Abstract
A study was undertaken on the pathology and associated schizont morphology of apicomplexan species of avian haematozoa. Some 32 birds from the families Artamidae, Meliphagidae, Oriolidae, Podargidae, Columbidae, Alcedinidae and Psittacidae were identified as having schizonts in various tissues. Based on blood stages observed, the probable relationship to tissue stages was considered. The majority of schizonts were referable to the genera Leucocytozoon and Haemoproteus. The comparative morphology of tissue stages previously described in the literature is discussed and the involvement of protozoa other than haematozoa considered. The naturally occurring infections in wild birds described in this study represent previously unreported data on the life-cycle stages involved. Some schizonts measured up to 640 μm. While pathological changes in some hosts were noticeable, in others no significant findings were observed. The role of endogenous stages in avian morbidity is discussed briefly.
1 Introduction
Apicomplexan parasites of the genera Plasmodium, Haemoproteus and Leucocytozoon have endogenous tissue stages as part of their life cycle, giving rise to merozoites that invade either erythrocytes or leucocytes. Descriptions of endogenous stages are primarily limited to studies in domestic birds and comparable descriptions from infections in wild birds are comparatively unknown (Bennett et al., Citation1993). As part of a survey of avian haematozoa in wild birds in southeast Queensland (Adlard et al., Citation2004), the opportunity presented itself to examine histopathology from a number of infected individuals. These individuals demonstrated a range of endogenous stages from a variety of birds that have been previously unreported in the literature. The pathology associated with these endogenous stages is herein described, most for the first time.
2 Materials and Methods
All the birds examined for histopathology were the result of road casualties or unexplained morbidity and were presented for clinical examination at the Currumbin Wildlife Sanctuary Hospital (Gold Coast, Queensland). The birds were examined clinically, bled from the Vena ulnaris or Vena jugularis (for thin blood films) and were euthanazed intravenously with pentobarbitone sodium (Lethabarb® Virbac). Giemsa-stained thin blood films were prepared for identification of haematozoa in circulating blood (see Adlard et al., Citation2004). Tissue samples of the lung, liver, spleen, kidney, skeletal and myocardial muscle were taken immediately after euthanasia, fixed in 10% buffered formalin and processed histologically. The tissue sections were stained with haematoxylin and eosin (HE) and examined under light microscopy using a Zeiss Photomicroscope II under magnification from ×40 to ×1000. Endogenous stages were identified and measured using an eyepiece graticule calibrated against a stage micrometer. Photomicrographs were taken using a microscope-mounted digital video camera (Panasonic) connected to a capture device (Snappy, Play Inc.) on a personal computer. Pathological changes were noted and recorded. Avian systematics used throughout this study follows that of Christidis & Boles (Citation1994).
3 Results
Endogenous stages of haematozoan parasites were identified in a variety of tissues from 32 individual birds belonging to 10 species from six avian families. Details of the blood parasites identified in these birds and associated tissue pathology are presented in .
Pathology associated with endogenous development of avian haematozoa
3.1 Artamidae
Thirteen artamids were found to exhibit schizonts of various sizes in their tissues (). Schizonts ranging from 25 to 360×30 to 160 μm2 were found in the spleen, kidneys, liver and lung (a to d) and smaller schizonts (10 to 180×10 to 35 μm2) of similar morphology were found in cardiac muscle of several Australian Magpies, Pied Currawongs and one Grey Butcherbird. The number of schizonts varied between one and 100 per section. An inflammatory reaction (with macrophages, lymphocytes and granulocytes) was apparent in two of these birds with a high number of schizonts. Schizonts were mostly located in the lumen of vessels and were believed to have infected endothelial cells and possibly renal tubular cells. Pathological effects due to these schizonts could be observed in the myocardium and kidneys of two birds with an inflammatory infiltrate (macrophages, plasma cells, granulocytes, syncytia) in the myocardium and pericardium, as well as myocardial haemorrhage and inflammation in the kidneys (consisting of granulocytes, macrophages and lymphocytes).
