Abstract
Four waterfowl were collected in the Tri-State Mining District (Oklahoma, Kansas and Missouri, USA), an area known to be contaminated with lead, cadmium and zinc (Zn). They were part of a larger group of 20 waterfowl collected to determine the exposure of birds to metal contamination at the site. The four waterfowl (three Branta canadensis, one Anas platyrhynchos) had mild to severe degenerative abnormalities of the exocrine pancreas, as well as tissue (pancreas, liver) concentrations of Zn that were considered toxic. The mildest condition was characterized by generalized atrophy of exocrine cells that exhibited cytoplasmic vacuoles and a relative lack of zymogen. The most severe condition was characterized by acini with distended lumens and hyperplastic exocrine tissue that completely lacked zymogen; these acini were widely separated by immature fibrous tissue. Because the lesions were nearly identical to the lesions reported in chickens and captive waterfowl that had been poisoned with ingested Zn, and because the concentrations of Zn in the pancreas and liver of the four birds were consistent with the concentrations measured in Zn-poisoned birds, we concluded that these waterfowl were poisoned by Zn. This may be the first reported case of zinc poisoning in free-ranging wild birds poisoned by environmental Zn.
1 Introduction
Although zinc (Zn) poisoning has been reported in captive wild birds that have ingested bits of Zn as galvanized hardware or coins (Droual et al., Citation1991; Zdziarski et al., Citation1994), it has not been described convincingly in free-ranging wild birds. Gasaway & Buss (Citation1972) suggested that waterfowl in the Coeur d'Alene River Basin might be dying from exposure to Zn; although this remains a possibility, later studies at that site (Sileo et al., Citation2001) identified lead (Pb) as the cause of death in metal-poisoned waterfowl. Three Canada geese (Branta canadensis) and a mallard (Anas platyrhynchos) recently collected from the Tri-State Mining District had pancreatic lesions similar to those reported in captive waterfowl experimentally or accidentally poisoned with Zn (Dewar et al., Citation1983; Kazacos & Van Vleet, 1989; Zdziarski et al., Citation1994). These four wild waterfowl are the subject of this report. Dr James Carpenter of the College of Veterinary Medicine at Kansas State University has recently diagnosed zinc poisoning in a trumpeter swan (Cygnus buccinator) from the Tri-State Mining District (James Carpenter, personal communication, 2003.
The Tri-State Mining District covers about 3000 km2 and includes parts of Ottawa County in Oklahoma, Cherokee County in Kansas, and Jasper and Newton Counties in Missouri (Gibson, Citation1972). The District extends from the northwestern edge of the Ozark Uplift across rolling prairie west to the Neosho River. The area has been mined from about the 1850s to the 1970s with the peak of activity in the first half of the twentieth century. The sulfide forms of lead (galena) and zinc (sphalerite) and to a lesser extent zinc carbonate (smithsonite), lead carbonate, lead phosphate (pyromorphite) and other less abundant ores were mined (Gibson, Citation1972; Weidman, Citation1932). Phillips & Lincoln (Citation1930) reported the deaths of many mallards, pintails and teal in February 1923, on Spring River, near Riverton, KS. These authors concluded that the well-filled crops and other signs observed in the waterfowl were consistent with Pb poisoning. The suggested source of the Pb was mine refuse that had been dumped into the river. Two samples of sediment from Spring River, taken in 1987 near the mouth of Short Creek, contained an average of 23 000 mg/kg Zn and 1600 mg/kg Pb (Ferrington, Citation1989), suggesting that waterfowl might have been exposed to toxic concentrations of Zn near Riverton.
Although Zn poisoning has not yet been reported in free-ranging wild birds, it has been studied in other birds. Dewar et al. (Citation1983) experimentally poisoned chickens and reported lesions of the exocrine pancreas including dilation of acinar lumina, cytoplasmic vacuolation, cytoplasmic globule formation, necrosis, numerous mitotic figures and interparenchymal fibrosis. In an ultrastructure study of Zn toxicity in Pekin ducklings, Kazacos & Van Vleet (1989) reported apoptosis; attenuated, cuboidal, atrophic acinar cells; interstitial fibrosis; and the formation of duct-like structures embedded in fibrous connective tissue. They found only minimal inflammatory response and the islets were normal. Similar lesions were reported in four species of captive diving ducks that had ingested pennies (Zdziarski et al., Citation1994).
