, the following books may be consulted.
Agar, J. (2013). ‘Science in the 20th Century and Beyond’. Cambridge: History of Science Series, Polity Press. ISBN-10 : 9780745634708
Crowther, J.G. (1974). ‘The Cavendish Laboratory 1874–1974’. London: Macmillan and Co.
Hughes, J. A. (1993). ‘Radioactivists: community, controversy and the rise of nuclear physics’ (Doctoral thesis). https://doi.org/10.17863/CAM.43377
Hunt, B.J. (1994) ‘The Maxwellians’. Cornell University Press, ISBN13: 9780801482342
Kim, D-W (2002). ‘Leadership and creativity : a history of the Cavendish Laboratory, 1871-1919’. Dordrecht, London: Archimedes, Kluwer Academic Publishers.
Navarro, J. (2006). ‘Early Attempts to Detect the Neutrino at the Cavendish Laboratory’. Physics Perspectives, 8, 64–82. https://doi.org/10.1007/s00016-005-0249-z
Steuwer, R.H. (2018). ‘The Age of Innocence: Nuclear Physics between the First and Second World Wars’. Oxford, Oxford University Press.
Photographic credits
All the photographs in this essay are copyright of the Cavendish Laboratory, University of Cambridge. These and many others relevant to this essay may be viewed on the Cavendish Laboratory's PhotoArchive at https://cudl.lib.cam.ac.uk/collections/cavendish/.
Disclosure statement
No potential conflict of interest was reported by the author.
Notes
1 This essay is based upon the Rutherford material contained in my scientific history of the Cavendish Laboratory (Longair Citation2016), which contains much more complete descriptions of the work described here, as well as much more complete references. The book also provides more of the historical and social background to the research achievements.
References
- Blackett P. 1925. The ejection of protons from nitrogen nuclei, photographed by the Wilson method. Proceedings of the Royal Society of London. A107:349–360.
- Blackett P. 1964. Patrick M.S. Blackett – nobel lecture 1948: cloud chamber researches in nuclear physics and cosmic radiation. In: Nobel Lectures, Physics 1942–19620. Amsterdam: Elsevier Publishing Company. p. 97–119.
- Blackett PMS, Champion FC. 1931. The scattering of slow alpha-particles by Helium. Proceedings of the Royal Society of London. A130:380–388.
- Blackett PMS, Occhialini GPS. 1933. Some photographs of the tracks of penetrating radiation. Proceedings of the Royal Society of London. A139:699–722.
- Chadwick J. 1932. The existence of a neutron. Proceedings of the Royal Society of London. A136:692–708.
- Chadwick J. 1933. Bakerian lecture: the neutron. Proceedings of the Royal Society of London. A142:1–25.
- Chadwick J. 1984. Some personal notes on the discovery of the neutron. In: Hendry J. editor. Cambridge physics in the thirties. Bristol: Adam Hilger Ltd. p. 42–45. This paper was read at the Tenth International Congress of the History of Science, 1962 and first published in volume 1 of the proceedings (Paris: Hermann et Cie, 1964).
- Chadwick J, Goldhaber M. 1934. A ‘Nuclear photo-effect’: disintegration of the diplon by γ-rays. Nature. 134:237–238.
- Cockcroft JD, Walton ETS. 1930. Experiments with high velocity positive ions. Proceedings of the Royal Society of London. A129:477–489.
- Cockcroft JD, Walton ETS. 1932. Experiments with high velocity positive ions. II. The disintegration of elements by high velocity protons. Proceedings of the Royal Society of London. A137:229–242.
- Crowther JG. 1974. The Cavendish laboratory, 1874–1974. London: Macmillan and Co.
- Dee PI, Walton ETS. 1933. A photographic investigation of the transmutation of lithium and Boron by Protons and of lithium by ions of the heavy isotope of hydrogen. Proceedings of the Royal Society of London. A141:733–742.
- Ellis CD, Mott NF. 1933. Energy relations in the β-type of radioactive disintegration. Proceedings of the Royal Society of London. A141:502–511.
- Ellis CD, Wooster WA. 1927. The average energy of disintegration of radium E. Proceedings of the Royal Society of London. A117:109–123.
- Feather N. 1984. The experimental discovery of the neutron. In: Hendry J. editor. Cambridge physics in the thirties. Bristol: Adam Hilger Ltd. p. 31–41.
- Hendry J. 1984. Introduction to part 3: underlying themes. In: Hendry J. editor. Cambridge physics in the thirties. Bristol: Adam Hilger Ltd. p. 103–124.
- Lewis WB. 1984. The development of electrical counting methods in the Cavendish. In: Hendry J. editor. Cambridge physics in the thirties. Bristol: Adam Hilger Ltd. p. 133–136.
- Longair MS. 2016. Maxwell's enduring legacy: a scientific history of the Cavendish Laboratory. Cambridge: Cambridge University Press.
- Marsden E. 1914. The passage of α-particles through hydrogen. Philosophical Magazine. 27:824–830.
- Mott NF. 1928. The solution of the wave equation for the scattering of particles by a Coulombian centre of force. Proceedings of the Royal Society of London. A118:542–549.
- Mott NF. 1984. Theory and experiment at the Cavendish circa 1932. In: Hendry J. Cambridge physics in the thirties. Bristol: Adam Hilger Ltd. p. 125–132.
- Neary GJ. 1940. The β-ray spectrum of Radium-E. Proceedings of the Royal Society of London. A175:71–87.
- Oliphant M. 1984. Working with rutherford. In: Hendry J. editor. Cambridge physics in the thirties. Bristol: Adam Hilger Ltd. p. 184–188.
- Oliphant MLE, Kinsey BB, Rutherford E. 1933. The transmutation of lithium by protons and by ions of the heavy isotope of hydrogen. Proceedings of the Royal Society of London. A141:722–733.
- Rutherford E. 1899. Uranium radiation and the electrical conduction produced by it. Philosophical Magazine (5). 47:109–163.
- Rutherford E. 1919a. Collisions of α particles with light atoms, I. Hydrogen. Philosophical Magazine, Series 6. 37:537–561.
- Rutherford E. 1919b. Collisions of α particles with light atoms, IV. An anomalous effect in Nitrogen. Philosophical Magazine, Series 6. 37:581–587.
- Rutherford E. 1920. Bakerian lecture: nuclear constitution of atoms. Proceedings of the Royal Society of London. A97:374–400.
- Rutherford E. 1928. Address of the president, Sir Ernest Rutherford, O.M., at the anniversary meeting, November 30, 1927. Proceedings of the Royal Society of London. B102:239–255.
- Rutherford E, Chadwick J. 1921. The artificial disintegration of light elements. Philosophical Magazine, Series 6. 42:809–825.
- Taylor G. 1934. The mechanism of plastic deformation in crystals. I. Theoretical. Proceeedings of the Royal Society of London. A145:362–387.
- Wynn-Williams CE. 1931. The use of thyratrons for high speed automatic counting of physical phenomena. Proceedings of the Royal Society of London. A132:295–310.
- Wynn-Williams CE. 1932. A thyratron ‘Scale of two’ automatic counter. Proceedings of the Royal Society of London. A136:312–324.
- Wynn-Williams CE. 1984. The scale-of-two counter. In: Hendry J. editor. Cambridge physics in the thirties. Bristol: Adam Hilger Ltd. p. 141–149.