Abstract
The aim of the present study was to investigate the pathology in normal or immunosuppressed chickens followed intravenous or intraperitoneal inoculation with a well-characterized strain of Gallibacterium anatis. Two groups of 30 15-week-old commercial brown laying chickens were used, having been screened and found negative for Gallibacterium organisms. One group was treated with 5-fluorouracil to promote heterophil depletion, while the other was saline treated. Ten days later 15 chickens from each group were inoculated either intravenously or intraperitoneally with 3.3×107 colony-forming units of G. anatis strain 12656-12. Subsets of chickens were sacrificed at 3, 12 or 24 h post-infection and examined for lesions. Livers and spleens were examined by culture and by fluorescent in situ hybridization. Intravenously infected birds showed severe septicaemic lesions in both the normal and immunosuppressed birds. Mortality was recorded only in the latter, with an overall rate of 73%. The intraperitoneally infected chickens of normal immune status showed various degrees of localized purulent peritonitis at the inoculation site, but in the immunosuppressed birds the entire peritoneum tended to be involved along with the abdominal organs. This was similar to previous descriptions of natural infections and may represent a useful infection model for detailed analysis of Gallibacterium virulence factors and pathogenesis.
Résumé
Études in vivo de l'infection à Gallibacterium anatis chez le poulet
Le but de la présente étude a été d'investiguer la pathologie chez le poulet normal ou immunodéprimé après inoculation intraveineuse ou intrapéritonéale avec une souche bien caractérisée de Gallibacterium anatis. Deux groupes de 30 poulettes futures pondeuses rousses, âgées de 15 semaines, ont été sélectionnées et ont été contrôlées négatives vis à vis de Gallibacterium anatis. Un groupe a été traité avec du 5-fluorouracil pour provoquer une déplétion en hétérophiles, alors que l'autre groupe a reçu une solution saline. Dix jours plus tard, 15 poulets de chaque groupe ont été inoculés par voie intraveineuse ou intrapéritonéale avec 3,3×107 colonies formant unité de la souche 12656-12 de G. anatis. Des sous-groupes de poulets ont été sacrifiés à 3, 12 ou 24 heures après infection et les lésions ont été recherchées. Les foies et les rates ont été mis en culture et ont fait l'objet d'un test de fluorescence par hybridation in situ. Les animaux normaux et immunodéprimés, infectés par voie intraveineuse ont présenté des lésions septicémiques graves. La mortalité enregistrée chez les immunodéprimés a été de 73%. Les animaux normaux infectés par voie intrapéritonéale ont présenté une péritonite purulente, plus ou moins grave, localisée au point d'inoculation mais chez les animaux immunodéprimés tout le péritoine a été concerné avec les organes abdominaux. Les observations sont similaires à celles décrites antérieurement dues à des infections spontanées, ainsi elles peuvent servir de modèle d'infection pour des études ultérieures concernant les facteurs de virulence de Gallibacterium et la pathogénie.
Zusammenfassung
In vivo- Untersuchungen von Gallibacterium anatis– Infektionen bei Hühnern
Ziel der vorliegenden Studie war es, in normalen und immunsuppremierten Hühnern die Pathologie nach intravenöser oder intraperitonealer Inokulation mit einem gut charakterisierten Gallibacterium anatis-Stamm zu untersuchen. Zwei Gruppen von je 30 15-Wochen-alten kommerziellen braunen Legehühnern, die vorher untersucht und als Gallibakterium-Organismen-negativ befunden worden waren, wurden verwendet. Eine Gruppe wurde zur Verminderung der Heterophilen mit 5-Fluoruracil behandelt, während die andere Kochsalzlösung erhielt. Zehn Tage später wurden jeweils 15 Hühner aus jeder Gruppe entweder intravenös oder intraperitoneal mit 3,3×107 Kolonie-bildenden Einheiten des G. anatis-Stamms 12656-12 inokuliert. 3, 12 oder 24 Stunden post infectionem wurden jeweils einige Tiere getötet und seziert. Lebern und Milzen wurden bakteriologisch mittels Kultivierung und in situ-Fluoreszenz-Hybridisierung untersucht. Sowohl die normalen als auch die immunsuppremierten Tiere wiesen nach der intravenösen Infektion schwere septikämische Läsionen auf. Mortalität trat nur bei den letzteren mit einer Todesrate von insgesamt 73% auf. Die intraperitoneal infizierten Hühner mit normalem Immunstatus zeigten verschiedene Grade einer lokalisierten purulenten Peritonitis im Bereich der Inokulationsstelle; die immunsuppremierten Tiere dagegen tendierten zu einer generalisierten Peritonitis mit Einbeziehung der abdominalen Organe. Dieses Infektionsbild ähnelte den vorherigen Beschreibungen natürlicher Infektionen und kann somit ein nützliches Infektionsmodell für detaillierte Untersuchungen der Virulenzfaktoren und der Pathogenese von Gallibakterien darstellen.