2 Methods
The birds were collected under the authority of permits from the US Fish and Wildlife Service and from the states of Oklahoma and Kansas. The geese were collected on 8 February 2001 and the mallard on 9 February 2001.
The four waterfowl collected were part of a larger study to determine whether birds from the site were exposed to toxic concentrations of Zn, Pb, and cadmium. The primary target organs (liver, kidney, spleen, and pancreas) of these metals were collected for histopathology examination and metal analyses. This study was not designed to determine other potential causes of morbidity in wild birds and did not include ancillary microbiological, virological, and parasitological laboratory tests.
One of the geese was weak and was captured alive by a bird dog. The two other geese and the mallard were active and were collected by shotgun and steel pellets. The impaired goose (number 1) was captured near an area of surface subsidence about 3 km west of Baxter Springs, Cherokee County, KS. It was examined clinically at the collection site and then was euthanized with carbon dioxide. Geese 2 and 3 were collected at Doubthat Bridge in Ottawa County, OK. The mallard was collected near the Atlas Chat Pile, also in Ottawa County, OK. The term ‘chat’ refers to the crushed rock remaining after the recoverable Zn and Pb have been removed.
Immediately after death, about 1 ml blood was taken by cardiac puncture with a needle and a vacutainer (both treated with lithium heparin) from each of the four birds. The haematocrit was measured in microhaematocrit tubes after centrifugation and the remaining blood was divided into two portions. One portion was frozen in liquid nitrogen and stored in an ultralow freezer until it was analyzed for activity of delta aminolevulinic dehydratase (ALAD), as in Pain (Citation1989) and Henny et al. (Citation2000). ALAD activity is a sensitive indicator of exposure to Pb. A unit of ALAD activity was defined as a 0.001 increase in absorbance at 555 nm with a 1.0 cm light path per milliliter of erythrocytes per hour at 38°C.
The second portion of blood was frozen and saved for metal analysis. Portions of the liver, pancreas, kidney and spleen of each bird were preserved in formalin and the remainders of the liver, pancreas and kidney were saved for chemical analysis. The gizzard contents were examined for ingested shot and other metallic artifacts but none were found. Concentrations of metals were determined by inductively coupled plasma-atomic emission spectrometry (Zn, cadmium and copper (Cu)) or by inductively coupled plasma-mass spectrometry (Pb) at the Research Triangle Institute (Research Triangle Park, NC, USA). Concentrations measured in reference materials, spiked samples and replicates were found to be within acceptable limits by the Patuxent Analytical Control Facility (US Fish and Wildlife Service, Laurel, MD). The formalinized tissues were submitted to the College of Veterinary Medicine, University of Wisconsin for histological processing. Sections stained by the haematoxylin and eosin method were examined by light microscope.
3 Results and Discussion
The impaired goose (Goose 1) was collected in Kansas within a few kilometers of Spring River, close to Riverton, where the waterfowl die-off was reported in 1923 (Phillips & Lincoln, Citation1930). This goose was a hatch-year male with a bursa of Fabricius. Its legs had reduced motor function; a foot prick did not produce a response. Its wings drooped and it had reduced wing proprioception. Gasaway & Buss (Citation1972) and Zdziarski et al. (Citation1994) reported paralysis of the legs as one of the few clinical signs of Zn poisoning in mallards. Grandy et al. (Citation1968) reported that mallards poisoned with Zn shot showed reduced wing proprioception as well as a loss of control of their leg muscles. The goose's breastbone was prominent and it had very little subcutaneous or intra-abdominal fat, which is consistent with the weight loss observed by Levengood et al. (Citation1999) and Zdziarski et al. (Citation1994). The goose's mouth, tongue and phonations were normal. An enlarged spleen was the only internal lesion. Its ALAD activity was 208 u and its haematocrit was 54.
Goose 2 was a female in normal body condition with no bursa of Fabricius. It had ALAD activity of 89, and a haematocrit of 39. There was a healed wound in its intestines, probably a penetrating gunshot wound, but no other gross lesions. Goose 3 was a male in normal body condition with no bursa of Fabricius, an ALAD activity of 38 and a haematocrit of 45. The only gross lesion noted at necropsy was a firm and fibrotic pancreas with a haemorrhagic surface. The mallard was a female with ALAD activity of 5 and a haematocrit of 51. It had a granuloma in the proventricular adventitia, but no other gross lesions.
