Abstract
Australian broiler breeders were screened for avian leukosis viruses (ALVs) (May 2001 to December 2003) as surveillance of measures to reduce the prevalence of ALV-J. Samples of blood (4233), albumen (1122), meconium (99) and tumours (16) were obtained from 93 flocks in six Australian states. Virus isolation was performed in C/O chick embryo fibroblast cultures, which were initially screened by group-specific antigen enzyme-linked immunosorbent assay, with follow-up confirmation using polymerase chain reaction. The chronology of isolations reveals the circulation of both ALV-J and ALV-A during this period. On 16 occasions single isolations were found to contain both ALV-A and ALV-J. This is the first report of dual infections with two subgroups of ALV occurring in the same chicken. The effectiveness of ALV-J eradication measures is indicated by the absence of any ALV-J isolations in late 2003. ALV-A however, continued to be isolated from the broiler population. The detection of dual infections, as well as the ongoing occurrence of ALV-A in meat-type birds, is discussed in the context of ongoing potential for recombinations and the associated threat for the emergence of avian leukosis virus with changes in host range and pathogenicity.
Infections simples et concomitantes par les virus ALV-J et ALV-A de la leucose aviaire chez des poulets de chair australiens
Des reproducteurs de type chair ont fait l'objet d'un dépistage des virus de la leucose aviaire (de mai 2001 à décembre 2003), dans le cadre d'un plan de surveillance pour réduire la prévalence de l'ALV-J. A partir de 93 troupeaux répartis dans six états australiens, il a été prélevé 4233 échantillons de sang, 1122 d'albumen, 99 de méconium, et 16 tumeurs. L'isolement du virus a été réalisé sur cultures de fibroblastes d'embryon de poulet (CEF) C/O. les cultures ont fait l'objet, en premier d'un screening par ELISA, antigène spécifique de groupe (gsa) et ensuite d'une PCR de confirmation. La chronologie des isolements a révélé la circulation des deux virus ALV-J et ALV-A durant cette période. Dans 16 cas, l'isolement viral a mis en évidence la présence des deux virus ALV-A et ALV-J. C'est la première publication qui fait état d'infections doubles par deux sous-groupes d'ALV, chez un même poulet. L'efficacité des mesures d’éradication de l'ALV-J est mise en évidence par l'absence d'isolement d'ALV-J, fin 2003. Cependant, l'ALV-A continue d’être isolé chez les poulets. La détection d'infections doubles, tout comme l'existence de l'ALV-A chez les animaux de type chair, est discutée dans le contexte d'un potentiel pour des recombinaisons et la menace associée pour l’émergence de virus de la leucose avec des changements d'hôtes et de pathogénicité.
Einzel- und Doppelinfektionen mit den aviäre Leukoseviren ALV-J und ALV-A bei australischen Masttyp-Hühnern
Als Überwachungsmaßnahme zur Reduzierung des Vorkommens von ALV-J wurden von Mai 2001 bis Dezember 2003 australische Broilereltern auf aviäre Leukoseviren überprüft. Aus 93 Herden in sechs australischen Staaten wurden Blut (4233)-, Eiweiß (1122)- Mekonium (99)- und Tumor (16)-proben entnommen. Die Virusisolierung wurde in C/O-HEF-Kultur, die vorher mittels gruppenspezifischem (gsa)-ELISA mit nachfolgender Absicherung durch PCR überprüft worden waren, durchgeführt. Die Chronologie der Isolierungen ließ die Zirkulation sowohl von ALV-J als auch ALV-A während des Untersuchungszeitraums erkennen. In sechzehn Fällen enthielten die Isolate sowohl ALV-A als auch ALV-J. Dies ist die erste Beschreibung einer gleichzeitigen Infektion mit zwei ALV aus verschiedenen Subgruppen in einem Huhn. Die Effektivität der ALV-J-Eradikationsmaßnahmen wurde durch ausbleibende ALV-J-Isolierungen zum Ende des Jahres 2003 nachgewiesen. ALV-A wurde jedoch weiterhin aus der Broilerpopulation isoliert. Der Nachweis der Doppelinfektionen sowie das anhaltende Vorkommen von ALV-A bei Masthühnern wird im Zusammenhang mit dem vorhandenen Rekombinationspotential und der damit verbundenen Bedrohung durch die Entstehung von aviärem Leukosevirus mit Veränderungen des Wirtsspektrums und der Pathogenität diskutiert.
