Abstract
Coccidiosis and necrotic enteritis (NE) are globally common, sometimes intercurrent, diseases of poultry. The risk of NE, due to the Gram-positive bacterium Clostridium perfringens, has increased in recent years because of the voluntary or legally required withdrawal of the use of certain in-feed antibiotic growth promoters with anticlostridial activity. In-feed ionophorous anticoccidial drugs incidentally also possess anticlostridial activity. Such ionophores, although not banned, are usually precluded when live anticoccidial vaccines are used, potentially increasing yet further the risk of NE. This review provides information for the design of rational, integrated management strategies for the prevention and control of coccidiosis and NE in chickens by maintaining gut integrity. Because of differences in local availability of feed ingredients and national legislations regarding antibiotic growth promoters and anticoccidial vaccine licensing, no universal strategy is applicable. The diseases and their interactions are described under the headings of forms of disease, diagnosis, sources of infection, pathophysiological effects, predisposing factors, and control methods. Elements of gut integrity, which influences host predisposition and clinical responses to disease, include physical development, immune competence, gut enzyme activity, mucin production, gut flora and epithelial damage. Experimental studies of coccidiosis and NE are compared, and where possible reconciled, with field observations. Gaps in knowledge and necessary further experiments are identified. Insights are provided regarding interactions between coccidiosis, NE, and the use of live anticoccidial vaccines. Recent changes in NE prevalence in commercial flocks, and their possible causes, are discussed. The necessarily wide range of topics reviewed emphasizes the enormous complexity of this disease combination, and indicates the importance of a multidisciplinary approach in order to reduce its harmful impact on the world's poultry industry.
Coccidiose et entérite nécrotique intercurrentes du poulet: gestion intégrée et rationnelle de la maladie par le maintien de l'intégrité de l'intestin
La coccidiose et l'entérite nécrotique (NE) sont des maladies des volailles, communes dans le monde, quelquefois intercurrentes. Le risque de NE, due à une bactérie Gram positif Clostridium perfringens, a augmenté ces dernières années à cause du retrait, volontaire ou imposé par la législation, de certains antibiotiques facteurs de croissance (AGPs) dans les aliments pour animaux ayant une activité anticlostridienne. Les molécules anticoccidiennes ionophores incorporées dans l'aliment possèdent aussi incidemment une activité anticlostridienne. De tels ionophores, bien que non interdits, ne sont pas incorporés quand des vaccins anticoccidiens sont utilisés, ce qui pourrait augmenter d'avantage le risque de NE. Cette synthèse fournit des informations sur l’établissement d'un plan de stratégies de management intégré et rationnel pour prévenir et contrôler la coccidiose et la NE chez le poulet en maintenant l'intégrité de l'intestin. En raison des différences des composés de l'aliment disponibles localement et des réglementations nationales concernant les AGPs, aucune stratégie universelle n'est applicable. Les maladies et leurs interactions sont décrites sous les rubriques, types de maladie, diagnostic, sources d'infection, effets pathophysiologiques, facteurs prédisposants et méthodes de contrôle. Les éléments de l'intégrité de l'intestin, qui influencent la prédisposition de l'hôte et les réponses cliniques à la maladie, incluent le développement physique, la compétence immunitaire, l'activité enzymatique de l'intestin, la production de mucine, la flore de l'intestin et les lésions de l’épithélium. Des études expérimentales de coccidiose et de NE ont été comparées et quand cela a été possible fusionnés à des observations faites sur le terrain. Des lacunes dans les connaissances ont été mises en évidence et des expérimentations complémentaires indispensables sont proposées. Des idées sont données en ce qui concerne les interactions entre coccidiose et NE ainsi que pour l'utilisation des vaccins vivants anticoccidiens. Les changements récents de la prévalence de NE dans les troupeaux commerciaux, et leurs possibles causes, font l'objet de discussion. La diversité forcement importante des thèmes, passés en revue, souligne l’énorme complexité des combinaisons de la maladie, et indique l'importance de l'approche multidisciplinaire dans le but de réduire son impact nuisible sur l'aviculture industrielle mondiale.
Interkurrierende Kokzidiose und nekrotisierende Enteritis: Rationelles, integriertes Krankheitsmanagement unter Aufrechterhaltung der Funktionsfähigkeit des Darms
Sowohl die Kokzidiose als auch die nekrotisierende Enteritis (NE) sind weltweit vorkommende, manchmal interkurrierende Erkrankungen beim Geflügel. Die Gefahr des Auftretens der durch das grampositive Bakterium Clostridium perfringens induzierten NE hat sich in den letzten Jahren aufgrund des freiwilligen oder legal verordneten Absetzens bestimmter antibiotischer Wachstumsförderer (AGPs) mit anticlostridieller Wirkung im Futter erhöht. Ionophore Antikokzidia im Futter haben ebenfalls anticlostridielle Wirkung. Diese Ionophore werden, obwohl nicht verboten, nicht dem Futter zugesetzt, wenn Kokzidien-Lebendvakzinen angewendet werden, wodurch das Risiko für NE noch weiter ansteigt. Dieser Übersichtsartikel informiert über das Konzept einer rationellen, integrierten Managementstrategie zur Verhinderung und Bekämpfung von Kokzidiose und NE bei Hühnern unter Aufrechterhaltung der Funktionsfähigkeit des Darms. Aufgrund von Unterschieden in der lokalen Verfügbarkeit von Futterkomponenten und in der nationalen Gesetzgebung hinsichtlich der AGPs ist eine allgemeingültige Strategie nicht anwendbar. Die Krankheiten und ihre Interaktionen werden beschrieben in den Kapiteln Krankheitsformen, Diagnose, Infektionsquellen, pathophysiologische Effekte, prädisponierende Faktoren und Bekämpfungsmethoden. Elemente der Funktionsfähigkeit des Darms, die die Wirtstierprädisposition und die klinische Antworten auf die Krankheiten beeinflussen, umfassen die physikalische Entwicklung, die Immunkompetenz, Aktivität der Darmenzyme, die Schleimproduktion, die Darmflora und die Epithelbeschädigung. Experimentelle Untersuchungen zur Kokzidiose und NE werden verglichen und, wo möglich, mit Feldbeobachtungen in Übereinstimmung gebracht. Wissenslücken und notwendige weitere Experimente werden aufgeführt. Erkenntnisse hinsichtlich der Interaktionen zwischen Kokzidiose und NE sowie der Anwendung von Kokzidien-Lebendvakzinen werden vorgestellt. Neuerliche Veränderungen im Auftreten von NE in kommerziellen Herden sowie ihre möglichen Ursachen werden diskutiert. Der notwendigerweise umfangreiche Themenbereich in dieser Übersicht unterstreicht die enorme Komplexität dieser Erkrankungskombination und weist auf die Wichtigkeit einer multidisziplinären Vorgehensweise zur Reduzierung seiner schädlichen Auswirkung auf die weltweite Geflügelindustrie hin.
Concurrencia de enteritis necrótica y coccidiosis: manejo racional e integrado de la enfermedad mediante el mantenimiento de la integridad intestinal
La coccidiosis y la enteritis necrótica (NE) son enfermedades aviares comunes en todo el mundo y algunas veces concurrentes. El riesgo de NE, a causa de la bacteria Gram-positiva Clostridium perfringens, ha aumentado en los últimos años debido a la retirada voluntaria o legalmente obligatoria del uso en el pienso de ciertos antibióticos promotores del crecimiento (AGPs) con actividad anticlostridial. Estos ionóforos, aunque no prohibidos, se excluyen cuando se usan vacunas frente a la coccidiosis, incrementando potencialmente aún más el riesgo de NE. Esta revisión proporciona información para el diseño de estrategias de manejo integradas y racionales para la prevención y el control de la coccidiosis y la NE en los pollos mediante el mantenimiento de la integridad intestinal. A causa de las diferencias en la disponibilidad local de ingredientes del pienso y en la legislación nacional referente a los AGPs, no se puede aplicar una estrategia universal. Se describen las enfermedades y sus interacciones bajo los apartados de cuadros de la enfermedad, diagnóstico, fuentes de infección, efectos patofisiológicos, factores predisponentes, y métodos de control. Aquellos elementos de la integridad intestinal que influyen en la predisposición del huésped y la respuesta clínica a la enfermedad incluyen el desarrollo físico, la inmunocompetencia, la actividad enzimática intestinal, la producción de moco, la flora intestinal y el daño epitelial. Se comparan estudios experimentales de coccidiosis y NE, y en aquellos casos donde es posible se concuerdan con observaciones de campo. Se identifican las lagunas en el conocimiento y los experimentos necesarios todavía. Se aportan elementos respecto a las interacciones entre coccidiosis, NE, y el uso de vacunas vivas contra la coccidiosis. Se discuten cambios recientes en la prevalencia de NE en lotes comerciales, y sus posibles causas. El amplio y necesario abanico de tópicos revisados destaca la enorme complejidad de la combinación de estas enfermedades, e indica la importancia de un enfoque multidisciplinar para reducir su impacto perjudicial en la industria avícola mundial.
Introduction
Coccidioses of domesticated animals have been studied for over a century (Hagenmüller, Citation1899; Becker, Citation1934; Davies et al., Citation1963; Hammond & Long, Citation1973; Pellérdy, Citation1974; Long, Citation1982, Citation1990) and, as the world's poultry industry has developed, have continued to be of major economic importance (Biggs, Citation1982; Williams, Citation1999a). Furthermore, interactions occur between poultry coccidioses and other diseases, caused by various pathogens or nutritional imbalances (Ruff, Citation1986, Citation1989; Yvoré, Citation1986). Among those other diseases, clostridioses intercurrent with coccidioses are an increasing health risk to poultry (Williams, Citation2002a, Citation2003a; Dhawale, Citation2004; Shane, Citation2004a).
A frequent, although sporadic, poultry clostridiosis (necrotic enteritis [NE]) was first recorded by Bennetts (Citation1930) in Australia, and fully characterized by Parish (Citation1961a,Citationb,Citationc) in the United Kingdom. Interestingly, NE did not appear in a 1964 guide to common poultry diseases in the UK (British Oil & Cake Mills, Citation1964), nor in a 1979 global disease survey (Biggs, Citation1982). Even as recently as 1997, it did not merit special attention in an international poultry disease directory (van der Sluis, Citation1997). Soon afterwards, however, NE emerged as a worldwide problem (van der Sluis, Citation2000a, Citationb; Van Immerseel et al., Citation2004). Furthermore, the occurrence of the causative agent of NE, Clostridium perfringens, in poultry meat is an important threat to public health (Van Immerseel et al., Citation2004).
NE, especially in broilers, has long been controlled incidentally by some in-feed antibiotic growth promoters (AGPs), which include well-known antibacterial drugs like virginiamycin, bacitracin, and so on. However, legislation in the European Union (EU) now precludes the use of certain AGPs, and the remainder will be banned in 2006 (Van Immerseel et al., Citation2004). Some in-feed ionophorous anticoccidial drugs, which additionally possess Gram-positive antibacterial activity, have also incidentally contributed to NE control in broilers, but they are usually omitted from poultry diets if a live, anticoccidial vaccine is administered. Furthermore, ionophores and other anticoccidial drugs for broiler breeders are being superseded by live anticoccidial vaccines (Ross Breeders, Citation1995). Hence, the potential for interactions between coccidia (wild-type or vaccinal) and clostridia is becoming much greater than previously.
The nature of a possible relationship between coccidiosis and NE in the field is controversial (Williams, Citation1999b; Anonymous, Citation2000). Furthermore, the increasing market penetration of the broiler sector by live anticoccidial vaccines has raised the question of whether such vaccines might exacerbate clostridial diseases (Williams, Citation2003a). An assessment of relationships between coccidiosis, NE and anticoccidial vaccination in chickens, with rational proposals for integrated disease management, is therefore timely.
Coccidioses
The fowl coccidia (Eimeria spp.) and the diseases they cause are well documented (Davies et al., Citation1963; Joyner & Long, Citation1974; Pellérdy, Citation1974; Long et al., Citation1976; Joyner, Citation1978; Long & Reid, Citation1982). Seven Eimeria species, Eimeria acervulina, Eimeria brunetti, Eimeria maxima, Eimeria mitis, Eimeria necatrix, Eimeria praecox and Eimeria tenella, are now accepted (Shirley, Citation1986), but sometimes only six (Long et al., Citation1976) or as many as nine (Long & Reid, Citation1982) were recognized in the recent past.
Each species causes a separate disease, each exhibiting a characteristic degree of pathogenicity. Unlike bacteria and viruses, which potentially multiply infinitely until checked by immune responses or the host's death, coccidia have a genetically fixed, self-limiting life cycle (Johnson, Citation1923; Tyzzer, Citation1929). Therefore, the severity of each coccidiosis is positively correlated with the number of infective oocysts ingested (Johnson, Citation1927; Tyzzer, Citation1932; Dickinson, Citation1941).
Forms of disease in chickens
Each disease may exhibit three increasing levels of severity (see Williams, Citation1999a): (1) coccidiasis, a mild infection, causing no adverse effects (Levine, Citation1961); (2) subclinical coccidiosis, resulting in slight but economically important reductions of growth and feed utilization; and (3) clinical coccidiosis (frank disease).
The most serious effects of the less severe clinical coccidioses (caused by E. praecox, E. mitis or E. acervulina) are diarrhoea, morbidity, reduction of weight gain and poor feed conversion. The more severe coccidioses (E. brunetti, E. maxima, E. necatrix, and E. tenella) produce similar signs and, with heavier infections, various degrees of intestinal haemorrhage and perhaps death.
Diagnoses
Coloured illustrations of coccidial lesions are available (Long et al., Citation1976; Conway & McKenzie, Citation1991). While the diagnostic methods described by Joyner & Long (Citation1974), Long et al. (Citation1976), Joyner (Citation1978) and Long & Reid (Citation1982) are adequate in experienced hands, misdiagnoses are still possible. Perhaps the most common results from confusion between subclinical coccidiosis and coccidiasis (see Catchpole, Citation2000).
NE intercurrent with coccidiasis is sometimes misdiagnosed as primary coccidiosis. Conversely, E. brunetti coccidiosis might be misdiagnosed as NE (Helmboldt & Bryant, Citation1971; Wages & Opengart, Citation2003). P. L. Long (Citation1973) considered that the enteritis regarded as characteristic of E. brunetti infection (see Gregory, Citation1990) is not associated with Clostridium perfringens, the causative agent of true NE.
Intestinal intussusceptions, involving severe congestion and necrosis, are occasionally misdiagnosed as coccidiosis, particularly when concomitant with coccidiasis (see Williams, Citation1986; Catchpole, Citation2000). There is, however, no evidence that coccidiosis causes intussusceptions (Williams, Citation1986).
Sources of infection
All seven species of fowl Eimeria have been discovered wherever they have been sought—for example, Argentina (McDougald et al., Citation1997), Australia (Lew et al., Citation2003), the Czech Republic (Kucera, Citation1989, Citation1990, Citation1991), France (Williams et al., Citation1996), India (Mandal, Citation1980), Japan (Tsuji et al., Citation1997), Sweden (Thebo et al., Citation1998), the UK (Williams, Citation2001), and the USA, whence all the valid species, except for E. tenella, were originally described.
Coccidia are spread by the faecal-oral route. Unsporulated oocysts are expelled from the intestinal mucosa and excreted in the faeces. Excreted oocysts must sporulate to become infective, for which oxygen, moisture and warmth are necessary (Kheysin, Citation1972). Birds reared on litter may be exposed to coccidia throughout their lives.
Oocysts in the environment are practically ubiquitous where poultry occur, and may be transported by the definitive host, paratenic avian hosts, rodent vermin, flying insects, other invertebrate pests, contaminated feed, old litter, human agency and the general paraphernalia of the poultry industry (Fayer & Reid, Citation1982). New poultry houses quickly become contaminated, even on farms where poultry have not previously been kept. The dramatic effect of infection of naïve birds in new buildings, which do not have a pre-existing background contamination, has been termed “new house coccidiosis syndrome” (Reid, Citation1989).
Some pathophysiological effects of coccidioses
Reduced weight gain and poor feed conversion efficiency. The commonest effects of poultry coccidioses are reduction of weight gain and a concomitant adverse effect on feed conversion ratio. In 1995, economic losses by the UK chicken industry due to coccidiosis were estimated as £38.6 million, 46% of which resulted from reduced weight gain of broilers while a poor feed conversion ratio accounted for 34% (Williams, Citation1999a). Contrary to widespread belief, E. mitis and E. praecox may adversely affect commercial performance similarly to E. acervulina (Williams, Citation1997a, Citation1998; Shirley, Citation2003).
Reduced feed and water intake
Moderate to heavy coccidial infections reduce water and feed intake during acute disease (Reid & Pitois, Citation1965). The water to feed ratio is about 1.9:1, both in sick and healthy birds (Williams, Citation1996). Only about 70% of the reduced weight gain of chickens infected with E. acervulina is accounted for by lowered feed intake (Preston-Mafham & Sykes, Citation1967a). Hence, reduced body weight gain cannot result simply from anorexia (Preston-Mafham & Sykes, Citation1970; Michael & Hodges, Citation1971, Citation1972; Allen et al., Citation1973; Takhar & Farrell, Citation1979).
