Abstract
Iron overload is a very frequent finding in several animal species and a genetic predisposition is suggested. In one of the most commonly reported species with susceptibility for iron overload (mynah bird), it was recently shown that the cause of this pathophysiology is high uptake and retention of dietary iron. Here we compare susceptible (mynahs) with non-susceptible avian species (chickens) by evaluating iron uptake at the intestinal absorptive cell level. Enterocytes from mynahs and chickens were isolated and uptake of Fe(II) and Fe(III) was studied in vitro. It was found that Fe(III) uptake is much lower than Fe(II) uptake for both species. Although liver iron, present only in hepatocytes, was at least 10-fold higher in mynahs than chickens, enterocyte Fe(II) uptake was considerably higher in mynahs. Fe(II) uptake showed saturation at the studied concentrations in both species. Kinetic studies revealed a three-fold increase in V max for mynahs. Calculated values for the uptake kinetics of the probable membrane transporter suggest that mynah bird enterocytes have a significantly higher limiting uptake rate, due to the possible increase in the number of transporters when compared with chicken enterocytes. The susceptibility of this species is due to intestinal iron uptake despite hepatic iron accumulation, implicating a ‘mis-sensing’ of body iron similarly to human hereditary haemochromatosis.
1 Introduction
Due to the lack of an active excretory mechanism, iron overload develops when iron intake exceeds needs. The proximal small intestine has been the subject of attention over the past few decades since this is the site of iron absorption and thus the site of the major regulatory pathway of homeostasis of iron metabolism. Under normal physiology, regulation is by means of an inverse relation between body iron stores and dietary absorption, such that absorption increases when body iron stores are depleted (Marx, Citation1979). It is essential to understand the factors playing a role in the whole iron cycle in order to maintain such a balance, since both deficiency and overload of iron can be detrimental. Several mechanisms have been postulated to elucidate the feedback sensing of body iron status by the duodenal absorptive cells. Currently, the most accepted view is that sensing occurs at the crypt cell level where newly formed epithelial cells are receiving information on body iron stores via various iron-regulating proteins. As these cells migrate and mature into absorptive enterocytes, they are considered to have been ‘programmed’ to take up adjusted amounts of iron at the absorptive part of the villi (Crichton, Citation2001).
Increased awareness of iron overload disease has resulted from the high prevalence of hereditary haemochromatosis (HH). In humans, HH is one of the most common inherited disorders in people of northern European descent. Excess iron accumulates in parenchymal organs, mainly in the liver, causing tissue damage and resulting in fibrosis, cirrhosis and hepatocellular carcinoma. The cause of excess iron is the relatively high absorption of iron from the diet with respect to needs (Cox & Peters, Citation1978). Mutations in genes encoding for iron proteins responsible in the ‘sensing’ mechanism have been identified to be the causes of the condition (Pietrangelo, Citation2002).
A high incidence of iron overload is also seen in animals. Progressive iron deposition in the liver occurs in many species (Lowenstine & Munson, Citation1999). However, observations in captive animals have shown that some species are especially susceptible to iron overload: some bird families such as toucans, birds of paradise (Cork, Citation2000), New World monkeys (Spelman et al., Citation1989; Miller et al., Citation1997) and the black rhinoceros (Smith et al., Citation1995). In general, fruit-eating and insect-eating birds have a predisposition to develop the disease (Dierenfeld et al., Citation1994; Cork et al., Citation1995), in which excess liver iron is found primarily in hepatocytes and the amount can be as high as in HH (Gosselin & Kramer, Citation1983; Ward et al., Citation1988). It is believed that high levels of iron in the diet in captivity is the major determining factor in the development of iron overload (Taylor, Citation1984; Crissey et al., Citation2000). This view does not explain why iron accumulation is less severe in other species under the same conditions, and why susceptible species are unable to adequately down-regulate the uptake of iron to prevent iron storage disease.
Excess iron overload is the most common metabolic disorder of mynahs, a bird species from the family Sturnidae (Panigraphy & Senne, Citation1991). It is one of the most frequently reported bird species to have haemochromatosis. Severity and pathology of iron loading resembles that described in human HH (Panigraphy & Senne, Citation1991; Dorrestein et al., Citation1992; Iancu, Citation1993). A previous in vivo study revealed that mynah birds can down-regulate or up-regulate uptake of iron, but take-up and retain four-fold to seven-fold more than a non-susceptible species (Mete et al., Citation2001). This indicates that the high prevalence of iron storage in this species is due to high absorption of dietary iron relative to body iron needs. However, the cellular and molecular mechanisms operating behind this observation are still unclear. The present study addresses this gap by investigating iron uptake in mynahs at the proximal small intestinal absorptive cell level. Chickens (which normally do not develop overload) serve as controls. Enterocytes were isolated from mynah birds and chickens, and uptake of Fe(II) ascorbate and Fe(III) citrate was studied in vitro.