Fig. 1 Schizonts from species of Artamidae. 1a: Gymnorhina tibicen (Australian Magpie) spleen with schizont with central body and cytomeres in vessel. 1b: G. tibicen with schizont causing embolism in the spleen. 1c: Strepera graculina (Pied Currawong) kidney with large schizont and prominent cytomeres. 1d: Cracticus torquatus (Grey Butcherbird) liver with developing schizont in hepatic vessel. All scalebars, 100 μm, and tissue sections stained with HE.
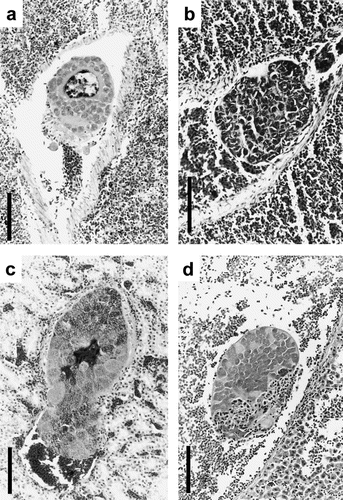
It is believed that displacement of tissue, blockage of vessels, destruction of renal tubuli and inflammation in the kidney and myocardium were the cause of clinical disease in two birds that displayed either ataxia and convulsions or weakness and splenomegaly without any signs of trauma or other obvious infections. In all other artamids the displacement of tissue, blockage of vessels, thrombosis and emboli as well as slight inflammation surrounding the schizonts appeared to have had no obvious impact on the overall health of the infected birds.
The general morphology of the schizonts showing cytomere formation corresponds closely to the descriptions of Leucocytozoon spp. schizonts in various stages of development. Evidence from examination of corresponding material showing blood stages indicates that in some birds only gametocytes of Leucocytozoon were present while others displayed peripheral stages of different taxa of haemosporidia. In the latter instances (for example, d, J98023), schizonts were more clearly referable to Leucocytozoon than to those of Plasmodium.
Large, multilocular schizonts found in skeletal muscle of Pied Currawongs and described elsewhere (Lederer et al., Citation2002) were not thought to represent schizonts of haemosporidian origin.
No species of Leucocytozoon has hitherto been described from the Artamidae and material is currently being collated to address this, of which the endogenous stages described here will form a significant component.
3.2 Meliphagidae
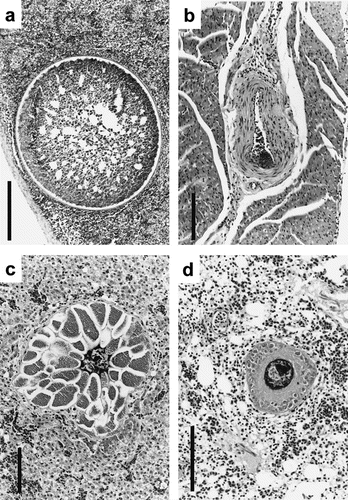
Schizonts of haematozoan origin were found in the spleen, lung, skeletal muscle, liver and heart of meliphagids (). Large, round, unsegmented schizonts with a clear cell wall measuring up to 640 μm were observed in the spleen of one Noisy Miner (a). These appeared to be degenerating schizonts of Leucocytozoon, probably Leucocytozoon anellobiae (the only leucocytozoid described from Meliphagidae) and were the largest found during the study. Smaller schizonts (25 to 35×15 to 30 μm2) found in some of the tissues were clearly referable to other taxa; for example, developing schizont of Haemoproteus spp. (probably Hamoproteus ptilotis) in the blood vessel of cardiac muscle of Noisy Friarbird (K98105, b). Intact schizonts were especially numerous in the spleen of one Noisy Miner, together with numerous degenerate large, round schizonts, and surrounded by a wavy fibrous capsule, and inflammatory cells (macrophages, lymphocytes and syncytia). The schizonts therefore not only caused marked displacement of functional tissue, but also (especially deteriorating) local inflammation, and fibrous reparation processes.