Although erosion of the lining of the gizzard of captive birds experimentally or accidentally poisoned with Zn has been reported (Dewar et al, Citation1983; Droual et al., Citation1991; Zdziarski et al., Citation1994), the linings of the gizzards of these four waterfowl were unremarkable.
Henny et al. (Citation2000) reported ALAD activity as 156±68 (mean±standard deviation) in adult reference mallards and 183±54 in adult reference Canada geese. These reference values suggest that the mallard and geese 2 and 3 had had elevated exposure to Pb. Both Zn poisoning and Pb poisoning have been reported to depress mean haematocrits of waterfowl slightly, but responses of individual waterfowl are variable. Haematocrits of adult reference Canada geese are 43±3.3 and those of adult reference mallards are 45±2.5 (Henny et al., Citation2000). Judged by these values, Canada goose 2 had a slightly lower than normal haematocrit.
Microscopic examination revealed mild to severe degenerative abnormalities of the exocrine pancreas in each of the four birds. The mildest condition, which occurred in Goose 1, was characterized by generalized atrophy of exocrine cells, a mild lack of zymogen, and empty cytoplasmic vacuoles approximately 5 μm in diameter; also present were a few scattered, prominent, individual cytomegalic exocrine cells with larger (approximately 10 to 15 μm diameter) but otherwise similar cytoplasmic vacuoles. The most severe condition occurred in the mallard and was characterized by widely separated acini of hyperplastic exocrine tissue that completely lacked zymogen and had distended lumens. Separating these acini were prominent bands of immature fibrous tissue. Mitotic figures were apparent ().
Fig. 1 Mallard pancreas that had a concentration of 440 mg/kg dry weight of Zn. Note widely separated acini with disorganized, misshapen cells that lack zymogen. Mitotic figures, marked variation in nuclei size, and prominent acinar lumens are evident. Scale bar=40 μm.
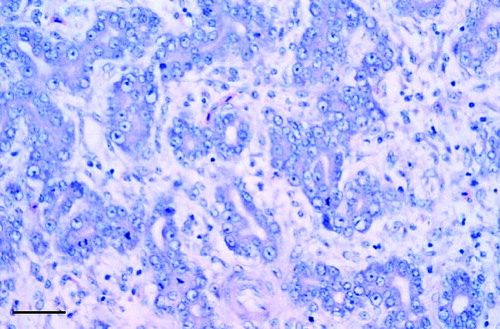
The severity of the lesions in the other two geese (geese 2 and 3) was intermediate, with subcapsular and interacinar fibrosis being a prominent change; also present were a mild lack of zymogen, increasingly prevalent karymegaly, and cytoplasmic vacuolation ( ). Inflammation was limited to a few interstitial granulocytes and lymphocytes. Apoptosis was not a prominent feature and islets were not affected. Although the lesions in the geese were less severe than those in the mallard, they may have been more chronic. In a study with ducklings of the relation between dietary selenium and vitamin E and silver, Cu, cobalt, tellurium cadmium and Zn, Van Vleet et al. (1981) reported that excessive Zn caused pancreatic necrosis and fibrosis, but the other metals did not. Several other microscopic lesions of minimal to mild severity occurred in the geese and mallard. The cause of these was unknown; they might have been a consequence of Zn intoxication, or unrelated. Goose 2 had moderately severe multifocal hepatic necrosis, minimal nephrosis, mild renal haemosiderosis, and fibrinoid necrosis of the media of a splenic artery; although these changes are not specific to any particular etiology, they are sometimes associated with Pb poisoning in waterfowl (Sileo et al., Citation2001). However, the concentrations of Pb in the tissues of this goose were not remarkable (). The mallard had marked hepatocellular vacuolar degeneration, also of undetermined cause.
Concentrations of Zn, Pb, Cd and Cu detected in tissues of Canada geese and a mallard from the Tri-State Mining District of Kansas, Oklahoma and Missouri
Fig. 2 Canada goose pancreas that had a concentration of 2400 mg/kg dry weight of Zn. Note the disorganized acini and prominent subcapsular and interacinar fibrosis. Scale bar=150 μm.
