?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
On the occasion of the 150th anniversary of Ernest Rutherford's birth I discuss how indistinguishabilty profoundly affects the collisions of identical particles. In the case of a Coulombic interaction this leads to a dramatic modification of Rutherford's famous scattering formula as predicted by Nevill Mott. An even more extreme example is provided by a low-energy scattering experiment of identical fermions interacting via a short range potential. Here indistinguishability will make or break the observation of scattered particles. This fascinating phenomenon has been observed using a laser-based collider for ultracold fermionic potassium atoms.
1. Prologue
In 1909 Hans Geiger and Ernest Marsden published a paper entitled ‘On a Diffuse Reflection of the α-Particles’ in Proceedings of the Royal Society of London, ‘Communicated by Prof. E. Rutherford, FRS’ (Geiger and Marsden Citation1909). The paper carried the acknowledgement:
We are indebted to Prof. Rutherford for his kind interest and advice throughout this research.
In essence, the research reported by Geiger and Marsden and conducted under Rutherford's patronage was concerned with bombarding a very thin gold foil with α-particles. A mere 4 microns thick, the foil had a stopping power equivalent to 1.6 mm of air and most α-particles would hardly notice its presence and only deflect by small angles. But, crucially, a few α-particles would be reflected back from the foil and be scattered at angles exceeding with respect to their incoming direction. In a lecture given at Cambridge some thirty years later, Rutherford would recount (Rutherford Citation1938):
It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back to hit you.
2. Introduction
In this contribution to the Special Issue ‘150th anniversary of the birth of Ernest Rutherford’ of the Journal of the Royal Society of New Zealand, I aim to showcase some manifestations of quantum mechanical identity as observed in atomic scattering experiments at the University of Otago. These experiments can ‘whakapapa’ back to the seminal ur-scattering experiments by Rutherford and co-workers, and are powered by the same idea: colliding material particles to observe angular distributions of scattered particles and thereby gain insight into the governing physics at a ‘microscopic’ level.
The experimental observation of Geiger and Marsden described in the above prologue inspired Rutherford to (Rutherford Citation1911):
Consider an atom which contains a charge at its centre surrounded by a sphere of electrification containing a charge
supposed uniformly distributed throughout a sphere of radius R.
Today this might not sound like a particular radical assumption, but the prevailing thinking of the time assumed the atom to be a ‘number N of negatively charged corpuscles, accompanied by an equal quantity of positive electricity uniformly distributed throughout a sphere’ (Rutherford Citation1911), and one has to keep in mind that within the framework of classical physics, the Rutherford atom would collapse and that a quantum mechanical description (yet to emerge) is required to stabilise the charge configuration.
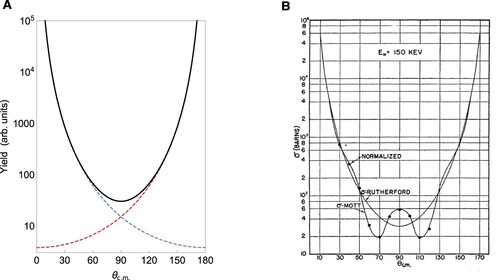
Once Rutherford's ad hoc assumption of a central charge in the atom has been made it is not particularly hard to work out the classical trajectory of an incoming point-like α-particle for a given impact parameter b (Brandt Citation2009). Indeed, Coulomb's inverse square law for the electric force gives rise to a hyperbolic trajectory for a light incoming point charge scattering off a heavy (effectively fixed) point charge. For such trajectories one can find the angle of deflection as a function of b and from this deflection function,in turn, infer the so-called differential scattering cross section, which for an incoming pencil of particles describes how many particles per unit area go out in a particular direction. What Rutherford showed was that the differential cross section should, classically, have a dependence [see blue dashed line in Figure A;
denotes the centre-of-mass scattering angle]. This was exactly the dependence found by Geiger and Marsden (Citation1913) in their follow-up experiment (Trenn Citation1974). Here it is in order to remark that the agreement between theoretical prediction and experiment was a fortuitous coincidence (Schattschneider Citation1986):
Experimental results were completely explained by the angular distribution of scattered particles obtained from their classical trajectories. In 1911, when the classical model came forth, no one knew about wave mechanics or probability amplitudes. Nor did anyone know that there is nothing like a trajectory on the atomic scale. Though the theory was successful–today we know it was good luck, since the classical and quantum mechanical cross sections coincide, by chance, for the Coulomb potential. So, it can be said that a wrong theory, applied onto a correct model, made an early emergence and an early success of atomic physics possible.
Figure 1. A, Rutherford cross section in the centre-of-mass frame valid for scattering of distinguishable particles interacting via a Coulomb potential. Dashed lines show the angular yield for each type of particle, while the solid line is the total yield. B, Measured angular distribution of α-α scattering, i.e. scattering between identical bosons. The data points do not follow the Rutherford cross section but agree perfectly with Mott's formula. Reprinted figure with permission from N. P. Heydenburg and G. M. Temmer, Phys. Rev. 104, 123 (1956). Copyright 1956 by the American Physical Society.
The perfect agreement between experiment and prediction did, however, not only rest on the fact that the quantum mechanical and classical formulas serendipitously coincide for the Coulomb potential in 3D (Friedrich Citation2013). As we shall see, it also crucially relied on that the collisions involved distinguishable particles–α-particles and gold nuclei.
3. Twice lucky
What if, rather than bombarding gold atoms with α-particles, the Manchester team had decided to bombard helium atoms with α-particles to verify Rutherford's hypothesis? Now, following Rutherford's assumption quoted above, the helium atom should be considered as of charge at its centre, surrounded by a spherical distribution of the same amount of charge but of the opposite sign. Furthermore, in deriving his angular scattering formula Rutherford disregarded the surrounding dilute charge sphere and considered the trajectories of point charges. Hence the problem reduces to that of an α-particle considered to be a point charge, which scatters off a helium nucleus also considered to be a point charge. From the point of view of classical physics this situation is completely equivalent to that of α-particles scattering off gold nuclei and one would naïvely expect a centre-of-mass angular distribution
following the Rutherford formula. But surprisingly, the scattering of alpha particles on helium nuclei spectacularly deviates from the Rutherford formula as was predicted by Nevill Mott in 1930 (Mott Citation1930). The reason is that α-particles are themselves helium nuclei so one is dealing with collisions between identical particles. By identical we mean that all intrinsic properties of two helium nuclei are the same: every helium nucleus in the world consists of two protons and two neutrons and all helium nuclei have exactly the same mass, the same electric charge and the same spin (S = 0). Mott would late in life recall (Mott Citation1972):
My own first researches were involved with showing that quantum mechanics gave the Rutherford scattering formula (I never heard his comments on that), and then the prediction that if the nucleus was helium, so that the two particles were identical, this was no longer so. Chadwick did the experiment and I remember vividly the day he showed me his results, in his typically pessimistic way which left me till the last moment thinking that he had disproved my prediction. Then he took me along to Rutherford who said ‘If you think of anything else like this, come and tell me’ – enormously high praise, which I shall never forget.
Indeed, following Mott's paper Chadwick conducted measurements (Chadwick Citation1930) showing that twice as many particles would be scattered into a -angle (centre-of-mass frame) as predicted by Rutherford's formula. Figure B shows the results of subsequent and more refined experiments by Heydenburg and Temmer covering a range of angles for
-scattering (Heydenburg and Temmer Citation1956). We note how the Rutherford formula is off by a factor of two at
, while Mott's formula, based on quantum mechanics and taking into account the particles' indistinguisbility, captures the behaviour perfectly.