Fig. 2 Schizonts from species of Meliphagidae and Oriolidae. 2a: Manorina melanocephala (Noisy Miner) spleen with large degenerating schizont with distinct cell wall. Scalebar, 250 μm. 2b: Philemon corniculatus (Noisy Friarbird) cardiac muscle with small schizont in blood vessel. Scalebar, 100 μm. 2c: Sphecotheres viridis (Southern Figbird) liver with large septate schizont. Scalebar, 100 μm. 2d: S. viridis lung with developing schizont; note cytomeres. Scalebar, 100 μm. All tissue sections stained with HE.
3.3 Oriolidae
In the oriolids, schizonts associated with pathology were found in the liver, lung and skeletal muscle of three Southern Figbirds. Mature septate schizonts (170 to 290×120 to 160 μm2) were seen in the liver of one bird (K98410, c), occasionally surrounded by granulocytes, lymphocytes and macrophages. The schizonts were situated in lumina of small vessels; host cells most probably were of endothelial origin. Maturing schizonts with distinct cytomere development were observed in lung tissue of the same bird (d). Leucocytozoon oriolis gametocytes were found in the peripheral blood of all three birds but in bird K98410 no other haemosporidian taxa were observed.
Obvious histopathological findings included displacement of tissue, and focal inflammation with granulocytes, lymphocytes and macrophages associated with a schizont in the liver. Thrombosis caused by several schizonts in the lung and complete or partial obstruction of the affected vessels were observed in two Southern Figbirds, one with ataxia and severe splenomegaly.
3.4 Podargidae
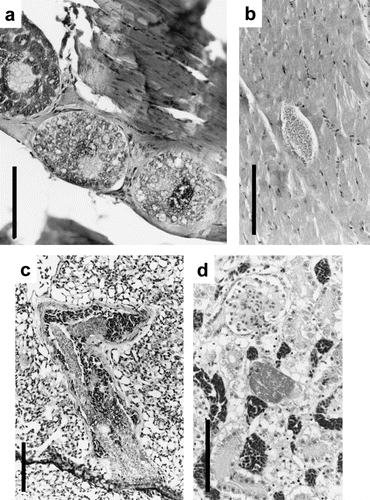
In all four Tawny Frogmouths () only Leucocytozoon podargii gametocytes were observed in the peripheral blood. Schizonts in lung and spleen tissue measuring up to 163 μm in diameter and characterized by cytomere formation were observed commonly (see Adlard et al., Citation2002). Concentrations of developing schizonts in skeletal muscle were observed particularly in bird K98207 (a). Schizonts in muscle tissue were surrounded by collagenous layers and erythrocytes, macrophages and some granulocytes. A slight inflammatory response and encapsulation with fibrous tissue was seen in association with one group of muscle schizonts.
Fig. 3 Schizonts from species of Podargidae, Alcedinidae, Columbidae and Psittacidae.3a: Podargus strigoides (Tawny Frogmouth) skeletal muscle with concentration of developing schizonts. 3b: Todirhamphus sanctus (Sacred Kingfisher) skeletal muscle with a small ovoid schizont. 3c: Columba livia (Domestic Pigeon) lung with concentrated foci of elongate schizonts. 3d: Cacatua galerita (Sulphur-crested Cockatoo) kidney with developing schizont. All scalebars, 100 μm, and tissue sections stained with HE.
Smaller, immature schizonts (40 to 60×35 to 45 μm2) were found in the wall of a small arteriole in the liver and in cardiac and skeletal muscle of three birds. No significant pathological changes were associated with schizonts observed in podargids ().