Increased intestinal passage time
Coccidiosis usually increases the passage time of digesta during the acute phase (Schildt & Herrick, Citation1955; Aylott et al., Citation1968). Only Joyner et al. (Citation1975) concluded that gut passage time is decreased by E. acervulina infection. Shane et al. (Citation1985) found otherwise, and considered that reduced intestinal motility is caused by gut pH values ≤5.6, adversely affecting smooth-muscle cell membrane permeability. The mechanism must be different in the case of E. tenella (Schildt & Herrick, Citation1955), which does not affect gut pH (Ruff & Reid, Citation1975). There is no relation between generalized stasis and feed intake reduction (McKenzie et al., Citation1982).
Decreased digesta viscosity
E. acervulina, E. tenella and E. praecox reduce digesta viscosity in chickens, associated with poor performance (Morgan & Catchpole, Citation1996; Waldenstedt et al., Citation2000).
Intestinal malabsorption
Coccidiosis-related malabsorption was reviewed by Turk (Citation1978) and Ruff (Citation1978, Citation1986). Infections of the duodenum, jejunum or ileum cause reduced absorption of nutritional elements by the intestinal epithelium. E. acervulina causes malabsorption of zinc (Turk & Stephens, Citation1967a, Citation1970), calcium (Turk, Citation1973), selenium (Pesti & Combs, Citation1976), oleic acid (Turk & Stephens, Citation1967a, Citation1970), vitamin A (Kouwenhoven & van der Horst, Citation1969, Citation1972), glucose (Preston-Mafham & Sykes, Citation1967b, Citation1970; Giese et al., Citation1971), amino-acids (Preston-Mafham & Sykes, Citation1970; Joyner et al., Citation1975; Patterson et al., Citation1975; Ruff et al., Citation1976), lipids (Sharma & Fernando, Citation1975) and pigments, particularly carotenes (Kouwenhoven & van der Horst, Citation1969, Citation1972; Yvoré & Mainguy, Citation1972; Yvoré et al., Citation1972; Ruff & Fuller, Citation1975; Tyczkowski et al., Citation1991).
Similarly, E. necatrix causes malabsorption of zinc (Turk & Stephens, Citation1966, Citation1970), calcium (Turk, Citation1973), selenium (Pesti & Combs, Citation1976), oleic acid (Turk & Stephens, Citation1966, Citation1967b, Citation1970), glucose (Giese et al., Citation1971) and methionine (Ruff, Citation1974). E. maxima causes selenium malabsorption (Pesti & Combs, Citation1976). With E. mitis, malabsorption of glucose and L-methionine was observed simultaneously with depigmentation of the host's plasma (Ruff & Edgar, Citation1982). No malabsorption was observed following E. tenella infection of the caeca (Turk & Stephens, Citation1970; Pesti & Combs, Citation1976; Ruff et al., Citation1976) or E. brunetti infection of the distal intestine (Pesti & Combs, Citation1976).
Reduced nutrient digestion
Reduced activities of digestive enzymes, such as disaccharidases associated with the epithelial brush border, have been reported (Enigk & Dey-Hazra, Citation1976; Major & Ruff, Citation1978a). In chickens infected with E. acervulina, E. maxima or E. necatrix, amylolytic activity in the pancreas was decreased, associated with pancreatic weight loss (Major & Ruff, Citation1978b). A general impairment of protein digestion occurs in birds infected with coccidia (Turk, Citation1972).
Villous atrophy
Villous atrophy occurs especially in E. acervulina infections (Pout, Citation1967, Citation1968; Fernando & McCraw, Citation1973), contributing to malabsorption (Pout, Citation1968). It also occurs during E. maxima infections (Pout, Citation1967). Its association with other coccidioses is uncertain; it may be masked by epithelial destruction.
Intestinal leakage of plasma proteins
Increases in mucosal permeability are indicated by leakage of plasma proteins (Preston-Mafham & Sykes, Citation1967b; Rose & Long, Citation1969; Enigk et al., Citation1970; Joyner et al., Citation1975). Leakage due to E. acervulina is caused by the sexual stages damaging the mucosa (Schalm et al., Citation1975; Shane et al., Citation1985).
Increased intestinal acidity
Increased acidity of digesta, which probably increases gut passage time (see earlier), occurs in chicks infected with E. acervulina, E. brunetti, E. maxima and E. necatrix (Kouwenhoven & van der Horst, Citation1969, Citation1972; Stephens et al., Citation1974; Ruff et al., Citation1974; Ruff & Reid, Citation1975; Shane et al., Citation1985). E. tenella, however, has no effect on intestinal pH (Ruff & Reid, Citation1975). Kouwenhoven & van der Horst (Citation1969, Citation1972) suggested that increased intestinal acidity reduces absorption of vitamin A and xanthophylls.
Predisposing factors
Association with bacteria. Although coccidioses in chickens appear to occur in the absence of other organisms, Johansson & Sarles (Citation1948) considered that bacteria such as Clostridium perfringens and coliforms might be involved in the pathology of E. tenella. Similar findings regarding E. tenella and E. brunetti were reported by other workers (see Ohe & Arakawa, Citation1975). However, Clark et al. (Citation1962) showed that the gross pathology of E. tenella was similar in gnotobiotic and conventional chickens, and Hegde et al. (Citation1969) found the same for E. brunetti. It is often difficult to be sure which is the primary or predisposing infection in cases of intercurrent coccidioses and bacterioses or other diseases (see Quiroz, Citation2003).
Litter conditions
Since moisture is essential for oocyst sporulation, higher moisture content is commonly believed to facilitate greater sporulation rates. Temperature, however, is not limiting in commercial poultry houses, which are maintained close enough to optimum sporulation temperatures (Edgar, Citation1954, Citation1955). Neither litter moisture nor temperature had a marked effect on E. acervulina sporulation within the ranges of 40% to 80% relative humidity and 21 to 33°C (Graat et al., Citation1994). However, sporulation rates were higher in dry or clammy litter (22.6% or 19.5% sporulation, respectively) than in pure faeces (11.6% sporulation) This was unexpected, considering the high faecal moisture content.
Litter containing 31% to 62% moisture at 25 to 28°C initially allowed rapid, high sporulation rates of E. acervulina oocysts, but after about 5 days exerted a lethal effect (Williams, Citation1995). Viable oocysts are continually detectable in the litter of commercial flocks only because they are replenished by repeated autoreinfections of birds. Waldenstedt et al. (Citation2001) found that sporulation of E. maxima oocysts is negatively correlated with litter moisture between 16% and 62%. High moisture levels encourage bacterial growth leading to high ammonia levels and low oxygen levels, so the results of Williams (Citation1995) and Waldenstedt et al. (Citation2001) probably account for the unexpected results of Graat et al. (Citation1994).
Host immune status
Coccidia-free chickens exhibit no age-related differences in innate susceptibilities to coccidiosis (Pellérdy, Citation1974). However, exogenous factors influencing immune responses have significant effects. Marek's disease virus is immunosuppressive, increasing susceptibility to coccidiosis (Biggs et al., Citation1968, Citation1969; Rice & Reid, Citation1973). Infectious bursal disease virus has a similar effect (Anderson et al., Citation1977; McDougald et al., Citation1980). There are also interactions, possibly involving immune responses, between coccidiosis and reticuloendothelial virus (Motha & Egerton, Citation1984) or reoviruses (Ruff & Rosenburger, Citation1985a, Citationb).
Breed of chicken
Coccidial pathogenicity is influenced by the breed of chicken, since differences in innate immunity occur (Patterson et al., Citation1961; Long, Citation1968; Lillehoj et al., Citation1986; Lillehoj, Citation1988; Bumstead & Millard, Citation1992; Smith et al., Citation2002). Among commercial hybrids, layers are frequently more susceptible to coccidiosis than are broilers (Marshall et al., Citation1995; Williams & Catchpole, Citation2000), but results vary.
Host nutrition
Higher crude protein levels increase coccidial pathogenicity (Britton et al., Citation1964; Sharma et al., Citation1973), possibly because increased tryptic activity in the host leads to more efficient excystation of oocysts in the intestine.
E. tenella is more pathogenic in chickens fed wheat-based diets than in those fed maize-based diets (Williams, Citation1992a). It was suggested by Morgan & Catchpole (Citation1996) that the difference in susceptibility to coccidiosis is related to different levels of soluble arabinoxylans in the cereals and the resulting effects on digesta viscosity (see van der Klis & van Voorst, Citation1993). Hence, because wheat contains much higher amounts of soluble non-starch polysaccharides (NSPs), which increase digesta viscosity, than does maize, supplementing a wheat-based diet with enzymes that digest NSPs should reduce coccidial pathogenicity by virtue of lowered viscosity. In fact, the results of Morgan & Catchpole (Citation1996) showed that, in birds fed a wheat-based diet, coccidial infection per se reduced digesta viscosity by the same amount as xylanase supplementation of the diet in uninfected birds, and the infected birds performed significantly worse than the enzyme treated birds. Furthermore, the lowest viscosity occurred in infected birds fed the enzyme-supplemented diet, but coccidial pathogenicity was no lower than in the infected, untreated control birds.
Waldenstedt et al. (Citation2000) supplemented a maize and soya-based diet with an indigestible polysaccharide to study the graded effects of increasing digesta viscosity in chickens infected with coccidia. The increasing viscosity induced in uninfected birds proportionally reduced their performance, but the effect in infected birds fed the graded supplemented diet was considerably less, and the viscosity was barely different from that in uninfected birds fed a non-supplemented diet. The marked pathogenic effects of the infection had no clear association with the level of dietary supplementation. The fundamental basis of cereal-related effects on coccidial pathogenicity is therefore probably not related to digesta viscosity. It may rest upon differences in micronutrients in cereal grains, as originally suggested by Williams (Citation1992a).
Established control methods
Dietary modifications. Cereals that predispose birds to coccidiosis can be reduced in diets; but different wheat varieties and batches vary in effect (Waldron et al., Citation1993; Gutiérrez, Citation2004). Adverse effects of wheat and barley on performance can be ameliorated by reducing dietary energy and protein content, thus minimizing undigested protein utilizable by bacteria (Wallach & Waldenstedt, Citation1999). The resulting early slowing of growth may ameliorate ascites and leg problems (Williams, Citation2003b).
The complex effects of feed structure and nutrition on susceptibility to coccidiosis, some of which may also apply to NE, were discussed by Allen et al. (Citation1998), Waldenstedt (Citation1998), Langhout (Citation1999), Banfield & Forbes (Citation1999), Silversides & Remus (Citation1999), Duffy & Tucker (Citation2003) and Van Immerseel et al. (Citation2004). Vitamin E and selenium enhance viability and immune responses of poultry and increase resistance to infection (Colnago et al., Citation1983, Citation1984; El-Boushy, Citation1988). Vitamin A also protects against coccidiosis (Taylor & Russell, Citation1947; Erasmus et al., Citation1960; Singh & Donovan, Citation1973), perhaps because of beneficial effects on the intestinal epithelium and immune status (Gerriets, Citation1961; Coles et al., Citation1970; Lillehoj et al., Citation2003).
Anticoccidial drugs
Drugs for the prevention and treatment of coccidiosis in chickens have been available since the 1940s (McDougald, Citation1982). Reviews of chemotherapy include those by Ryley & Betts (Citation1973), Fayer & Reid (Citation1982) and McDougald (Citation1982, Citation1990, Citation1993). McDougald (Citation1982) described various strategies, such as prophylaxis, shuttle and rotation programmes, restricted feeding programmes, drug combinations, and therapy, and he also (McDougald, Citation2003) provided a comprehensive historical list of commercially available drugs.
Anticoccidial drugs are classed as either synthetic drugs or ionophores. Because clostridia are sensitive to certain ionophores (Liu, Citation1982), those primarily used as in-feed prophylactic anticoccidial drugs may also protect against clostridioses, while synthetic anticoccidials do not (Williams, Citation2002a). In the absence of coccidial drug resistance, any anticoccidial drug should contribute indirectly to protection against NE by preventing clinical coccidiosis from acting as a predisposing factor.
When live, anticoccidial vaccines are administered to chickens, anticoccidial drugs are not usually used concomitantly because they may kill some of the vaccinal parasites (Williams, Citation2002a). Nevertheless, in some countries vaccines containing ionophore-tolerant coccidia are available, so that ionophores may be included in the feed of vaccinated chickens, thus providing some protection against NE while the vaccine is stimulating immunity to coccidiosis. Such vaccines may be non-attenuated (Schetters et al., Citation1999; Vermeulen et al., Citation2001) or attenuated by selection for precocity (Li et al., Citation2004, Citation2005).
Drug resistance
Drug resistance, a long-standing problem, was reviewed by Cuckler et al. (Citation1969), Joyner (Citation1970), McLoughlin (Citation1970), Ryley & Betts (Citation1973), Chapman (Citation1978, Citation1982, Citation1984, Citation1986, Citation1997), Jeffers (Citation1978, Citation1989), Ryley (Citation1980) and Geary et al. (Citation1985). The rotation of anticoccidial vaccines with in-feed anticoccidial drugs in successive flocks (Mathis & McDougald, Citation1989; Chapman, Citation1994, Citation1996; Oliveira, Citation2001) can restore the sensitivities of coccidial populations to those drugs, thus extending their commercial life, as proposed by Chapman (Citation2000a,Citationb), Erník & Bedrník (Citation2001), Chapman et al. (Citation2002) and Mathis (Citation2003). Probably drug-sensitive parasites introduced by live vaccines interbreed with local drug-resistant populations (Williams, Citation2002a). Not only does this ameliorate drug resistance, but an attenuated vaccine might also reduce local population pathogenicity (Williams, Citation1992b).
Anticoccidial vaccines
Vaccines have been available since 1952, but have taken a long time to become accepted as an alternative to chemotherapy (Williams, Citation2002b, Citation2003c). Vaccination has been reviewed by Williams (Citation1992b, Citation1998, Citation1999b, Citation2002a, Citation2003a,Citationb), Bedrník (Citation1993), Yvoré et al. (Citation1993), Shirley & Bedrník (Citation1997), Danforth (Citation1998), Chapman (Citation2000a), Vermeulen et al. (Citation2001) and Chapman et al. (Citation2002). So far, all vaccines developed include live oocysts, except for one, a subunit vaccine of purified proteins isolated from E. maxima gametocytes, injected into hens to stimulate maternal antibodies (Wallach et al., Citation1995). Williams (Citation2003c) provided a comprehensive historical list of commercially available vaccines.
In many European countries, nearly all broiler breeders are vaccinated against coccidiosis with a multivalent, live, attenuated vaccine (Pattison, Citation2000); elsewhere, non-attenuated or attenuated live vaccines may be used in breeders (Williams, Citation1999a). Vaccine use in layer replacement flocks is increasing (Shapiro, Citation2000), since live vaccines are particularly effective in birds reared to maturity on litter, and improvements are being made in vaccine administration to cage-reared layers (Soares et al., Citation2004).
Vaccines are also used to some extent in broilers (Williams, Citation2002a) but, compared with the breeder and layer sectors, worldwide market penetration is relatively limited. The poor understanding of relationships between coccidioses and clostridioses is a major factor limiting anticoccidial vaccine use in broilers (Williams, Citation2003a). Hence, most broilers are still fed diets containing anticoccidial drugs (Williams, Citation1999a).
Disinfectants
Apparently, only methyl bromide, carbon disulphide, ammonia or phenols can kill oocysts, and only the latter two can safely be used under commercial conditions (Williams, Citation1997b). Their use is probably worthwhile only in cases of extreme contamination. Litter treatment with an enzyme that hydrolyses oocyst walls may help to control coccidiosis (van der Sluis, Citation1996a).
Necrotic enteritis
Forms of clostridiosis in chickens
Clostridium perfringens is the most important cause of clostridial enteritides in domesticated animals (Songer, Citation1996). According to Köhler (Citation2000) and Schuring & van Gils (Citation2001), chicken clostridioses include NE, ulcerative enteritis, necrotic dermatitis and botulism; to these may be added necrotic hepatitis and cholangiohepatitis (Løvland & Kaldhusdal, Citation2001). NE may be fatal or subclinical, leading to reduction of commercial performance (Kaldhusdal & Hofshagen, Citation1992; Løvland & Kaldhusdal, Citation2001), with cholangiohepatitis causing downgrading at the processing plant (Løvland & Kaldhusdal, Citation2001).
NE is a globally important welfare and economic problem (van der Sluis, Citation2000a, Citationb). It is an enterotoxaemia, caused by types A and C of the enteric Gram-positive bacterium C. perfringens (=Clostridium welchii) (Köhler, Citation2000; Wages & Opengart, Citation2003), which occurs when high numbers of bacteria coincide with a damaged intestinal mucosa (Al-Sheikhly & Truscott, Citation1977a). Disease results from the high frequency of adhesion by C. perfringens to the damaged mucosa (Kageyama et al., Citation1987; Baba et al., Citation1992a), facilitating bacterial proliferation and toxin production.
The characteristic lesion of NE is an intestinal mucosal necrosis, caused by the α-toxin of types A and C of C. perfringens and the β-toxin of type C. The α-toxin produces lesions and death in conventional (Al-Sheikhly & Truscott, Citation1977a,Citationb) or germ-free chickens (Fukata et al., Citation1988); α-toxin is by far the most potent in poultry (Köhler, Citation2000). The epizootiology and pathogenesis of NE are still rather poorly understood (see Kaldhusdal, Citation2000; Hermans & Morgan, Citation2003).