Infecciones simples y combinadas con virus de leucosis aviar, ALV-J y ALV-A, en pollos de engorde
Se analizaron los reproductores de pollos de engorde australianos para detectar virus de leucosis (de mayo 2001 a diciembre 2003), para evaluar las medidas utilizadas para reducir la prevalencia de ALV-J. Se recogieron muestras de sangre (4233), albumen (1122), meconio (99) y tumores (16) de 93 lotes de seis estados australianos. El aislamiento vírico se realizó en cultivos celulares CEF C/O, que fueron inicialmente analizados mediante un ELISA con un antígeno específico de grupo (gsa), con confirmación posterior mediante PCR. La cronología de los aislamientos revela la circulación de ALV-J y ALV-A durante este periodo. En dieciséis ocasiones se aislaron conjuntamente ALV-A y ALV-J. Esta es la primera descripción de infecciones duales con dos subgrupos de ALV en el mismo animal. La efectividad de las medidas de erradicación de ALV-J viene indicada por la ausencia de aislamientos de ALV-J a finales del 2003. Aún así, ALV-A sigue siendo aislado de la población de pollos de engorde. La detección de infecciones duales, así como la existencia de ALV-A en pollos de carne, se discute en el contexto de recombinaciones potenciales y el riesgo asociado de emergencia de nuevos virus de leucosis aviar con cambios en la patogenicidad y rango de huéspedes.
Introduction
Avian leukosis virus (ALV) is the most common naturally occurring avian retroviral infection, causing neoplastic diseases and other production problems in chickens (Fadly & Payne, Citation2003). There are six well-characterized chicken subgroups of ALV (A to E and J), which are defined on the basis of host range, antibody neutralization, and receptor interference studies (Fadly & Payne, Citation2003). Viruses of subgroups A, B and J occur as common pathogenic exogenous viruses in the field, but those of subgroups C and D have rarely been reported in the field (Sandelin & Estola, Citation1975). Subgroup E virus includes the ubiquitous endogenous leukosis virus of low to no pathogenicity (Smith, Citation1987). ALV infections with subgroup A and subgroup B have had a significant impact in commercial layer flocks, both from lymphoid leukosis (B-cell lymphoma) mortality after sexual maturity and from lowered egg production (Fadly & Payne, Citation2003). Subgroup J (ALV-J) was first reported in the UK in 1991 (Payne et al., Citation1991a) and was found to be associated with myeloid leukosis (Payne et al., Citation1991b) and other production problems in meat-type chickens (Stedman & Brown, Citation1999).
ALV-J is believed to have arisen by recombination between exogenous ALVs and the EAV family of endogenous retroviruses (Bai et al., Citation1995a,Citationb; Smith et al., Citation1999). Sequence analysis of more recent isolates of ALV-J has revealed multiple changes in the env gene leading to antigenic variation (Venugopal et al., Citation1998). Susceptibility to tumour formation has been found to vary markedly between different genetic lines of chickens (Payne et al., Citation1992), but meat-type chickens are particularly prone to tumours.
A serological survey revealed the occurrence of ALV-J infection within broiler breeder flocks in Australia (Bagust, Citation2000). To control ALV-J and reduce economic losses being incurred through ALV-J-associated mortalities and production losses in broiler breeders, a clear understanding of the ALV-J status in Australian breeding flocks was required. The establishment of detection technology was successfully undertaken (Bagust et al., Citation2004) and has ultimately led to the implementation of measures to reduce the prevalence of ALV-J in Australian broiler stocks.
Simultaneous infections with more than one subgroup have not been previously reported for ALV. This paper presents evidence that infections by both ALV-J and ALV-A have been circulating within various broiler breeder flocks in Australia, and in a number of cases dual infections in the same chicken have been detected. Also, while industry measures to reduce the prevalence of ALV-J appear to be taking effect, active (viraemic) ALV-A infections in broiler breeder stocks in Australia continue to be readily detectable.
Materials and methods
Experimental samples
Between May 2001 and December 2003 broiler grandparent and parent flocks belonging to two different breeds, designated A and B, from commercial breeding companies were sampled for the isolation of ALV, as part of a research programme targeted at reduction and then removal of ALV-J from Australian poultry stocks. The breeding stocks for breeds A and B are both imported at GGP level from Europe or the USA. Breeder sites were visited and necropsies performed on the farm in order to assess the disease status of flocks and to collect specimens for laboratory examinations for ALV infections. Samples of blood (4233), albumen (1122), meconium (99) and tumours (16) were obtained from 93 flocks in six Australian states using procedures detailed recently (Bagust et al., Citation2004). The most recent samples were taken in December 2003, when 50 whole blood samples from breed A were obtained from eight commercial broiler parent flocks aged between 18 and 25 weeks, situated on sites in Queensland, New South Wales, Victoria and Tasmania. Virus isolation and polymerase chain reaction (PCR) was performed to determine the subgroups of ALV present.