4. Scattering of identical particles
The idea that our material world is assembled from a number of fundamental building blocks has been around at least since Democritus and is probably something most modern people would feel quite comfortable about. It also echoes our experience with many everyday objects that are built in a modular fashion. The notion that distinguishablity of material objects should play a role in the outcome of their collisions is, however, difficult to reconcile with. In truth, one would be very surprised if the head-on collision between two cars of the same make and model would depend on whether they were painted in the same colour or not. In quantum mechanics, however, distinguishability of two particles on a collision course is crucial in determining the outcome. Before providing an illustration of this let us note that (Hilborn and Yuca Citation2002):
In quantum mechanics, unlike everyday discourse, indistinguishability is a contingent condition dependent upon the quantum state of the system. Two particles that share the same intrinsic properties can in quantum mechanics also share the same dynamic properties (as described by the quantum state). But they may also occur in states in which they have different dynamical properties, say spin orientation, which may be used to distinguish the particles under appropriate conditions.
4.1. Distinguishable versus indistinguishable particle collisions
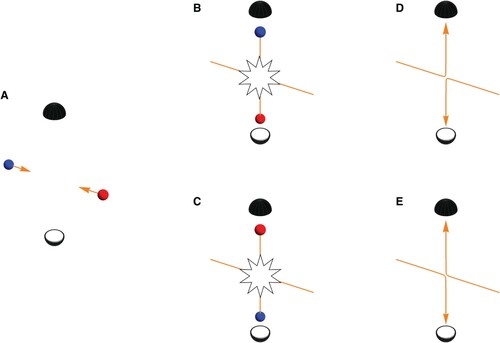
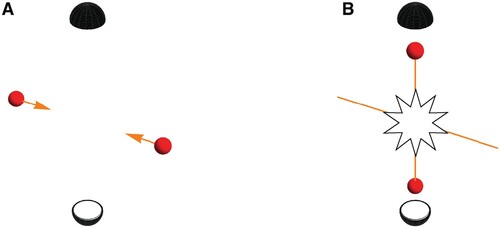
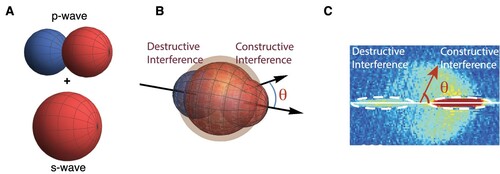
To set the scene, let us consider two particles of the same mass and charge, but which can be distinguished via some other property. For the sake of the argument we will take this to be their colour (Dickhoff and Van Neck Citation2008). In Figure A we show a blue particle coming in from the left and a red particle coming in from the right at the same speed. We will assume particles interact via a potential that has no reference to their colour. Figure B shows a possible outcome of the collision where the blue particle is captured by the top detector. Because of conservation of energy and momentum, a red particle is captured by the bottom detector. Figure C shows the equally (because of symmetry) likely scenario of a red particle being captured by the top detector while the blue is captured by the bottom detector. In both scenarios Figure B,C it is, a posteriori, possible to assign a trajectory to each particle based on the results recorded by the detectors: in the case of Figure B we can make the statement that the particle coming in from the left went up and the one coming in from the right went down as shown in Figure D. Likewise, Figure C corresponds to the situation shown in Figure E where the particle coming in from the left went down while the one coming in from the right went up.
But what about the situation depicted in Figure , where two red and therefore indistinguishable particles collide? Because of their indistinguishability, there is no way, not even in principle, to tell if the particle coming in from the left went up or down. This loss of ‘which-way’ information, means we should brace ourselves for something dramatic to be happening in the quantum mechanical realm. Case in point, the lack of ‘which-way’-information is what causes a single particle to – borrowing from Dirac – ‘interfere with itself’ in the famous double slit experiment (Feynman et al. Citation1965). More accurately, it is the probability amplitudes for the two possible pathways of the particle that interfere (Glauber Citation1995).
Readers of Physics World voted ‘Young's double-slit experiment applied to the interference of single electrons’ the most beautiful experiment in physics of all time (Rosa Citation2012) ahead of ‘Rutherford's discovery of the nucleus’, which came in 9th place. One can only speculate what placement this latter experiment might have gained, had it been concerned with the collisions of indistinguishable particles such as two helium nuclei. It would not only have discovered the nucleus but also two-particle quantum interference.
4.2. Quantum scattering
In quantum scattering of two particles of mass and
one goes into the centre-of-mass frame to solve the time-independent Schrödinger equation
(1)
(1) where
is the separation of particles which enter along the z-axis with relative momentum
, to collide at the centre-of-mass energy
, with
denoting the reduced mass. One looks for solutions which at large distances can be written as a sum of an incoming plane wave and an outgoing spherical wave
(2)
(2) In (Equation2
(2)
(2) ),
is the so-called scattering amplitude and the squared modulus of this complex-valued quantity is the aforementioned differential cross section – a direct measure of the number of particles of type 1 scattered out in a direction
. Particles of type 2 could also be scattered out in a direction
and because in the centre-of-mass frame every particle is scattered back-to-back with another particle this will be determined by the number of particles of type 1 to be scattered in direction
.
For in (Equation1
(1)
(1) ) being the Coulomb potential
, one finds that the scattering amplitude has no ϕ-dependence and that
(Friedrich Citation2013). Let us begin by considering collisions between two types of particles, 1 and 2. In Figure A we plot
as a dashed blue line which describes scattering of particles of type 1 (the direct flux). We also plot
as dashed red line, which as mentioned is the corresponding recoil flux of scattered particles of type 2. We finally plot
as solid black line, which describes the angular probability of any particle, 1 or 2, going out in a direction θ–the resulting flux is the sum of the direct and recoil fluxes.
4.3. Scattering of bosons and fermions
In the quantum description of systems of particles two categories are encountered: particles with half-integer spin, called fermions, and particles with integer spin, called bosons. The quantum mechanical wave function for a system of identical bosons is required to be symmetric under the permutations of two particles. In contrast, the quantum mechanical wave function for a system of identical fermions is required to be antisymmetric under the permutations of two particles. This is the basis of the Pauli exclusion principle which forbids two identical fermions to occupy the same quantum state and for example accounts for the ordering of electrons into shells in atoms: the electron has spin 1/2 (and is consequently a fermion) with the two possible spin projections (spin-up) and
(spin-down) – hence there can be exactly two in the innermost shell.
For colliding identical fermions in the same spin state (which makes them indistinguishable), the spatial wave function describing their scattering must respect antisymmetrisation. Bosons, on the other hand require a symmetric spatial wave function. Equation (Equation2(2)
(2) ) displays no particular symmetry and the prescription for dealing with indistinguishable particles is to first solve the problem as if the particles were distinguishable to obtain
and then subsequently impose the required symmetrisation as (Friedrich Citation2013):
(3a)
(3a)
(3b)
(3b) The requirement of appropriate symmetrisation of the spatial wave function radically changes the scattering when comparing to the case of distinguishable particles. This can easily be seen by considering side-ways scattering into an angle
. Suppose for example that a given rotationally invariant potential V (possibly a Coulomb potential) in (Equation1
(1)
(1) ) gave rise to a scattering amplitude so that
for distinguishable particles. Equation (Equation3b
(3b)
(3b) ) then tells us that for indistinguishable bosons interacting via the same potential one has
. This is twice as much as the
one would get from distinguishable particles (
from each kind) and a detector at
will experience twice the (total) particle flux in the indistinguishable boson case. As mentioned in Section 3, this is exactly what is observed experimentally with α-α collisions [see Figure B] – the S=0
nucleus is a bosonic particle.