Diagnosis of NE
NE is potentially fatal, and flock mortality rates may reach up to 1% per day (Julian, Citation1969; Helmboldt & Bryant, Citation1971), total mortalities attaining 30% (Köhler et al., Citation1974a). Chickens are most commonly affected at 2 to 6 weeks old (Gardiner, Citation1967; Bains, Citation1968; Julian, Citation1969; Bernier & Filion, Citation1971; J. R. Long, Citation1973; Williams et al., Citation1999). However, NE may occur in birds 7 to 16 weeks old (Nairn & Bamford, Citation1967; Helmboldt & Bryant, Citation1971; Broussard et al., Citation1986; Frame & Bickford, Citation1986), or even up to 6 months (Wages & Opengart, Citation2003). Onset of disease is sudden, and clinical illness before death is of short duration (Wages & Opengart, Citation2003).
Gross lesions occur in the jejunum (Parish, Citation1961a; Kaldhusdal & Hofshagen, Citation1992), sometimes extending into the duodenum (Helmboldt & Bryant, Citation1971; Broussard et al., Citation1986; Kaldhusdal & Hofshagen, Citation1992) or the ileum (Parish, Citation1961a; Broussard et al., Citation1986). Rarely, lesions also occur in the colorectum, or caecal tonsils or necks (Long et al., Citation1974; Pedersen et al., Citation2004). Typical focal lesions are sharply demarcated from the surrounding mucosa (Parish, Citation1961a; Riddell, Citation1987; Kaldhusdal & Hofshagen, Citation1992). In severe cases, lesions are longitudinally expanded along the gut, and the epithelium is eroded and detached. Gram-positive rods occur in the lesions (Nairn & Bamford, Citation1967; Kaldhusdal & Hofshagen, Citation1992; Kaldhusdal et al., Citation1995) and can be isolated by differential anaerobic culture (Parish, Citation1961a,Citationb; Helmboldt & Bryant, Citation1971; Wages & Opengart, Citation2003). A diphtheritic membrane of dead mucosal enterocytes trapped in fibrin (Helmboldt & Bryant, Citation1971; Riddell, Citation1987), coloured yellow or green (Bains, Citation1968; Wages & Opengart, Citation2003) or brownish-orange (Broussard et al., Citation1986) may cover the mucosa (the “Turkish towel” effect). The gut is friable, dilated and gas-filled, with foul-smelling brown liquid contents (Parish, Citation1961a; Bains, Citation1968; Helmboldt & Bryant, Citation1971; Broussard et al., Citation1986).
Sources of infection
C. perfringens occurs naturally in a healthy chicken's gut (Timms, Citation1968; Barnes et al., Citation1972), but the burden in individual birds is very variable (Wages & Opengart, Citation2003). In broilers up to 5 weeks old, there is a positive correlation between age and C. perfringens burden (Hofshagen & Kaldhusdal, Citation1992; Kaldhusdal et al., Citation2001). C. perfringens may also be found in the farm environment (Wages & Opengart, Citation2003) and in poultry processing plants (Craven et al., Citation2001a). Poultry feed is a major source of infective spores (Wijewanta & Seneviratna, Citation1971; Char et al., Citation1986; Frame & Bickford, Citation1986; Köhler, Citation2000), which can tolerate 100°C for 2 h (Parish, Citation1961b). Fish-meal in diets is a particular source of C. perfringens contamination (Wijewanta & Seneviratna, Citation1971; Char et al., Citation1986). It is extremely difficult to ensure that poultry feed is spore-free, because the pellet and extrusion processes typically involve exposure to about 85°C for only a few minutes (Heijnen, Citation2004). Other extreme forms of ingredient treatments, such as hexane extraction and oven-heating, also do not kill all spores (Schuring & van Gils, Citation2001). However, Henry et al. (Citation1995) and Köhler (Citation2000) found about 10% of feed samples tested were contaminated, so contamination is not necessarily inevitable (Craven et al., Citation2001a).
Newly hatched chicks may already be infected; Shane et al. (Citation1984) have shown that vertical transmission from hens is possible. Whatever the primary source, C. perfringens is a frequent hatchery contaminant (Barnes et al., Citation1980; Craven et al., Citation2001a,Citationb; Sasaki et al., Citation2003). Moreover, Craven et al. (Citation2001a) found that swabs taken on farms from wild birds’ faeces, live insects, soil and puddles outside broiler houses, from the water pipes, nipple-drinker drip-cups, floors, walls and fans inside the houses, from chick delivery-tray liners, from litter material, and from the boots of farm staff all yielded C. perfringens before chicks were placed. Although feed delivered before chick placement was found to be uncontaminated, feed samples taken within 2 weeks of birds being introduced yielded C. perfringens.
Some pathophysiological effects of NE
Considerably less is known of the pathophysiological effects of NE than of those of coccidioses. In the field, subclinical disease is not easily recognized, and “diagnosis by therapy” may be practised on-farm. Thus, if sick birds respond to treatment with a penicillin analogue, they are often deemed to have had NE. This is not a reliable conclusion, since penicillins may be active against many bacterial species.
General clinical signs
Chickens suffering from clinical NE appear depressed, anorexic and relatively immobile. If death occurs, it may come so quickly that no reduction of body weight gain is apparent (Nairn & Bamford, Citation1967; Helmboldt & Bryant, Citation1971; Broussard et al., Citation1986), although loss of condition may occur in chronic cases, and C. perfringens may certainly cause growth depression (Lev & Forbes, Citation1959; Smith, Citation1959; George et al., Citation1982; Stutz et al., Citation1983; Stutz & Lawton, Citation1984; Hofshagen & Kaldhusdal, Citation1992; Köhler, Citation2000; van der Sluis, Citation2000a). Although “sticky droppings” adhering to a bird's cloaca may be a sign of NE (van der Sluis, Citation2000a), Kaldhusdal & Hofshagen (Citation1992) found no association in a controlled trial. Dietary rye, a predisposing factor for NE, can itself cause sticky droppings (Pettersson, Citation1987). Atrophy of the bursa of Fabricius, possibly indicating immunosuppression, may occur in birds suffering NE (Frame & Bickford, Citation1986), but cause and effect has not been established.
Diarrhoea and associated wet litter
Kaldhusdal & Hofshagen (Citation1992) noted “dark and moist” wood-shaving litter when birds had subclinical NE, and Elwinger & Teglöf (Citation1991) found a direct correlation between sticky droppings and poor litter condition. Diarrhoea may be associated with acute NE (Helmboldt & Bryant, Citation1971), but not always (Nairn & Bamford, Citation1967), although the water to food intake ratio may be increased (van der Sluis, Citation2000a). Most descriptions of NE field outbreaks do not mention the typical slippery, wet litter seen during chronic disease, but associations between diarrhoea or wet litter and NE were identified during world (van der Sluis, Citation2000b) and UK (Hermans & Morgan, Citation2003) field surveys. Such effects are so well known to UK poultry managers that any sudden increase in litter moisture is often taken to signal imminent NE, and antibiotic therapy is immediately initiated (R. B. Williams, personal observations).
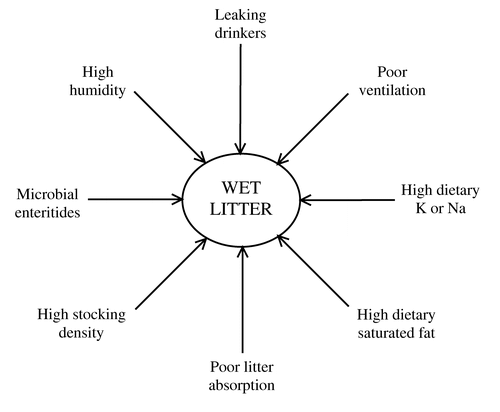
Although such action may sometimes be justified, wet litter is not always clostridial in origin. Apart from leaking drinkers, it may result from many adverse effects related to feed quality, mycotoxins, house temperature and ventilation, and infection with bacteria, viruses or protozoa (Babu, Citation1992), as well as too high a stocking density. illustrates some factors known to contribute to wet litter. When Collins et al. (Citation1989) observed diarrhoea and increased mortality rates in chickens, examination of the litter and faeces revealed virus particles resembling picornaviruses, parvoviruses and caliciviruses, but similar particles were not found in healthy flocks. Wet litter may also arise from spirochaete infections (Stephens & Hampson, Citation2002), which are exacerbated by wheat-based diets (Phillips et al., Citation2004). Recent anecdotal evidence from the field in the UK seems to implicate variant infectious bronchitis virus in wet litter problems, but confirmation is so far lacking.
Predisposing factors
Types of dietary cereal. Feed composition is most important during early life of chicks (Hofshagen & Kaldhusdal, Citation1992; Kaldhusdal & Hofshagen, Citation1992). Birds fed diets based on wheat, rye, oats or barley suffer more severe NE than birds fed maize-based diets (Branton et al., Citation1987, Citation1997; Hofshagen & Kaldhusdal, Citation1992; Kaldhusdal & Hofshagen, Citation1992; Riddell & Kong, Citation1992; Kaldhusdal & Skjerve, Citation1996). In commercial trials, outbreaks of NE and coccidiosis occurred in broilers fed a wheat-based pellet diet supplemented with 5% whole wheat, despite inclusion of an ionophore and virginiamycin in the feed (Williams et al., Citation1999). Annett et al. (Citation2002) found that in vitro proliferation of C. perfringens was greater in extracts of digested wheat or barley than in an extract of digested maize, but there were no differences between extracts of the undigested cereals. Rye increases the adhesion of bacteria to the intestinal mucosa (Untawale & McGinnis, Citation1979). Furthermore, rye and barley cause wet and sticky litter conditions (Petersen, Citation1997), predisposing birds to infectious diseases. Abrupt changes in feed texture may also cause problems (Ross Breeders, Citation1999).
Dietary animal products
Animal proteins are favourable substrates for clostridial growth, and high concentrations in broiler feeds are often associated with NE (Kaldhusdal & Skjerve, Citation1996; Kocher, Citation2003). Experimentally, fish-meal exacerbates NE (Truscott & Al-Sheikhly, Citation1977; Prescott, Citation1979). Drew et al. (Citation2004) found a positive association between crude protein derived from fish-meal and numbers of ileal and caecal C. perfringens, but no such association existed for soya-derived protein. Glycine and methionine levels in fish-meal are higher than in soya concentrate, and these amino acids are known to stimulate C. perfringens growth in vitro (Ispolatovskaya, Citation1971; Muhammed et al., Citation1975; Nakamura et al., Citation1978). Increased gizzard pH, stimulated by animal protein, encourages C. perfringens proliferation (Smith, Citation1965). Nevertheless, interactions between animal or plant protein sources and cereals are poorly understood (Kaldhusdal, Citation2000).
Animal fat (lard or tallow) may lead to higher ileal counts of C. perfringens than does soya oil (Knarreborg et al., Citation2002).
Dietary zinc
Relatively high zinc concentrations occur in wheat, meat-meal and fish-meal (National Research Council US, Citation1984), all of which are predisposing factors to NE. In vitro production of α-toxin is increased by zinc, which also protects α-toxin from destruction by trypsin (Baba et al., Citation1992b).
Low intestinal acidity
Low acidity may be a predisposing factor because C. perfringens growth in vitro is inhibited at low pH (Kmet et al., Citation1993) and toxin production is increased at high pH (Taylor & Stewart, Citation1941). Smith (Citation1965) found that by increasing dietary animal protein the gizzard pH approached 7, with concomitant increase of the proportion of intestinal C. perfringens.
Intestinal stasis
Cereals containing large amounts of NSPs increase digesta viscosity, which increases the gut passage time (van der Klis & van Voorst, Citation1993), which in turn may allow anaerobic bacteria to proliferate and produce enterotoxins (Parish, Citation1961a; Vahjen et al., Citation1998). Thus, gut stasis due to increased digesta viscosity in wheat-fed birds or to clinical coccidiosis may predispose them to NE (Riddell, Citation1987). This is likely to be influenced by the increase in nutrients available to the clostridia, rather than by the effect of viscosity per se, because Waldenstedt et al. (Citation2000) found no change in numbers of caecal C. perfringens when digesta viscosity was increased by dietary supplementation with carboxymethyl cellulose.
Birds dying of NE may sometimes have intussusceptions (R. B. Williams, personal observations). Although intussusceptions may be caused by dysperistalsis due to hunger (Okoye, Citation1985), there is usually no time for this to happen before a bird dies of acute NE. NE is therefore unlikely to cause intussusceptions, but may itself result from intestinal stasis due to a prior intussusception. However, intestinal necrosis in birds with intussusceptions may also be caused by an interrupted blood supply (M. Kaldhusdal, personal observations).
Damage to the intestinal mucosa
Damage to the mucosa facilitates proliferation of C. perfringens and toxin production (Al-Sheikhly & Truscott, Citation1977a). Damage might be caused by mechanical action, such as litter-eating by birds (Köhler et al., Citation1974b; Branton et al., Citation1997) or fibrous materials in the diet, but the evidence is rather weak. A healthy gizzard grinds feed so efficiently that large particles do not normally enter the duodenum (Hetland et al., Citation2002). Hammer-milled (finely ground) feed caused higher mortality attributed to NE than did roller-milled (coarsely ground) feed (Branton et al., Citation1987), but Riddell & Kong (Citation1992) found no correlation between NE mortality and particle sizes of ground wheat between 1.5 mm and 6 mm. However, Petersen (Citation1997) discussed adding whole wheat to broiler diets in Denmark, following which increases in NE and coccidiosis occurred, although cause and effect were not established. Conversely, Engberg et al. (Citation2004) found that feeding whole wheat reduced intestinal counts of C. perfringens, as well as reducing gizzard pH, although Hetland et al. (Citation2002) found no consistent effect on gizzard pH by various forms of cereals, including wheat. Engberg et al. (Citation2002) found no effect of feed grinding on intestinal counts of C. perfringens, but numbers were reduced if birds were fed pellets rather than mash.
Gut damage caused by coccidial infection sometimes predisposes birds to clostridial infection and NE, which is apparently supported by a UK field survey (Hermans & Morgan, Citation2003). Certainly, coccidial infections stimulate proliferation of intestinal C. perfringens (Johansson & Sarles, Citation1948; Bradley & Radhakrishnan, Citation1973; Arakawa & Ohe, Citation1975; Kimura et al., Citation1976; Dykstra & Reid, Citation1978; Waldenstedt et al., Citation1998). Leakage of plasma proteins due to coccidiosis can provide growth factors for the proliferation of C. perfringens (Van Immerseel et al., Citation2004). However, the links between coccidia and NE are still uncertain (Williams, Citation1999b; Anonymous, Citation2000), and field associations are inconsistent. Recent insights will be discussed later.
Immunosuppression
Exposure to infectious bursal disease, chick infectious anaemia virus and Marek's disease, as well as non-specific stress, may predispose birds to NE (Schuring & van Gils, Citation2001).
Climate
Little information is available, but Kaldhusdal & Skjerve (Citation1996) noted peak incidence of NE during the Norwegian winter, while in Canada high prevalence occurred in late summer in Ontario (J. R. Long, Citation1973) or throughout the summer in Quebec (Bernier et al., Citation1974). In Australia, peak incidence occurred during the winter in Victoria (Nairn & Bamford, Citation1967) and in late summer in Queensland (Bains, Citation1968), but in Western Australia there was no apparent seasonal effect (Gardiner, Citation1967). Research into the interactions of temperature, humidity and ventilation may reconcile these observations.
Potential control methods
Methods for controlling NE are not so well established as those for coccidiosis. Some of the following are poorly understood and may give inconsistent results.
Dietary modifications
Dietary cereals that predispose birds to NE should be reduced if possible. Combinations of enzymes such as β-glucanase, pentosanase and cellulase (Jansson et al., Citation1990), or β-glucanase, xylanase, pectinase, amylase and cellulase (Elwinger & Teglöf, Citation1991), may decrease NE risk by improving digestion of NSPs in wheat, barley, rye or oats. However, pentosanase alone had no effect in a wheat-based diet (Riddell & Kong, Citation1992). Phillips et al. (Citation2004) found that soluble NSP contents of various cereals did not consistently predict ileal digesta viscosity.
Animal protein, particularly fish-meal, should be omitted from or included at low levels in poultry diets, since this may be a source of C. perfringens contamination, as well as being a source of nutrients for the bacterium. Plant oils may be preferable to animal fats.
Prebiotics are feed ingredients serving as substrates for bacteria that are beneficial to the host by suppressing more pathogenic bacteria (see Kaldhusdal, Citation2000; Van Immerseel et al., Citation2004). Thus, glucose (but not sucrose or fructose) may reduce ileal counts of C. perfringens and maintain body weight gain (Stutz & Lawton, Citation1984); when it is replaced by starch in vitro, an increase in C. perfringens α-toxin results (Logan et al., Citation1945). Riddell & Kong (Citation1992) found that glucose in a maize-based diet improved body weight, but slightly increased mortality (not statistically significantly); that work should be repeated. Dietary lactose reduces C. perfringens colonization (Takeda et al., Citation1995). However, results from experiments on complex carbohydrates such as pectin and guar gum were confounded because of severe reductions of food consumption and growth rate (Riddell & Kong, Citation1992; Branton et al., Citation1997); nevertheless, those compounds did not prevent NE lesions (Branton et al., Citation1997). Dietary fructo-oligosaccharides or manno-oligosaccharides were also ineffective against experimentally induced NE in broilers (Hofacre et al., Citation2003).