Resumen
Estudios in vivo de la infección por Gallibacterium anatis en pollos
El objetivo de este estudio fue investigar la patología en pollos normales o inmunosuprimidos ocasionada por la inoculación intravenosa o intraperitoneal de una cepa bien caracterizada de Gallibacterium anatis. Se usaron dos grupos de 30 pollitas ponedoras marrones comerciales de 15 semanas de edad, que se habían analizado y encontrado negativas para organismos del tipo Gallibacterium. Un grupo se trató con 5-fluorouracil para provocar una depleción heterofílica, mientras que el otro se trató con una solución salina. Diez días después, 15 pollitas de cada grupo se inocularon intravenosa o intraperitonealmente con 3.3×107 unidades formadoras de colonias de G. anatis cepa 12656-12. Se sacrificaron subgrupos de pollitas a las 3, 12 o 24 horas post-infección y se examinaron sus lesiones. Los hígados y bazos se examinaron mediante cultivo e hibridación in situ fluorescente. Las aves infectadas por vía intravenosa mostraron lesiones septicémicas graves tanto en el grupo de aves normales como inmunosuprimidas. La mortalidad se contabilizó solamente en este último grupo, con un valor final del 73%. Las pollitas infectadas por vía intraperitoneal con un estado inmunitario normal mostraron varios grados de peritonitis purulenta localizada en el punto de inoculación, pero en las aves inmunosuprimidas se observó una tendencia a la afectación del peritoneo en su totalidad junto con los órganos abdominales. Este hallazgo fue similar a descripciones previas de infecciones naturales y podría representar un modelo de infección útil para un análisis detallado de los factores de virulencia y la patogénesis de Gallibacterium.
Introduction
Gallibacterium was recently established as a new genus within the family Pasteurellaceae Pohl 1981 (Christensen et al., Citation2003). Bacteria belonging to this genus have previously been reported as Pasteurella anatis, avian Pasteurella haemolytica-like organisms or Actinobacillus salpingitidis.
The pathogenic potential reported for Gallibacterium is highly variable. These organisms have been isolated from clinically healthy birds, in which they have been suggested to constitute a part of the upper respiratory tract and lower genital tract flora (Harry, Citation1962; Bisgaard, Citation1977; Mushin et al., Citation1980; Bojesen et al., Citation2003a). However, others have isolated Gallibacterium in pure culture from diseased chickens affected with salpingitis, oophoritis, peritonitis, septicaemia, pericarditis, hepatitis and upper respiratory tract lesions, indicating that at least some strains of Gallibacterium possess a pathogenic potential (Kjos-Hanssen, Citation1950; Greenham & Hill, Citation1962; Harbourne, Citation1962; Gerlach, Citation1977; Bisgaard & Dam, Citation1981; Addo & Mohan, Citation1984; Shaw et al., Citation1990; Mirle et al., Citation1991; Suzuki et al., Citation1996, Citation1997a,Citationb). Although no specific lesions have been assigned Gallibacterium infections, some reports have indicated that these bacteria may be involved in infections of the salpinx and the peritoneum in particular (Kohlert, Citation1968; Hinz, Citation1969; Matthes et al., Citation1969; Gerlach, Citation1977). This view was substantiated by Mirle et al. (Citation1991) who, in a postmortem investigation including 496 hens, isolated Gallibacterium as one of the most frequent bacterial agents from lesions in the reproductive tract.
Previous experimental infections with Gallibacterium have shown substantial variation with regard to mortality, ranging from low or non-pathogenic (Harbourne, Citation1962; Bisgaard, Citation1977; Mushin et al., Citation1980) to highly pathogenic, with mortality rates reaching 85 to 90% (Matthes & Löliger, Citation1976; Gerlach, Citation1977). However, differences in the experimental set-up as well as uncertainty with regard to correct classification of organisms now classified as genus Gallibacterium have placed some doubt upon interpretation of these results (Bisgaard, Citation1993).