2 Materials and Methods
All procedures were approved by the animal experiments committee of the Faculty of Veterinary Medicine, Utrecht University, The Netherlands.
2.1 Animals
Four adult male Hill Mynahs (Gracula religiosa) and five domestic female chickens (Gallus domesticus) from the same hatching were obtained from a retailer and placed in an animal facility. Housing conditions were 2×2×4 m wire mesh cages with each species held together with controlled lighting and humidity. The birds were fed a standard pellet diet consisting of 54 mg Fe/kg as Fe(II) sulphate (see Mete et al. (Citation2001) for dietary ingredients) for at least 2 weeks to establish constant iron conditions. Routine parasitic treatments (fenbendazole) for prevention of possible complications in these conventional animals were performed as soon as they arrived at the animal facility. Feed and water were provided ad libitum. Every animal was brought to the experimental facility the night before the experimental procedures. One bird was used per experiment.
The animals were weighed and heparinized blood was collected to determine haematocrit, white blood cell count, total protein, plasma iron and total iron binding capacity. After blood sampling from the wing vein or jugular vein, the birds were euthanized either by intravenous Euthesate (Ceva Sante Animale, Libourne, France) injection or intramuscular T61 (Hoechst Roussel Vet GmbH, Mycofarm, De Bilt, The Netherlands) injection in chickens and mynahs, respectively. Complete necropsy was performed on each animal, including routine histopathology on paraffin-embedded sections of the liver, the kidney, the lung, the heart, the gonads, the spleen, the parathyroid, the thyroid, the ventriculus, the proventriculus, the bursa of Fabricius, the pancreas and the intestines. Sections were cut at 3 μm and stained with haematoxylin and eosin and Perl's iron stain. Sections of liver were stored (–20°C) for iron analysis by atomic absorption spectrometry (Cobas Bio auto-analyzer; Roche, Basel, Switzerland).
2.2 Enterocyte isolation and solutions
Proximal intestinal villus epithelial cells were isolated as previously described with few modifications (Kimmich, Citation1990). The incubation medium (pH 7.4) used in preparation of cell suspensions consisted of 10 mM Hepes, 137 mM NaCl, 5 mM KCl, 10 mM mannose, 2.5 mM glutamine and 0.008% trypsin inhibitor Type I-S (Merck Co., Amsterdam, The Netherlands and Sigma-Aldrich Chemie, Zwijndrecht, The Netherlands). The isolation medium was the same as the incubation medium, with the addition of sodium citrate (27 mM) for detachment of cells and β-hydroxybutyrate (0.5 mM) as an extra source of energy. Immediately after death of the animal, the duodenal loop, routinely from the pylorus to the pancreatic duct opening, was freed from the pancreas and the remaining tissues and was excised. The lumen was rinsed with ice-cold 0.9% NaCl containing 1 mM dithiothreitol to remove all contents and cut open lengthwise. These intestinal segments, approximately 17 cm for chickens and 10 cm for mynahs, were cut further into 2 to 3 cm pieces, placed in pre-warmed isolation medium and incubated for 35 min in a 40°C shaking water bath at 50 shakes per min. The segments were moderately agitated two to three times during the incubation using a plastic pipette tip to improve release of villus cells. At the end of the incubation, cells were filtered through nylon stockings into tubes and washed twice by centrifugation (100 g, 5 min). Final pellets were suspended in incubation medium at room temperature. Homogeneous samples were distributed into flat-bottomed plastic vials, ready to be used for iron uptake studies.
The initial and final cell membrane integrity and functionality was evaluated by the percentage of lactate dehydrogenase (LDH) released in the extracellular medium at the onset and end of experimental procedures. To assess the origin and characterize the cells, histological examination of the remaining intestinal segment and electron microscopy of the isolated cells were performed after the isolation procedures.
2.3 Iron solutions
Iron-55 as FeCl3 in 0.1 M HCl (Perkin–Elmer Life Sciences, Boston, Massachusetts, USA) was used as the radio-iron tracer as a 300 kBq/ml cell suspension in the highest concentration. For Fe(II) uptake studies, Fe(II) ammonium sulphate was combined with a 10 times molar excess of ascorbic acid and then combined with 55Fe freshly before the start of each experiment. The mixture was always used within 15 min to standardize the conditions and stabilize the initial rapid reactive state of Fe(II) (Goddard et al., Citation1997). For Fe(III) uptake studies, Fe(III) citrate (1:20 molar ratio) was prepared by combining Fe(III) chloride hydrate with citric acid and labelling with 55Fe. Further dilutions were made to reach the desired concentrations (1 to 200 μM for Fe(II) and 50 μM for Fe(III)) with incubation medium. Thus, the final pH was always between 7.1 and 7.3, and the specific activity was kept constant throughout all series of experiments. Washing solution for removal of non-specifically bound radioactive iron was prepared as 100-fold molar excess of non-labelled Fe(III) citrate solution compared with the studied concentrations.