For indistinguishable fermions, (Equation3a(3a)
(3a) ) shows that destructive interference will happen at
so that no particles whatsoever will be scattered into a sideways directions. This ‘no-sideways-rule’ is a direct consequence of the Pauli exclusion principle and does not depend on the collision energy or the range of the potential.
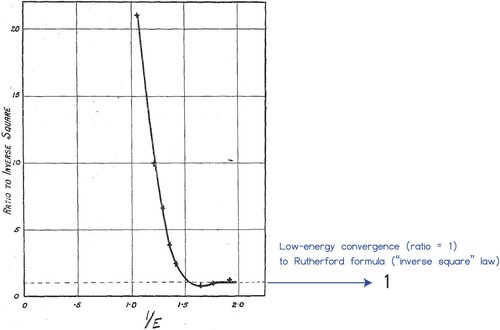
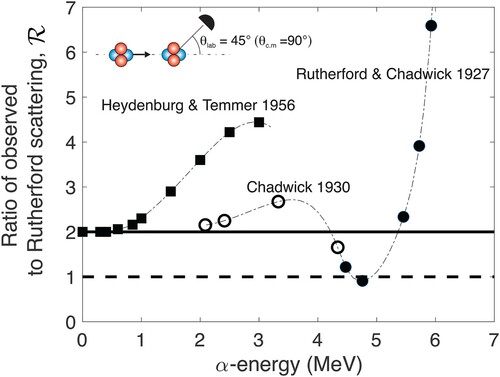
I opened Section 3 by posing the question: What if the Manchester team had decided to bombard helium atoms with alpha particles to verify Rutherford's hypothesis? In fact, in 1927 – by which time Rutherford had moved to Cambridge (Longair Citation2021) – and prior to Mott's prediction, Rutherford together with Chadwick did decide to do just that (Rutherford and Chadwick Citation1927). Figure shows their result for a measurement of the sideways scattered particles (centre-of-mass frame). For high energies they found a clear deviation from the Rutherford formula for scattering in a pure Coulomb potential echoing what had previously been found for collisions of α-particles with hydrogen nuclei (Chadwick and Bieler Citation1921; Barrette Citation2021). In this high-energy domain ‘the scattering of alpha particles by gases and light atoms show that the law of inverse squares breaks down when the alpha particle comes very close to, or penetrates the nucleus’ (Rose Citation1926). For low energies, however, the experiments on α-hydrogen collisions showed excellent agreement with the Rutherford formula and based on their results presented in Figure , Rutherford and Chadwick were led to believe this was also true for the α-α case. But it turns out that at the lowest energy of their experiments, nuclear effects are still important: to achieve pure point-particle Coulomb interactions, one needs to go even lower in energy. By calling it a day, they missed out on discovering the consequences of quantum mechanical identity (Temmer Citation1989): had they continued down in energy the number of sideways scattered particles would have been found to exceed the prediction of the Rutherford formula by a factor of 2.
Figure 4. α-α scattering reported in Rutherford and Chadwick (Citation1927). The observed number of scattered particles at a angle (centre-of-mass frame) is plotted as the ratio to the number predicted by Rutherford's formula versus 1/E (the inverse of the collision energy E). From this Rutherford and Chadwick concluded that agreement was reached (ratio=1) in the low-energy limit (1/E large). The graph appeared as Figure in ‘The scattering of α-particles by helium’, E. Rutherford and J. Chadwick, The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 4(22):605, 1927, reprinted by permission of the publisher (Taylor & Francis Ltd, http://www.tandfonline.com).
As mentioned in Section 3, following Mott's 1930 prediction that identical particles should pick up this factor, Chadwick went back to the lab and extended the measurements to lower energies where he found this to hold true (Chadwick Citation1930). Or did he? As summarised in Appendix, Chadwick's confirmation of Mott's prediction was arguably a false positive: the subsequent precision measurements by Heydenburg and Temmer (Citation1956) show that to reach the factor of 2, half of Chadwick's lowest energy is needed.
5. Collision experiments at Otago
Over the past decade a laser-based collider for ultracold atoms has been constructed at the University of Otago (Rakonjac et al. Citation2012; Thomas et al. Citation2016; Horvath et al. Citation2017; Thomas et al. Citation2018; Chilcott et al. Citation2021). This optical collider makes use of the fact that, generally, polarisable neutral particles can be confined and manipulated by laser light (Ashkin Citation1997). We use such ‘optical tweezers’ to ‘trap’ small clouds of neutral atoms. A trapped atom minimises its energy by residing at the high intensity point of a light field: for a focused laser beam this means the focal point; for two crossed beams this means their intersection (see Figure ). Our setup incorporates the atomic species rubidium (R) and potassium (K), and by steering the optical tweezer beams, atomic clouds can be accelerated and guided into a collision as shown in Figure for the case of .
Figure 5. Crossed dipole trap. Atoms congregate at the laser beam intersection where the light intensity is the highest.
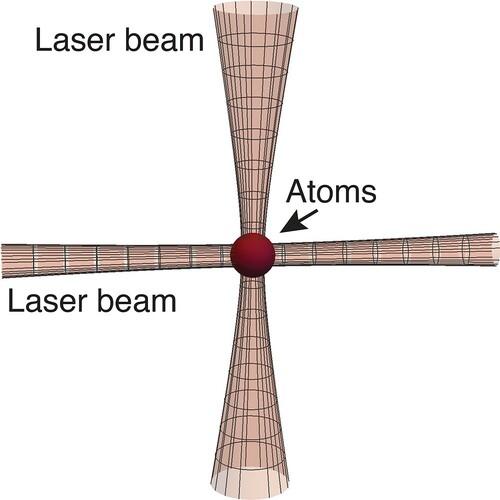
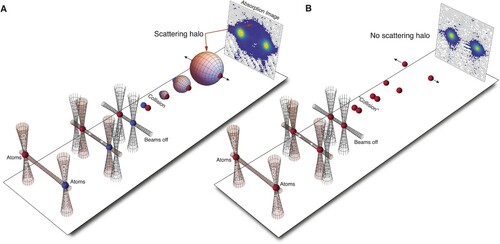
Figure 6. Collisions at K. A, Optical collider procedure.
atoms prepared in the internal quantum states
(red) and
(blue) are initially held by two separate cross beam traps. By steering the vertical tweezer laser beams the two clouds are accelerated along a horizontal guide beam into a collision, where a halo of scattered particle emerges. The distribution of scattered particles is detected using absorption imaging. B, Same procedure as A with the crucial difference that both clouds are in
. No scattering halo forms as result of combined action of Pauli exclusion and Wigner threshold suppression.
Particle accelerators such as the Large Hadron Collider (LHC) at CERN typically operate in a regime where particles travel close to the speed of light (Evans Citation2011). In contrast, the optical collider at Otago is constructed to study collisions at a pedestrian speed (). Measuring the collision energies in units of the Boltzmann constant
J/K, these attain typical values between 2 microkelvin and 2 millikelvin. When colliding atomic clouds at such low energies, the temperature of each cloud, which is a direct measure of the mean kinetic energy of a particle within a cloud, needs to be correspondingly low. We use established techniques of laser cooling and evaporative cooling to produce samples of
in the nanokelvin domain (Fallani and Kastberg Citation2015). Laser cooled
atoms can be taken to similar low temperatures by sympathetically cooling them with
as coolant. The
isotope of rubidium is made up by an even number (124) of constituent spin-
particles (37 protons, 50 neutrons, and 37 electrons) and is therefore a composite boson, while
, on the other hand, consists of an odd number (59) of particles (19 protons, 21 neutrons, and 19 electrons) making it a composite fermion.