Consequently, the aim of the present study was to provide a basic understanding of bacteria–host interaction through a controlled infection study using a well-characterized Gallibacterium strain in chickens of different immune status. Immunosuppression was achieved by depleting the heterophil cells, which play a major role in the innate cellular immune response in chickens (Bounous & Stedman. Citation2000). Two inoculation routes were used. Intravenous (i.v.) inoculation was included to provide basic information on virulence, whereas inoculation by intraperitoneal (i.p.) route was included to mimic peritoneal infection, from which Gallibacterium have often been isolated after natural infection (Gerlach, Citation1977; Bisgaard & Dam, Citation1981; Mirle et al., Citation1991).
Materials and methods
Experimental animals and housing facilities
Fifteen-week old chickens (Lohmann Brown layers) were obtained from a commercial farm that had a high biosecurity level. A random sample of cloacal swabs from 30 chickens, which allows detection of infection levels greater than 10%, with 95% certainty (Martin et al., Citation1987), was collected for bacterial cultivation on blood agar base (Oxoid, Hampshire, UK) with 5% bovine blood (BA) to ensure freedom from Gallibacterium infection. Birds were allowed to acclimatize for 1 week and were provided water and food ad libitum and kept together under free-range housing conditions without outdoor access. All work on experimental animals was carried out with the approval of the Danish National Animal Ethics Committee.
Bacterial strains, media and preparation of inoculum
Gallibacterium anatis strain 12656-12 originally isolated from the liver of a chicken with septicaemia. This strain has been characterized in detail by phenotypical and genotypical methods and was therefore found suitable for further studies of pathogenicity (Bojesen et al., Citation2003b). Bacteria were stored at –80°C and cultivated overnight on BA. Single colonies were incubated with shaking in Brain Heart Infusion broth (Oxoid) at 37°C and subsequently stored at 4°C, in order to synchronize the population in stationary growth phase. Prior to use, 12 μl primary culture of each tube was added to 10 ml Brain Heart Infusion following incubation at 37°C until the culture reached late log-phase. Bacterial numbers and growth phase were estimated at OD600 and adjusted to a concentration of approximately 108 CFU/0.5 ml with 1×phosphate buffered saline prior to inoculation. The bacterial concentration in each inoculum was verified by plate counts on BA plates in duplicate.
Immunosuppressive treatment
5-Fluorouracil (5-FU) (Faulding Pharmaceuticals, Warwickshire, UK) (50 mg/ml, pH 9.0) was given at a dosage of 200 mg/kg body weight by a single i.v. injection into the jugular vein. The control group received an equal volume of sterile, pyrogen-free saline.
Heterophil counts
Blood samples from each bird were collected at the time of 5-FU/saline treatment, day 0 (D0) and at the time of inoculation with G. anatis 12656-12, 10 days after 5-FU treatment (D10). Blood samples (0.3 to 0.4 ml) were collected from each bird with a syringe (27G needle) into Vacutainer™ blood collection tubes containing ethylenediamine tetraacetic acid (Becton & Dickinson, Franklin Lakes, NJ, USA). The blood samples were gently inverted five to 10 times to ensure mixing with ethylenediamine tetraacetic acid and kept at 4°C for further use. The blood was subsequently diluted 1:20 to 1:40 in an eosin:acetone solution (eosin, 100 mg; sodium citrate hydrate, 300 mg; acetone, 15 g; made up to 100 ml with distilled water) for the heterophil count. The number of heterophil granulocytes was counted in a Fuchs–Rosenthal haemocytometer. The counts were completed within 48 h of blood collection. All eosin-stained cells were counted and no attempts were made to differentiate heterophils from eosinophilic granulocytes. The average heterophil count for the chickens in each treatment group, at D0 and D10, was compared using Students t test for significance of differences between means of paired data.
Experimental infection of heterophil-depleted and non-depleted chickens
Sixty 15-week-old chickens were divided randomly into two groups of 30, and on experimental day 0 (D0) one group was treated with 5-FU and the other with saline. Ten days later (D10), each treatment group was randomly divided into two further subgroups of 15. One subgroup of 5-FU-treated birds and one of saline-treated birds were inoculated i.v. with G. anatis while the other two subgroups were inoculated i.p.
Five birds from each of these subgroups were then selected randomly and killed by decapitation 3 h (D10+3 h), 12 h (D10+12 h) or 24 h (D10+24 h) after bacterial inoculation, in order to evaluate the pathological lesions developing over time. All were subjected to postmortem examination. Bacterial counts from spleen and liver samples were obtained by streaking tissue swabs onto plates of blood agar base (BA) (Oxoid, Ballerup, Denmark) supplemented with 5% citrated bovine blood. Isolates were identified as Gallibacterium if they exhibited greyish, semi-transparent colonies surrounded by a wide β-haemolytic zone (1 to 2 mm), had a butyrous consistency, were smooth, shiny and circular and raised with an entire margin and a size of 1 to 2 mm in diameter after 24 h at 37°C, and met the genus criteria outlined by Christensen et al. (Citation2003).