2.4 Uptake studies
Cell suspensions were placed in a 40°C incubator on a shaking block and experiments started after 5 min to enable warming up of suspensions. Fe(II) ascorbate whole cell uptake was studied at 1, 10, 25, 50, 100 and 200 μM concentrations, and Fe(III) citrate at 50 μM concentration. For investigating temperature effects, experiments were also conducted on ice, cooling the cell suspension and solutions for 5 min prior to start. Studies at 4°C were performed with one concentration, 50 μM. The radioactively labelled iron solution was presented to cells (time, t=0) and uptake followed up to 30 min. At each time point (1, 3, 5, 7, 10 and 30 min), the cell suspension was gently swirled, a 0.5 ml sample was taken and the reaction stopped in 4 ml ice-cold 0.9% NaCl. After centrifugation for 3 min at 328 g, cell pellets were suspended in washing solution for 4 min for exchange of surface-bound radio-iron with cold iron and re-centrifuged. Final pellets were dissolved in NaOH (0.1 M) and placed in a liquid scintillation counter preset for counting in the 55Fe window. Aliquots were saved for protein determinations (Pierce-Biorad Protein Assay). Whole cell uptake measurements are expressed as picomoles of iron per microgram of protein.
2.5 Kinetic analysis
The kinetic constants (K m and V max) of the putative enterocyte membrane transporter were calculated using non-linear regression fitting of data to a simple Michaelis–Menten equation. All data points were used and the fit converged to all data sets.
2.6 Statistical analysis
Comparisons between the liver and plasma iron values for each species were performed using the unpaired two-tailed t test. Analyses of iron uptake measurements were carried out by repeated-measures analysis of variance, taking the species as the between factor and the time, iron form or temperature as within factors (SPSS for Windows, version 10.0). Differences were considered significant when P<0.05.
3 Results
3.1 Haematology and histopathology
Necropsy revealed no significant lesions. Haematology data are presented in . Haematocrit, white blood cell count and total protein values were in the normal range in all animals according to the species. All iron parameters in the blood were higher in the chicken but comparisons of total iron binding capacity and saturation (%) were hindered due to the small amount of material obtained from two mynahs. Plasma iron values were significantly higher in chickens compared with in mynahs (P<0.02).
Haematology and liver iron values: haematocrit, total white blood cell, total protein, plasma iron, total iron binding capacity, iron saturation and total liver iron value for chickens and mynahs
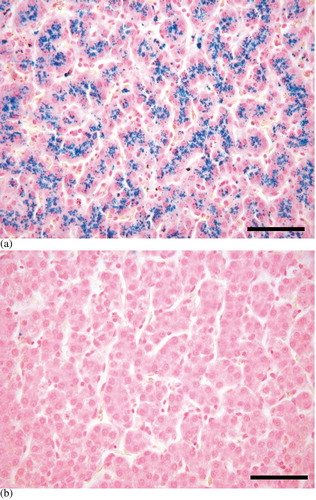
Light microscopy evaluation with Perl's iron stain showed heavy pigmentation in the liver of the mynah birds, with predominant loading in the cytoplasm of hepatocytes throughout the whole organ, while there was no detectable iron in chickens ( a,b). In addition, staining of trace amounts of iron were observed in the tubular epithelium of the kidney in three of the mynahs, and in one mynah discrete staining of iron was evident in testis tubular epithelium. All other examined tissues of mynahs and all tissues of chickens stained negative for iron. Liver iron contents, determined by atomic absorption spectrometry, were at least 10-fold greater for mynahs, where the highest value for chicken was 701 μg Fe/g dry weight and the lowest for mynah was 6683 μg Fe/g dry weight (P<0.01) ().
3.2 Enterocyte suspensions
LDH release at the beginning and end of uptake experiments was measured to determine the percentage intact cell population. No differences in initial and end-stage values were observed in our conditions, showing that the major disruption of the cells occurred during isolation and that they were in a stable state throughout the experimental period. Values of LDH release were 15 to 35% in mynahs and 7 to 15% in chickens. Thus, mynah enterocytes seem to be more sensitive to experimental influences compared with chickens. Histological examination of the intestine after the isolation procedures to assess the origin of the cells showed remaining intact crypt regions and mostly villus structures consisting of lamina propria and interstitial tissue, stripped off from the epithelium. Electron microscopy characterization of isolated cells showed small groups of four or five cells or single cells with microvilli bordering the apical membrane, indicating that these were villus epithelial cells.