Short-chain carboxylic acids included in feed or water have direct antibacterial effects, also reducing digesta pH and increasing pancreatic secretion (Kmet et al., Citation1993; Dibner, Citation2004). Essential oils reduced C. perfringens numbers in the intestine early in a chick's life, but did not have dramatic effects against clinical NE (Waldenstedt, Citation2003; Mitsch et al., Citation2004). Duffy & Tucker (Citation2003) provided further information on the effects of natural products, such as plant extracts, ω-3 fatty acids, minerals and carbohydrates, on disease resistance and gut integrity.
Anticlostridial drugs
No medicinal prophylactic drug specifically aimed at clostridial diseases is employed, but AGPs have long played an incidental part in clinical NE suppression. Thus, AGPs such as virginiamycin, bacitracin, avoparcin, avilamycin, etc. are effective against clostridioses (for example, Prescott, Citation1979; Elwinger & Teglöf, Citation1991; Elwinger et al., Citation1998). Furthermore, some ionophorous anticoccidial drugs have similar effects (for example, Elwinger et al., Citation1998; Brennan et al., Citation2001). summarizes some studies on the in vivo efficacy of various AGPs and ionophores against C. perfringens isolated from chickens. None had outstanding efficacy, but the diets were usually suboptimal for gut integrity.
Table 1. In vivo efficacy of antibiotic growth promoters and ionophores included alone in various diets against clinical NE in chickens
Some AGPs and ionophores have equivocal effects on clostridioses in vivo (Nairn & Bamford, Citation1967; Wang & Davidson, Citation1992; Elwinger et al., Citation1992a, Citation1994, Citation1998; Brennan & Cheng, Citation1997; Bolder et al., Citation1999; Williams et al., Citation1999). At growth promotional concentrations, AGPs cannot be relied upon invariably to protect against NE under commercial conditions (Miller, Citation1983). NE may occur in broilers when drug shuttles including an ionophore are used, even with an additional AGP (Williams et al., Citation1999; Erník & Bedrník, Citation2001). If clinical NE should occur, penicillin analogues administered in the drinking water are normally extremely effective (Long & Truscott, Citation1976; Ross Breeders, Citation1999), although not always (see later).
However, AGPs have not been used in poultry diets in Sweden since 1986, in Norway since 1995 or in Denmark since 1998 (Johansson et al., Citation2004). Avoparcin was banned in all EU countries in 1997 (Elwinger et al., Citation1998). In the EU, the continuing process of mandatory (Corpet, Citation2000; Van Immerseel et al., Citation2004) and voluntary cessation of use of AGPs has led to the withdrawal of most of them from poultry diets, but anticoccidial ionophores are still used. According to Elwinger et al. (Citation1998) and Elwinger & Teglöf (Citation1991), broilers should perform well under good hygienic conditions if fed an ionophore without any additional AGP.
Drug resistance
summarizes in vitro sensitivities to AGPs and ionophores of C. perfringens strains from poultry, mainly chickens, in various countries. Penicillins, avilamycin, avoparcin and ionophores were generally efficacious, but resistance to bacitracin, lincomycin, flavophospholipol, virginiamycin and tetracyclines was widespread. Efficacy of therapeutics in vitro may not always be predictive for the field (Long & Truscott, Citation1976; Rada et al., Citation1994; Watkins et al., Citation1997; Bolder et al., Citation1999).
Table 2. In vitro sensitivities of C. perfringens isolated from chickens (C) or turkeys (T) to antibiotic growth promoters and ionophores
In UK broiler trials (Williams et al., Citation1999), NE was observed in flocks medicated with either salinomycin or monensin in addition to virginiamycin; resistant C. perfringens was possibly implicated, but no sensitivity tests were carried out. In other trials, NE sometimes occurred in broilers given ionophores, with or without avilamycin (Williams, Citation2002a).
Sometimes, one or even two periods of treatment with amoxycillin failed to control outbreaks of NE in UK flocks (R. B. Williams, personal observations). Development of penicillin resistance by C. perfringens is known (Gardiner, Citation1967; Nairn & Bamford, Citation1967).
Probiotics and competitive exclusion products
Maintenance of a stable gut flora is essential to prevent dysbacteriosis, a general overgrowth of the intestinal microflora, which may predispose to NE by reducing oxygen tension to a level favourable for C. perfringens proliferation (Schuring & van Gils, Citation2001). This objective may be achieved with probiotics or competitive exclusion products (CEPs). Probiotic definitions have evolved considerably since 1954 (Fuller, Citation2004), the best known being “A live microbial feed supplement which beneficially affects the host animal by improving its intestinal microbial balance” (Fuller, Citation1989). Some workers regard CEPs as distinct from probiotics, the former comprising orally administered mixtures of undefined bacterial species, and the latter usually being monospecific in-feed preparations. The latest redefinition of a probiotic (Fuller, Citation2004) subsumes CEPs: “A probiotic is a preparation of viable micro-organisms which is consumed by humans or other animals with the aim of inducing beneficial effects by qualitatively or quantitatively influencing their gut microflora and/or modifying their immune status”. Hence, there are no distinctions regarding specific compositions and routes of administration, but the term CEP is retained here for convenience.
Bolder et al. (Citation1999), summarizing current literature, concluded that transferring normal intestinal contents from adult hens to chicks decreases susceptibility to pathogenic bacteria. Mechanisms may include competition for attachment sites, production of volatile fatty acids and reduction of pH. Experimentally, conventional or monoflora (Lactobacillus acidophilus or Streptococcus faecalis) chickens are more resistant to C. perfringens infections than are germ-free chicks (Fukata et al., Citation1991). Barnes et al. (Citation1980), Kmet et al. (Citation1993) and La Ragione & Woodward (Citation2003) also found C. perfringens susceptible to competitive exclusion. Furthermore, a Lactobacillus probiotic can increase resistance to coccidia (Lillehoj et al., Citation2003).
Elwinger et al. (Citation1992b) found that a commercial CEP reduced NE and caecal carriage of C. perfringens, and improved bird performance, but simultaneous inclusion of narasin in the feed masked those effects. Kaldhusdal et al. (Citation2001) showed only slight, statistically non-significant, benefits of the same CEP in a diet containing narasin. Other CEPs may also be effective (Hofacre et al., Citation1998; Craven et al., Citation1999). Generally, benefits of CEPs were greater if diets were suboptimal for NE control; for example, containing high levels of animal protein or rye, or lacking an AGP (Elwinger et al., Citation1992b; Craven et al., Citation1999)
Surprisingly, in a UK field survey, there was apparently an association between NE and the use of CEPs (Hermans & Morgan, Citation2003). However, questionnaire data may be less reliable than those resulting from controlled experiments, and it was not clear whether any factors incompatible with CEPs may have been involved. Certain ionophores may reduce the effectiveness of probiotics containing lactobacilli (Rada et al., Citation1994).
Manipulation of litter conditions
An adequate depth of material with good absorptive capacity is essential. Leaking drinkers must be avoided, and litter moisture kept down to 20% to 25% (Anonymous, Citation2003). Litter moisture can be lowered by inclusion of dietary carob-pod-meal to reduce the birds’ water to feed intake ratio (Best, Citation2004); by rearing birds on special perforated floors through which air is forced in order to dry the litter (van der Sluis, Citation1996b); or simply by efficient traditional ventilation. Treatment of litter with organic acids to reduce the pH helps generally to control microbes in the litter (Ivanov, Citation2001) and C. perfringens in birds’ intestines (Garrido et al., Citation2004). Essential oils in certain softwoods that may be used for litter possess anticlostridial activity (Johnston et al., Citation2001). Because clostridial spores are so resistant to many disinfectants (Russell, Citation1990), it is best to remove all litter during a complete physical clean-out of the house between crops (Norton & Hoerr, Citation1999). Perhaps an enzymic method to attack bacterial spores and viruses in litter could be developed, as done for coccidial oocysts (cf. van der Sluis, Citation1996a).
Anticlostridial vaccines
Although maternally derived antibodies to α-toxin are detectable in 1-day-old chicks (Heier et al., Citation2001), and vaccination against clostridia may be feasible (Løvland et al., Citation2004), no vaccine is yet commercially available for poultry.
Gut integrity in poultry
Gut integrity may be regarded as satisfactory when the intestinal epithelium with its mucous coat, its vascular supply and the gut-associated lymphoid tissue (GALT) are mature and sound. Gut integrity has considerable effects on the reactions of the host to invasion by micro-organisms (see Sklan, Citation2001, Citation2004). Kenny & Kemp (Citation2003) described a nutritional approach to maintaining gut integrity. Further crucial aspects, potentially affecting susceptibility to coccidial and clostridial diseases, are briefly addressed here.
Physical development of the gut
During the period immediately after hatching, increase in size of a chick's gut is much faster than increase in body weight. Rapid relative growth of the small intestine in broiler chicks is maximal at 6 to 10 days of age (Uni et al., Citation1999). The duodenum shows earlier rapid growth than the jejunum and ileum (Uni et al., Citation1999). While this early spurt of intestinal growth depends to some extent upon the yolk as an energy source, it is greatly enhanced by food ingestion (Noy & Sklan, Citation1999; Sklan, Citation2001).
Development of the crypts of Lieberkühn in the small intestine is maximal during the first 2 days after hatch, although those in the duodenum and jejunum continue to develop after those in the ileum have matured (Geyra et al., Citation2001). Villus growth is rapid during the first 2 days, reaching a plateau in the duodenum after 6 to 8 days, and in the jejunum and ileum after about 10 days (Geyra et al., Citation2001). Starvation of hatchlings delays early villus development, the effect being longer lasting in the jejunum than in the duodenum (Sklan, Citation2004). Enterocytes migrate from the crypts, progressing up the villi until shed from the tips, taking 2 to 3 days in chicks 2 to 4 days old, and taking about 4 days in older birds (Imondi & Bird, Citation1966; Uni et al., Citation1998; Geyra et al., Citation2001).
Development of immune competence
The GALT is a component of the mucosal immune system, protecting against enteric micro-organisms. The avian GALT includes the bursa of Fabricius, the caecal tonsils, Meckel's diverticulum, Peyer's patches and intraepithelial lymphocytes (Lillehoj & Trout, Citation1996). At hatch, GALT contains T lymphocytes and B lymphocytes that do not mature for about 2 weeks; cellular immune responses develop earlier than, and are a prerequisite for, humoral responses (Bar-Shira et al., Citation2003). There is a crucial relationship between immune system development and initiation of feeding, because gut development is integrated with that of the immune system. Starvation slightly and temporarily impairs GALT development of the small intestine, but lymphocyte colonization and maturation in the caeca and colon are delayed by at least 35 days (Sklan, Citation2004). Starvation of older birds severely depresses the whole immunological system and slows bacteria clearance from the bloodstream (Ben-Nathan et al., Citation1981).
While innocuous antigens such as nutrients absorbed by enterocytes are immunologically tolerated, antigens derived from pathogens penetrating the mucosa by transcellular pathways or phagocytes are immunogenic. Gut protection is achieved by generating, in the distal portion and bursa, immune responses disseminated systemically throughout the bird. Because the GALT takes time to mature, early exposure to immunogens may compromise health more than later exposure, although early foraging promotes development of immune functions by increasing access to environmental flora via the cloaca (see Sklan, Citation2004).
Development of gut enzyme activity
Pancreatic enzymes are present in the gut of the late embryo (Marchaim & Kulka, Citation1967), but significant increases in luminal trypsin and amylases occur in the hatchling only when food ingestion commences (see Sklan, Citation2004). However, lipases have high luminal activity before food ingestion, since they hydrolyse yolk triglycerides (Noy & Sklan, Citation1998).
Mucosal enzymes, including sucrase-isomaltase, peptidases and phosphatases, are anchored to membranes at the brush border of the epithelium (Semenza, Citation1986). After 2 days of age, enzymatic activity is correlated with the number of enterocytes per villus (see Sklan, Citation2004).
Mucin production
Mucus is secreted by goblet cells, which increase in number as the villi grow (Sklan, Citation2004). A mucus layer adheres to the enterocytes, protecting them from irritants and bacteria, but functional details are poorly understood (Sklan, Citation2004). However, mucolytic bacteria doubtlessly impair its barrier effect.
Development of gut flora
The gut of a newly hatched chick is often stated to be normally sterile, but this is not always true (Barnes et al., Citation1980; Shane et al., Citation1984), and micro-organisms are in any case rapidly acquired from the environment. Many anaerobes colonize the caeca of a hatchling within 3 to 6 h, while streptococci and enterobacteria invade the small intestine and caeca during the first 2 to 4 days. After 1 week, lactobacilli predominate in the small intestine and the caeca contain mainly Escherichia coli and Bacteroides spp. (Mead & Adams, Citation1975).
The small intestinal flora stabilizes by 2 weeks but the caecal flora, comprising obligate anaerobes, develops in about 30 days, when bifidobacteria and bacteroides types predominate (Barnes et al., Citation1972). Absence of a normal caecal flora is a major influence on the susceptibility of chicks to bacterial infection (Barrow, Citation1992).
Epithelial damage
Mucosal abrasion and parasitic lesions potentially compromise gut integrity by preventing efficient digestion and allowing the proliferation of pathogens.
What is the nature of interactions between coccidioses and NE?
Controversy regarding relationships between coccidia and NE has been stimulated by several considerations. First, AGPs and ionophores afford some control of clostridioses, and ionophores also control coccidioses. Second, administration of an anticoccidial vaccine normally precludes inclusion of ionophores in poultry feed, thereby possibly leading to an increased risk of NE (Ross Breeders, Citation1999). Third, clinical coccidiosis predisposes birds to NE. Fourth, it is feared that if mild coccidial infections (as opposed to clinical coccidioses) might also predispose birds to NE, live anticoccidial vaccines might do the same. However, these multifactorial relationships are seriously under-researched, and are discussed here.
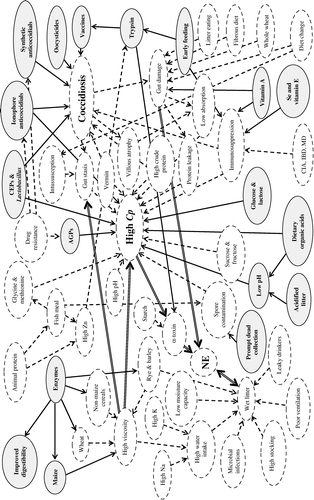
NE and some Eimeria species may independently cause acute, fatal diseases in chickens and turkeys. NE seems unlikely to predispose birds to coccidiosis, since destruction of enterocytes would remove potential development sites for coccidia, and the diphtheritic membrane would impede intraluminal dissemination of extracellular coccidial stages. However, various laboratory trials and anecdotal cases suggest that coccidiosis may predispose birds to NE (for example, Bennetts, Citation1930; Bradley & Radhakrishnan, Citation1973; Kimura et al., Citation1976; Al-Sheikhly & Al-Saieg, Citation1980; Baba et al., Citation1997), but other observations have not necessarily corroborated such evidence (for example, Parish, Citation1961a; Nairn & Bamford, Citation1967; Julian, Citation1969; Helmboldt & Bryant, Citation1971; J. R. Long, Citation1973; Bernier et al., Citation1974; Long et al., Citation1974; Kaldhusdal & Hofshagen, Citation1992; van der Stroom & van der Sluis, Citation1999; Kaldhusdal et al., Citation2001; Répérant & Humbert, Citation2002). This may be because some minimum degree of gut damage or a threshold clostridial infection is necessary for disease to become patent. Other factors may also be involved, as presented in , which shows that some pathophysiological features of coccidioses might have opposing effects on clostridial development. Wider ranging interactions are elucidated in .
Table 3. The impact of some pathophysiological effects of coccidioses on the development of clostridial infections in the chicken's intestine
Figure 2. The intercurrent coccidiosis-NE syndrome: a network of potentially important pathophysiological, medicinal, nutritional and husbandry factors. Those with solid-line arrows and ellipses are beneficial in controlling disease, those with dashed-line arrows and ellipses impart high disease risk. Major high-risk relationships are shown by double-line arrows. AGP, antibiotic growth promoter; CIA, chick infectious anaemia; CEP, competitive exclusion product; Cp, Clostridium perfringens; IBD, infectious bursal disease; MD, Marek's disease; NE, necrotic enteritis.
summarizes some randomly observed associations of coccidia and NE in field cases, which demonstrate the difficulty of drawing any generalized conclusion regarding cause and effect, probably because of uncontrolled differences in natural challenges and husbandry. The innumerable cases of coccidiosis recorded worldwide without associated NE should also be borne in mind. In microscopical studies of spontaneous NE, clostridia always occurred in the lesions but the presence of coccidia in tissues or faeces was often inconsistent (Bernier et al., Citation1974; Long et al., Citation1974; Kaldhusdal et al., Citation1995). Long et al. (Citation1974) found coccidia most commonly in the upper intestine, whereas in the same birds NE lesions were more caudad. Controlled laboratory experiments are therefore necessary.
Table 4. Occurrence in chickens (C) or turkeys (T) of spontaneous coccidial infections and NE cases in circumstances where susceptible birds were exposed to a contaminated environment, but no experimental challenges were administered
Williams (Citation1999b) pointed out that it had not been established whether coccidial infection per se predisposes chickens to NE; or whether only severe coccidiosis that damages the mucosa sufficiently to allow the establishment of a clostridial infection leads to NE. Consideration of the methods used in attempts to reproduce NE by using coccidial challenges provides some insights. In laboratory experiments, concurrent heavy infections of coccidia and clostridia produced more severe pathogenic effects than either infection alone when the coccidium was E. acervulina (Al-Sheikhly & Al-Saieg, Citation1980; Shane et al., Citation1985), E. maxima (Williams et al., Citation2003) or E. necatrix (Al-Sheikhly & Al-Saieg, Citation1980; Baba et al., Citation1997). Smaller inocula of either organism or the random exposure to infected litter have often failed to reproduce NE.