Cells and viruses
The DF-1 chicken embryo fibroblast cell line (ATCC CRL-12203), known to be resistant to ALV-E, was obtained from the American Type Culture Collection (Manassas, Virginia, USA). C/O chicken embryonic fibroblast cell culture (CEFs), which are susceptible to all ALVs, were prepared from specific pathogen free embryos initially obtained from Intervet Pty. Ltd (Newcastle NSW, Australia), and then subsequently from a derivative flock that is currently maintained at the University of Melbourne Veterinary Precinct at Werribee. The ALV-J strains HPRS-103 (UK prototype; Payne et al., Citation1991a) and ADOL-Hc1 (USA reference strain; Fadly & Smith, Citation1999) were obtained through Dr Guillermo Zavala, University of Georgia, USA. Dr Gordon Firth (Intervet Australia) kindly supplied the Australian reference strain of ALV-J designation J-98290/191. This virus was isolated in 1998 and is the first confirmed isolate of ALV-J obtained in Australia.
Virus isolation
Virus isolation was achieved from buffy-coat cells, egg albumen, meconium and tumour homogenates using standard techniques (Fadly & Witter, Citation1998). Briefly, samples were inoculated on to CEF cell cultures prepared from specific pathogen free C/O embryos and samples were then incubated for two serial passages each of 5 to 7 days at 37°C. After incubation, cell lysates were prepared after three cycles of freeze thawing and tested for ALV group specific antigen (gsa) (p27) by an antigen capture enzyme-linked immunosorbent assay (AC-ELISA) using anti-p27 antibody-coated plates (IDEXX laboratories Pty. Ltd, Zetland, NSW, Australia). For the most recent sampling (December 2003) of eight commercial parent flocks as an additional screening procedure, the mean S/P ratios for each flock were calculated and any sample showing significant deviation above this mean was selected to undergo PCR testing. Any of the selected gsa-ELISA potential positives that were found to be negative by PCR were then further passaged in DF-1 cells. Genomic DNA from gsa-ELISA-positive CEF cultures was extracted and ALVs identified by PCR. In order to monitor for the possible cross-contamination of samples, negative tissue culture controls (C/O CEFs with no sample added) were included in each 24-well plate used during an isolation procedure. These controls were incubated and processed under the same conditions as the samples. These negative tissue culture control samples were subjected to ALV-J, ALV-A and β-actin PCR. Only when this control was negative for gsa-ELISA, ALV-J PCR and ALV-A PCR and positive for β-actin PCR would a corresponding sample from the isolation be designated an ALV status; that is, ALV-negative, ALV-J-positive, ALV-A-positive, or positive for both ALV-A and ALV-J.
Polymerase chain reaction
The PCR used to test genomic DNA from cultured CEFs for the presence of proviral envelope sequence specific for ALV-J and ALV-A is described by Smith et al. (Citation1998a,Citationb). Before DNA extraction, cultured cells were lysed in tissue lysis buffer (4 M guanidine hydrochloride, 25 mM sodium citrate, 1% triton×-100) and then extracted twice with phenol–chloroform–iso-amyl alcohol (25:24:1). The DNA was precipitated with absolute isopropanol, washed with 70% isopropanol and dried at room temperature. The DNA was then resuspended in nuclease-free water and stored at 4°C.
The oligonucleotide primers used in PCR detection of ALV-J and ALV-A are presented in . These include the forward primer H5 that anneals just upstream from the 3′ region of the pol gene and is conserved across several ALV subgroups. The ALV-J reverse primer H7b (1 base pair [bp] shift of H7 primer; Smith et al., Citation1998a) anneals specifically in the gp85 region of ALV-J, while Env-A anneals specifically to ALV-A envelope sequence (position 694 to 718 of the RAV-1 sequence; accession number, M19113).
Table 1. PCR primer sequences
ALV proviral DNA was amplified in a 25 μl reaction volume, using a mixture composed of 10 mM Tris–HCl (pH 8.8), 50 mM KCl, 1.0 mM MgCl2, 200 μM dNTPs, 20 pmol each primer and 0.5 U Taq polymerase. Amplification was performed in a iCycler thermal cycling system (Bio-Rad) with preheating at 94°C for 4 min, and 35 cycles with denaturation at 94°C for 30 sec, primer annealing at 58°C for ALV-J primers (55°C for ALV-A and all other primers) for 30 sec, and amplification at 72°C for 30 sec with a 5 sec increment in each cycle, followed by a final elongation at 72°C for 10 min. Primers designed to specifically amplify the entire ALV-J envelope sequence J5′ and J3′ (Zavala et al., Citation2002) were used to amplify the ALV-J env region. The primers EnvAF (the complement of EnvA) and A3′ (designed towards a 3′ sequence in ALV-A) were also used to amplify the majority of the ALV-A env region. Primers toward β-actin, Bar and Baf () were used in a similar PCR and amplified a fragment of 401 bp as a control to ensure the presence of chicken genomic DNA when negative PCR results were observed for ALV sequences. Amplification products were resolved by electrophoresis through 1% agarose gels containing 10 ng/ml ethidium bromide and examined under ultraviolet light transillumination. Samples were considered ALV-J-positive if the expected 544 bp product (694 bp for ALV-A) could be visualized after gel electrophoresis.