3.3 Uptake studies
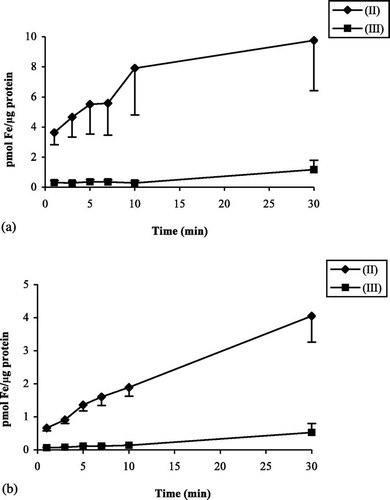
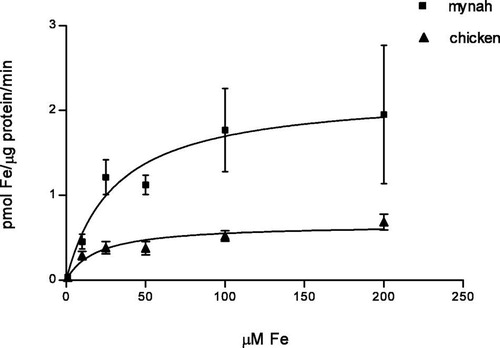
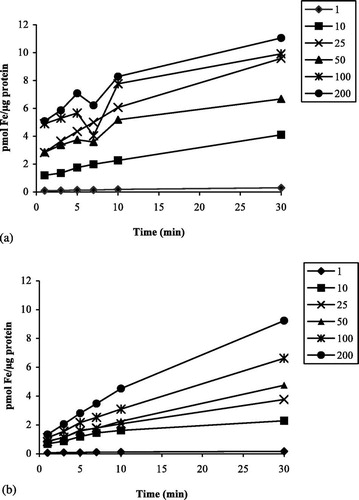
Uptake of Fe(II) ascorbate increased with increasing substrate concentrations and did not equilibrate for both bird species during 30 min. a,b shows the time course of Fe(II) uptake, measured at different concentrations, in mynah and chicken enterocytes. Mynahs showed higher iron uptake than chickens for all concentrations studied, significant differences being observed at 1, 10 and 25 μM Fe(II) ascorbate (P<0.01). The initial uptake of iron by mynah enterocytes, already at the 1 min time point, was always higher than in chickens. Thus, the initial rapid uptake of iron may provide the higher overall incorporation. Comparison of uptake at 40 and 4°C showed 50% decreased uptake at the lower temperature for both species but this difference was only significant in chickens (P<0.02) (data not shown). For both species, Fe(III) citrate incorporation was significantly lower when compared with the uptake of Fe(II) ascorbate at the same concentration (P<0.01) ( a,b). Uptake of Fe(II) ascorbate as a function of concentration gave significantly higher results in mynahs when compared with chickens (P<0.05). Observation of saturation curves suggested the presence of an active transport system, and thus kinetic constants following saturation properties (K m and V max) were determined (). Values of the apparent affinity constant K m were not significantly different whereas the limiting uptake rate (V max) was three-fold higher in mynahs (K m=30.5±14 and 20.3±8, V max=2.21±0.34 and 0.66±0.08, for mynahs and chickens, respectively).
Fig. 2 Fe(II) ascorbate uptake. Uptake of Fe(II) ascorbate by enterocytes, six concentrations, 30 min. 2a: Mynahs (n=4). 2b: Chickens (n=5).
4 Discussion
High absorption of dietary iron relative to body iron stores is observed in iron overload susceptible bird species and in HH. In the present work, we have evaluated this issue at the duodenal absorptive cell level in a susceptible species, the mynah bird. Working with iron overload susceptible bird species is a problem since they are not easily bred and obtained. Also, there is no sexual dimorphism in these birds, which results in a limited possibility of choosing the sex to work with. Moreover, using animals with different dietary histories can cause discrepancies, as can be seen in the present work, where two pairs of mynahs with different backgrounds showed variation in body weight and liver iron content. However, such animals are closer representatives of natural conditions, and the standardization of iron status in body and cells by the diet proved to be sufficient for our purposes. Our technique appeared to be efficient and reliable for investigating cellular iron uptake directly and enabling comparisons while avoiding the involvement of underlying tissue or mucus.