Histopathology and 16S rRNA in situ hybridization
Samples of liver and spleen were kept in 10% phosphate-buffered formalin for up to 24 h, followed by dehydration, embedding in paraffin wax and preparation of 3 to 4 μm cross-sections. Sections were mounted on adhesive slides (Super Frost/Plus, Menzel-Gläser, Germany) and stained with haematoxylin and eosin (HE) stain.
Fluorescent in situ hybridization (FISH) on liver and splenic tissue was performed with a Gallibacterium-specific probe GAN850 as reported previously (Bojesen et al., Citation2003).
Results
Heterophil counts
The average heterophil count at D0, in the 5-FU-treated chickens, was 3950 cells/mm3 (standard deviation±1430) compared with 90 cells/mm3 (standard deviation±5) at D10, corresponding to a 44-fold and highly significant reduction (P<0.001). There was no significant reduction (P>0.25) in the average heterophil counts from D0 (4030±1070 cells/mm3) to D10 (3910±970) cells/mm3) in the saline-treated chickens.
Intravenously infected chickens
The results from the postmortem examinations are summarized in . The infection induced severe depression of the chickens, which were reluctant to move. In addition, a number of birds developed acutely inflamed bursa presternalis, probably as a consequence of unnaturally long periods of resting on their breasts. Both the 5-FU-treated and untreated chickens showed severe vascular lesions as a consequence of the induced septicaemia. Red discolouration of fat and extensive serohaemorrhagic exudation into all body cavities was apparent as early as 3 h post-infection (p.i.). Histopathology of the spleens demonstrated non-identifiable necrotic cells, proteinaceous fluid, eosinophilic and basophilic aggregates in the ellipsoids (). The basophilic aggregates were shown to be microcolonies of Gallibacterium by the positive signal obtained with FISH (). The liver lesions were less apparent and included basophilic aggregates (Gallibacterium microcolonies) bordered by necrotic hepatocytes in the treated birds.
Table 1. Number of culture positive animals, mortality and lesions at different times post i.v. inoculation of G. anatis in chickens
A number of chickens from the treated group had to be killed before the scheduled sampling at 12 h p.i., due to their very poor condition, but both the 5-FU-treated and saline-treated chickens exhibited excessive vascular changes and multiple necrotic foci in the spleens and livers. However, the gross pathological changes were more profound in the 5-FU-treated than the saline-treated chickens. The splenic lesions in the saline-treated birds were similar to those shown in . In the 5-FU-treated birds, the splenic lesions had larger necrotic areas involving the peri-arterial lymphatic sheath, as well as pronounced oedema in the organ. The histological changes in the livers were less apparent than the splenic lesions, although bacterial colonies bordered by necrotic hepatocytes were found in the 5-FU-treated chickens.
Figure 1. 1A: The spleen from an intravenously inoculated and heterophil-depleted chicken, 12 h p.i. The splenic penicilium arteriole (thick arrow) surrounded by ellipsoids filled with degranulated heterophils, eosinophilic aggregates and proteinaceus fluid (thin arrow) (HE stain). 1B: The spleen section (as 1A) demonstrating a signal distribution corresponding to the eosinophilic aggregates observed in 1A showing microcolonies of Gallibacterium (FISH). 1C: The liver from an i.p. inoculated and heterophil-depleted chicken, 12 h p.i. Blood-filled sinousoids and a thick perihepatic layer of pus (arrow) including Gallibacterium and erythrocytes (FISH). 1D: The liver from an i.p. inoculated and heterophil-depleted chicken, 12 h p.i. Single cells (thin arrow) and microcolonies (thick arrow) of Gallibacterium visualized intracapsularly and subcapsularly (FISH).
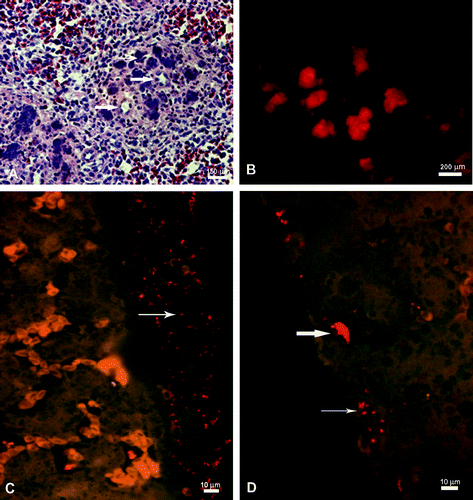
At 24 h p.i. the macroscopic changes were mostly similar to those observed after 12 h p.i. including vascular changes and multiple necrotic foci. The histopathological changes were also similar to those observed at 12 h p.i. However, the formation of multinucleated giant cells was apparent around some of the ellipsoid lesions in the spleen.