5.1. Colliding atoms in different internal states
Figure A shows schematically how two clouds of are initially trapped at separate location by crossed laser beams. By site-selectively addressing the clouds with radio frequency radiation (Sawyer et al. Citation2019), the atoms in one cloud (red) have been prepared in the internal hyperfine state
while the atoms of the other cloud (blue) are in the
state. By steering the vertical laser beams the two clouds are accelerated towards each other. Just before the clouds encounter, all confining laser beams are turned off so that they collide in free space at an energy
K. The difference in internal states for the colliding particles means that the collisions can be treated as for distinguishable particles (Feynman et al. Citation1965; Thomas Citation2017; Walraven Citation2017). Scattered particles are detected from the shadow they cast on a CCD chip when probed with laser light that is resonant with an atomic optical transition (Kjærgaard Citation2015). Figure A shows that for our collision experiments with
atomic clouds prepared in different internal states, such absorption images reveal a clear scattering halo.
5.2. Wigner threshold laws
In Section 4.3 I stated that indistinguishable fermions cannot scatter sideways irrespective of the collision energy or the range of the potential. Unlike Rutherford scattering of charged particles via a long range Coulomb potential (), neutral
atoms interact via a short range potential, where by short range we mean a potential that falls off with distance faster than
. Particles with a relative motion described by an angular momentum quantum number
will therefore experience a centrifugal barrier which dominates when the atomic separation is large. A certain energy is hence needed to ‘overcome’ the centrifugal barrier associated with a particular
. This is summarised in so-called threshold laws (Sadeghpour et al. Citation2000), usually credited to Wigner (Citation1948). The bottom line is that when the collision energy approaches zero, all components but
– so-called s-wave scattering – will be suppressed. The absorption image in Figure A shows an isotropic s-wave halo because the experiment is conducted at such a low collision energy that angular momentum states with
are ‘frozen’ out.
Figure B shows the result of colliding two clouds of fermions in the same internal state. The experiment is conducted at the same collision energy as Figure A and with a similar number of atoms in each cloud, but remarkably no scattered particles are to be seen for the indistinguishable particle case. The requirement of antisymmetrisation for fermions stipulates the total wave function to be antisymmetric. Because the particles are in the same spin state, the spin part of the wave function is symmetric and it is left to the spatial part to ensure antisymmetry, cf. (Equation3a
(3a)
(3a) ). When decomposing the spatial wave function into angular momentum components only those with odd
are antisymmetric. So on one hand Pauli exclusion strictly forbids s-wave scattering while on the other hand Wigner's threshold law strongly suppresses everything but s-wave scattering. As a result, there is no observable effect in our absorption image when colliding two indistinguishable clouds at low energy. Indeed, the difference in the outcome of the experiment in Figure B to that of Figure A is striking, and as if you ‘fired a 15 inch shell at a 15 inch shell and nothing happened’.
5.3. Fermionic p-wave scattering above threshold
The reason no scattering is observed in Figure B is that all even- states – s, d, g,…– are strictly forbidden by the Pauli exclusion principle while odd-
states are all suppressed by their Wigner threshold law. As we raise the collision energy, the odd-
channels will increasingly contribute, beginning with the
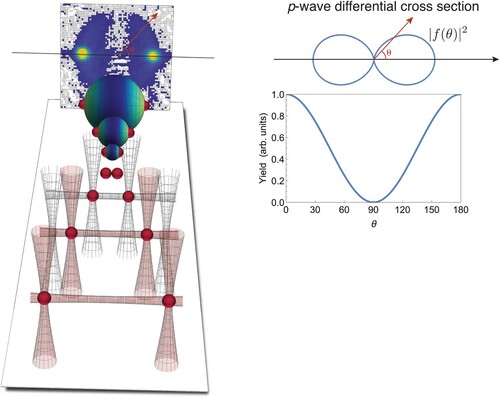
p-state and its characteristic
dumbbell scattering pattern. Figure shows the result of an experiment with indistinguishable fermions conducted at an energy
K. For this increased energy [as compared to Figure B], we see massive scattering form, but, notably, no atoms are scattered sideways into
as predicted by (Equation3a
(3a)
(3a) ). A detailed analysis of the angular scattering shows that virtually all scattering is p-wave in this energy domain (Thomas et al. Citation2016).
Figure 7. Collisions at K between two
atomic clouds using the procedure described in Figure . As the particles are indistinguishable fermions, a clear p-wave scattering halo forms as the collision energy is above the domain of
Wigner threshold suppression. The angular p-wave distribution governing the scattering halo is shown ar the right side.
5.4. Bosonic scattering
Our optical collider at Otago also makes it possible to study indistinguishable particle effects in bosonic . In fact, working with these bosons holds less complications than the fermionic
because evaporative cooling can take them directly to the nanokelvin regime. Evaporative cooling works by selectively removing the hottest atoms while letting the remainders rethermalise through elastic collisions. For a sample of fermionic atoms all in the same state, this rethermalisation process cannot take place efficiently because of the suppression of p-wave scattering at threshold; in the case of
this means around
K (DeMarco et al. Citation1999). In our experiments on fermions we therefore resort to using bosonic rubidium as a coolant to get fermionic potassium to the ultracold domain (Roati et al. Citation2002). The need to employ such sympathetic cooling arises exactly due to the vanishing of low-energy scattering described in Section 5.2. In contrast, the rethermalisation via s-wave interaction works so well for
that a trapped atomic cloud can be evaporatively cooled to form a Bose-Einstein condensate (BEC) (Anderson et al. Citation1995). In the results presented below, the evaporative cooling of
was terminated just above the temperature for which a BEC would form. Scattering halos can therefore be considered the result of many binary collision events between incoming individual atoms.
5.4.1. Indistinguishable boson case
Figure shows the result of colliding two ultracold clouds of bosonic with both prepared in the internal state
. This makes two colliding atoms indistinguishable and renders their joint spin state symmetric. To obtain a totally symmetric wave function for colliding particles, as required for identical bosons, their spatial wave function must be symmetric, cf. (Equation3b
(3b)
(3b) ). Hence only even-
angular momentum states will contribute to scattering. In particular,
– s-wave scattering – is, unlike the indistinguishable fermionic case, not only allowed, but will completely dominate at low energies due to Wigner threshold suppression of states with
. Moreover, because of the constructive interference represented by (Equation3b
(3b)
(3b) ), the amount of scattered particles observed going into any direction will be twice that of what would be found if the clouds were in different and distinguishable internal states.
Figure 8. s-wave and d-wave scatering for in the internal state
(indistinguishable bosons). See main text for description. Figure reprinted from (Kjærgaard Citation2015) with permission.
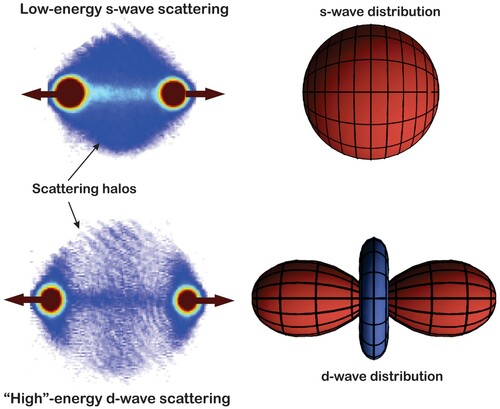
By increasing the collision energy, components to the scattering will rise. However, with the particles being indistinguishable bosons all odd-
will be strictly forbidden and the first
component encountered will be
– the so-called d-wave. Figure shows the pattern resulting from
d-wave scattering. In general, the s-wave and d-wave will interfere, i.e. their respective scattering amplitudes must be added up before the modulus square is taken to obtain the differential cross section (Kjærgaard et al. Citation2004; Buggle et al. Citation2004). The collision experiment presented in the bottom panel of Figure was conducted at an energy around
K, where the d-wave contribution happens to be resonantly enhanced and therefore dominates the overall scattering pattern. Below the d-wave resonance, so-called partial-wave interference gives rise to intriguing scattering patterns that depend sensitively on the magnitude and relative phase of the s-wave and d-wave components (Thomas et al. Citation2004).