Laboratory tests revealed a normal health status for all animals. Blood iron indices of plasma iron, total iron binding capacity and transferrin iron saturation values were higher in the chickens than in the mynahs. The plasma iron levels were lower in mynahs but were similar to previous reports on mynahs (Mete et al., Citation2001), starlings (Garcia et al., Citation1984) and chickens (Marti et al., Citation1980; Sanchez & Planas, Citation1981), whereas feeding with higher quantities of iron had no influence. Biochemical analysis of the liver iron content showed much higher values for the mynahs, the difference being at least 10-fold. A previous study in another susceptible species (starlings) states that progressive liver iron loading eventually occurs, especially when high amounts of iron are given (Crissey et al., Citation2000). Plasma iron values do not correspond with the liver iron values in the species studied here. An explanation for this discrepancy is that different bird species have different body iron stores and plasma iron values due to the various factors that influence the metabolic state, such as migration, egg laying or moulting in birds (Garcia et al., Citation1984; Cork, Citation2000). In mynahs, it can be postulated that a different mechanism may be responsible for the difference in low plasma and high liver iron levels, where iron coming in is rapidly cleared from the plasma and deposited in the liver.
Histopathology revealed intense iron staining in the livers of mynahs, with trace findings in the tubular epithelium of kidneys. Iron was diffusely present throughout the liver and in hepatocytes only. This is expected to be due to the absorbed iron, since iron of dietary origin is found in hepatocytes (Borch-Iohnsen et al., Citation1991; Whittaker et al., Citation1997; Basclain et al., Citation1998; Dorrestein et al., Citation2000). Our findings are in accord with an earlier report on mynah birds (Gosselin & Kramer, Citation1983). Hepatic lesions were absent in our study although the iron content was very high and parenchymal cells were loaded; perhaps time for these mynah birds to develop fibrosis, cirrhosis or neoplasms has been too short. It is also possible that mynahs have a hepatocyte threshold before toxic effects occur.
We report here the uptake of radio-labelled Fe(II) ascorbate and Fe(III) citrate by avian enterocytes. Since iron in the reduced form is constantly oxidized, ascorbic acid at the ratio used enables a more steady state of Fe(II) and the proper comparison of uptake measurements. Iron in the Fe(III) state, on the other hand, has a poor bioavailability and uptake is much lower compared with Fe(II). Studies using enterocyte suspensions and tissue segments have determined that iron uptake is maximal at pH 7.4 and at physiological temperature for the organism (Goddard et al., Citation1997). We have studied whole cell uptake of Fe(II) ascorbate at physiological (40°C) and non-physiological (4°C) temperatures and of Fe(III) citrate at physiological temperature. At 40°C, uptake of Fe(II) ascorbate was linear in time and increased with concentration for both species. At 4°C, uptake of Fe(II) ascorbate decreased by 50% for both species. However, results are not conclusive as being those of an active/passive uptake mechanism since this difference was significant only for chickens, implying that more tests need to be carried out. Previous studies on iron uptake by isolated human enterocytes and duodenal biopsies have found a 20 to 30% decrease at low temperatures, concluding that temperature dependency of Fe(II) uptake is low (Duane et al., Citation1992; Goddard et al., Citation1997). Fe(III) citrate uptake increased slightly in time and was much lower than Fe(II) iron uptake in both species of birds. In man, Fe(III) is reduced to Fe(II) prior to be taken up by the absorptive cell and an abnormality in this process can lead to higher uptake of iron, just as is suggested with HH (Raja et al., Citation1996). Recently, a ferri-reductase (Dcytb) has been identified at the brush border membrane of enterocytes for the purpose of reducing dietary Fe(III), and its role in all body cells is under further investigation (McKie et al., Citation2001, 2002Citation). This reductase activity can also be valid in avian species.
Whole cell uptake of Fe(II) ascorbate levelled off to a plateau in the concentration range studied, expressing saturation and suggesting a carrier-mediated transport process. The present data revealed similar apparent transporter affinity (K m) for both species but a three-fold higher limiting uptake rate (V max) in mynah bird enterocytes, implying that the number of the transporter is increased. Earlier data in intestinal segments of chickens were obtained from Fe(III) uptake, much higher concentrations were used, and saturation was not observed (Marti et al., Citation1980; Sanchez & Planas, Citation1981). Since conditions may have been less than physiological, the results are not comparable. Human studies on uptake kinetics have shown higher affinity of the transporter for iron rather than higher capacity in HH patients with both iron nitrilo-triacetic acid and Fe(II) ascorbate (Cox & Peters, Citation1978, 1980Citation; Duane et al., Citation1992). However, these experiments were performed on tissue segments and very high concentrations of iron or the Fe(III) form was used. Similar results to ours were obtained in a recent study on a mouse model for HH. Uptake was higher in these mice compared with wild-type mice and kinetics revealed twice the capacity, confirming the increase in the number of functional carriers (Griffiths et al., Citation2001).