Results
Clinical findings
During farm visits made to commercial breeding operations, examination of flock records for commercial breed B showed some of these had regularly experienced lower egg production, higher mortality and flock uniformity problems relative to breed standards and operator parameters. Some flocks of breed A had experienced similar problems. Characteristic myeloid tumours were observed in both breeds. In December 2003 whole blood samples were obtained from eight commercial parent flocks of strain A at 18 to 25 weeks of age, which had no obvious clinical signs or production problems to that time.
ALV isolations made during May 2001 to December 2003
The mixture of ALV subgroups that were detected during May 2001 to December 2003 is shown schematically in . Over this period ALV-J, ALV-A and dual infections with both of these subgroups were detected. The last positive ALV-J isolation was recorded in August 2003, while isolations of ALV-A have continued to be detected.
Figure 1. Time-line of ALV isolations made in Australian broilers between May 2001 and December 2003. The shading of a shape depicts the subgroup of the ALV that was isolated: ALV-J (black) or ALV-A (grey)-negative samples are not coloured. The time scale shows the month in which the isolation was made. The different symbols indicate the type of sample from which an isolation was made. Diagonal dual shading indicates dual infection in an individual bird. Vertical dual shading indicates flocks with both subgroups present (not in individual birds). * The single occasion when a flock has been sampled twice, being first at 30 weeks of age (June 2003) and then at 40 weeks of age. The majority of samples are from breed A; those isolations performed from breed B birds are indicated within the shape with the letter B.
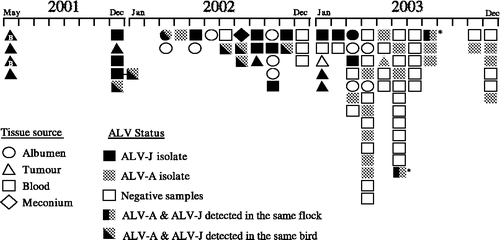
Initial isolations made were of ALV-J alone and were obtained from flocks that had been suspected to contain ALV-J, by the presence of myeloid tumours in livers and sternal region. In December 2001 isolations of ALV-J were made from buffy-coat and tumour samples, but at this time dual infections by ALV-J and ALV-A from buffy-coat samples were also identified. During the next 12-month period a total of 16 samples, from seven different flocks of breed A, revealed dual infections with ALV-J and ALV-A (). These isolations were predominantly made from buffy-coat samples, but on one occasion (March 2002) both subgroups were isolated simultaneously from albumen samples. During this period flocks that were infected with only ALV-J or ALV-A were also detected. In one flock examined (December 2001), one bird was found to be infected with ALV-J only, while birds with dual infections of ALV-J and ALV-A were also detected. Conversely, flocks with birds infected with ALV-A only, and birds with dual infections of ALV-J and ALV-A were not detected. A 30-week-old flock of breeders sampled in June 2003 and again in August 2003 showed the continuing presence of ALV-J and ALV-A at both samplings, but no individual birds showing dual infections of ALV-A and ALV-J subgroups were detectable.
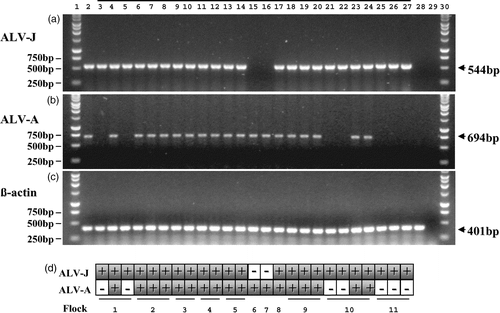
Figure 2. PCR detection of ALV-J, ALV-A and dual infections of both subgroups. Twenty-five of the samples depicted in have been selected to demonstrate the PCR results recorded for these samples. This includes seven isolates of ALV-J alone, two isolates of ALV-A alone and the entire 16 dual infections of both ALV-J and ALV-A made during the period May 2001 to December 2003. 2a: Samples tested by ALV-J specific PCR using the primers H5 and H7b: lane 1, 1 kb ladder; lane 2, ALV-J-positive control; lane 3 to lane 27, over-lined are 25 ALV samples isolated using C/O CEFs during the period May 2001 to December 2003; lane 28, Genomic DNA extracted from a tissue culture negative control; lane 29, PCR negative control (H5/H7b); lane 30, 1 kb ladder. The 544 bp band amplified from ALV-J-positive samples is indicated. 2b: Samples tested by ALV-A specific PCR using the primers H5 and EnvA: lane 1, 1 kb ladder; lane 2, ALV-A-positive control; lane 3 to lane 27, 25 ALV samples isolated using C/O CEFs during the period May 2001 to December 2003; lane 28, genomic DNA extracted from a tissue culture negative control; lane 29, PCR negative control (H5/EnvA); lane 30, 1 kb ladder. The 694 bp band amplified from ALV-A positives samples is indicated. 2c: Samples subject to chicken β-actin control PCR using the primers Baf and Bar: lane 1, 1 kb ladder; lane 2, chicken genomic DNA control; lane 3 to lane 27, 25 ALV samples isolated using C/O CEFs during the period May 2001 to December 2003; lane 28, tissue culture negative control; lane 29, PCR negative control (Baf/Bar); lane 30, 1 kb ladder. The 401 bp band amplified from ALV positive samples is indicated. Importantly the tissue culture negative control (lane 28, from which no product was seen with both ALV-J and ALV-A PCR) did generate this 401 bp fragment, demonstrating the presence of chicken genomic DNA. 2d: Tabulated results of the PCR tests indicating the source of the flock of the samples.