The severity of the gross and microscopic lesions differed markedly between the 5-FU-treated and the saline-treated group, which was underlined by the difference in mortality between the groups, where 11 out of 15 (73%) died in the 5-FU-treated group, compared with 0/15 (0%) among the saline-treated chickens.
Intraperitoneally infected chickens
The i.p. inoculated chickens showed only minor macroscopic lesions at 3 h p.i. However, bacteria had already gained access to the lymphatic system in two of the 5-FU-treated birds, as shown by positive cultures obtained from the spleens. No histopathological lesions were observed at this time in either spleen or liver tissue from any of the birds.
By 12 h p.i., a diffuse purulent peritonitis and fibrinous perihepatitis was apparent in 4/5 of the 5-FU-treated birds, some of which showed signs of systemic infection by positive bacterial culture from both livers and spleens. However, no histological reaction was observed in those organs in any of the birds examined. One bird was culturally negative in the liver and spleen; however, it was possible to identify basophilic aggregates by HE stain that later were confirmed to be G. anatis. The only histological changes observed in the spleen of this bird were thrombosis in some of the central veins.
Generally, purulent masses containing bacteria and erythrocytes were demonstrated by FISH on the serosal surface of the livers of the 5-FU-treated birds (). Additionally, microcolonies and single cells of Gallibacterium were demonstrated intracapsularly and subcapsularly in the livers by FISH ().
At 24 h p.i. none of the birds cultured positive from the liver or spleen and only one of the 5-FU-treated chickens had diffuse purulent peritonitis. No histopathological changes were observed in the livers or spleens from either treated or untreated birds.
Discussion
Although avian heterophils have been shown to generate relatively low amounts of oxygen free radicals and have less phagocytic capability than human and canine neutrophils, their bactericidal abilities seems to be at a similar level (Kogut et al., Citation1993; Brooks et al., Citation1996). The anti-cancer drug, 5-FU, has been used extensively to generate neutropaenic mice as models for the study of human bone marrow transplantation and cancer treatment (Ardalan & Glazer, Citation1981; Donowitz & Quesenberry, Citation1986). In the present study, 5-FU treatment resulted in an approximately 44-fold reduction of circulating heterophils, confirming previous observations in chickens (Kogut et al., Citation1993; Raj et al., Citation1997). Chickens do not possess a reservoir of preformed granulocytes extravascularly (Glick & Rosse, Citation1976; Toth & Siegel, Citation1986), and as this cell line has a high turn-over rate and is dependent on a continuous in-flow of newly formed cells from the bone marrow, a single administration of 5-FU promotes a reversible reduction of the granulocytic cells in the chicken. We did not investigate whether blood cells other than granulocytic cells were affected by the cytotoxic effects of 5-FU and our cell staining method did not differentiate between subgroups of granulocytic cells. However, previous studies including differential counts have shown that 5-FU primarily acts on the granulocytic precursors in the bone marrow of chickens (Kogut et al., Citation1993). In addition, adverse effects on eosinophils and basophils are unlikely to be of significance in the present study, since the numbers of these cells are low in the normal chicken (Bounous & Stedman, Citation2000).
The onset of pathological lesions and mortality happened very quickly in the present study, resulting in a high number of severely affected individuals within 5 to 6 h p.i. We have previously observed a similar response in a preliminary study using 50-week-old layers, inoculated i.v. with the same dose and strain of G. anatis, where seven out of nine chickens succumbed within 4 h p.i. with lesions very similar to those described for the i.v. inoculated birds in the present work (A.M. Bojesen and M. Bisgaard, unpublished results). The chickens from the preliminary study were obtained from a commercial flock still in production, and were healthy according to clinical observations. However, we did not attempt to define their immune status specifically, and it may have been partly impaired, explaining the high mortality rate and the severe pathological lesions. The results from the preliminary and present studies indicate that at least certain strains of Gallibacterium may play a role as a pathogen in apparently healthy chickens. This contradicts some earlier investigations that suggested only severely debilitated chickens are likely to be affected by a Gallibacterium infection (Bisgaard, Citation1977; Gerlach, Citation1977).