Various proteins are involved in the regulation of iron uptake, transport and storage (Griffiths & Cox, Citation2000). The uptake protein DMT1 (Gunshin et al., Citation1997) is localized at the brush border membrane of enterocytes from the upper villus region where iron absorption takes place (Cannone-Hergaux et al., Citation1999). Its expression is highly up-regulated in iron deficiency (Schumann et al., Citation1999) and in HH, both in man (Zoller et al., Citation2001) and in rodent models (Fleming et al., Citation1999; Griffiths et al., Citation2001; Moos et al., Citation2002). In the present work, finding the affinity constant (K m) in the same range for both species suggests that if DMT1 was playing a role in iron transport in avian species as well, it would be conserved like in mammals and the properties would not be changed. Finding a higher limiting uptake rate (V max) in the mynah may indicate that their enterocytes behave more as iron-deficient cells rather than replete cells and express more transporters, which results in taking up more iron just as in human HH (Marx & Santos, Citation2001). While knockout animal models of HH are being produced, some in the wild are found to have similar properties, such as the black rhinoceros (Beutler et al., Citation2001). The high variation in underlying genetic alterations affecting any of the proteins involved in iron transport, also in combinations, causes high variation in HH phenotype (Levy et al., Citation2000; Santos et al., Citation2000).
The liver iron overload and high intestinal iron uptake in mynah birds mimics HH in man. These features of iron metabolism can be a consequence of the low iron in the natural habitat of these birds, where the duodenal epithelia are ‘programmed’ to take up all the iron available. Then, when confronted with high levels of available iron, such as in captivity, they are not able to program for adaptation sufficiently. It is highly possible that the same mechanism developed in man, as an evolutionary advantage when iron was scarce and deficiency was a greater threat.
Acknowledgments
The authors would like to thank A.G. Lemmens, for the liver and dietary iron measurements by AAS, Prof. M. Egmond for his guidance in kinetic analysis, and Dr E.P. Martens for his contribution in the statistical analyses.
References
- Basclain , K.A. , Shilkin , K.B. , Withers , G. , Reed , W.D. and Jeffrey , G.P. 1998 . Cellular expression and regulation of iron transport and storage proteins in genetic haemochromatosis . Journal of Gastroenterology and Hepatology , 32 : 624 – 634 .
- Beutler , E. , West , C. , Speir , J.A. , Wilson , I.A. and Worley , M. 2001 . The HFE gene of browsing and grazing rhinoceroses: a possible site of adaptation to a low-iron diet. Blood Cells . Molecules and Diseases , 32 : 342 – 350 .
- Borch-Iohnsen , B. , Holm , H. , Jorgensen , A. and Norheim , G. 1991 . Seasonal siderosis in female eider nesting in Svalbard . Journal of Comparative Pathology , 32 : 7 – 15 .
- Cannone-Hergaux , F. , Gruenheid , S. , Ponka , P. and Gros , P. 1999 . Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron . Blood , 32 : 4406 – 4417 .
- Cork , S.C. 2000 . Iron storage disease in birds . Avian Pathology , 32 : 7 – 12 .
- Cork , S.C. , Alley , M.R. and Stockdale , P.H. 1995 . A quantitative assessment of haemosiderosis in wild and captive birds using image analysis . Avian Pathology , 32 : 239 – 254 .
- Cox , T.M. and Peters , T.J. 1978 . Uptake of iron by duodenal biopsy specimens from patients with iron-deficiency anaemia and primary haemochromatosis . The Lancet , 32 : 123 – 124 .
- Cox , T.M. and Peters , T.J. 1980 . In vitro studies of duodenal iron uptake in patients with primary and secondary iron storage disease . Quarterly Journal of Medicine , 32 : 249 – 257 .
- Crichton R. Iron absorption in mammals with particular reference to man, Inorganic Biochemistry of Iron Metabolism: From Molecular Mechanisms to Clinical Consequences 2nd edn, Crichton R. (ed.), John Wiley & Sons: West Sussex 2001 191 204
- Crissey , S.D. , Ward , A.M. , Block , S.E. and Maslanka , M.T. 2000 . Hepatic iron accumulation over time in european starlings (Sturnus vulgaris) fed two levels of iron . Journal of Zoo and Wildlife Medicine , 32 : 491 – 496 .