Confirmation of ALV-J infection
In , some 25 representative examples of ALV isolates across the sampling period illustrate PCR detection of ALV-J, ALV-A and also co-isolations of ALV-J and ALV-A. Each of these samples has been tested by PCR for ALV-J, ALV-A and β-actin using the protocol outlined. ALV-J is the single subgroup present in seven samples from three different flocks, as demonstrated by the amplification of a 544 bp fragment when using the ALV-J specific primers H5 and H7b (), while a PCR reaction using the ALV-A specific primers H5 and Env-A () failed to amplify any product in these seven ALV-J-positive samples (lanes 3, 5, 21, 22, 25, 26 and 27).
Confirmation of ALV-A infection
Referring again to , ALV-A is the single subgroup present in only two samples taken from two different flocks. This is demonstrated by the amplification of a 694 bp product when using the ALV-A specific primers H5 and Env-A (), while the PCR reaction using the ALV-J specific primers H5 and H7b failed to amplify any product in these two ALV-A positive samples (, lanes 15 and 16).
Detection of concurrent infection with ALV-A and ALV-J
The presence of both subgroups is demonstrated by the amplification of a 544 bp product when using the ALV-J specific primers H5 and H7b (), and also a 694 bp product when using the ALV-A specific primers H5 and Env-A (). Twenty-five gsa-ELISA positive samples are shown in ; 16 of these can be seen to contain both ALV-A and ALV-J. These 16 represent all the dual isolations that have been obtained in the present study.
ALV-J and ALV-A envelope sequence amplification
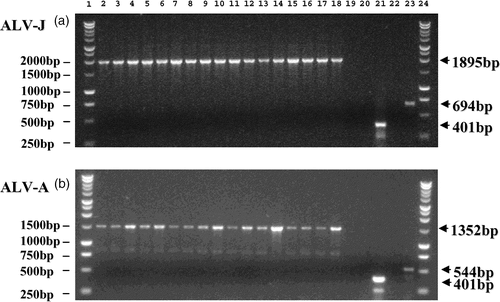
The complete env sequence of ALV-J was amplified using the primers J5′ and J3′ from genomic DNA isolated from all 16 dual infection samples (). Similarly the majority of the ALV-A env region (all env sequence 3′ of the EnvA primer) was amplified using the primers EnvAF (the reverse and complement of EnvA) and A3′ (situated in the 3′ region of ALV-A) in all 16 dual infection samples (). This combined with the ALV-J specific H5/H7b PCR and the ALV-A specific H5/EnvA PCR () demonstrates the presence of the complete and unaltered env sequence for both ALV-J and ALV-A in these dual infected samples, and would indicate the absence of a recombinant that would be unlikely to carry both complete env regions.
Figure 3. Amplification of ALV-J and ALV-A envelope sequences. The 16 samples identified as having dual infections of ALV-J and ALV-A were amplified with primers designed to specifically amplify the envelope sequences for both ALV-J (J5′/J3′) and ALV-A (EnvAF/A3′). 3a: Dual infected samples subject to ALV-J envelope specific PCR using the primers J5′ and J3′: lane 1, 1 kb ladder; lane 2, ALV-J-positive control; lane 3 to lane 18, 16 dual infected samples (J5′/J3′); lane 19, PCR negative control (J5′/J3′); lane 20, tissue culture negative control (J5′/J3′); lane 21, tissue culture negative control (Baf/Bar); lane 22, ALV-A control (J5′/J3′); lane 23, ALV-A-positive control (H5/EnvA); lane 24, 1 kb ladder. 3b: Dual infected samples subject to ALV-A envelope specific PCR using the primers EnvAF and A3′: lane 1, 1 kb ladder; lane 2, ALV-A-positive control; lane 3 to lane 18, 16 dual infected samples (EnvAF/A3′); lane 19, PCR negative control (EnvAF/A3′); lane 20, tissue culture negative control (EnvAF/A3′); lane 21, tissue culture negative control (Baf/Bar); lane 22, ALV-J control (EnvAF/A3′); lane 23, ALV-J control (H5/H7b); lane 24, 1 kb ladder.