5.4.2. Distinguishable boson case
The scattering pattern resulting from partial wave interference encountered for indistinguishable particles will fulfil . For colliding clouds coming in from the left and the right, this implies that absorption images of scattering halos will be left-right symmetric.
The mirror symmetry encountered for scattered indistinguishable particles is broken for distinguishable particles when multiple partial waves are in play. Figure presents a case of partial-wave interference when an s-wave and a p-wave simultaneously contribute. If the right lobe of the p-wave is in phase with the s-wave, the left lobe will be out of phase [Figure A]. The result is constructive interference of (partial wave) scattering amplitudes to the right and destructive interference to the left as illustrated in Figure B. Figure C shows a manifestation of this phenomenon in a collision experiment with in different internal states, namely
and
(Mellish et al. Citation2007). Only
atoms are detected and a clear left-right asymmetry can be seen with
atoms predominantly scattered to the right (forward direction of the incoming
cloud). A scattered
atom going in a direction
will be matched by a
counterpart which travels in direction
, but will remain unobserved as a result of the state-selective detection scheme.
Figure 9. A, p-wave and s-wave angular distributions. The blue and red colours illustrate the antisymmetric and symmetric nature, respectively, of the two, with the amplitude of the p-wave changing sign under the operation of reflection in the origin. B, When adding up the s-wave and p-wave amplitudes one can obtain constructive inference in the forward or backward direction and destructive interference in the opposite direction. C, Experimental observation of s+p-wave interference for scattering of two clouds of in different internal states (
and
) at an energy
K (Mellish et al. Citation2007). Only atoms in the
are state-selectively imaged and we see that these are predominantly scattered in the forward direction. Of course,
atoms give rise to an additional scattering halo – invisible to the detection scheme – which predominantly travels in the opposite direction.
I should note that partial wave interference is not an effect due to the particles being identical. Indeed, Thomas et al. (Citation2017) presents a case of s+p partial-wave interference for colliding rubidium and potassium atoms. As such, partial-wave interference is brought about by two ‘pathways’ – s and p – for a single atom, akin to Young's double slit experiment.
6. Discussion
Using the pioneering experiment by Geiger, Marsden, and Rutherford as a springboard, we have delved into the profound consequences that indistinguishability of identical particles has on their scattering. Along with Chadwick, Rutherford was at some point very close to stumbling over this fact, but it was not until Mott predicted that something was amiss that the effect was identified in experiment.
In Section 4.3 statements were made that the quantum mechanical wave function for a system of identical bosons or fermions is required to be symmetric and antisymmetric, respectively, under the permutations of two particles. This aligns with the treatment given in standard textbooks on quantum mechanics such as Landau and Lifshitz (Citation1977). The meaning and validity of the language surrounding this symmetrisation postulate can be debated both on philosophical (Hilborn and Yuca Citation2002) and more technical (Mirman Citation1973; Leinaas and Myrheim Citation1977; Kaplan Citation2017) grounds, but I shall not digress into a discussion on this. Rather, I shall limit myself to consider the somewhat unsettling consequence of the symmetrisation postulate: ‘The wave function representing any state of the whole universe should in principle be properly symmetrised with respect to all particle of all species’ (Taylor Citation2006). The two electrons in a helium atom need to form an antisymmetric state (and spectroscopy experiments show they do), but when dealing with this problem in a first course on atomic physics no care is taken to ensure the correct symmetry with respect to other electrons in the world, be it an electron in another helium atom or an electron on the Moon. And it so happens that discarding particles outside of the domain of interest is completely fine for practical purposes. In the words of Messiah (Citation1969):
In practice, the electrons of a system are all inside a certain spatial domain D, and the dynamical properties in which we are interested all correspond to measurements to be made inside this domain. It turns out that the other electrons may simply be ignored so long as they remain outside D and so long as their interaction with the electrons of the system remains negligible. This is a general result and applies to bosons as well as to fermions.
One might argue that ‘the practical issue of when we can safely ignore indistinguishability should not deflect our attention from the underlying philosophical issues surrounding indistinguishability’ (Hilborn and Yuca Citation2002). Irrespective of this, it is an experimental fact that when two indistinguishable particles collide, their scattering is radically different to that of distinguishable particles. Moreover, this remains true even if the particles are initially far apart and reside in completely separate domains. In the case of Rutherford scattering – the scattering of particles interacting via a Coulomb potential – indistiguishability introduces Mott's modification of the angular scattering probability. In the Otago experiment presented in Figure of the present paper, the effect of indistinguishability becomes even more extreme: for fermions colliding at low energies it becomes a matter of ‘to scatter or not to scatter’.
In our comparison of the scattering between indistinguishable identical fermions to that of ‘distinguishable’ fermions, we established the latter scenario by taking identical fermions and preparing them in different and thus distinguishable states. For the purpose of obtaining scattering cross sections and angular scattering these identical fermions in distinguishable states can be treated as distinguishable particles. In one aspect their scattering is, however, crucially different to that of two non-identical particles: when two identical fermions in different internal states collide, these internal states become quantum mechanically entangled.
To see how quantum entanglement emerges from the collision, we can consider the scenario of Figure with the blue particle representing an electron (a spin- fermion) in the spin state
and the red particle representing
, where the spin projection
is with respect to a quantisation axis
. When examining the case of sideways scattering we see that the top detector has an equal chance of detecting ↑ and ↓. This generally holds true, also for nonidentical spin-
fermions.
For identical fermions, antisymmetrisation requires the spin part to be antisymmetric as scattering sideways into implies that the spatial part of the wave function is symmetric. Hence sideways scattered particles form a spin singlet state
. A particle going towards the top detector is in an equal quantum mechanical superposition of ↑ and ↓, compatible with an equal probability of either state being detected. If ↑ is detected, we know for certain that ↓ will be detected by the bottom detector and vice versa. We would encounter the same correlation had we used two nonidentical particles, say a proton in ↑ and an electron in ↓: again, if ↑ is detected by the top detector, ↓ will be detected by the bottom detector and vice versa. However, if we measure the spin projection at the top detector with respect to an axis
(perpendicular to
), the outcome from the bottom detector depends crucially on whether the particles are identical or not.
For nonidentical particles the spin projection measured at the top detector will be genuinely random and ↑ will be found with 50% probability as before, but, unlike the measurement with respect to , given the top detector measurement we are not any wiser in regards to what will be detected subsequently at the bottom detector. The two outcomes are completely uncorrelated. In stark contrast, because the identical particle case is represented by a singlet state, which is rotationally invariant (Basdevant and Dalibard Citation2010), it does not matter if we perform the two measurements with respect to
or
(or any other axis): at either the top or bottom detector ↑ or ↓ will be randomly found with equal probability, but once a spin projection has been determined by one of the detectors, the outcome at the other detector is fixed. The two measurements are always perfectly correlated.