- Dierenfeld , E.S. , Pinis , M.T. and Sheppard , C.D. 1994 . Hemosiderosis and dietary iron in birds . Journal of Nutrition,, S , 32 : 2685S – 2686 .
- Dorrestein, G.M., Grinwis, G.M., Dominguez, L., Jagt, v. d. E. & Beynen, A.C. (1992). An induced iron storage disease syndrome in doves and pigeons: a model for genetic hemochromatosis in mynah birds? In: Proceedings of the Association of Avian Veterinarians (pp. 108–112). New Orleans, LA, USA.
- Dorrestein G.M. Sa L. de Ratiarison S. Mete A. Iron in the liver of animals in the zoo; a pathologist's point of view, Zoo Animal Nutrition Nijboer J., Hatt J., Kaufmanns W., Beynen A., Ganslosser U. (eds), Filander Verlag: Fuerth 2000 291 300
- Duane , P. , Raja , K.B. , Simpson , R.J. and Peters , T.J. 1992 . In vitro uptake of iron from iron-ascorbate by human duodenal biopsies from control subjects and patients with idiopathic haemochromatosis . European Journal of Gastroenterology and Hepatology , 32 : 661 – 666 .
- Fleming , R.E. , Migas , M.C. , Zhou , X.Y. , Jiang , J. , Britton , R.S. , Brunt , E.M. , Tomatsu , S. , Waheed , A. , Bacon , B.R. and Sly , W.S. 1999 . Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: Increased duodenal expression of the iron transporter DMT1 . Proceedings of the National Academy of Science , 32 : 3143 – 3148 .
- Garcia , F. , Ramis , J. and Planas , J. 1984 . Iron content in starlings, Sturnus vulgaris L . Comparative Biochemistry and Physiology , 32 : 651 – 654 .
- Goddard , W.P. , Coupland , K. , Smith , J.A. and Long , R.G. 1997 . Iron uptake by isolated human enterocyte suspensions in vitro is dependent on body iron stores and inhibited by other metal cations . Journal of Nutrition , 32 : 177 – 183 .
- Gosselin , S.J. and Kramer , L.W. 1983 . Pathophysiology of excessive iron storage in mynah birds . Journal of the American Veterinary Medical Association , 32 : 1238 – 1240 .
- Griffiths , W. and Cox , T. 2000 . Haemochromatosis: novel gene discovery and the molecular pathophysiology of iron metabolism . Human Molecular Genetics , 32 : 2377 – 2382 .
- Griffiths , W.J. , Sly , W.S. and Cox , T.M. 2001 . Intestinal iron uptake determined by Divalent Metal Transporter is enhanced in HFE-deficient mice with hemochromatosis . Gastroenterology , 32 : 1420 – 1429 .
- Gunshin , H. , Mackenzie , B. , Berger , U.V. , Gunshin , Y. , Romero , M.F. , Boron , W.F. , Nussberger , S. , Gollan , J.L. and Hediger , M.A. 1997 . Cloning and characterization of a mammalian proton-coupled metal-ion transporter . Nature , 32 : 482 – 487 .
- Iancu T.C. Animal models in liver research: iron overload, Advances in Veterinary Science and Comparative Medicine 32 Cornelius C.E. (ed.), Academic Press: San Diego, CA 1993 379 401
- Kimmich G.A. Isolation of intestinal epithelial cells and evaluation of transport functions, Methods in Enzymology: Biomembranes Part W Cellular and Subcellular Transport: Epithelial Cells 32 Fleischer S., Fleischer B. (eds), Academic Press: San Diego, CA 1990 324 340
- Levy , J.E. , Montross , L.K. and Andrews , N.C. 2000 . Genes that modify the hemochromatosis phenotype in mice . Journal of Clinical Investigations , 32 : 1209 – 1216 .
- Lowenstine L.J. Munson L. Iron overload in the animal kingdom, Zoo and Wild Animal Medicine, Current Therapy 4th edn, Fowler M.E., Miller R.E. (eds), WB Saunders: Philadelphia, PA 1999 260 268
- Marti , M.T. , Saiz , M.P. , Mitjavila , M.T. and Planas , J. 1980 . Intestinal iron absorption in chickens I. Experimental conditions . Biological Trace Element Research , 32 : 255 – 267 .
- Marx , J.J.M. 1979 . Iron absorption and its regulation . Haematologica , 32 : 479 – 493 .