The G. anatis strain 12656-12 used in the present study was originally isolated from a hen dying from septicaemia in a commercial flock. Another isolate from a hen in the same flock with similar lesions was subjected to genomic fingerprinting, which revealed that the two isolates represented identical clonal types, indicating that this strain could be particularly virulent (Bojesen et al., Citation2003b). This may also explain the serious lesions in the birds from the present study compared with previous studies that may have involved less virulent strains. The basis for differences in pathogenic potential is not known, but is probably governed by the possession and expression of virulence factors. No investigations have, however, to our knowledge, been performed to characterize specific factors that could be of significance to G. anatis. Some apparent candidates do exist as most clinical isolates of G. anatis from chickens are strongly haemolytic when grown on media containing bovine, horse, rabbit or chicken blood (Greenham & Hill, Citation1962; Christensen et al., Citation2003). Closely related bacteria also encode haemolytic toxins, and those of the RTX toxin family seem to be especially widespread among members of the family Pasteurellaceae, whereas the toxins LktA in Mannheimia haemolytica (Lo et al., Citation1989) and ApxI-IV in Actinobacillus pleuropneumoniae (Chang et al., Citation1989, Citation1993; Frey et al., Citation1991; Schaller et al., Citation1999) have been shown to be important for virulence. A similar role may be ascribed to Aqx in Actinobacillus equuli, but this remains to be shown in vivo (Kuhnert et al., Citation2000). Certain strains of Gallibacterium also produce a capsule, which may be of importance as a virulence factor as has been shown for Pasteurella multocida (Boyce & Adler, Citation2000). The roles of these putative virulence factors remain, however, pure speculation and a subject for further analysis.
Several host and environmental factors may also influence the outcome of a Gallibacterium infection. Hormonal imbalances (Gerlach, Citation1977), co-infections with other microorganisms (Shaw et al., Citation1990) and ‘cold stress’ (Matthes & Löliger, Citation1976) have all been indicated as being of importance. Matthes & Löliger (Citation1976) inoculated Gallibacterium into the crop of normal and ‘cold stressed’ chickens, which after 48 h showed a mortality rate of 0% (0/20) and 36% (9/25), respectively. Their results indicated that even though Gallibacterium may be a harmless inhabitant under normal conditions, adverse effects may be initiated by various stressors permitting invasion and even mortality as seen in heterophil depleted chickens in the present study.
Although no specific syndrome has been associated with Gallibacterium infection, a number of reports have indicated salpingitis and peritonitis as the dominant lesions (Hinz, Citation1969; Gerlach, Citation1977; Bisgaard & Dam, Citation1981; Mirle et al., Citation1991). We inoculated the chickens with Gallibacterium i.p. in order to mimic a peritoneal infection following an ascending bacterial infection through the oviduct. All chickens developed some degree of purulent peritonitis within 12 h p.i., which in the 5-FU-treated birds developed to involve the entire peritoneum. Furthermore, there was evidence of bacterial invasion of the spleen and liver as shown by positive culture and by FISH. These results indicate that at least G. anatis strain 12656-12 has the ability to promote peritonitis and gain systemic access corresponding to naturally occurring cases of Gallibacterium infections. This is of importance as this is the first controlled study of Gallibacterium infection where both the inoculated strain and the immune status of the host were defined in detail. Our results may therefore have important implications for future work attempting to elucidate the nature of the virulence factors discussed earlier, where intraperitoneal infection of 5-FU-treated chickens may act as an infection model for this purpose.
In one of the chickens inoculated i.p., Gallibacterium was identified in the liver by FISH although bacterial cultivation was negative. This indicates that FISH may be more sensitive for identifying Gallibacterium in infected tissue than the traditional methods based on bacterial cultivation. Similar results were demonstrated for the identification of Haemophilus somnus from naturally infected bovine lungs (Tegtmeier et al., Citation2000), which emphasizes the need for alternative detection methods to complement culture when establishing a definitive diagnosis.
In conclusion, we confirmed that 5-FU is a potent drug for mediating heterophil depletion in chickens in order to study the pathogenesis of bacteria with a low to moderate virulence. We showed that i.v. and, in particular, i.p. infections resulted in pathological lesions comparable with those reported previously from naturally infected chickens, indicating that the present protocol may act as a model for future studies of the G. anatis pathogenesis.
Acknowledgments
Technical staff Johnny Jensen and Thomas Bernau Kristiansen are thanked for their great help with the practical parts of this work. The Danish Agricultural and Veterinary Research Council financed this work (grant no. 9702797).
References
- Addo , PB and Mohan , K . 1984 . Atypical Pasteurella haemolytica Type A from poultry . Avian Diseases , 29 : 214 – 217 .