What we have just described is that spin-independent scattering of identical particles can create a very particular form of correlation between the spins of collisional partners if they are initially in different and distinguishable spin states (Gottfried and Yan Citation2003; Lamata and León Citation2006; Kouzakov Citation2019). This form of correlation, which has no classical counterpart, is called quantum entanglement and it lies at the heart of quantum mechanics. It is tempting to think that at the point of the collision the particles already ‘decided’ the correlated outcomes that would eventually be measured by the detectors. This temptation does not get any less when considering the prospect of letting the particles separate for a long distance before conducting a measurement. If one particle is measured on the Moon to give ↑ along some axis, how would a second particle on Earth instantaneously ‘know’ it should answer ↓? Albert Einstein certainly did not like the prospects of this and stated in a paper co-authored with Poldolsky and Rosen that ‘No reasonable definition of reality could be expected to permit this’ (Einstein et al. Citation1935), arguing that quantum mechanics cannot completely describe the physical world. He believed ‘that physics should represent a reality in time and space free from spooky actions at a distance’ as he wrote in a letter to Max Born in 1947 (Born-Einstein Letters Citation1971).
In 1964 the paradox of Einstein, Podolsky and Rosen led John Bell to ‘Consider a pair of spin one-half particles formed somehow in the singlet spin state and moving freely in opposite directions’ (Bell Citation1964). In a groundbreaking paper he showed that it is possible to disprove experimentally that the correlated outcome in the spin projection measurements on such two entangled particles are in some way predetermined ‘behind the scenes’. Such measurements have been conducted in a number of settings (Blaylock Citation2010), all permitting the ‘unreasonable’ and vindicating quantum mechanics as a theory.
I should stress that spin entanglement is not reserved to identical particles. Nonidentical particles, such as a proton and an electron, can also be prepared in a spin entangled state, but not via a spin-independent collisional interaction. What makes the outcome of the experiment in Figure A remarkable is that without spin-dependent interactions, spin entangled pairs are produced oppositely on the spherical scattering halo.
So far, the Otago experiments have, regrettably, been unable to verify any level of spin-entanglement from colliding identical atoms in distinguishable states because of an insufficient signal-to-noise ratio. While this should, in principle, be possible with Rb or K atoms using absorption imaging (Greiner et al. Citation2005), the extraordinary properties of helium in its metastable state makes this a much more suitable candidate for capturing correlations in an expanding scattering halo. For a so-called microchannel plate can be employed for the detection of atoms (Fitzgerald Citation2001) – a technique offering an exceptional signal-to-noise ratio. By taking this approach, a recent Australian collision experiment using ultracold
, much in the spirit of the experiments presented in Section 5, succeeded in detecting Bell correlations between spatially separated pairs of atoms (Shin et al. Citation2019).
7. Conclusion
The scattering experiment has been hailed as the ‘most important experimental technique in quantum physics’ (Taylor Citation2006). To be sure, ‘The importance of observations made in physics by analysing the scattering of particles and waves can hardly be exaggerated’ (Newton Citation1982). As discussed in the prologue of this article it was, indeed, the analysis of an angular distribution of scattered particles that led to the discovery of the atomic nucleus.
In 1913 Niels Bohr published a paper entitled ‘On the constitution of atoms and molecules’ in The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, ‘Communicated by Prof. E. Rutherford, FRS’ (Bohr Citation1913). Here Bohr would lay the ground work for what would become a quantum mechanical description of the atom. Similarly to Geiger and Marsden (Citation1909), from which my prologue departed, Bohr's paper carried an acknowledgement:
I wish here to express my thanks to Prof. Rutherford for his kind and encouraging interest in this work.
While a proper formulation of quantum mechanics – as, e.g. captured by (Equation1(1)
(1) ) – would only follow about a decade later, the work of Rutherford and Bohr was truly pioneering (Weinberger Citation2014; Kragh Citation2021) and heralded a new era in physics. Schrödingers wave equation (Equation1
(1)
(1) ) opens up the possibility for probability amplitudes to interfere and it was armed with this machinery that Mott attacked the problem of identical particle quantum scattering.
Quantum interference radically modifies the Rutherford formula and notably forbids scattering into for indistinguishable fermions. In this paper, I have showcased some examples of scattering experiments conducted at Otago using an optical collider and involving identical particles. These include collisions of bosonic rubidium and fermionic potassium, which both display a compelling discrepancy between colliding clouds prepared in distinguishable and indistinguishable internal states.
The schematics showing the ultracold collider operation in Figure somewhat belies the complexity of the apparatus involved in preparing two atomic clouds at a temperature of 200 nanokelvin at the crossings of laser beams with breadth of a hair (Chilcott and Kjærgaard Citation2021). According to Massey and Feather (Citation1976), ‘Rutherford had a horror of complex apparatus. Having obtained his major results with comparatively simple equipment ….’ I think there is a fair chance Rutherford would have been horrified by the Otago experimental setup, but I am optimistic he might have appreciated the simplicity of the experimental scenario established once all the fancy equipment is turned off and what remains are two clouds of atoms, prepared in particular quantum states and travelling on a collision course with a well-defined relative energy. Here we encounter the quintessential scattering experiment and because of the pristine setting we can illuminate paradigms of quantum collision theory such as resonances, partial wave interference, and identical particle effects.
8. Epilogue
Of course, at some point after the atoms collide in the Otago experiments, the fancy equipment is turned on again: the scattered atoms are detected using laser light via the shadow they cast on a CCD chip and the resulting absorption image is processed by a computer. In closing, I would like to note how all of these technologies build on an understanding of quantum mechanics as do many devices we encounter in everyday life. This may hold increasingly true for tomorrow's technologies emerging from a current strong push to harness and exploit quantum entanglement as a resource for quantum computation, quantum communications, and quantum metrology (Dowling and Milburn Citation2003; Jaeger Citation2018).
The focus of this paper on the occasion of Rutherford's sesquicentennial has been to highlight the pedigree of present-day scattering experiments at Otago and elsewhere, and pay a particular tribute to their Manchester legacy: the original gold foil experiment. However, the impact of Rutherford's work go far beyond that, and the part he played in setting off the quantum revolution a century ago is incalculable. Figure shows Rutherford seated centrally among some of the brilliant physicists who partook in developing quantum mechanics. These not least include Bohr, Chadwick, Dirac, Fermi, Mott, Pauli, and Schrödinger, all of whom appeared in my telling of this story of quantum mechanical identity.
Figure 10. Rutherford at the 7th Solvay Conference on Physics held in Bruxelles, Belgium, October 22-29, 1933. The theme was the structure and properties of atomic nuclei. Previously (beginning 1911) he had been a participant at the 1st, 2nd, 3rd, and 4th Solvay Conferences on Physics (Mehra Citation1975; Hughes Citation1999). Seated left to right: E. Schrödinger, I. Joliot, N. Bohr, A. Joffe, M. Curie, P. Langevin, O.W. Richardson, Lord Rutherford, Th. DeDonder, M. deBroglie, L. deBroglie, L. Meitner, J. Chadwick. Standing left to right: E. Henriot, F. Perrin, F. Joliot, W. Heisenberg, H.A. Kramers, E. Stahel, E. Fermi, E. T. S. Walton, P. A. M. Dirac, P. Debye, N. F. Mott, B. Cabrera, G. Gamow, W. Bothe, P. Blackett (at back), M.S. Rosenblum, J. Errera, Ed. Bauer, W. Pauli, J. E. Verschaffelt, M. Cosyns (at back), E. Herzen, J. D. Cockcroft, C. D. Ellis, R. Peierls, Aug. Piccard, E. O. Lawrence, L. Rosenfeld. Photograph by Benjamin Couprie, Institut International de Physique Solvay, courtesy AIP Emilio Segrè Visual Archives, Leon Brillouin Collection. Copyright by Solvay Institutes. Reprinted with permission.