An observation that has cast doubt on any consistent relationships between coccidioses and NE is that when those diseases simultaneously occur in a commercial flock, the respective lesions may be mutually exclusive in individual birds (Williams et al., Citation1999; R. B. Williams, personal observations). Williams et al. (Citation2003) provided a possible explanation of this, showing that severe coccidial lesions can occur alone before NE lesions have developed; and that by the time NE lesions are observable, any coccidial lesions that facilitated clostridial invasion have regressed. However, other explanations are possible. Clinical coccidiosis is not necessarily followed by NE unless sufficient numbers of C. perfringens are already present. Moreover, in the absence of coccidiosis, NE can result from other predisposing factors. This should be remembered if NE occurs following administration of a live anticoccidial vaccine.
What effect does anticoccidial vaccination have on NE?
Since the use of live anticoccidial vaccines for broilers is increasing (Williams, Citation2002a), it is important to know what effect vaccination against coccidiosis might have on NE. This question elicits many opinions but few facts; controlled experimental results are sparse. Birds given an attenuated vaccine in facilities free from extraneous coccidia did not develop NE (Williams, Citation1994; Waldenstedt et al., Citation1999), despite the demonstration of a clostridial challenge in one of the trials (Waldenstedt et al., Citation1999). In some commercial broiler trials, birds receiving an attenuated vaccine did not suffer NE, although birds on the same farm fed ionophores with an AGP did (Williams et al., Citation1999). In other trials, either vaccinated or medicated broilers occasionally developed NE (Williams, Citation2002a).
Williams et al. (Citation2003) examined relationships between clinical coccidiosis, anticoccidial vaccination and NE. An attenuated anticoccidial vaccine was used to immunize birds before oral administration of a heavy E. maxima infection followed by a culture of C. perfringens administered per cloaca. A single, heavy E. maxima infection given to a control group exacerbated NE, but the light, mixed infection (including E. maxima) caused by the vaccine, followed by a heavy E. maxima infection, did not. Thus, immunization against the virulent coccidial challenge reduced the severity of the later clostridial challenge, probably because any coccidial lesions that might have predisposed birds to NE were prevented, as previously hypothesized by Williams (Citation1999b). This situation is analogous to indirect control of NE by anticoccidial drugs.
Vaccination itself, even with an attenuated vaccine, may cause mild coccidial lesions in some birds (Williams, Citation1994; Williams & Andrews, Citation2001). Such lesions, and also secondary lesions resulting from virulent challenges of immunized birds, contain very few, if any, endogenous parasites (Williams & Andrews, Citation2001; Williams, Citation2003d). Perhaps those lesions are not severe enough to predispose immunized birds to NE, as suggested by the results of Williams et al. (Citation2003). However, a further definitive experiment involving simultaneous vaccination and C. perfringens challenge is necessary to confirm this.
Administration of live, attenuated anticoccidial vaccines does not increase birds’ water consumption (Williams, Citation1994; Williams et al., Citation1999), nor does it adversely affect litter condition (Williams, Citation1994; Williams et al., Citation1999; Williams & Gobbi, Citation2002). In those particular respects, anticoccidial vaccination should not predispose birds to NE.
Changes in global incidence of NE
NE is becoming more common in Europe (Williams, Citation2003a), Asia (Dhawale, Citation2004) and North America (Shane, Citation2004a,Citationb). In EU broilers, NE has been associated variously with: changes from dietary maize to wheat in Italy, France and the Benelux countries; greater use of ionophores than of synthetic anticoccidial drugs, resulting in poorer coccidiosis control and hence predisposition to NE in Spain; and the widespread banning of AGPs (Williams, Citation2003a; Van Immerseel et al., Citation2004). In 1995, NE constituted 4% of reported diseases in France, increasing to 12.4% in 1999 (Drouin, Citation1999). Following prohibition of AGPs in Nordic countries, NE increased, but was not associated with any increase in coccidiosis (van der Stroom & van der Sluis, Citation1999). However, in Norway the abrupt increase in NE immediately after the prohibition of avoparcin use in 1995 was followed by a reduction to previous levels when an ionophore was substituted (Grave et al., Citation2004). Replies to a questionnaire in 2001 indicated that 31% of broiler flocks in the UK suffered NE (Hermans & Morgan, Citation2003).
To reduce production costs, broiler producers in the USA have increased withdrawal periods of ionophores, leading to poorer protection against both coccidia and clostridia. Therefore, acute clostridial enteritis and chronic cholangiohepatitis have increased (Shane, Citation2004a). American broiler producers who have ceased using in-feed AGPs have also seen increases in NE, cholangiohepatitis, gangrenous dermatitis and botulism (Shane, Citation2004b). In Canada, caged layers currently suffer more coccidiosis, sometimes NE-associated, because of changes in the manure collection system (Soares et al., Citation2004).
The situation is different for breeding stock, however. In France, Portugal and the UK, the NE incidence fell after introduction of an anticoccidial vaccine, despite AGPs not being used (Williams, Citation1999b). Perhaps this was due to a protective effect against NE of improved coccidiosis control, as demonstrated by Williams et al. (Citation2003).
Rational management strategies for control of coccidioses and NE
Van Immerseel et al. (Citation2004) have emphasized that few tools are currently available for the control of C. perfringens in poultry; there is little time to develop strategies before the remaining AGPs are banned in Europe in 2006. While AGPs, ionophorous anticoccidial drugs and anticoccidial vaccines may all, in certain circumstances, help to control NE, none of them alone is invariably effective. The most successful control strategy is likely to incorporate elements of direct or indirect control of coccidia and clostridia, together with improved hygiene, husbandry and nutrition (Williams, Citation1999b, Citation2002a; Kaldhusdal, Citation2000; Köhler, Citation2000; Van Immerseel et al., Citation2004).
Based on this review, rational, integrated management strategies may be designed to prevent or reduce infection, or enhance host protection, incorporating methods of maintaining gut integrity. summarizes some significant factors for field testing. Beneficial features may be introduced or retained; others that increase disease risk should be avoided. Supporting references are given under the appropriate foregoing headings, but some in vitro effects are as yet unconfirmed in vivo, or may be modified by other uncontrolled factors in the field. Experimental results may often be conflicting.
When developing new management strategies, only one factor should be changed at a time, otherwise effects will be confounded. Obviously incompatible factors should be avoided; for example, the simultaneous use of a probiotic and an antibacterial drug, or of an in-feed anticoccidial drug and a drug-sensitive vaccine. Similar trials should be carried out simultaneously on several problem farms in the same geographical area, to account for climatic differences, and to ensure that observed effects are general rather than peculiar to one farm. National legislations regarding the use of dietary animal products, anticoccidial vaccines and AGPs must be complied with.
Testing feed ingredients for spore contamination (Köhler, Citation2000) and checking droppings for the “fluid zone” area (van der Sluis, Citation2000a) may be useful for monitoring management programmes.
Translations of the abstract in French, German and Spanish are available on the Avian Pathology website.
Acknowledgments
The author is grateful to Dr M. Kaldhusdal and P. McMullin for their constructive criticism of an early draft of this review, and for suggesting useful literature. The author also thanks Dr R. Fuller for helpful advice on probiotics, and G. Heijnen for information on poultry feed production.
References
- Allen , PC , Danforth , HD and Augustine , PC . (1998) . Dietary modulation of avian coccidiosis . International Journal for Parasitology , 28 : 1131 – 1140 .
- Allen , WM , Berrett , S , Hein , H and Hebert , CN . (1973) . Some physiopathological changes associated with experimental Eimeria brunetti infection in the chicken . Journal of Comparative Pathology , 83 : 369 – 375 .
- Al-Sheikhly , F and Al-Saieg , A . (1980) . Role of coccidia in the occurrence of necrotic enteritis of chickens . Avian Diseases , 24 : 324 – 333 .
- Al-Sheikhly , F and Truscott , RB . (1977a) . The interaction of Clostridium perfringens and its toxins in the production of necrotic enteritis of chickens . Avian Diseases , 21 : 256 – 263 .
- Al-Sheikhly , F and Truscott , RB . (1977b) . The pathology of necrotic enteritis of chickens following infusion of crude toxins of Clostridium perfringens into the duodenum . Avian Diseases , 21 : 241 – 255 .
- Anderson , WI , Reid , WM , Lukert , PD and Fletcher , OJ . (1977) . Influence of infectious bursal disease on the development of immunity to Eimeria tenella . Avian Diseases , 21 : 637 – 641 .
- Annett , CB , Viste , JR , Chirino-Trejo , M , Classen , HL , Middleton , DM and Simko , E . (2002) . Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A . Avian Pathology , 31 : 598 – 601 .
- Anonymous (2000) Link between coccidiosis and necrotic enteritis still uncertain Poultry Digest 59, 6, 32, 34
- Anonymous . (2003) . Watering system is the key to healthy poultry flocks . Poultry International , 42 : 36 – 38 .
- Arakawa , A and Ohe , O . (1975) . Reduction of Clostridium perfringens by feed additive antibiotics in the ceca of chickens infected with Eimeria tenella . Poultry Science , 54 : 1000 – 1007 .
- Aylott , MV , Vestal , OH , Stephens , JF and Turk , DE . (1968) . Effect of coccidial infection upon passage rates of digestive tract contents of chicks . Poultry Science , 47 : 900 – 904 .
- Baba , E , Wakeshima , H , Fukui , K , Fukata , T and Arakawa , A . (1992a) . Adhesion of bacteria to the cecal mucosal surface of conventional and germ-free chickens infected with Eimeria tenella . American Journal of Veterinary Research , 53 : 194 – 197 .
- Baba , E , Fuller , AL , Gilbert , JM , Thayer , SG and McDougald , LR . (1992b) . Effects of Eimeria brunetti infection and dietary zinc on experimental induction of necrotic enteritis in broiler chickens . Avian Diseases , 36 : 59 – 62 .
- Baba , E , Ikemoto , T , Fukata , T , Sasai , K , Arakawa , A and McDougald , LR . (1997) . Clostridial population and the intestinal lesions in chickens infected with Clostridium perfringens and Eimeria necatrix . Veterinary Microbiology , 54 : 301 – 308 .
- Babu , M . (1992) . Diarrhoea and wet litter in poultry . Poultry Adviser , 25 (9) : 41 – 45 .
- Bains , BS . (1968) . Necrotic enteritis of chickens . Australian Veterinary Journal , 44 : 40
- Banfield , MJ and Forbes , JM . (1999) . Feed content and structure effects on coccidiosis . World Poultry, Special Supplement Coccidiosis , 3 : 34 – 36 .
- Barnes , EM , Mead , GC , Barnum , DA and Harry , EG . (1972) . The intestinal flora of the chicken in the period 2 to 6 weeks of age, with particular reference to the anaerobic bacteria . British Poultry Science , 13 : 311 – 326 .
- Barnes , EM , Impey , CS and Cooper , DM . (1980) . Manipulation of the crop and intestinal flora of the newly hatched chick . American Journal of Clinical Nutrition , 33 : 2426 – 2433 .
- Barrow PA (1992) Probiotics for chickens In R. Fuller (Ed.) Probiotics: The Scientific Basis pp. 225–257 London Chapman & Hall
- Bar-Shira , E , Sklan , D and Friedman , A . (2003) . Establishment of immune competence in the avian GALT during the immediate post-hatch period . Developmental and Comparative Immunology , 27 : 147 – 157 .
- Becker ER (1934) Coccidia and Coccidiosis of Domesticated, Game and Laboratory Animals and of Man Ames IA Collegiate Press
- Bedrník P (1993) Livacox, A Vaccine against Coccidiosis of Domestic Fowl Jilové Czech Republic Research Institute of Feed Supplements and Veterinary Drugs
- Ben-Nathan , B , Drabkin , N and Heller , D . (1981) . The effect of starvation on the immune response of chickens . Avian Diseases , 25 : 214 – 217 .
- Bennetts , HW . (1930) . Bacillus welchii and bowel lesions. With special reference to a case of coccidiosis . Australian Veterinary Journal , 6 : 153 – 154 .
- Benno , Y , Endo , K , Shiragami , N and Mitsuoka , T . (1988) . Susceptibility of fecal anaerobic bacteria from pigs and chickens to five polyether antibiotics for growth promotion . Japanese Journal of Veterinary Science , 50 : 783 – 790 .
- Bernier , G and Filion , R . (1971) . Necrotic enteritis in broiler chickens . Journal of the American Veterinary Medical Association , 158 : 1896 – 1897 .
- Bernier , G , Phaneuf , J-B and Filion , R . (1974) . Entérite nécrotique chez le poulet de gril. I. Aspect clinico-pathologique . Canadian Journal of Comparative Medicine , 38 : 280 – 285 .
- Best , P . (2004) . Novel ingredients: seeds into feeds . Feed International , 25 (11) : 26 – 27 .
- Biggs , PM . (1982) . The world of poultry disease . Avian Pathology , 11 : 281 – 300 .
- Biggs , PM , Long , PL , Kenzy , SG and Rootes , DG . (1968) . Relationship between Marek's disease and coccidiosis. II. The effect of Marek's disease on the susceptibility of chickens to coccidial infection . Veterinary Record , 83 : 284 – 298 .
- Biggs , PM , Long , PL , Kenzy , SG and Rootes , DG . (1969) . Investigations into the association between Marek's disease and coccidiosis . Acta Veterinaria, Brno , 38 : 65 – 75 .
- Bolder , NM , Wagenaar , JA , Putirulan , FF , Veldman , KT and Sommer , M . (1999) . The effect of flavophospholipol (Flavomycin) and salinomycin sodium (Sacox) on the excretion of Clostridium perfringens, Salmonella enteritidis, and Campylobacter jejuni in broilers after experimental infection . Poultry Science , 78 : 1681 – 1689 .
- Bradley , RE and Radhakrishnan , CV . (1973) . Coccidiosis in chickens: obligate relationship between Eimeria tenella and certain species of cecal microflora in the pathogenesis of the disease . Avian Diseases , 17 : 461 – 476 .
- Branton , SL , Reece , FN and Hagler , WM . (1987) . Influence of a wheat diet on mortality of broiler chickens associated with necrotic enteritis . Poultry Science , 66 : 1326 – 1330 .
- Branton , SL , Lott , BD , Deaton , JW , Maslin , WR , Austin , FW , Pote , LM , Keirs , RW , Latour , MA and Day , EJ . (1997) . The effect of added complex carbohydrates or added dietary fiber on necrotic enteritis lesions in broiler chickens . Poultry Science , 76 : 24 – 28 .
- Brennan JJ Cheng T (1997) Efficacy of lasalocid for prevention of necrotic enteritis in broiler chickens In Proceedings of the VIIth International Coccidiosis Conference (p. 118) Oxford UK
- Brennan , J , Bagg , R , Barnum , D , Wilson , J and Dick , P . (2001) . Efficacy of narasin in the prevention of necrotic enteritis in broiler chickens . Avian Diseases , 45 : 210 – 214 .
- British Oil & Cake Mills (1964) Common Diseases of Poultry Erith British Oil & Cake Mills
- Britton , WM , Hill , CH and Barber , CW . (1964) . A mechanism of interaction between dietary protein levels and coccidiosis in chicks . Journal of Nutrition , 82 : 306 – 310 .
- Broussard , CT , Hofacre , CL , Page , RK and Fletcher , OJ . (1986) . Necrotic enteritis in cage-reared commercial layer pullets . Avian Diseases , 30 : 617 – 619 .
- Bumstead , N and Millard , BJ . (1992) . Variation in susceptibility of inbred lines of chickens to seven species of Eimeria . Parasitology , 104 : 407 – 413 .
- Catchpole J (2000) The diagnosis and misdiagnosis of coccidiosis in chickens In Proceedings of Coccidiosis Conference (7th International Poultry Health Conference) Hannover, Germany (4 pp., unpaginated) Driffield UK Positive Action Publications
- Chapman HD (1978) Drug resistance in coccidia In P.L. Long, K.N. Boorman & B.M. Freeman (Eds.) Avian Coccidiosis pp. 387–412 Edinburgh British Poultry Science
- Chapman HD (1982) Anticoccidial drug resistance In P.L. Long (Ed.) The Biology of the Coccidia pp. 429–452 London Edward Arnold
- Chapman , HD . (1984) . Drug resistance in avian coccidia (a review) . Veterinary Parasitology , 15 : 11 – 27 .
- Chapman HD (1986) Drug resistance in coccidia: recent research In L.R. McDougald, L.P. Joyner & P.L. Long (Eds.) Research in Avian Coccidiosis pp. 330–347 Athens University of Georgia
- Chapman , HD . (1994) . Sensitivity of field isolates of Eimeria to monensin following the use of a coccidiosis vaccine in broiler chickens . Poultry Science , 73 : 476 – 478 .
- Chapman , HD . (1996) . Restoration of drug sensitivity following the use of live coccidiosis vaccines . World Poultry, Special Supplement Coccidiosis , 2 : 20 – 21 .
- Chapman , HD . (1997) . Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl . Avian Pathology , 26 : 221 – 244 .
- Chapman , HD . (2000a) . Practical use of vaccines for the control of coccidiosis in the chicken . World's Poultry Science Journal , 56 : 7 – 20 .