- Ardalan , B and Glazer , R . 1981 . An update on the biochemistry of 5-fluorouracil . Cancer Treatment Review , 8 : 157 – 167 .
- Bisgaard , M . 1977 . Incidence of Pasteurella haemolytica in the respiratory tract of apparently healthy chickens and chickens with infectious bronchitis. Characterisation of 213 strains . Avian Pathology , 6 : 285 – 292 .
- Bisgaard , M and Dam , A . 1981 . Salpingitis in poultry. II. Prevalence, bacteriology, and possible pathogenesis in egg-laying chickens . Nordisk Veterinaer Medicin , 33 : 81 – 89 .
- Bisgaard , M . 1993 . Ecology and significance of pasteurellaceae in animals . Zentralblat für Bakteriologie , 279 : 7 – 26 .
- Bojesen , AM , Nielsen , SS and Bisgaard , M . 2003a . Prevalence and transmission of haemolytic Gallibacterium species in chicken production systems with different biosecurity levels . Avian Pathology , 32 : 503 – 510 .
- Bojesen , AM , Torpdahl , M , Christensen , H , Olsen , JE and Bisgaard , M . 2003b . Genetic diversity of Gallibacterium anatis isolates from different chicken flocks . Journal of Clinical Microbiology , 41 : 2737 – 2740 .
- Bojesen , AM , Christensen , H , Nielsen , OL , Olsen , JE and Bisgaard , M . 2003 . Detection of Gallibacterium spp. in chickens by fluorescent 16S rRNA in situ hybridization . Journal of Clinical Microbiology , 41 : 5167 – 5172 .
- Bounous DI Stedman NL 2000 Normal avian hematology: chicken and turkey In Feldman B.H., Zinkl J.G., Jain N.C. (Eds) Schalm's Veterinary Haematology pp. 1147–1154 Philadelphia PA Lippincott, Williams & Wilkins
- Boyce , JD and Adler , B . 2000 . The capsule is a virulence determinant in the pathogenesis of Pasteurella multocida M1404 (B:2) . Infection & Immunity , 68 : 3463 – 3468 .
- Brooks , RL , Bounous , DI and Andreasen , CB . 1996 . Functional comparison of avian heterophils and human and canine neutrphils . Comparative Haematology International , 6 : 153 – 159 .
- Chang , CF , Shi , J , Ma , DP , Shin , SJ and Lein , DH . 1993 . Molecular analysis of the Actinobacillus pleuropneumoniae RTX toxin-III gene cluster . DNA and Cell Biology , 12 : 351 – 362 .
- Chang , CF , Young , RY and Struck , DK . 1989 . Cloning and characterisation of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae . DNA , 8 : 635 – 647 .
- Christensen , H , Bisgaard , M , Bojesen , AM , Mutters , R and Olsen , JE . 2003 . Genetic relationships among strains of biovars of avian isolates classified as ‘Pasteurella haemolytica’, ‘Actinobacillus salpingitidis’ or Pasteurella anatis with proposal of Gallibacterium anatis gen. nov., comb. nov. and description of additional genomospecies within Gallibacterium gen. nov . International Journal of Systematic and Evolutionary Microbiology , 53 : 275 – 287 .
- Donowitz , GR and Quesenberry , P . 1986 . 5-Fluorouracil effect on cultured murine stem cell progeny and peripheral leukocytes . Experimental Hematology , 14 : 207 – 214 .
- Frey , J , Meier , R , Gygi , D and Nicolet , J . 1991 . Nucleotide sequence of the haemolysin I gene from Actinobacillus pleuropneumoniae . Infection and Immunity , 59 : 326 – 332 .
- Gerlach , H . 1977 . Significance of Pasteurella haemolytica in poultry . Der Praktische Tierartz , 58 : 324 – 328 .
- Glick , B and Rosse , C . 1976 . Cellular composition of the bone marrow in the chicken. Identification of cells . Anatomical Record , 185 : 235 – 246 .
- Greenham , LW and Hill , TJ . 1962 . Observations on an avian strain of Pasteurella haemolytica . The Veterinary Record , 74 : 861 – 863 .
- Harbourne , JF . 1962 . A haemolytic coccobacillus recovered from poultry . The Veterinary Record , 74 : 566 – 567 .
- Harry , EG . 1962 . A haemolytic coccobacillus recovered from poultry . The Veterinary Record , 74 : 640
- Hinz KH 1969 Bakteriologische Befunde bei Erkrankung der Atmungsorgane von Junghennen 9th Congress of the World Veterinary Poultry Associasion 713 pp Beograd Yugoslavia
- Kjos-Hanssen , B . 1950 . Oviduct peritonitis in hens due to pathogenic ‘cloacal bacteria’ . Nordisk Veterinaer Medicin , 2 : 523 – 531 .