Apparent continuing presence of ALV-A
In December 2003, as part of routine screening for ALV, eight flocks of broiler parents of commercial breed A were sampled at 18 to 25 weeks of age. A total of 409 blood samples were cultured and screened by gsa-ELISA. 25/409 (6%) were selected on gsa screening results after 2×5 day passages in C/O CEFs, and were then examined by PCR (). ELISA S/P ratios of these 25 samples were found to range from as low as 0.11 up to 3.24 (). Of these 25 samples, 14 of the gsa-positive samples were identified as ALV-A, while no ALV-J-positive samples were identified. The remaining 11 samples selected based on gsa-ELISA in which no ALV-J or ALV-A was detected were generally in the lower S/P ratio range, but there were a number of exceptions. An S/P ratio of 0.18 was identified as ALV-A-positive by PCR and demonstrates that S/P ratios below the gsa-ELISA cut-off of 0.20 may contain replicating exogenous ALV. Conversely, S/P ratios of 0.22, 0.23, 0.27, 0.35, 0.41 and 0.59 were found to negative for both ALV-A and ALV-J. These initial positive gsa-ELISA reactions were attributed to endogenous ALV-E elements as a passage in DF-1 (C/E) cells yielded negative gsa-ELISA results.
Table 2. Summary of ALV isolations made in December 2003 from parent broiler testing (Breed A)
Table 3. Comparison of gsa-ELISA S/P ratios with PCR detection
Discussion
The chronology of ALV isolations, which has been demonstrated here (), reflects the changing dynamics of ALV-J and ALV-A infection in the Australian broiler industry during the period May 2001 to December 2003. We wish to emphasize, however, that our data have been influenced by early selective sampling and later by efforts to reduce ALV-J. During 2001 and 2002 initial testing had been targeted towards flocks in which ALV-J infection was indicated by the presence of visceral and sternal tumours. Many of these flocks were terminated by industry on proof of ALV-J infection. More recent testing has been more widespread, reflecting our efforts to detect and then reduce the prevalence of ALV-J. A decline in ALV-J appears to be occurring in 2003, reflected by an increasing prevalence of negative flocks. During the period May 2001 to December 2003 isolations of either ALV-J or ALV-A were regularly being observed, as well as dual infections by both subgroups in individual birds, reflecting the presence of these two subgroups in broiler breeder stocks during late 2001 and 2002. The more recent decline in detection of dual infections is likely to be directly attributable to the active steps being taken to reduce ALV-J.
Models for both homologous and non-homologous recombination between retroviral sequences and also between retroviral sequences and cellular RNA have been described (Hajjar & Linial, Citation1993; Girod et al., Citation1996). The potential of ALV for undergoing recombinations is no surprise, as emergence of the original ALV-J isolate has been attributed to recombination occurring between an exogenous virus and an endogenous retroviral sequence (Bai et al., Citation1995b). Eradication of exogenous ALVs has become a priority in recent years. This is necessary to reduce the potential for exogenous viruses to undergo recombination with other exogenous virus, or with endogenous retroviral sequences. Interestingly, elimination of one potential source of recombination, the endogenous ALV-E sequences, has recently become a more realistic possibility (Bacon et al., Citation2004). A number ALV recombinations have been described, including an ALV-J virus encoding an ALV-A envelope (Lupiani et al., Citation2000), an acutely transforming isolate of ALV-J (strain 966) that induces rapid-onset tumours (Chesters et al., Citation2001), and Gingerich et al. (Citation2002) have described a layer flock that exhibited myeloid leukosis and the presence of a recombinant ALV containing the long terminal repeat (LTR) of ALV-J and the envelope of ALV-B.
Simultaneous viraemic infections by multiple subgroups of ALV occurring within the same bird have not been reported elsewhere to date, although the co-occurrence of ALV-A and ALV-J has been recognized as likely to have been possible in Europe in the past decade (Venugopal Nair, Institute for Animal Health, Compton, UK, unpublished data). Eradication of ALV-A in meat-type chickens may not have been as strictly followed as it had been in layer stocks. Hence ALV-A infections persisted in some broiler breeders while ALV-J was emerging in the early 1990s. The observation of dual infections is strengthened by the fact that there is no evidence of receptor interference between ALV-A and ALV-J (Payne et al., Citation1991b) and that they have distinct cell tropism (Arshad et al., Citation1997).
Tissue culture propagation is the most sensitive method for virus detection, thus we routinely rely on it for our ALV-J surveillance. While direct detection from tissue by PCR or RT-PCR is a useful diagnostic tool, it can be less reliable than culture and should not be relied upon as a primary screening tool for the eradication of ALV-J (Davidson & Borenshtain, Citation2002; Zavala et al., Citation2002). Using tissue cultures for isolation, however, can raise the possibility of cross-contamination between samples and thus is it necessary to monitor a range of tissue culture negative controls. Close examination of gsa-ELISA S/P ratios detected during ALV isolations here during December 2003 () revealed that an ALV-A isolate, confirmed by PCR analysis, had an S/P ratio of 0.18 (negative cut-off point is 0.2). This raises the question as to whether the current screening programmes based on gsa-ELISA alone can be relied upon, especially if they are performed only once and always at one designated age.