- Marx J. J. M. Santos M. Pathophysiology of iron deficiency and iron overload in man, Inorganic Biochemistry of Iron Metabolism: From Molecular Mechanisms to Clinical Consequences 2nd edn, Crichton R. (ed.), John Wiley & Sons: West Sussex 2001 207 233
- McKie , A.T. , Barrow , D. , Latunde-Dada , G.O. , Rolfs , A. , Sager , G. , Mudaly , E. , Mudaly , M. , Richardson , C. , Barlow , D. , Bomford , A. , Peters , T.J. , Raja , K.B. , Shirali , S. , Hediger , M.A. , Farzaneh , F. and Simpson , R.J. 2001 . An iron-regulated ferric reductase associated with the absorption of dietary iron . Science , 32 : 1755 – 1759 .
- McKie , A.T. , Latunde-Dada , G.O. , McGregor , J.A. , Anderson , G.J. , Vulpe , C.D. , Wrigglesworth , J.M. and Simpson , R.J. 2002 . Molecular evidence for the role of a ferric reductase in iron transport . Biochemical Society Transactions , 32 : 722 – 724 .
- Mete , A. , Dorrestein , G.M. , Marx , J.J.M. , Lemmens , A.G. and Beynen , A.C. 2001 . A comparative study of iron retention in mynahs, doves and rats . Avian Pathology , 32 : 479 – 486 .
- Miller , G.F. , Barnard , D.E. , Woodward , R.A. , Flynn , B.M. and Bulte , J.W.M. 1997 . Hepatic hemosiderosis in common marmosets, Callithrix jacchus: effect of diet on incidence and severity . Laboratory Animal Science , 32 : 138 – 142 .
- Moos , T. , Trinder , D. and Morgan , E.H. 2002 . Effect of iron status on DMT1 expression in duodenal enterocytes from B2-microglobulin knockout mice . American Journal of Physiology Gastrointestinal and Liver Physiology , 32 : G687 – G694 .
- Panigraphy , B. and Senne , D.A. 1991 . Diseases of mynahs . Journal of the American Veterinary Medical Association , 32 : 378 – 381 .
- Pietrangelo , A. 2002 . Physiology of iron transport and the hemochromatosis gene . American Journal of Physiology Gastrointestinal and Liver Physiology , 32 : G403 – G414 .
- Raja , K.B. , Pountney , D. , Bomford , A. , Przemioslo , R. , Sherman , D. , Simpson , R.J. , Williams , R. and Peters , T.J. 1996 . A duodenal mucosal abnormality in the reduction of Fe(III) in patients with genetic haemochromatosis . Gut , 32 : 765 – 769 .
- Sanchez , J. and Planas , J. 1981 . Iron absorption in the everted chicken intestine . Revista Espanola de Fisiologia , 32 : 241 – 248 .
- Santos , M. , Sousa , M. de and Marx , J. J. M. 2000 . Regulation of intracellular iron levels in iron-acceptor and iron-donor cells . Transfusion Science , 32 : 225 – 235 .
- Schumann , K. , Elsenhans , B. and Forth , W. 1999 . Kinetic analysis of 59Fe movement across the intestinal wall in duodenal rat segments ex vivo . American Journal of Physiology Gastrointestinal and Liver Physiology , 32 : G431 – G440 .
- Smith , J.E. , Chavey , P.S. and Miller , R.E. 1995 . Iron metabolism in captive black (Diceros bicornis) and white (Ceratotherium simum) rhinoceroses . Journal of Zoo and Wildlife Medicine , 32 : 525 – 531 .
- Spelman , L.H. , Osborn , K.G. and Anderson , M.P. 1989 . Pathogenesis of hemosiderosis in Lemurs: role of dietary iron, tannin, and ascorbic acid . Zoo Biology , 32 : 239 – 251 .
- Taylor , J.J. 1984 . Iron accumulation in avian species in captivity. Dodo . Journal of the Jersey Wildlife Preservation Trust , 32 : 126 – 131 .
- Ward , R.J. , Iancu , T.C. , Henderson , G.M. , Kirkwood , J.R. and Peters , T.J. 1988 . Hepatic iron overload in birds: analytical and morphological studies . Avian Pathology , 32 : 451 – 464 .
- Whittaker , P. , Dunkel , V.C. , Bucci , T.J. , Kusewitt , D.F. , Thurman , J.D. , Warbritton , A. and Wolff , G.L. 1997 . Genome-linked toxic responses to dietary iron overload . Toxicologic Pathology , 32 : 556 – 564 .
- Zoller , H. , Koch , R.O. , Theurl , I. , Obrist , P. , Pietrangelo , A. , Montosi , G. , Haile , D.J. , Vogel , W. and Weiss , G. 2001 . Expression of the duodenal iron transporters Divalent-Metal Transporter 1 and Ferroportin 1 in iron deficiency and iron overload . Gastroenterology , 32 : 1412 – 1419 .