3.5 Columbidae
One domestic pigeon was infected with large numbers of elongate schizonts in the lung (c). These schizonts were clearly referable to previous descriptions of Haemoproteus columbae (see Baker, Citation1966), which was the only parasite identified from peripheral blood. Schizonts ranged in size from 50 to 400×50 to 120 μm2 and often appeared branched. Schizonts caused displacement in lung tissue and restricted pulmonary blood flow. Inflammatory responses were not evident.
3.6 Alcedinidae
One Sacred Kingfisher with a moderate intensity of Haemoproteus halcyonis gametocytes in the peripheral blood also showed an ovoid schizont in the skeletal muscle (b) with small merozoites (1 μm in diameter) morphologically similar to schizonts described from other hosts with Haemoproteus infections. No significant pathological changes were observed.
3.7 Psittacidae
Blood smears were examined from 15 species of psittacids (see Adlard et al., Citation2004), and only two individuals (Rainbow Lorikeet and Sulphur-crested Cockatoo) out of 1199 examined exhibited low levels of parasitaemia (<1%) with gametocytes of Leucocytozoon identified from peripheral blood. Histopathological examination of the Sulphur-crested Cockatoo revealed small developing schizonts in the kidney tissue only (d), which caused obstruction of tubuli. Schizonts lacked distinct outlines and varied between 35 to 65×25 to 30 μm2. Merozoites were not morphologically typical of those normally associated with Leucocytozoon spp. Thus, the morphological characteristics of both merozoites and schizonts suggest these were more probably due to a species of Plasmodium (or possibly Haemoproteus) rather than Leucocytozoon. Investigations are continuing in an attempt to elucidate the precise nature and host specificity of parasites found in wild psittacids.
All material has been deposited in the IRCAH Collection in the Queensland Museum.
4 Discussion
In general, the life cycles of haematozoa of the apicomplexan genera Plasmodium, Haemoproteus and Leucocytozoon are poorly known. Of the three genera, Plasmodium is perhaps the better documented due to the relatively easier experimental studies made possible by blood inoculation into recipient hosts without the need for intermediate vectors. Thus, for the majority of the more common Plasmodium spp., both blood and tissue stages are known (Garnham, Citation1966). All three genera have similar life cycles in as much as endogenous development, including schizonts, occur in the hosts' tissues. These schizonts give rise to the release of merozoites on maturity and can in many instances be identified on morphological characteristics. Life-cycle studies on species of Haemoproteus and Leucocytozoon are limited due to the need for the appropriate vectors in order to conduct experimental studies. Thus, published data are generally limited to those species having an impact on game and domestic species (Huff, Citation1942; Akiba et al., Citation1971; Miura et al., Citation1973; Atkinson & Forrester, Citation1987). In the genus Leucocytozoon there are two types of schizonts occurring in some species. First-generation schizonts are usually small and give rise to merozoites that develop into round gametocytes, and larger second-generation megaloschizonts give rise to elongate gametocytes (Fallis et al., Citation1974). The occurrence of both round and elongate gametocytes is generally a characteristic of Leucocytozoon spp., which occur in larger non-passerine hosts; raptors, galliforms and water fowl. There are a few exceptions known where both types of morphs occur in smaller hosts or where megaloschizonts occur in the absence of elongate morphs (i.e. Leucocytozoon marchouxi) (Peirce et al., Citation1997). There are descriptions in the literature that purport to describe various types of schizonts found in postmortem histopathology examinations where the identification has been based solely on the presence of gametocytes in peripheral blood. Such claims should be viewed with caution as, in many instances, parasites of other genera may have been present in a latent phase and the endogenous stages described may not have been related to the observed blood stages. In other instances, protozoal cysts of non-haematozoan genera have been misinterpreted, giving rise to much confusion in the literature (see later).