- Chapman HD (2000b) Long-term anticoccidial strategy: chemotherapy plus vaccination Feed International, March 18 19
- Chapman , HD , Cherry , TE , Danforth , HD , Richards , G , Shirley , MW and Williams , RB . (2002) . Sustainable coccidiosis control in poultry production: the role of live vaccines . International Journal for Parasitology , 32 : 617 – 629 .
- Char , NL , Khan , DI , Rao , MRK , Gopal , V and Narayana , G . (1986) . A rare occurrence of clostridial infections in poultry . Poultry Adviser , 19 (11) : 59 – 62 .
- Clark , DT , Smith , CK and Dardas , RB . (1962) . Pathological and immunological changes in gnotobiotic chickens due to Eimeria tenella . Poultry Science , 41 : 1635 – 1636 .
- Coles , B , Biely , J and March , BE . (1970) . Vitamin A deficiency and Eimeria acervulina infection in the chick . Poultry Science , 49 : 1295 – 1301 .
- Collins , MS , Gough , RE , Alexander , DJ and Parsons , DG . (1989) . Virus-like particles associated with a “wet litter” problem in chickens . Veterinary Record , 124 : 641
- Colnago , GL , Gore , T , Jensen , LS and Long , PL . (1983) . Amelioration of pale bird syndrome in chicks by vitamin E and selenium . Avian Diseases , 27 : 312 – 316 .
- Colnago , GL , Jensen , LS and Long , PL . (1984) . Effect of selenium and vitamin E on the development of immunity to coccidiosis in chickens . Poultry Science , 63 : 1136 – 1143 .
- Conway DP McKenzie ME (1991) Poultry Coccidiosis Diagnostic and Testing Procedures 2nd edn New York Pfizer
- Corpet , DE . (2000) . Mécanismes de la promotion de croissance des animaux par les additifs alimentaires antibiotiques . Revue de Médecine Vétérinaire , 151 : 99 – 104 .
- Cowen , BS , Schwartz , LD , Wilson , RA and Ambrus , SI . (1987) . Experimentally induced necrotic enteritis in chickens . Avian Diseases , 31 : 904 – 906 .
- Craven , SE , Stern , NJ , Cox , NA , Bailey , JS and Berrang , M . (1999) . Cecal carriage of Clostridium perfringens in broiler chickens given Mucosal Starter Culture . Avian Diseases , 43 : 484 – 490 .
- Craven , SE , Stern , NJ , Bailey , JS and Cox , NA . (2001a) . Incidence of Clostridium perfringens in broiler chickens and their environment during production and processing . Avian Diseases , 45 : 887 – 896 .
- Craven , SE , Cox , NA , Stern , NJ and Mauldin , JM . (2001b) . Prevalence of Clostridium perfringens in commercial broiler hatcheries . Avian Diseases , 45 : 1050 – 1053 .
- Cuckler , AC , McManus , EC and Campbell , WC . (1969) . Development of resistance in coccidia . Acta Veterinaria, Brno , 38 : 89 – 99 .
- Danforth , HD . (1998) . Use of live oocyst vaccines in the control of avian coccidiosis: experimental studies and field trials . International Journal for Parasitology , 28 : 1099 – 1109 .
- Davies SFM Joyner LP Kendall SB (1963) Coccidiosis Edinburgh Oliver and Boyd
- Devriese , LA , Daube , G , Hommez , J and Haesebrouck , F . (1993) . In vitro susceptibility of Clostridium perfringens isolated from farm animals to growth-enhancing antibiotics . Journal of Applied Bacteriology , 75 : 55 – 57 .
- Dhawale , A . (2004) . Necrotic enteritis—a silent profit robber . Poultry International , 43 (4) : 42 – 43 .
- Dibner , J . (2004) . Organic acids: can they replace antibiotic growth promoters? . Feed International , 25 (12) : 14 – 16 .
- Dickinson , EM . (1941) . The effects of variable dosages of sporulated Eimeria acervulina oocysts on chickens . Poultry Science , 20 : 413 – 424 .
- Drew , MD , Syed , NA , Goldade , BG , Laarveld , B and Van Kessel , AG . (2004) . Effects of dietary protein source and level on intestinal populations of Clostridium perfringens in broiler chickens . Poultry Science , 83 : 414 – 420 .
- Droual , R , Shivaprasad , HL and Chin , RP . (1994) . Coccidiosis and necrotic enteritis in turkeys . Avian Diseases , 38 : 177 – 183 .
- Drouin P (1999) Retour en force de l'entérite nécrotique Filières Avicoles (for 1999) 78 79
- Duffy , CF and Tucker , LA . (2003) . Natural treatments for avian coccidiosis . World Poultry, Special Supplement Coccidiosis , 4 : 9 – 10 .
- Dykstra , DD and Reid , WM . (1978) . Monensin, Eimeria tenella infection, and effects on the bacterial populations in the ceca of gnotobiotic chickens . Poultry Science , 57 : 398 – 402 .
- Edgar , SA . (1954) . Effect of temperature on the sporulation of oocysts of the protozoan Eimeria tenella . Transactions of the American Microscopical Society , 73 : 237 – 242 .
- Edgar , SA . (1955) . Sporulation of oocysts at specific temperatures and notes on the prepatent period of several species of avian coccidia . Journal of Parasitology , 41 : 214 – 216 .
- El-Boushy , AR . (1988) . Vitamin E affects viability, immune response of poultry . Feedstuffs , 60 : 20–26, 39
- Eleazer , TH and Harrell , JS . (1976) . Clostridium perfringens infection in turkey poults . Avian Diseases , 20 : 774 – 776 .
- Elwinger , K and Teglöf , B . (1991) . Performance of broiler chickens as influenced by a dietary enzyme complex with and without antibiotic supplementation . Archiv für Geflügelkunde , 55 : 69 – 73 .
- Elwinger K Engström B Berndtson E Fossum O Teglöf B (1992a) The effects of narasin on Clostridium perfringens in caeca and the occurrence of necrotic enteritis in broiler chickens In Proceedings XIX World's Poultry Congress, Amsterdam 3 pp. 580–584 Amsterdam The Netherlands
- Elwinger , K , Schneitz , C , Berndtson , E , Fossum , O , Teglöf , B and Engström , B . (1992b) . Factors affecting the incidence of necrotic enteritis, caecal carriage of Clostridium perfringens and bird performance in broiler chicks . Acta Veterinaria Scandinavica , 33 : 369 – 378 .
- Elwinger , K , Engström , B , Fossum , O , Hassan , S and Teglöf , B . (1994) . Effect of coccidiostats on necrotic enteritis and performance in broiler chickens . Swedish Journal of Agricultural Research , 24 : 39 – 44 .
- Elwinger , K , Berndtson , E , Engström , B , Fossum , O and Waldenstedt , L . (1998) . Effect of antibiotic growth promoters and anticoccidials on growth of Clostridium perfringens in the caeca and on performance of broiler chickens . Acta Veterinaria Scandinavica , 39 : 433 – 441 .
- Engberg , RM , Hedemann , MS and Jensen , BB . (2002) . The influence of grinding and pelleting of feed on the microbial composition and activity in the digestive tract of broiler chickens . British Poultry Science , 43 : 569 – 579 .
- Engberg , RM , Hedemann , MS , Steenfeldt , S and Jensen , BB . (2004) . Influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract . Poultry Science , 83 : 925 – 938 .
- Enigk , K and Dey-Hazra , A . (1976) . Activity of disaccharidases of the intestinal mucosa of the chicken during infection with Eimeria necatrix . Veterinary Parasitology , 2 : 177 – 185 .
- Enigk , K , Schanzel , E , Scupin , E and Dey-Hazra , A . (1970) . Der enterale plasmaproteinverlust bei der coccidiose des Huhnes . Zentralblatt für Veterinärmedizin, Reihe B , 17 : 522 – 526 .
- Erasmus , J , Scott , ML and Levine , PP . (1960) . A relationship between coccidiosis and vitamin A nutrition of chickens . Poultry Science , 39 : 565 – 572 .
- Erník , F and Bedrník , P . (2001) . Controlling coccidiosis in broiler growing . Poultry International , 40 (4) : 36 – 42 .
- Fayer R Reid WM (1982) Control of coccidiosis In P.L. Long (Ed.) The Biology of the Coccidia pp. 453–487 London Edward Arnold
- Fernando , MA and McCraw , BM . (1973) . Mucosal morphology and cellular renewal in the intestine of chickens following a single infection of Eimeria acervulina . Journal of Parasitology , 59 : 493 – 501 .
- Frame , DD and Bickford , AA . (1986) . An outbreak of coccidiosis and necrotic enteritis in 16-week-old cage-reared layer replacement pullets . Avian Diseases , 30 : 601 – 602 .
- Fukata , T , Hadate , Y , Baba , E , Uemura , T and Arakawa , A . (1988) . Influence of Clostridium perfringens and its toxin in germ-free chickens . Research in Veterinary Science , 44 : 68 – 70 .
- Fukata , T , Hadate , Y , Baba , E and Arakawa , A . (1991) . Influence of bacteria on Clostridium perfringens infections in young chickens . Avian Diseases , 35 : 224 – 227 .
- Fuller , R . (1989) . Probiotics in man and animals . Journal of Applied Bacteriology , 66 : 365 – 378 .
- Fuller , R . (2004) . What is a probiotic? . Biologist , 51 : 232
- Gardiner , MR . (1967) . Clostridial infections in poultry in Western Australia . Australian Veterinary Journal , 43 : 359 – 360 .
- Garrido , MN , Skjervheim , M , Oppegaard , H and Sorum , H . (2004) . Acidified litter benefits the intestinal flora balance of broiler chickens . Applied and Environmental Microbiology , 70 : 5208 – 5213 .
- Geary TG Edgar SA Jensen JB (1985) Drug resistance in Protozoa In W.C. Campbell & R.S. Rew (Eds.) Chemotherapy of Parasitic Diseases pp. 209–236 New York Plenum Press
- George , BA , Quarles , CL and Fagerberg , DJ . (1982) . Virginiamycin effects on controlling necrotic enteritis infection in chickens . Poultry Science , 61 : 447 – 450 .
- Gerriets , E . (1961) . The prophylactic action of vitamin A in caecal coccidiosis by protection of the epithelium . British Veterinary Journal , 117 : 507 – 515 .
- Geyra , A , Uni , Z and Sklan , D . (2001) . Enterocyte dynamics and mucosal development in the posthatch chick . Poultry Science , 80 : 776 – 782 .
- Giese , W , Stoll , U , Dey-Hazra , A and Enigk , K . (1971) . Der Einfluss verschiedener Eimeria-Arten auf absorption und Stoffwechsel von 14C-Glucose bei Hühnerküken . Experimental Parasitology , 29 : 440 – 450 .
- Graat , EAM , Henken , AM , Ploeger , HW , Noordhuizen , JPTM and Vertommen , MH . (1994) . Rate and course of sporulation of oocysts of Eimeria acervulina under different environmental conditions . Parasitology , 108 : 497 – 502 .
- Grave , K , Kaldhusdal , M , Kruse , H , Harr , LMF and Flatlandsmo , K . (2004) . What has happened in Norway after the ban of avoparcin? Consumption of antimicrobials by poultry . Preventive Veterinary Medicine , 62 : 59 – 72 .
- Gregory MW (1990) Pathology of coccidial infections In P.L. Long (Ed.) Coccidia of Man and Domestic Animals pp. 235–261 Boca Raton FL CRC Press
- Gutiérrez , Á. del Á. O . (2004) . Wheat for broilers . Feed International , 25 (12) : 22 – 24 .
- Hagenmüller P (1899) Bibliographie générale et spéciale des travaux concernant les sporozoaires Annales du Musée d'Histoire Naturelle de Marseille, série II Bulletin, tome I supplément 1 233
- Hammond DM Long PL (Eds.) (1973) The Coccidia Eimeria, Isospora, Toxoplasma, and Related Genera Baltimore MD University Park Press
- Hegde , KS , Reid , WM , Johnson , J and Womack , HE . (1969) . Pathogenicity of Eimeria brunetti in bacteria free and conventional chickens . Journal of Parasitology , 55 : 402 – 405 .
- Heier , BT , Løvland , A , Soleim , KB , Kaldhusdal , M and Jarp , J . (2001) . A field study of naturally occurring specific antibodies against Clostridium perfringens alpha toxin in Norwegian broiler flocks . Avian Diseases , 45 : 724 – 732 .
- Heijnen , G . (2004) . Conditioning and pelleting: the new generation . Feed International , 25 (7) : 22 – 24 .
- Helmboldt , CF and Bryant , ES . (1971) . The pathology of necrotic enteritis in domestic fowl . Avian Diseases , 15 : 775 – 780 .
- Henry CW Murphy BD Norton RA (1995) Incidence of Clostridia [sic] perfringens on commercial broiler farms with a history of necrotic enteritis Poultry Science 74 (supplement 1) 51
- Hermans PG Morgan KL (2003) The epidemiology of necrotic enteritis in broiler chickens Research in Veterinary Science 74 (supplement A) 19
- Hetland , H , Svihus , B and Olaisen , V . (2002) . Effect of feeding whole cereals on performance, starch digestibility and duodenal particle size distribution in broiler chickens . British Poultry Science , 43 : 416 – 423 .
- Hofacre , CL , Froyman , R , Gautrias , B , George , B , Goodwin , MA and Brown , J . (1998) . Use of Aviguard and other intestinal bioproducts in experimental Clostridium perfringens-associated necrotizing enteritis in broiler chickens . Avian Diseases , 42 : 579 – 584 .
- Hofacre , CL , Beacorn , T , Collett , S and Mathis , G . (2003) . Using competitive exclusion, mannan-oligosaccharide and other intestinal products to control necrotic enteritis . Journal of Applied Poultry Research , 12 : 60 – 64 .
- Hofshagen , M and Kaldhusdal , M . (1992) . Barley inclusion and avoparcin supplementation in broiler diets. 1. Effect on small intestinal bacterial flora and performance . Poultry Science , 71 : 959 – 969 .
- Imondi , AR and Bird , FH . (1966) . The turnover of intestinal epithelium in the chick . Poultry Science , 45 : 142 – 147 .
- Ispolatovskaya MV (1971) Type A Clostridium perfringens toxin In A.S. Kadis, T.C. Montie & S.J. Ajl (Eds.) Microbial toxins 2 Biology (pp. 108–158) New York Academic Press
- Ivanov , IE . (2001) . Treatment of broiler litter with organic acids . Research in Veterinary Science , 70 : 169 – 173 .
- Jansson L Elwinger K Engström B Fossum O Teglöf B (1990) Test of the efficacy of virginiamycin and dietary enzyme supplementation against necrotic enteritis (NE) disease in broilers In Proceedings of the VIII Conferencia Europea de Avicultura Memoria 2 pp. 556–559 Barcelona Spain
- Jeffers TK (1978) Genetics of coccidia and the host response In P.L. Long, K.N. Boorman & B.M. Freeman (Eds.) Avian Coccidiosis pp. 51–125 Edinburgh British Poultry Science
- Jeffers TK (1989) Anticoccidial drug resistance: a review with emphasis on the polyether ionophores In P. Yvoré (Ed.) Coccidia and Intestinal Coccidiomorphs pp. 295–308 Paris INRA
- Johansson , A , Greko , C , Engström , BE and Karlsson , M . (2004) . Antimicrobial susceptibility of Swedish, Norwegian and Danish isolates of Clostridium perfringens from poultry, and distribution of tetracycline resistant genes . Veterinary Microbiology , 99 : 251 – 257 .
- Johansson , KR and Sarles , WB . (1948) . Bacterial population changes in the ceca of young chickens infected with Eimeria tenella . Journal of Bacteriology , 56 : 635 – 647 .
- Johnson , WT . (1923) . Avian coccidiosis . Poultry Science , 2 : 146 – 163 .
- Johnson , WT . (1927) . Two basic factors in coccidial infection of the chicken . Journal of the American Veterinary Medical Association , 70 : 560 – 584 .
- Johnston , WH , Karchesy , JJ , Constantine , GH and Craig , AM . (2001) . Antimicrobial activity of some Pacific Northwest woods against anaerobic bacteria and yeast . Phytotherapy Research , 15 : 586 – 588 .
- Joyner , LP . (1970) . Coccidiosis: problems arising from the development of anticoccidial drug resistance . Experimental Parasitology , 28 : 122 – 128 .
- Joyner LP (1978) The identification and diagnosis of avian coccidiosis In P.L. Long, K.N. Boorman & B.M. Freeman (Eds.) Avian Coccidiosis pp. 29–49 Edinburgh British Poultry Science
- Joyner , LP and Long , PL . (1974) . The specific characters of the Eimeria with special reference to the coccidia of the fowl . Avian Pathology , 3 : 145 – 157 .
- Joyner , LP , Patterson , DSP , Berrett , S , Boarer , CDH , Cheong , FH and Norton , CC . (1975) . Amino-acid malabsorption and intestinal leakage of plasma-proteins in young chicks infected with Eimeria acervulina . Avian Pathology , 4 : 17 – 33 .
- Julian , RJ . (1969) . Necrotic enteritis in chickens . Veterinary Services Branch, Ontario Department Agriculture and Food Quarterly Bulletin , 4 (2) : 2
- Kageyama , A , Fukata , T , Baba , E and Arakawa , A . (1987) . The influence of various bacteria on the cecal mucosa of monoflora chickens infected with Eimeria tenella. A scanning electron microscopic study . Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene , A265 : 353 – 359 .