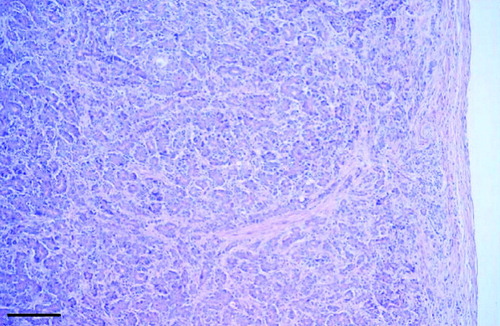
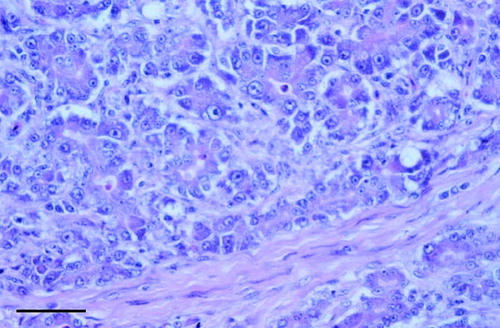
Fig. 3 Close up of Figure 2. Note disorganized, misshapen cells, marked variation in nuclei size, cytoplasmic vacuoles, and interacinar fibrosis. Scale bar=40 μm.
Zn is bound to metallothioneins found in the liver, kidney, intestinal mucosa and especially in the pancreas (Oh et al., Citation1979). Animals usually regulate Zn effectively, and consequently hepatic concentrations of Zn do not vary proportionately with dietary exposure. For example, the whole body concentrations of Zn in songbirds from a site severely contaminated with Zn from smelting were less than 20% greater than those of songbirds from a reference site, although the A1 soil horizon of that contaminated site had more than 10 times the Zn concentration of the reference site (Beyer et al., Citation1985). In experimental studies on chickens, hepatic Zn concentrations (wet weight) remained constant as the dietary concentration was increased from 37 to about 110 mg/kg (Stahl et al., Citation1989). Homeostatic mechanisms fail, however, at extremely high Zn concentrations. Hepatic Zn increased more than 10-fold in that study, when the dietary concentration was increased to about 2200 mg/kg (Stahl et al., Citation1989). In mallards, liver concentrations (wet weight) increased from 54 to 401 mg/kg Zn as the dietary concentration increased from control concentrations to 3000 mg/kg (Gasaway & Buss, Citation1972). The proportional increase in Zn concentrations in kidneys and pancreases was greater than that in livers of dosed birds (Gasaway & Buss, Citation1972; Levengood et al., Citation1999). Zn concentrations were higher and more variable in the pancreas than in the liver of Zn-poisoned birds and, when dietary concentrations of Zn were reduced, Zn concentrations remained longer in the pancreas compared with other tissues examined (Williams et al., Citation1989). Tissue Zn concentrations in chickens drop rapidly after exposure returns to normal (Oh et al., Citation1979). The half-lives of Zn in metallothionein were estimated as 2.3 days in the pancreas, 1.5 days in the liver and 0.9 days in the kidney (Oh et al., Citation1979).
Although tissue concentrations of metals reported in wild waterfowl are quite variable (Di Giulio & Scanlon, Citation1984) we can draw some conclusions about the concentration detected in the four waterfowl in our study. The concentrations of Zn in the tissues of these geese were many times the values for control birds in toxicological studies () and the hepatic concentrations were comparable with those in waterfowl killed by Zn in laboratory studies or accidentally killed by ingesting zinc pennies in zoos (). The concentrations of Pb (Pain, Citation1996) and cadmium (Furness, Citation1996) in the geese tissues were well below those associated with histological lesions. The concentrations of Zn in the tissues of the mallard were elevated, but were not in the range associated with death. Concentrations of Pb in the kidney and blood of the mallard also were elevated.