The concurrent infection with ALV-A and ALV-J demonstrated here would indicate a potential for host range extension to occur by viral recombination. Dual infections of ALV-A and ALV-J would present an ideal opportunity for these viruses to extend their host range to form new receptors for viral entry to cells (Jonah et al., Citation2003). However, our PCR results show the specific amplification of both partial () and full-length () env sequences from ALV-J and ALV-A. The size and specificity of these bands demonstrates the absence of any new recombinant ALVs arising during this period. Furthermore, findings here of isolation of single infections of both ALV-J and ALV-A, the decline of ALV-J over time and the persistence of ALV-A all support a period of co-infection occurring, and serve to highlight the danger for potential recombination.
Historically ALV-A is known to cause major disease and production problems in layer birds, while ALV-J has caused significant economic losses in meat-type chickens (Payne, Citation1998). It would be of concern, however, if either ALV-A or ALV-J were to increase its host range, via receptor acquisition, to infect genetic stocks that were previously resistant. Sporadic but confined reports of field cases of myeloid leukosis caused by ALV-J in commercial egg-type chickens have been described (Gingerich et al., Citation2002; Xu et al., Citation2004). The presence of ALV-A in meat-type chickens is undesirable, as these flocks may act as a potential source of re-infection for egg-type birds. This would further highlight the dangers of mixed incubation and hatching of broiler and layer stocks as a route of ALV transmission. Ongoing persistence of ALV-A is also a continuing concern because of the potential for recombination. Hence, eliminating all exogenous ALV in meat strains at the primary breeder level would seem to be highly desirable in the control of this disease.
The authors are grateful to all those who assisted with this work, including Ms D. O'Rourke, Ms M. Barraza and Mr P. Cowling of these International Avian Health Laboratories of the University of Melbourne. They also thank Dr Gordon Firth for supplying the original Australian isolate of ALV-J (J98290/191), and Dr Guillermo Zavala for supplying UK and US prototypes of ALV-J and antiserum for ADOL Hc1. The authors particularly wish to acknowledge the Australian poultry industry veterinarians for their cooperation and providing samples, Dr J. Ignjatovic for her editorial comments, and the Rural Industry Research and Development Corporation Chicken meat Program for their continued encouragement and financial support of these studies.
References
- Arshad , SS , Howes , K , Barron , GS , Smith , LM , Russel , PH and Payne , LN . (1997) . Tissue tropism of the HPRS-103 strain of J subgroup avian leukosis virus and of a derivative acutely transforming virus . Veterinary Pathology , 34 : 127 – 137 .
- Bacon , LD , Fulton , JE and Kulkarni , GB . (2004) . Methods for evaluating and developing commercial chicken strains free of endogenous subgroup E avian leukosis virus . Avian Pathology , 33 : 233 – 243 .
- Bagust TJ (2000) Avian leukosis virus-J in Australia: scenarios and approaches for control In Proceedings of International Symposium on ALV-J and other Retroviruses, World Veterinary Poultry Association and Institute fur Geflugelkrankheiten, Justus Liebig University Giesen (pp. 256–265) Rauischholzhausen Germany
- Bagust , TJ , Fenton , SP and Reddy , MR . (2004) . Detection of subgroup J avian leukosis virus infection in Australian meat-type chickens . Australian Veterinary Journal , 82 : 701 – 706 .
- Bai , J , Howes , K , Payne , LN and Skinner , M . (1995a) . Sequence of host range determinants in the env gene of a full-length, infectious virus proviral clone of exogenous avian leucosis virus HPRS-103 confirms that it represents a new subgroup (designated J) . Journal of General Virology , 76 : 181 – 187 .
- Bai , J , Payne , LN and Skinner , MA . (1995b) . HPRS-103 (exogenous avian leukosis virus, subgroup J) has an env gene related to those of endogenous elements EAV-0 and E51 and an E element found previously only in sarcoma viruses . Journal of Virology , 69 : 779 – 784 .
- Chesters , PM , Howes , K , McKay , JC , Payne , LN and Venugopal , K . (2001) . Acutely transforming avian leukosis virus subgroup J strain 966: defective genome encodes a 72-kilodalton gag-myc fusion protein . Journal of Virology , 75 : 4219 – 4225 .
- Davidson , I and Borenshtain , R . (2002) . The feather tips of commercial chickens are a favourable source of DNA for the amplification of Marek's disease virus and avian leukosis virus, subgroup J . Avian Pathology , 31 : 237 – 240 .
- Fadly AM Payne LN (2003) Leukosis/sarcoma group In Y.M. Saif, H.J. Barnes, J.R. Glisson, A.M. Fadly & L.R. McDougald (Eds.) Diseases of Poultry 11th edn (pp. 465–516) Ames Iowa USA Iowa State Press, Blackwell Publishing Company
- Fadly , AM and Smith , EJ . (1999) . Isolation and some characteristics of a subgroup-J-like avian leukosis virus associated with myeloid leukosis in meat-type chickens in the United States . Avian Diseases , 43 : 391 – 400 .