With few exceptions, only limited data on pathological changes associated with endogenous development have been described (Desser, Citation1967; Miura et al., Citation1973; Fallis et al., Citation1974; Earlé et al., Citation1993), as most descriptions have been restricted to the morphology of the schizonts. The degree to which haematozoa contribute to mortality and pathogenicity in wild birds is difficult to determine since most sick and dying birds are rapidly scavenged by predators (Bennett et al., Citation1993). Most species of Haemoproteus and Leucocytozoon are considered to be host specific at least to the avian family level, whereas many Plasmodium spp. infect a wide range of hosts. There is always the possibility that some species of Haemoproteus or Leucocytozoon may infect abnormal species due to a compromise in the recipient bird's immune status giving rise to aberrant infections as reported for some species (see Walker & Garnham, Citation1972; Earlé et al., Citation1993), but these may not in fact be due to the parasites the authors assumed (see later).
The current study presented a unique opportunity to evaluate the morphology and associated pathology of a variety of haematozoan species from wild birds, from which the schizonts have not been described previously. As indicated in , many of the birds had mixed infections and others only single infections based on examination of stained blood smears. However, the presence of a single infection based on blood film examination does not preclude the possibility that other species of parasites may have been present and that endogenous stages were related to a parasite not identified from blood. Thus, the interpretation of the schizonts observed in this study is cautiously based on a combination of blood stages identified and known morphological characteristics of the schizonts of the different parasite genera. As can be seen from , the pathological changes observed were variable and some species of the same genera caused more noticeable changes than others (i.e. Leucocytozoon in Artamidae and Podargidae).
The morphology of the schizonts identified here as those of H. columbae resemble those described in the literature by several authors including Baker (Citation1966). However, Earlé et al. (Citation1993) described a range of morphological forms including megaloschizonts, from what was claimed to be H. columbae from a Bleeding-heart Dove in South Africa. As discussed by Lederer et al. (Citation2002), the host from which Earlé et al. (Citation1993) described the schizonts is not endemic to Africa, and no such forms have been described from indigenous columbiform hosts. Thus, the conclusions drawn by Earlé et al. (Citation1993) should be interpreted with caution as other parasites may have been involved. Although large schizonts have been recorded, the only true megaloschizonts confirmed for a species of Haemoproteus based on controlled experimental studies are for Haemoproteus meleagridis in turkeys (Atkinson et al., Citation1986).
The status of schizonts in psittacid tissues is unclear. Large megaloschizonts described from tissues of imported psittacines in Europe were initially thought to be an aberrant form of Leucocytozoon, even though no blood forms were ever observed (Walker & Garnham, Citation1972; Peirce & Bevan, Citation1977). However, subsequent studies suggested that these large multilocular schizonts were in fact Besnoitia spp. (Bennett et al., Citation1993; Peirce, Citation1993). Schizonts with similar morphology have been observed in Pied Currawongs (Lederer et al., Citation2002).
With the exception of H. columbae and L. podargii, none of the endogenous stages of the parasite species presented here have previously been described. This aspect alone has added to the knowledge of the life cycles of these parasites. Clearly some of the parasites cause noticeable tissue changes during development and, where large numbers of schizonts occur, such damage may be considerable. While it is difficult to speculate definitively on the significance of these parasites on host morbidity, there are clear indications that in some species at least they may play a role in overall clinical manifestations, whether as sole causes or as concomitant infections with other disease agents that may increase pathological involvement (Peirce, Citation1989).
Translation of the abstract in French, German and Spanish are available on the Avian Pathology website.
Acknowledgments
The authors wish to thank the veterinary staff at the Currumbin Wildlife Sanctuary for their assistance in obtaining blood smears and for the provision of facilities. They thank Dr Roger Kelly for his comments on the pathology. R.L. was supported by a postgraduate scholarship by the Deutscher Akademischer Austauschdienst (DAAD, Germany). M.A.P. was supported by an Australian Biological Resources Study (ABRS) grant to R.D.A. and M.A.P.
References
- Adlard , R.D. , Peirce , M.A. and Lederer , R. 2002 . New species of Leucocytozoon from the avian families Otidae, Podargidae and Threskiornithidae . Journal of Natural History , 36 : 1261 – 1267 .