- Kaldhusdal , MI . (2000) . Necrotic enteritis as affected by dietary ingredients . World Poultry , 16 (6) : 42 – 43 .
- Kaldhusdal , M and Hofshagen , M . (1992) . Barley inclusion and avoparcin supplementation in broiler diets. 2. Clinical, pathological and bacteriological findings in a mild form of necrotic enteritis . Poultry Science , 71 : 1145 – 1153 .
- Kaldhusdal , M and Skjerve , E . (1996) . Association between cereal contents in the diet and incidence of necrotic enteritis in broiler chickens in Norway . Preventive Veterinary Medicine , 28 : 1 – 16 .
- Kaldhusdal , M , Evensen , Ø. and Landsverk , T . (1995) . Clostridium perfringens necrotizing enteritis of the fowl: a light microscopic, immunohistochemical and ultrastructural study of spontaneous disease . Avian Pathology , 24 : 421 – 433 .
- Kaldhusdal , M , Schneitz , C , Hofshagen , M and Skjerve , E . (2001) . Reduced incidence of Clostridium perfringens-associated lesions and improved performance in broiler chickens treated with normal intestinal bacteria from adult fowl . Avian Diseases , 45 : 149 – 156 .
- Kenny , M and Kemp , C . (2003) . The role of nutrition on the enteric health of broilers . Poultry International , 42 (13) : 24 – 32 .
- Kheysin YM (1972) Life Cycles of Coccidia of Domestic Animals London William Heinemann Medical Books
- Kimura , N , Mimura , F , Nishida , S , Kobayashi , A and Mitsuoka , T . (1976) . Studies on the relationship between intestinal flora and cecal coccidiosis in the chicken . Poultry Science , 55 : 1375 – 1383 .
- Kmet V Stachová M Nemcová R Jonecová Z Lauková A (1993) The interaction of intestinal microflora with avian enteric pathogens Acta Veterinaria, Brno 62 (supplement 6) S87 S89
- Knarreborg , A , Simon , MA , Engberg , RM , Jensen , BB and Tannock , GW . (2002) . Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages . Applied and Environmental Microbiology , 68 : 5918 – 5924 .
- Kocher , A . (2003) . Nutritional manipulation of necrotic enteritis outbreak in broilers . Recent Advances in Animal Nutrition in Australia , 14 : 111 – 116 .
- Köhler , B . (2000) . Clostridium perfringens intoxication affects bird performance . World Poultry , 16 (5) : 57 – 59 .
- Köhler , B , Marx , G , Kölbach , S and Böttcher , E . (1974a) . Untersuchungen zur nekrotischen Enteritis der Hühner. 1. Mitt.: Diagnostik und Bekämpfung . Monatshefte für Veterinärmedizin , 29 : 380 – 384 .
- Köhler , B , Kölbach , S and Meine , J . (1974b) . Untersuchungen zur nekrotischen Enteritis der Hühner. 2. Mitt.: Mikrobiologische Aspekte . Monatshefte für Veterinärmedizin , 29 : 385 – 391 .
- Kouwenhoven , B and Horst , CJGvan der . (1969) . Strongly acid intestinal content and lowered protein, carotene and vitamin A blood levels in Eimeria acervulina infected chickens . Zeitschrift für Parasitenkunde , 32 : 347 – 353 .
- Kouwenhoven , B and Horst , CJGvan der . (1972) . Disturbed intestinal absorption of vitamin A and carotenes and the effect of a low pH during Eimeria acervulina infection in the domestic fowl (Gallus domesticus) . Zeitschrift für Parasitenkunde , 38 : 152 – 161 .
- Kucera J (1989) Differentiation of poultry coccidia in mixed infections In P. Yvoré (Ed.) Coccidia and Intestinal Coccidiomorphs pp. 125–128 Paris INRA
- Kucera , J . (1990) . Identification of Eimeria species in Czechoslovakia . Avian Pathology , 19 : 59 – 66 .
- Kucera , J . (1991) . Enzyme variants of Eimeria parasitizing the domestic fowl and possibilities of species diagnostics . Folia Parasitologica , 38 : 193 – 199 .
- Langhout , DJ . (1999) . The role of nutrition on coccidial infections . World Poultry, Special Supplement Coccidiosis , 3 : 33 – 34 .
- La Ragione , RM and Woodward , MJ . (2003) . Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chicks . Veterinary Microbiology , 94 : 245 – 256 .
- Lev , M and Forbes , M . (1959) . Growth response to dietary penicillin of germ free chicks and of chicks with a defined intestinal flora . British Journal of Nutrition , 13 : 78 – 84 .
- Levine ND (1961) Protozoan Parasites of Domestic Animals and Man Minneapolis Burgess Publishing Company
- Lew , AE , Anderson , GR , Minchin , CM , Jeston , PJ and Jorgensen , WK . (2003) . Inter- and infra-strain variation and PCR detection of the internal transcribed spacer (ITS-1) sequences of Australian isolates of Eimeria species from chickens . Veterinary Parasitology , 112 : 33 – 50 .
- Li , GQ , Kanu , S , Xiang , FY , Xiao , SM , Zhang , L , Chen , HW and Ye , HJ . (2004) . Isolation and selection of ionophore tolerant Eimeria precocious lines: E. tenella, E. maxima and E. acervulina . Veterinary Parasitology , 119 : 261 – 276 .
- Li , GQ , Kanu , S , Xiao , SM and Xiang , FY . (2005) . Responses of chickens vaccinated with a live attenuated multi-valent ionophore-tolerant Eimeria vaccine . Veterinary Parasitology , 129 : 178 – 186 .
- Lillehoj , HS . (1988) . Influence of inoculation dose, inoculation schedule, chicken age, and host genetics on disease susceptibility and development of resistance to Eimeria tenella infection . Avian Diseases , 32 : 437 – 444 .
- Lillehoj , HS and Trout , JM . (1996) . Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites . Clinical Microbiology Reviews , 9 : 349 – 360 .
- Lillehoj HS Allen PC Ruff MD (1986) Comparative studies of humoral and cellular immune responses in two inbred strains of chickens showing different susceptibilities to coccidiosis In L.R. McDougald, L.P. Joyner & P.L. Long (Eds.) Research in Avian Coccidiosis pp. 470–481 Athens University of Georgia
- Lillehoj , HS , Dalloul , RA and Min , W . (2003) . Enhancing intestinal immunity to coccidiosis . World Poultry, Special Supplement Coccidiosis , 4 : 18 – 21 .
- Liu C-M (1982) Microbial aspects of polyether antibiotics: activity, production, and biosynthesis In J.W. Westley (Ed.) Polyether Antibiotics, Naturally Occurring Acid Ionophores Vol. 1 Biology (pp. 43–102) New York Marcel Dekker
- Logan , MA , Tytell , AA , Danielson , IS and Griner , AM . (1945) . Production of Clostridium perfringens alpha toxin . Journal of Immunology , 51 : 317 – 328 .
- Long , JR . (1973) . Necrotic enteritis in broiler chickens. I. A review of the literature and the prevalence of the disease in Ontario . Canadian Journal of Comparative Medicine , 37 : 302 – 308 .
- Long , JR and Truscott , RB . (1976) . Necrotic enteritis in broiler chickens. III. Reproduction of the disease . Canadian Journal of Comparative Medicine , 40 : 53 – 59 .
- Long , JR , Pettit , JR and Barnum , DA . (1974) . Necrotic enteritis in broiler chickens. II. Pathology and proposed pathogenesis . Canadian Journal of Comparative Medicine , 38 : 467 – 474 .
- Long , PL . (1968) . The effect of breed of chickens on resistance to Eimeria sp. infections . British Poultry Science , 9 : 71 – 78 .
- Long PL (1973) Pathology and pathogenicity of coccidial infections In D.M. Hammond & P.L. Long (Eds.) The Coccidia: Eimeria, Isospora, Toxoplasma, and Related Genera pp. 253–294 Baltimore MD University Park Press
- Long PL (Ed.) (1982) The Biology of the Coccidia London Edward Arnold
- Long PL (Ed.) (1990) Coccidiosis of Man and Domestic Animals Boca Raton FL CRC Press
- Long , PL and Reid , WM . (1982) . A guide for the diagnosis of coccidiosis in chickens . University of Georgia College of Agriculture Research Report , 404 : 1 – 17 .
- Long , PL , Joyner , LP , Millard , BJ and Norton , CC . (1976) . A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis . Folia Veterinaria Latina , 6 : 201 – 217 .
- Løvland , A and Kaldhusdal , M . (2001) . Severely impaired production performance in broiler flocks with high incidence of Clostridium perfringens-associated hepatitis . Avian Pathology , 30 : 73 – 81 .
- Løvland , A , Kaldhusdal , M , Redhead , K , Skjerve , E and Lillehaug , A . (2004) . Maternal vaccination against subclinical necrotic enteritis in broilers . Avian Pathology , 33 : 81 – 90 .
- Major , JR and Ruff , MD . (1978a) . Disaccharidase activity in the intestinal tissue of broilers infected with coccidia . Journal of Parasitology , 64 : 706 – 711 .
- Major , JR and Ruff , MD . (1978b) . Eimeria spp.: influence of coccidia on digestion (amylolytic activity) in broiler chickens . Experimental Parasitology , 45 : 234 – 240 .
- Mandal , AK . (1980) . Coccidia and coccidiosis of poultry and farm animals of India . Zoological Survey of India, Technical Monograph , 5 : 1 – 87 .
- Marchaim , U and Kulka , RG . (1967) . The non-parallel increase of amylase chymotrypsinogen and procarboxypeptidase in the developing chick pancreas . Biochimica et Biophysica Acta , 146 : 553 – 559 .
- Marshall , RN , Taylor , MA , Green , JA and Catchpole , J . (1995) . The pathogenic effects of a UK field isolate of Eimeria tenella in modern broiler and layer chickens . Journal of Protozoology Research , 5 : 66 – 76 .
- Martel , A , Devriese , LA , Cauwerts , K , De Gussem , K , Decostere , A and Haesebrouck , F . (2004) . Susceptibility of Clostridium perfringens strains from broiler chickens to antibiotics and anticoccidials . Avian Pathology , 33 : 3 – 7 .
- Mathis , GF . (2003) . Vaccine can decrease anticoccidial resistance . World Poultry, Special Supplement Coccidiosis , 4 : 22 – 23 .
- Mathis GF McDougald LR (1989) Restoration of drug sensitivity on turkey farms after introduction of sensitive coccidia during controlled-exposure immunization In P. Yvoré (Ed.) Coccidia and Intestinal Coccidiomorphs pp. 339–343 Paris INRA
- Maxey , BW and Page , RK . (1977) . Efficacy of lincomycin feed medication for the control of necrotic enteritis in broiler-type chickens . Poultry Science , 56 : 1909 – 1913 .
- McDougald LR (1982) Chemotherapy of coccidiosis In P.L. Long (Ed.) The Biology of the Coccidia pp. 373–427 London Edward Arnold
- McDougald LR (1990) Control of coccidiosis: chemotherapy In P.L. Long (Ed.) Coccidiosis of Man and Domestic Animals pp. 307–320 Boca Raton FL CRC Press
- McDougald LR (1993) Chemotherapy of coccidiosis In Proceedings of the VIth International Coccidiosis Conference pp. 45–47 Guelph Canada
- McDougald LR (2003) Coccidiosis In Y.M. Saif, H.J. Barnes, J.R. Glisson, A.M. Fadly, L.R. McDougald & D.E. Swayne (Eds.) Diseases of Poultry 11th edn pp. 974–991 Ames Iowa State Press
- McDougald , LR , Karlsson , T and Reid , WM . (1980) . Interaction of infectious bursal disease and coccidiosis in layer replacement chickens . Avian Diseases , 24 : 999 – 1005 .
- McDougald , LR , Fuller , L and Mattiello , R . (1997) . A survey of coccidia on 43 poultry farms in Argentina . Avian Diseases , 41 : 923 – 929 .
- McKenzie , ME , Colnago , GL , Lee , S and Long , PL . (1982) . Gut stasis induction by coccidiosis infections in chickens . Poultry Science , 61 : 1512
- McLoughlin , DK . (1970) . Coccidiosis: experimental analysis of drug resistance . Experimental Parasitology , 28 : 129 – 136 .
- Mead , GC and Adams , BW . (1975) . Some observations on the caecal microflora of the chick during the first two weeks of life . British Poultry Science , 16 : 169 – 176 .
- Michael , E and Hodges , RD . (1971) . The pathogenic effects of Eimeria acervulina: a comparison of single and repeated infections . Veterinary Record , 89 : 329 – 333 .
- Michael , E and Hodges , RD . (1972) . The pathogenic effects of Eimeria necatrix: a comparison of single and repeated infections . Veterinary Record , 91 : 958 – 962 .
- Miller CR (1983) Necrotic enteritis control with virginiamycin In Proceedings of the 32nd Western Poultry Disease Conference pp. 67–68 Davis CA USA
- Mitsch , P , Zitterl-Eglseer , K , Köhler , B , Gabler , C , Losa , R and Zimpernik , I . (2004) . The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens . Poultry Science , 83 : 669 – 675 .
- Morgan , A and Catchpole , J . (1996) . Do feed enzymes affect avian coccidial pathogenicity? . World Poultry, Special Supplement Coccidiosis , 2 : 38 – 39 .
- Motha , MXJ and Egerton , JR . (1984) . Influence of reticuloendotheliosis on the severity of Eimeria tenella infection in broiler chickens . Veterinary Microbiology , 9 : 121 – 129 .
- Muhammed , SI , Morrison , SM and Boyd , WL . (1975) . Nutritional requirements for growth and sporulation of Clostridium perfringens . Journal of Applied Bacteriology , 3 : 245 – 253 .
- Nairn , ME and Bamford , VW . (1967) . Necrotic enteritis of broiler chickens in Western Australia . Australian Veterinary Journal , 43 : 49 – 54 .
- Nakamura , M , Cook , J and Cross , W . (1978) . Lecithinase production by Clostridium perfringens in chemically defined media . Applied Microbiology , 16 : 1420 – 1421 .
- National Research Council US (1984) Nutrient Requirements of Poultry 8th edn Washington DC National Academy Press
- Norton RA Hoerr FJ (1999) Clostridial diseases of poultry Poultry Digest, August/September 14 20
- Noy , Y and Sklan , D . (1998) . Yolk utilisation in the newly hatched poult . British Poultry Science , 39 : 446 – 451 .
- Noy , Y and Sklan , D . (1999) . Energy utilization in newly hatched chicks . Poultry Science , 78 : 1750 – 1756 .
- Ohe , O and Arakawa , A . (1975) . Effect of feed additive antibiotics on chickens infected with Eimeria tenella . Poultry Science , 54 : 1008 – 1018 .
- Okoye , JOA . (1985) . Cases of intestinal intussusception in young fowls . Avian Pathology , 14 : 275 – 279 .
- Oliveira Cde (2001) Latest thinking on avian coccidiosis In Vaccination at Work in Commercial Broilers Part 2 (p. 9) Driffield UK Positive Action Publications
- Parish , WE . (1961a) . Necrotic enteritis in the fowl (Gallus gallus domesticus). I. Histopathology of the disease and isolation of a strain of Clostridium welchii . Journal of Comparative Pathology , 71 : 377 – 393 .
- Parish , WE . (1961b) . Necrotic enteritis in the fowl. II. Examination of the causal Clostridium welchii . Journal of Comparative Pathology , 71 : 394 – 404 .
- Parish , WE . (1961c) . Necrotic enteritis in the fowl. III. The experimental disease . Journal of Comparative Pathology , 71 : 405 – 413 .
- Patterson , DSP , Joyner , LP , Berrett , S , Boarer , CDH , Cheong , FH and Norton , CC . (1975) . In-vitro studies on intestinal malabsorption of amino-acids in young chicks infected with Eimeria acervulina . Avian Pathology , 4 : 11 – 16 .
- Patterson , LT , Johnson , LW and Edgar , SA . (1961) . The comparative resistance of several inbred lines of S.C. White Leghorns to certain infectious diseases . Poultry Science , 40 : 1442
- Pattison M (2000) Cost of coccidiosis In Proceedings of Coccidiosis Conference (7th International Poultry Health Conference) Hannover, Germany (17 pp., unpaginated) Driffield UK Positive Action Publications
- Pedersen , K , Kaldhusdal , M , Engström , B , Engberg , RM , Løvland , A , Nauerby , B and Bjerrum , L . (2004) . Clostridium perfringens: en lurende ræv i hønsegården? . Dansk Veterinærtidsskrift , 87 (11) : 18 – 22 .
- Pellérdy LP (1974) Coccidia and Coccidiosis 2nd edn Berlin Verlag Paul Parey
- Pesti , GM and Combs , GF . (1976) . Studies on the enteric absorption of selenium in the chick using localized coccidial infections . Poultry Science , 55 : 2265 – 2274 .
- Petersen CB (1997) Practical application of whole grain feeding In Proceedings of the Eleventh European Symposium on Poultry Nutrition pp. 6–15 Faaborg Denmark
- Pettersson , D . (1987) . Substitution of maize with different levels of wheat, triticale or rye in diets for broiler chickens . Swedish Journal of Agricultural Research , 17 : 57 – 62 .
- Phillips , ND , La , T , Pluske , JR and Hampson , DJ . (2004) . A wheat-based diet enhances colonization with the intestinal spirochaete Brachyspira intermedia in experimentally infected laying hens . Avian Pathology , 33 : 451 – 457 .