- Fadly AN Witter RL (1998) Oncornaviruses: leukosis/sarcoma and reticuloendotheliosis In J.R. Glisson, D.J. Jackwood, J.E. Pearson, W.M. Reed & D.E. Swayne (Eds.) A Laboratory Manual for the Isolation and Identification of Avian Pathogens 4th edn (pp. 185–196) Kennett Square PA American Association of Avian Pathologists
- Gingerich , E , Porter , RE , Lupiani , B and Fadly , AM . (2002) . Diagnosis of myeloid leukosis induced by a recombinant avian leukosis virus in commercial white leghorn egg laying flocks . Avian Diseases , 46 : 745 – 748 .
- Girod , A , Drynda , A , Cosset , F-L , Verdier , G and Ronfort , C . (1996) . Homologous and Nonhomologous retroviral recombinations are both involved in the transfer by infectious particles of defective avian leukosis virus-derived transcomplementing genomes . Journal of Virology , 70 : 5651 – 5657 .
- Hajjar , AM and Linial , ML . (1993) . A model system for nonhomologous recombinations between retroviral and cellular RNA . Journal of Virology , 67 : 3845 – 3853 .
- Jonah , G , Rainey , A , Natonson , A , Maxfield , LF and Coffin , JM . (2003) . Mechanisms of avian retroviral host range extension . Journal of Virology , 77 : 6709 – 6719 .
- Lupiani , B , Hunt , H , Silva , R and Fadly , A . (2000) . Identification and characterization of recombinant subgroup J avian leukosis viruses (ALV) expressing subgroup A ALV envelope . Virology , 276 : 37 – 43 .
- Payne , LN . (1998) . HPRS-103: a retrovirus strikes back. The emergence of subgroup J avian leukosis virus . Avian Pathology , 27 (suppl 1) : S36 – S45 .
- Payne , LN , Brown , SR , Bumstead , N , Howes , K , Frazier , JA and Thouless , ME . (1991a) . A novel subgroup of exogenous avian leukosis virus in chickens . Journal of General Virology , 72 : 801 – 807 .
- Payne , LN , Gillespie , AM and Howes , K . (1991b) . Induction of myeloid leukosis and other tumours with HPRS-103 strain of ALV . Veterinary Record , 129 : 447 – 448 .
- Payne , LN , Howes , K , Gillespie , AM and Smith , LM . (1992) . Host range of Rous sarcoma virus pseudotype RSV (HPRS-103) in 12 avian species: support for a new avian retrovirus envelope subgroup, designated J . Journal of General Virology , 73 : 2995 – 2997 .
- Sandelin , K and Estola , T . (1975) . Testing and management of a specific pathogen free chicken flock with special reference to avian leukosis virus infections . Acta Veterinaria Scandinavica , 16 : 341 – 356 .
- Smith EJ (1987) Endogenous avian leukemia viruses In G.F. Deboer (Ed.) (pp. 101–120) Avian Leukosis Boston MA Martinus Nijhoff
- Smith , EJ , Williams , SM and Fadly , AM . (1998a) . Detection of avian leukosis virus subgroup J using the polymerase chain reaction . Avian Diseases , 42 : 375 – 380 .
- Smith , LM , Brown , SR , Howes , K , McLeod , S , Arshad , SS , Barron , GS , Venugopal , K , McKay , JC and Payne , LN . (1998b) . Development and application of polymerase chain reaction (PCR) tests for the detection of subgroup J avian leukosis virus . Virus Research , 54 : 87 – 98 .
- Smith , LM , Toye , AA , Howes , K , Bumstead , N , Payne , LN and Venugopal , K . (1999) . Novel endogenous retroviral sequences in the chicken genome closely related to HPRS-103 (subgroup J) avain leukosis virus . Journal of General Virology , 60 : 261 – 268 .
- Stedman , NL and Brown , TP . (1999) . Body weight suppression in broilers naturally infected with avian leukosis virus subgroup . J Avian Diseases , 43 : 604 – 610 .
- Venugopal , K , Smith , LM , Howes , K and Payne , LN . (1998) . Antigenic variants of subgroup J avian leukosis virus: Sequence analysis reveals multiple changes in the env gene . Journal of General Virology , 79 : 757 – 766 .
- Xu , B , Dong , W , Yu , C , He , Z , Lv , Y , Sun , Y , Feng , X , Li , N , Lee , LF and Li , M . (2004) . Occurrence of avian leukosis virus subgroup J in commercial layer flocks in China . Avian Pathology , 33 : 13 – 17 .
- Zavala , G , Jackwood , MW and Hilt , DA . (2002) . Polymerase chain reaction for detection of avain leukoisis virus subgroup-J in feather pulp . Avian Diseases , 46 : 971 – 978 .