Acknowledgments
I gratefully acknowledge the contributions of past and present members of my research group towards building the laser-based collider at Otago. In particular, I thank Kris Roberts who acquired the absorption images in Figures and with support from Dr. Ryan Thomas and Dr. Amita Deb. I would also like to thank Emeritus Prof. Finn Folkmann (Aarhus University) for introducing me to the Rutherford foil experiment as an undergraduate student, where I had the privilege of verifying the -law using a small proton accelerator. This very much served as a source of inspiration for writing this paper. I finally thank Dr. Danny Schumayer, Anne Cathrine Petersen, and Susanne Otto for critically reading the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Anderson MH, Ensher JR, Matthews MR, Wieman CE, Cornell EA. 1995. Observation of Bose-Einstein condensation in a dilute atomic vapor. Science. 269(5221):198–201.
- Ashkin A. 1997. Optical trapping and manipulation of neutral particles using lasers. Proc Natl Acad Sci. 94(10):4853–4860.
- Ball P. 2015. The trouble with scientists. Nautilus. 24:Chapter 2. https://nautil.us/issue/24/error/the-trouble-with-scientists.
- Barrette J. 2021. Nucleus-nucleus scattering and the Rutherford experiment. J R Soc N Z. 51(3):1–10. https://doi.org/http://doi.org/10.1080/03036758.2021.1962368.
- Basdevant JL, Dalibard J. 2010. The quantum mechanics solver. 2nd ed. Berlin: Springer.
- Bell JS. 1964. On the Einstein Podolsky Rosen paradox. Physics. 1(3):195–200.
- Blaylock G. 2010. The EPR paradox, Bell's inequality, and the question of locality. Am J Phys. 78(1):111–120.
- Bohr N. 1913. I. On the constitution of atoms and molecules. Phil Mag. 26(151):1–25.
- Born-Einstein Letters. 1971. The Born-Einstein Letters: Correspondence between Albert Einstein and Max and Hedwig Born from 1916 to 1955 with commentaries by Max Born. Macmillan.
- Brandt S. 2009. The Harvest of a Century: Discoveries of Modern Physics in 100 Episodes. Oxford University Press. chap. 16. The Atomic Nucleus.
- Buggle C, Léonard J, von Klitzing W, Walraven JTM. 2004. Interferometric determination of the s and d-wave scattering amplitudes in 87Rb. Phys Rev Lett. 93(17):173202.
- Chadwick J. 1930. The scattering of α-particles in helium. Proc R Soc Lond A. 128(807):114–122.
- Chadwick J, Bieler ES. 1921. C. The collisions of α particles with hydrogen nuclei. Phil Mag. 42(252):923–940.
- Chilcott M, Kjærgaard N. 2021. Low-cost wireless condition monitoring system for an ultracold atom machine. Internet Things. 13:100345.
- Chilcott M, Thomas R, Kjærgaard N. 2021. Phys. Rev. Res. 3:033209.
- DeMarco B, Bohn JL, Burke JP, Holland M, Jin DS. 1999. Measurement of p-wave threshold law using evaporatively cooled fermionic atoms. Phys Rev Lett. 82(21):4208–4211.
- Dickhoff WH, Van Neck D. 2008. Many-body theory exposed! 2nd ed. Singapore: World Scientific.
- Dowling JP, Milburn GJ. 2003. Quantum technology: the second quantum revolution. Phil Trans R Soc A. 361(1809):1655–1674.
- Einstein A, Podolsky B, Rosen N. 1935. Can quantum-mechanical description of physical reality be considered complete? Phys Rev. 47(10):777–780.
- Evans L. 2011. The large hadron collider. Annu Rev Nucl Part Sci. 61(1):435–466.
- Fallani L, Kastberg A. 2015. Cold atoms: a field enabled by light. EPL (Europhysics Letters). 110(5):53001.
- Feynman RP, Leighton RB, Sands M. 1965. The Feynman Lectures on Physics. Vol. 3. Reading: Addison-Wesley.
- Fitzgerald R. 2001. Helium joins family of gaseous Bose-Einstein condensates. Phys Today. 54(5):13–14.
- Friedrich H. 2013. Scattering theory. Berlin: Springer.
- Geiger H, Marsden E. 1909. On a diffuse reflection of the α-particles. Proc R Soc Lond A. 82(557):495–500.
- Geiger H, Marsden E. 1913. LXI. the laws of deflexion of a particles through large angles. Phil Mag. 25(148):604–623.
- Glauber RJ. 1995. Dirac's famous dictum on interference: one photon or two? Am J Phys. 63(1):12–12.
- Gottfried K, Yan TM. 2003. Quantum mechanics: fundamentals. New York: Springer.
- Greiner M, Regal CA, Stewart JT, Jin DS. 2005. Probing pair-correlated fermionic atoms through correlations in atom shot noise. Phys Rev Lett. 94(11):110401.
- Heydenburg NP, Temmer GM. 1956. Alpha-alpha scattering at low energies. Phys Rev. 104(1):123–134.
- Hilborn RC, Yuca CL. 2002. Identical particles in quantum mechanics revisited. Br J Phil Sci. 53:355–389.
- Hoogerland MD. 2021. The size of the helium nucleus: then and now. J R Soc New Zealand. 51(3):1–10. https://doi.org/https://doi.org/10.1080/03036758.2021.1938146.
- Horvath MSJ, Thomas R, Tiesinga E, Deb AB, Kjærgaard N. 2017. Above-threshold scattering about a Feshbach resonance for ultracold atoms in an optical collider. Nat Commun. 8(1):452.
- Hughes J. 1999. Rutherford, the Cavendish laboratory and the solvay councils. In: The Solvay Councils and the Birth of Modern Physics. Basel: Birkhäuser; p. 24–34.
- Jaeger L. 2018. The second quantum revolution: from entanglement to quantum computing and other super-technologies. Cham: Springer International Publishing.
- Jeng M. 2006. A selected history of expectation bias in physics. Am J Phys. 74(7):578–583.
- Kaplan IG. 2017. The Pauli exclusion principle: origin, verifications, and applications. Chichester: Wiley.
- Kjærgaard N. 2015. Peeking and poking at atoms with laser light. New Zealand Sci Rev. 72:41.
- Kjærgaard N, Mellish AS, Wilson AC. 2004. Differential scattering measurements from a collider for ultracold atoms. New J Phys. 6:146–146.
- Kouzakov KA. 2019. Quantum entanglement in the nonrelativistic collision between two identical fermions with spin 1/2. Theoret Math Phys. 201(2):1664–1679.
- Kragh H. 2021. Chemical and other aspects of Rutherford's nuclear atom. J R Soc N Z. 51(3):1–15. https://doi.org/https://doi.org/10.1080/03036758.2020.1858879.
- Lamata L, León J. 2006. Generation of bipartite spin entanglement via spin-independent scattering. Phys Rev A. 73(5):052322.
- Landau LD, Lifshitz EM. 1977. Quantum mechanics (non-relativistic theory). 3rd ed. Oxford: Pergamon Press.
- Leinaas JM, Myrheim J. 1977. On the theory of identical particles. Il Nuovo Cimento B. 37(1):1–23.
- Longair M. 2021. Rutherford and the Cavendish laboratory. J R Soc NZ. 51(3):1–23. https://doi.org/https://doi.org/10.1080/03036758.2021.1885452.
- Massey HSW, Feather N. 1976. James Chadwick, 20 October 1891 – 24 July 1974. Biogr Mem Fellows R Soc. 22:10–70.
- Mehra J. 1975. The Solvay conferences on physics. D. Dordrecht: Reidel Publishing Company.
- Mellish AS, Kjærgaard N, Julienne PS, Wilson AC. 2007. Quantum scattering of distinguishable bosons using an ultracold-atom collider. Phys Rev A. 75(2):020701.