- Kogut , MH , Tellez , G , Hargis , BM , Corrier , DE and Deloach , JR . 1993 . The effect of 5-fluorouracil treatment of — a cell depletion model for the study of avian polymorphonuclear leukocytes and natural host defenses . Poultry Science , 72 : 1873 – 1880 .
- Kohlert , R . 1968 . Untersuchrungen zur Ätiologie der Eileiterentzündung beim Huhn . Monatshefte für Veterinärmedizin , 23 : 392 – 395 .
- Kuhnert , P , Heyberger-Meyer , B , Nicolet , J and Frey , J . 2000 . Characterization of PaxA and its operon: a cohemolytic RTX toxin determinant from pathogenic Pasteurella aerogenes . Infection and Immunity , 68 : 6 – 12 .
- Lo , RYC , Strathdee , CA and Shewen , PE . 1989 . Nucleotide sequence of the leucotoxin genes of Pasteurella haemolytica A1 . Infection and Immunity , 55 : 1987 – 1996 .
- Martin SW Meek AH Willeberg P 1987 Veterinary Epidemiology Ames IA Iowa State University Press
- Matthes , S and Löliger , HCh. 1976 . Beitrag zur kinetik Bakterieller Infektionen beim Huhn . Berliner und Munchener Tieraztliche Wochenschrift , 89 : 98 – 102 .
- Matthes , S , Löliger , HCh. and Schubert , HJ . 1969 . Enzootisches auftreten der Pasteurella haemolytica beim huhn . Deutche Tierärztliche Wochenschrift , 76 : 94 – 95 .
- Mirle , C , Schongarth , M , Meinhart , H and Olm , U . 1991 . Studies into incidence of Pasteurella haemolytica infections and their relevance to hens, with particular reference to diseases of the egg-laying apparatus . Monatshefte fur Veterinärmedizin , 46 : 545 – 549 .
- Mushin , R , Weisman , Y and Singer , N . 1980 . Pasteurella haemolytica found in respiratory tract of fowl . Avian Diseases , 24 : 162 – 168 .
- Raj , GD , Savage , CE and Jones , RC . 1997 . Effect of heterophil depletion by 5-fluorouracil on infectious bronchitis virus infection in chickens . Avian Pathology , 26 : 427 – 432 .
- Schaller , A , Kuhn , R , Kuhnert , P , Nicolet , J , Anderson , TJ , MacInnes , JI , Segers , RP and Frey , J . 1999 . Characterisation of apxIVA, a new RTX determinant of Actinobacillus pleuropneumoniae . Microbiology , 145 : 2105 – 2116 .
- Shaw , DP , Cook , DB , Maheswaran , K , Lindeman , CJ and Halvorson , DA . 1990 . Pasteurella haemolytica as a co-pathogen in pullets and laying hens . Avian Diseases , 34 : 1005 – 1008 .
- Suzuki , T , Ikeda , A , Shimada , J , Yanagawa , Y , Nakazawa , M and Sawada , T . 1996 . Isolation of ‘Actinobacillus salpingitidis’/avian Pasteurella haemolytica-like isolate from diseased chickens (abstract) . Journal of the Japan Veterinary Medical Association , 49 : 800 – 804 .
- Suzuki , T , Ikeda , A , Taniguchi , T , Nakazawa , M and Sawada , T . 1997a . Pathogenicity of an Actinobacillus salpingitidis/avian Pasteurella haemolytica-like isolate from laying hens (abstract) . Journal of the Japan Veterinary Medical Association , 50 : 381 – 385 .
- Suzuki , T , Ikeda , A , Shimada , J , Sawada , T and Nakazawa , M . 1997b . Pathogenicity of an Actinobacillus salpingitidis/avian Pasteurella haemolytica-like isolate from layer hens that died suddenly (abstract) . Journal of the Japan Veterinary Medical Association , 50 : 85 – 88 .
- Tegtmeier , C , Angen , O and Ahrens , P . 2000 . Comparison of bacterial cultivation, PCR, in situ hybridization and immunohistochemistry as tools for diagnosis of Haemophilus somnus pneumonia in cattle . Veterinary Microbiology , 76 : 385 – 394 .
- Toth , TE and Siegel , PB . 1986 . Cellular defenses of the respiratory tract: paucity of free residing macrophages in the normal chicken . Avian Diseases , 30 : 67 – 75 .