Concentrations of Zn in tissues of reference waterfowl and waterfowl experimentally or accidentally poisoned by Zn
Zn exerts its toxicity partially by interfering with Cu metabolism (National Research Council, Citation1980). Geese fed a diet adequate in Cu were reported to have hepatic Cu concentrations of 6.0 to 26 mg/kg wet weight (about 20 to 97 mg/kg dry weight) and renal Cu concentrations 3.0 to 9.0 mg/kg wet weight (about 10 to 30 mg/kg dry weight) (Puls, Citation1994). Stahl et al. (Citation1989) found that exposure to high Zn concentrations in chickens reduced hepatic Cu, and Levengood et al. (Citation1999) found that exposure to high Zn concentrations in mallards reduced hepatic Cu concentrations and increased renal Cu concentrations. Both an increase (Levengood et al., Citation1999) and a decrease (Stahl et al., Citation1989) in Cu concentrations in the pancreas have been reported in response to exposure to high Zn concentrations. Goose 3 had normal Cu concentrations in the liver and kidney, based on the criteria of Puls (1994). Goose 1, however, had an abnormally low hepatic Cu concentration of 19 mg/kg and the Cu concentration in its pancreas was below detection limits. Goose 2 had an abnormally low hepatic Cu concentration of 16 mg/kg and an abnormally high renal Cu concentration of 104 mg/kg. The hepatic Cu concentration in the mallard was low or normal (21 mg/kg) and its renal Cu concentration was much higher (150 mg/kg) than normal. These abnormal Cu concentrations in tissues of Goose 1, Goose 2, and the mallard are consistent with a diagnosis of Zn poisoning.
Vacuolar change, atrophy, and fibrosis of an exocrine pancreas are common in poultry and considered idiopathic or associated with selenium deficiency or zinc toxicity (Goodwin, Citation1996). However, in our experience, atrophy and fibrosis of exocrine pancreas is uncommon in free-ranging waterfowl. Because the lesions were virtually identical to the lesions reported in chickens (Dewar et al., Citation1983) and waterfowl (Grandy et al., Citation1968: Van Vleet et al., Citation1981; Kazacos & Van Vleet, Citation1989; Levengood et al., Citation1999; Zdziarski et al., Citation1994) that had been experimentally or accidentally poisoned with ingested Zn, and because the concentrations of Zn in the pancreases and livers of the four birds were significantly elevated (), we conclude that Zn was responsible for the pancreatic lesions and that these waterfowl were poisoned by Zn.
Acknowledgments
Our study would not have been possible without the assistance of John Dalgarn of the Bureau of Indian Affairs and John Miesner of the US Fish and Wildlife Service, who collected the waterfowl and shared their expertise on the Tri-State Mining District.
References
- Beyer , W.N. , Pattee , O.H. , Sileo , L. , Hoffman , D.J. and Mulhern , B.M. 1985 . Metal contamination in wildlife living near two zinc smelters . Environmental Pollution (Series A) , 32 : 63 – 86 .
- Dewar , W.A. , Wight , P.A.L. , Pearson , R.A. and Gentle , M.J. 1983 . Toxic effects of high concentrations of zinc oxide in the diet of the chick and laying hen . British Poultry Science , 32 : 397 – 404 .
- Di Giulio , R.T. and Scanlon , P.F. 1984 . Heavy metals in tissues of waterfowl from the Chesapeake Bay, USA . Environmental Pollution (Series A) , 32 : 29 – 48 .
- Droual , R. , Meteyer , C.U. and Galey , F.D. 1991 . Zinc toxicosis due to ingestion of a penny in a gray-headed Chachalaca (Ortalis cinereiceps) . Avian Diseases , 32 : 1007 – 1011 .
- Ferrington, L.C. (1989). Occurrence and biological effects of cadmium, lead, manganese and zinc in the Short Creek/Empire Lake aquatic system, in Cherokee County, Kansas. Kansas Water Resources Research Institute Report, Contribution No. 277. Manhattan, KS, USA.
- Furness R.W. Cadmium in birds, Environmental Contaminants in Wildlife, Interpreting Tissue Concentrations Beyer W. N., Heinz G.H., Redmon-Norwood A.W. (eds), Lewis Publishers: Boca Raton, FL 1996 389 404
- Gasaway , W.C. and Buss , I.O. 1972 . Zinc toxicity in the mallard duck . Journal of Wildlife Management , 32 : 1107 – 1117 .