- Messiah A. 1969. Quantum mechanics. Vol. 2. Amsterdam: North Holland Publishing Company.
- Mirman R. 1973. Experimental meaning of the concept of identical particles. Il Nuovo Cimento B. 18:110–122.
- Mott N. 1930. The collision between two electrons. Proc R Soc Lond A. 126(801):259–267.
- Mott NF. 1972. Rutherford and theory. Notes Rec R Soc Lond. 27(1):65–66.
- Newton RG. 1982. Scattering theory of waves and particles. New York: Springer-Verlag.
- Rakonjac A, Deb AB, Hoinka S, Hudson D, Sawyer BJ, Kjærgaard N. 2012. Laser based accelerator for ultracold atoms. Opt Lett. 37(6):1085.
- Roati G, Riboli F, Modugno G, Inguscio M. 2002. Fermi-Bose quantum degenerate 40K-87Rb mixture with attractive interaction. Phys Rev Lett. 89(15):150403.
- Rosa R. 2012. The Merli–Missiroli–Pozzi two-slit electron-interference experiment. Phys Perspect. 14(2):178–195.
- Rose DC. 1926. The scattering of alpha particles through small angles. Proc R Soc London A. 111(759):677–690.
- Rutherford E. 1911. LXXIX. The scattering of α and β particles by matter and the structure of the atom. Phil Mag. 21(125):669–688.
- Rutherford E. 1938. Background to modern science: ten lectures at Cambridge arranged by the history of science committee 1936. Cambridge. chap. III. Forty years of physics; p. 22.
- Rutherford E, Chadwick J. 1927. LII. The scattering of α-particles by helium. Phil Mag. 4(22):605–620.
- Sadeghpour HR, Bohn JL, Cavagnero MJ, Esry BD, Fabrikant II, Macek JH, Rau ARP. 2000. Collisions near threshold in atomic and molecular physics. J Phys B. 33(5):R93–R140.
- Sawyer BJ, Chilcott M, Thomas R, Deb AB, Kjærgaard N. 2019. Deterministic quantum state transfer of atoms in a random magnetic field. Eur Phys J D. 73(8):160.
- Schattschneider P. 1986. Classical scattering theory. In: Fundamentals of inelastic electron scattering. Vienna: Springer ; p. 1–14.
- Shin DK, Henson BM, Hodgman SS, Wasak T, Chwedeńczuk J, Truscott AG. 2019. Bell correlations between spatially separated pairs of atoms. Nat Commun. 10(1):4447.
- Taylor JR. 2006. Scattering theory: the quantum theory of nonrelativistic collisions. New York: Dover.
- Temmer GM. 1989. How Rutherford missed discovering quantum mechanical identity. Am J Phys. 57(3):235–237.
- Thomas NR, Kjærgaard N, Julienne PS, Wilson AC. 2004. Imaging of s and d partial-wave interference in quantum scattering of identical bosonic atoms. Phys Rev Lett. 93(17):173201.
- Thomas R. 2017. Cold Collisions of Ultracold Atoms [dissertation]. Unversity of Otago. http://hdl.handle.net/10523/7776.
- Thomas R, Chilcott M, Chisholm C, Deb AB, Horvath M, Sawyer BJ, Kjærgaard N. 2017. Quantum scattering in an optical collider for ultracold atoms. J Phys: Conf Ser. 875:012007.
- Thomas R, Chilcott M, Tiesinga E, Deb AB, Kjærgaard N. 2018. Observation of bound state self-interaction in a nano-eV atom collider. Nat Commun. 9(1):4895.
- Thomas R, Roberts KO, Tiesinga E, Wade ACJ, Blakie PB, Deb AB, Kjærgaard N. 2016. Multiple scattering dynamics of fermions at an isolated p-wave resonance. Nat Commun. 7(1):12069.
- Trenn TJ. 1974. The Geiger-Marsden scattering results and Rutherford's atom, July 1912 to July 1913: the shifting significance of scientific evidence. ISIS. 65(1):74–82.
- Walraven JTM. 2017. Quantum gases – lectures. Amsterdam: University of Amsterdam.
- Weinberger P. 2014. Niels Bohr and the dawn of quantum theory. Philos Mag. 94(27):3072–3087.
- Wigner EP. 1948. On the behavior of cross sections near thresholds. Phys Rev. 73:1002–1009.
Appendix
Experiments on α-α scattering
In this appendix, results from three experiments (A.1, A.2, and A.3) on α-α scattering are summarised. Specifically, Figure shows the ratio between the observed scattering at
and that predicted by the the Rutherford formula as a function of energy. I have chosen to include this, as I believe A.1 and A.2 provide instructive examples of what arguable are successive cases of expectation or confirmation bias – a problem that is as old as science and continues to remain relevant today (Jeng Citation2006; Ball Citation2015).
Figure A1. The result from three experiments on α-α scattering. ∙ Rutherford and Chadwick (Citation1927) reported that the ratio of observed scattering to that predicted by the Rutherford formula for would be unity for energies
MeV. ◦ Chadwick (Citation1930) found that the ratio would settle on a factor of 2 for energies
MeV. ■ Heydenburg and Temmer (Citation1956) found that Mott's prediction
only occurs for energies
MeV.
A.1. Rutherford and Chadwick (Citation1927)
Rutherford and Chadwick found that at high energies exceeds unity signifying a departure from the Rutherford formula (or ‘inverse square law’ as they called it) echoing results for the even lighter hydrogen atom.
It seems reasonable then to ascribe the abnormal scattering of α-particles by hydrogen and helium mainly to a departure from inverse square forces, and to seek an explanation …in the structure of the nuclei concerned in the collision.
The nucleus does indeed have structure and a non-zero size (Hoogerland Citation2021), and this will indeed introduce a deviation from the predication for a point charge for high collision energies. However, for the identical particle case of α-α scattering, this deviation should be measured against a base line of
(cf. Section 3) and not unity.
A.2. Chadwick (Citation1930)
Following Mott's prediction that , Chadwick makes a claim to observe this by going to lower energies
It is clear that the experimental results approach the value of twice the classical scattering as the energy of the incident α-particle decreases, that is, the results approach more and more closely to the quantum theory scattering. …The experiments thus provide a striking verification of Mott's calculation and of the assumption on which it is based, that it is impossible to distinguish one helium nucleus from another of the same velocity
A.3. Heydenburg and Temmer (Citation1956)
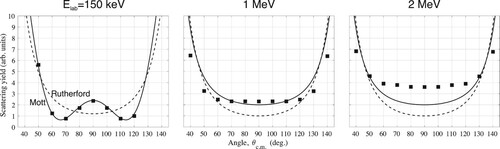
In their precision experiments (Heydenburg and Temmer Citation1956) measured angular scattering distribution for a range of energy. These showed that above a collision energy of MeV the data was not well-described by Mott's formula (see Figure ). In particular, at
MeV, where Chadwick claimed to observe
they found
. Hence it would seem that Chadwick under-counted the number of scattered α particles from He, which he compared to experiments with Ar. While his statement quoted in A.2 is very assertive, his paper does concede that
…when an α-particle is scattered through 45 by collision with a helium nucleus it loses half its energy. Consequently the scintillations observed in the helium experiments are due to particles of lower energy than those in the argon experiments. This fact is only of importance in the last two experiments in which α-particles of small initial range were used. In these experiments it may be possible that the efficiency of counting was less in the helium observations than in those with argon …
Figure A2. Angular scattering yield for collision energies (lab frame) 150 keV, 1 MeV, and 2 MeV. ■ are measurements from Heydenburg and Temmer (Citation1956) while lines show the predictions from Rutherford's fomula (dashed lines) and Mott's formula (full line.).