- Adlard, R.D., Peirce, M.A. & Lederer, R. (2004). Blood parasites of birds from southeast Queensland. Emu (in press)
- Akiba , K. , Inui , S. and Ishitani , R. 1971 . Morphology and distribution of intracellular schizonts in chickens experimentally infected with Akiba caulleryi . National Instiute of Animal Health , 11 : 109 – 121 .
- Atkinson , C.T. and Forrester , D.J. 1987 . Myopathy associated with megaloschizonts of Haemopoteus meleagridis in a wild turkey from Florida . Journal of Wildlife Diseases , 23 : 495 – 498 .
- Atkinson , C.T. , Greiner , E.C. and Forrester , D.J. 1986 . Pre-erythrocytic development and associated host responses to Haemoproteus meleagridis (Haemosporina: Haemoproteidae) in experimentally infected domestic turkeys . Journal of Protozoology , 33 : 375 – 381 .
- Baker , J.R. 1966 . Haemoproteus palumbis sp.nov. (Sporozoa, Haemosporina) of the English wood-pigeon Columba p.palumbus . Journal of Protozoology , 13 : 515 – 519 .
- Bennett , G.F. , Peirce , M.A. and Ashford , R.W. 1993 . Avian haematozoa: mortality and pathogenicity . Journal of Natural History , 27 : 993 – 1001 .
- Christidis, L. & Boles, W.E. (1994). The taxonomy and species of birds of Australia and its territories. Royal Australasian Ornithologists Union Monographs 2. Melbourne: RAOU.
- Desser , S.S. 1967 . Schizogony and gametogony of Leucocytozoon simondi and associated reactions in the avian host . Journal of Protozoology , 14 : 244 – 254 .
- Earlé , R.A. , Bastianello , S.S. , Bennett , G.F. and Krecek , R.C. 1993 . Histopathology and morphology of the tissue stages of Haemoproteus columbae causing mortality in Columbiformes . Avian Pathology , 22 : 67 – 80 .
- Fallis A.M. Desser S.S. Khan R.A. On species of Leucocytozoon, Advances in Parasitology 12 Dawes B. (ed.) Academic Press: New York 1974 1 67
- Huff , C.G. 1942 . Schizogony and gametocyte development in Leucocytozoon simondi and comparison with Plasmodium and Haemoproteus . Journal of Infectious Diseases , 71 : 18 – 32 .
- Garnham P.C.C. Malaria Parasites and Other Haemosporidia Blackwell Scientific: Oxford 1966
- Lederer , R. , Adlard , R.D. and O'Donoghue , P.J. 2002 . Severe pathology associated with protozoal schizonts in two Pied Currawongs (Strepera graculina) from Queensland . Veterinary Record , 150 : 520 – 522 .
- Miura , S. , Ohshima , K. , Itakura , C. and Yamagiwa , S. 1973 . A histopathological study on leucocytozoonosis in young hens . Japanese Journal of Veterinary Science , 35 : 175 – 181 .
- Peirce M.A. The significance of avian haematozoa in conservation strategies, Disease and Threatened Birds 10 Cooper J.E. (ed.) ICBP Technical Publication: Cambridge 1989 69 76
- Peirce M.A. Blood parasites and other protozoa of psittacines, Proceedings of the Psittivets Meeting Waine J. (ed.) London, UK 1993 20 26
- Peirce , M.A. and Bevan , B.J. 1977 . Blood parasites of imported psittacine birds . Veterinary Record , 100 : 282 – 285 .
- Peirce , M.A. , Greenwood , A.G. and Swinnerton , K. 1997 . Pathogenicity of Leucocytozoon marchouxi in the Pink Pigeon (Columba mayeri) in Mauritius . Veterinary Record , 140 : 155 – 156 .
- Walker , D. and Garnham , P.C.C. 1972 . Aberrant Leucocytozoon infection in parakeets . Veterinary Record , 15 : 70 – 72 .