- Gibson A.M. Wilderness Bonanza: The Tri-State District of Missouri, Kansas, and Oklahoma University of Oklahoma Press: Norman, OK 1972
- Goodwin M.A. Alimentary system, Avian Histopathology Riddell C. (ed.), American Association of Avian Pathologists: Tallahassee 1996 111 141
- Grandy , J.W. IV , Locke , L.N. and Bagley , G.E. 1968 . Relative toxicity of lead and five proposed substitute shot types to pen-reared mallards . Journal of Wildlife Management , 32 : 483 – 488 .
- Henny , C.J. , Blus , L.J. , Hoffman , D.J. , Sileo , L. , Audet , D.J. and Snyder , M.R. 2000 . Field evaluation of lead effects on Canada geese and mallards in the Coeur d'Alene River Basin, Idaho . Archives of Environmental Contamination and Toxicology , 32 : 97 – 112 .
- Hoffman , D.J. , Heinz , G.H. , Sileo , L. , Audet , D.J. , Campbell , J.K. , LeCaptain , L.J. and Obrecht , H.H. III . 2000 . Developmental toxicity of lead-contaminated sediment in Canada Geeose (Branta canadensis) . Journal of Toxicology and Environmental Health, Part A , 32 : 235 – 252 .
- Kazacos , E.A. and Van Vleet , J.F. 1989 . Sequential ultrastructural changes of the pancreas in zinc toxicosis in ducklings . American Journal of Pathology , 32 : 581 – 595 .
- Levengood , J.M. , Sanderson , G.C. , Anderson , W.L. , Foley , G.L. , Skowron , L.M. , Brown , P.W. and Seets , J.W. 1999 . Acute toxicity of ingested zinc shot to game-farm mallards . Illinois Natural History Survey Bulletin , 32 : 1 – 36 .
- National Research Council (1980). Mineral Tolerance in Domestic Animals. Washington, DC: National Academy of Sciences.
- Oh , S.H. , Nakaue , H. , Deagen , J.T. , Whanger , P.D. and Arscott , G.H. 1979 . Accumulation and depletion of zinc in chick tissue metallothioneins . Journal of Nutrition , 32 : 1720 – 1729 .
- Pain , D.J. 1989 . Hematological parameters as predictors of blood lead and indicators of lead poisoning in the black duck (Anas rubripes) . Environmental Pollution , 32 : 67 – 81 .
- Pain D.J. Lead in waterfowl, Environmental Contaminants in Wildlife, Interpreting Tissue Concentrations Beyer W.N., Heinz G.H., Redmon-Norwood A.W. (eds), Lewis Publishers: Boca Raton, FL 1996 251 264
- Phillips J.C. Lincoln F.C. American Waterfowl: Their Present Situation and the Outlook for their Future Houghton Mifflin: Boston, MA 1930
- Puls R. Mineral Levels in Animal Health: Diagnostic Data 2nd edn, Sherpa International: Clearbrook, BC 1994
- Sileo , L. , Creekmore , L.H. , Audet , D.J. , Snyder , M.R. , Meteyer , C.U. , Franson , J.C. , Locke , L.N. , Smith , M.R. and Finley , D.L. 2001 . Lead poisoning of waterfowl by contaminated sediment in the Coeur d'Alene River . Archives of Environmental Contamination and Toxicology , 32 : 364 – 368 .
- Stahl , J.L. , Greger , J.L. and Cook , M.E. 1989 . Zinc, copper and iron utilisation by chicks fed various concentrations of zinc . British Poultry Science , 32 : 123 – 134 .
- Van Vleet , J.F. , Boon , G.D. and Ferrans , V.J. 1981 . Induction of lesions of selenium-vitamin E deficiency in ducklings fed silver, copper, cobalt, tellurium, cadmium, or zinc: protection by selenium or vitamin E supplements . American Journal of Veterinary Research , 32 : 1206 – 1217 .
- Weidman, S. (1932). Miami-Picher Zinc–Lead District. Norman, OK:University of Oklahoma Press.
- Williams , S.N. , Miles , R.D. , Ouart , M.D. and Campbell , D.R. 1989 . Short-term high level zinc feeding and tissue zinc concentration in mature laying hens . Poultry Science , 32 : 539 – 545 .
- Zdziarski , J.M. , Mattix , M. , Bush , R.M. and Montali , R.J. 1994 . Zinc toxicosis in diving ducks . Journal of Zoo and Wildlife Medicine , 32 : 438 – 445 .