- Pout , DD . (1967) . Villous atrophy and coccidiosis . Nature, London , 213 : 306 – 307 .
- Pout DD (1968) The reaction of the small intestine of the chicken to infection with Eimeria spp In E.J.L. Soulsby (Ed.) The Reaction of the Host to Parasitism. Supplement to Veterinary Medical Review pp. 28–38 Marburg-Lahn N.G. Elwert Universitäts-und Verlagsbuchhandlung
- Prescott , JF . (1979) . The prevention of experimentally induced necrotic enteritis in chickens by avoparcin . Avian Diseases , 23 : 1072 – 1074 .
- Preston-Mafham , RA and Sykes , AH . (1967a) . Factors contributing to the weight loss during intestinal coccidiosis infections in the fowl . Proceedings of the Nutrition Society , 26 : 27 – 28 .
- Preston-Mafham , RA and Sykes , AH . (1967b) . Changes in permeability of the mucosa during intestinal coccidiosis infections in the fowl . Experientia , 23 : 972 – 973 .
- Preston-Mafham , RA and Sykes , AH . (1970) . Changes in body weight and intestinal absorption during infections with Eimeria acervulina in the chicken . Parasitology , 61 : 417 – 424 .
- Quiroz , MA . (2003) . Use of vaccines to prevent avian coccidiosis in broilers . International Poultry Production , 11 (8) : 7 – 11 .
- Rada , V , Rychlý , I and Voríšek , K . (1994) . Susceptibility of chicken intestinal lactobacilli to coccidiostats . Acta Veterinaria, Brno , 63 : 9 – 12 .
- Reid WM (1989) Recommending sanitary practices for coccidiosis control In P. Yvoré (Ed.) Coccidia and Intestinal Coccidiomorphs pp. 371–376 Paris INRA
- Reid , WM and Pitois , M . (1965) . The influence of coccidiosis on feed and water intake of chickens . Avian Diseases , 9 : 343 – 348 .
- Répérant , J-M and Humbert , F . (2002) . Interactions between Clostridium perfringens and Eimeria meleagrimitis on the development of clostridia in turkeys: experimental study . Turkeys , 50 : 53 – 54 .
- Rice , JT and Reid , WM . (1973) . Coccidiosis immunity following early and late exposure to Marek's disease . Avian Diseases , 17 : 66 – 71 .
- Riddell C (1987) Avian Histopathology Pennsylvania PA American Association of Avian Pathologists
- Riddell , C and Kong , XM . (1992) . The influence of diet on necrotic enteritis in broiler chickens . Avian Diseases , 36 : 499 – 503 .
- Rose , ME and Long , PL . (1969) . Immunity to coccidiosis: gut permeability changes in response to sporozoite invasion . Experientia , 25 : 183 – 184 .
- Ross Breeders (1995) Coccidiosis Control in Broiler Breeders Newbridge Ross Breeders
- Ross Breeders . (1999) . Necrotic enteritis and associated conditions in broiler chickens . World Poultry , 15 (8) : 44 – 47 .
- Ruff , MD . (1974) . Reduced transport of methionine in intestines of chickens infected with Eimeria necatrix . Journal of Parasitology , 60 : 838 – 843 .
- Ruff MD (1978) Malabsorption from the intestine of birds with coccidiosis In P.L. Long, K.N. Boorman & B.M. Freeman (Eds.) Avian Coccidiosis pp. 281–295 Edinburgh British Poultry Science
- Ruff MD (1986) Reasons for inadequate nutrient utilization during avian coccidiosis: a review In L.R. McDougald, L.P. Joyner & P.L. Long (Eds.) Research in Avian Coccidiosis pp. 169–185 Athens University of Georgia
- Ruff MD (1989) Interaction of avian coccidiosis with other diseases: a review In P. Yvoré (Ed.) Coccidia and Intestinal Coccidiomorphs pp. 173–181 Paris INRA
- Ruff , MD and Edgar , SA . (1982) . Reduced intestinal absorption in broilers during Eimeria mitis infection . American Journal of Veterinary Research , 43 : 507 – 509 .
- Ruff , MD and Fuller , HL . (1975) . Some mechanisms of reduction of carotenoid levels in chickens infected with Eimeria acervulina or E. tenella . Journal of Nutrition , 105 : 1447 – 1456 .
- Ruff , MD and Reid , WM . (1975) . Coccidiosis and intestinal pH in chickens . Avian Diseases , 19 : 52 – 58 .
- Ruff , MD and Rosenberger , JK . (1985a) . Concurrent infections with reoviruses and coccidia in broilers . Avian Diseases , 29 : 465 – 478 .
- Ruff , MD and Rosenberger , JK . (1985b) . Interaction of low-pathogenicity reoviruses and low levels of infection with several coccidial species . Avian Diseases , 29 : 1057 – 1065 .
- Ruff , MD , Johnson , JK , Dykstra , DD and Reid , WM . (1974) . Effects of Eimeria acervulina on intestinal pH in conventional and gnotobiotic chickens . Avian Diseases , 18 : 96 – 104 .
- Ruff , MD , Whitlock , DR and Smith , RR . (1976) . Eimeria acervulina and E. tenella: effect on methionine absorption by the avian intestine . Experimental Parasitology , 39 : 244 – 251 .
- Russell , AD . (1990) . Bacterial spores and chemical sporicidal agents . Clinical Microbiology Reviews , 3 : 99 – 119 .
- Ryley , JF . (1980) . Drug resistance in coccidia . Advances in Veterinary Science and Comparative Medicine , 24 : 99 – 120 .
- Ryley , JF and Betts , MJ . (1973) . Chemotherapy of chicken coccidiosis . Advances in Pharmacology and Chemotherapy , 11 : 221 – 293 .
- Sasaki , J , Goryo , M , Makara , M , Nakamura , K and Okada , K . (2003) . Necrotic hepatitis due to Clostridium perfringens infection in newly hatched broiler chicks . Journal of Veterinary Medical Science , 65 : 1249 – 1251 .
- Schalm OW Jain NC Carroll EJ (1975) Veterinary Hematology 3rd edn Philadelphia PA Lea and Febiger
- Schetters , TPM , Janssen , HAJM and Vermeulen , AN . (1999) . A new vaccination concept against coccidiosis in poultry . World Poultry, Special Supplement Coccidiosis , 3 : 26 – 27 .
- Schildt , CS and Herrick , CA . (1955) . The effect of cecal coccidiosis on the motility of the digestive tract of the domestic fowl . Journal of Parasitology , 41 ( supplement ) : 18 – 19 .
- Schuring , M and Gils , Bvan . (2001) . Clostridium perfringens — a nutritional challenge? . Feed Mix , 9 (1) : 26 – 28 .
- Semenza , G . (1986) . Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli . Annual Review of Cell Biology , 2 : 255 – 313 .
- Shane , SM . (2004a) . Clostridial diseases limit production efficiency in antibiotic-free broiler flocks . Poultry International , 43 (1) : 12 – 13 .
- Shane , SM . (2004b) . Update on the poultry disease situation in the USA . Poultry International , 43 (2) : 10 – 15 .
- Shane , SM , Koetting , DG and Harrington , KS . (1984) . The occurrence of Clostridium perfringens in the intestine of chicks . Avian Diseases , 28 : 1120 – 1124 .
- Shane , SM , Gyimah , JE , Harrington , KS and Snider , TG . (1985) . Etiology and pathogenesis of necrotic enteritis . Veterinary Research Communications , 9 : 269 – 287 .
- Shapiro D (2000) Coccidiosis control in replacement layers and breeders In Proceedings of Coccidiosis Conference (7th International Poultry Health Conference) Hannover, Germany (8 pp., unpaginated) Driffield UK Positive Action Publications
- Sharma , VD and Fernando , MA . (1975) . Effect of Eimeria acervulina infection on nutrient retention with special reference to fat malabsorption in chickens . Canadian Journal of Comparative Medicine , 39 : 146 – 154 .
- Sharma , VD , Fernando , MA and Summers , JD . (1973) . The effect of dietary crude protein level on intestinal and cecal coccidiosis in chickens . Canadian Journal of Comparative Medicine , 37 : 195 – 199 .
- Shirley MW (1986) New methods for the identification of species and strains of Eimeria In L.R. McDougald, L.P. Joyner & P.L. Long (Eds.) Research in Avian Coccidiosis pp. 13–35 Athens University of Georgia
- Shirley , MW . (2003) . The importance of E. mitis is underestimated . World Poultry, Special Supplement Coccidiosis , 4 : 6 – 7 .
- Shirley , MW and Bedrník , P . (1997) . Live attenuated vaccines against avian coccidiosis: success with precocious and egg-adapted lines of Eimeria . Parasitology Today , 13 : 481 – 484 .
- Silversides , FG and Remus , J . (1999) . Betaine improves performance of coccidia-challenged birds . World Poultry, Special Supplement Coccidiosis , 3 : 37 – 38 .
- Singh , SP and Donovan , GA . (1973) . A relationship between coccidiosis and dietary vitamin A levels in chickens . Poultry Science , 52 : 1295 – 1301 .
- Sklan , D . (2001) . Development of the digestive tract of poultry . World's Poultry Science Journal , 57 : 415 – 428 .
- Sklan D (2004) Early gut development: the interaction between feed, gut health and immunity In L.A. Tucker & J.A. Taylor-Pickard (Eds.) Interfacing Immunity, Gut Health and Performance pp. 9–31 Nottingham Nottingham University Press
- Smith , AL , Hesketh , P , Archer , A and Shirley , MW . (2002) . Antigenic diversity in Eimeria maxima and the influence of host genetics and immunization schedule on cross-protective immunity . Infection and Immunity , 70 : 2472 – 2479 .
- Smith , HW . (1959) . The effect of the continuous administration of diets containing tetracyclines and penicillin on the number of drug-resistant and drug-sensitive Clostridium welchii in the faeces of pigs and chickens . Journal of Pathology and Bacteriology , 77 : 79 – 93 .
- Smith , HW . (1965) . The development of the flora of the alimentary tract in young animals . Journal of Pathology and Bacteriology , 90 : 495 – 513 .
- Soares , R , Cosstick , T and Lee , EH . (2004) . Control of coccidiosis in caged egg layers: a paper plate vaccination method . Journal of Applied Poultry Research , 13 : 360 – 363 .
- Songer , JG . (1996) . Clostridial enteric diseases of domestic animals . Clinical Microbiology Reviews , 9 : 216 – 234 .
- Stephens , CP and Hampson , DJ . (2002) . Evaluation of tiamulin and lincomycin for the treatment of broiler breeders experimentally infected with the spirochaete Brachyspira pilosicoli . Avian Pathology , 31 : 299 – 304 .
- Stephens , JF , Borst , WJ and Barnett , BD . (1974) . Some physiological effects of Eimeria acervulina, E. brunetti, and E. mivati infections in young chickens . Poultry Science , 53 : 1735 – 1742 .
- Stutz , MW and Lawton , GC . (1984) . Effects of diet and antimicrobials on growth, feed efficiency, intestinal Clostridium perfringens, and ileal weight of broiler chicks . Poultry Science , 63 : 2036 – 2042 .
- Stutz , MW , Johnson , SL and Judith , FR . (1983) . Effects of diet and bacitracin on growth, feed efficiency, and populations of Clostridium perfringens in the intestine of broiler chicks . Poultry Science , 62 : 1619 – 1625 .
- Takeda , T , Fukata , T , Miyamoto , T , Sasai , K , Baba , E and Arakawa , A . (1995) . The effects of dietary lactose and rye on caecal colonisation of Clostridium perfringens in chicks . Avian Diseases , 39 : 375 – 381 .
- Takhar , BS and Farrell , DJ . (1979) . Energy and nitrogen metabolism of chickens infected with either Eimeria acervulina or Eimeria tenella . British Poultry Science , 20 : 197 – 211 .
- Taylor , AW and Stewart , J . (1941) . The toxins produced by Clostridium welchii in a simple medium . Journal of Pathology and Bacteriology , 53 : 87 – 94 .
- Taylor , MW and Russell , WC . (1947) . The provitamin A requirement of growing chickens . Poultry Science , 26 : 234 – 242 .
- Thebo , P , Lundén , A , Uggla , A and Hooshmand-Rad , P . (1998) . Identification of seven Eimeria species in Swedish domestic fowl . Avian Pathology , 27 : 613 – 617 .
- Timms , L . (1968) . Observations on the bacterial flora of the alimentary tract in three age groups of normal chickens . British Veterinary Journal , 124 : 470 – 476 .
- Truscott , RB and Al-Sheikhly , F . (1977) . Reproduction and treatment of necrotic enteritis in broilers . American Journal of Veterinary Research , 38 : 857 – 861 .
- Tsuji , N , Kawazu , S , Ohta , M , Kamio , T , Isobe , T , Shimura , K and Fujisaki , K . (1997) . Discrimination of eight chicken Eimeria species using the two-step polymerase chain reaction . Journal of Parasitology , 83 : 966 – 970 .
- Turk , DE . (1972) . Protozoan parasitic infections of the chick intestine and protein digestion and absorption . Journal of Nutrition , 102 : 1217 – 1222 .
- Turk , DE . (1973) . Calcium absorption during coccidial infections in chicks . Poultry Science , 52 : 854 – 857 .
- Turk DE (1978) The effects of coccidiosis on intestinal function and gut microflora In P.L. Long, K.N. Boorman & B.M. Freeman (Eds.) Avian Coccidiosis pp. 227–267 Edinburgh British Poultry Science
- Turk , DE and Stephens , JF . (1966) . Effect of intestinal damage produced by Eimeria necatrix infection in chicks upon absorption of orally administered zinc-65 . Journal of Nutrition , 88 : 261 – 266 .
- Turk , DE and Stephens , JF . (1967a) . Upper intestinal tract infection produced by E. acervulina and absorption of 65Zn and 1311I-labelled oleic acid . Journal of Nutrition , 93 : 161 – 165 .
- Turk , DE and Stephens , JF . (1967b) . Eimeria necatrix infections and oleic acid absorption in broilers . Poultry Science , 46 : 775 – 777 .
- Turk , DE and Stephens , JF . (1970) . Effects of serial inoculations with Eimeria acervulina or Eimeria necatrix upon zinc and oleic acid absorption in chicks . Poultry Science , 49 : 523 – 526 .
- Tyczkowski , JK , Hamilton , PB and Ruff , MD . (1991) . Altered metabolism of carotenoids during pale-bird syndrome in chickens infected with Eimeria acervulina . Poultry Science , 70 : 2074 – 2081 .
- Tyzzer , EE . (1929) . Coccidiosis in gallinaceous birds . American Journal of Hygiene , 10 : 269 – 383 .
- Tyzzer , EE . (1932) . Criteria and methods in the investigation of avian coccidiosis . Science, New York , 75 : 324 – 328 .
- Uni , Z , Ganot , S and Sklan , D . (1998) . Posthatch development of mucosal function in the broiler small intestine . Poultry Science , 77 : 75 – 82 .
- Uni , Z , Noy , Y and Sklan , D . (1999) . Posthatch development of small intestinal function in the poult . Poultry Science , 78 : 215 – 222 .
- Untawale , GG and McGinnis , J . (1979) . Effect of rye and levels of raw and autoclaved beans (Phaseolus vulgaris) on adhesion of microflora to the intestinal mucosa . Poultry Science , 58 : 928 – 933 .
- Vahjen , W , Glaser , K , Schäfer , K and Simon , O . (1998) . Influence of xylanase-supplemented feed on the development of selected bacterial groups in the intestinal tract of broiler chicks . Journal of Agricultural Sciences , 130 : 489 – 500 .
- van der Klis , JD and van Voorst , A . (1993) . The effect of carboxy methyl cellulose (a soluble polysaccharide) on the rate of marker excretion from the gastrointestinal tract of broilers . Poultry Science , 72 : 503 – 512 .
- van der Sluis , W . (1996a) . Controlling coccidiosis by means of enzymes, a new concept? . World Poultry, Special Supplement Coccidiosis , 2 : 40
- van der Sluis , W . (1996b) . Litter quality and coccidiosis . World Poultry, Special Supplement Coccidiosis , 2 : 41
- van der Sluis , W . (1997) . Poultry diseases around the world . World Poultry , 13 (7) : 32 – 44 .
- van der Sluis , W . (2000a) . Clostridial enteritis — a syndrome emerging worldwide . World Poultry , 16 (5) : 56 – 57 .
- van der Sluis , W . (2000b) . Clostridial enteritis is an often underestimated problem . World Poultry , 16 (7) : 42 – 43 .
- van der Stroom , JH and van der Sluis , W . (1999) . The effect of intercurrent diseases on coccidiosis . World Poultry, Special Supplement Coccidiosis , 3 : 15 – 17 .
- Van Immerseel , F , De Buck , J , Pasmans , F , Huyghebaert , G , Haesebrouck , F and Ducatelle , R . (2004) . Clostridium perfringens in poultry: an emerging threat for animal and public health . Avian Pathology , 33 : 537 – 549 .
- Vermeulen , AN , Schaap , DC and Schetters , TPM . (2001) . Control of coccidiosis in chickens by vaccination . Veterinary Parasitology , 100 : 13 – 20 .
- Wages DP Opengart K (2003) Necrotic enteritis In Y.M. Saif, H.J. Barnes, J.R. Glisson, A.M. Fadly, L.R. McDougald & D.E. Swayne (Eds.) Diseases of Poultry 11th edn pp. 781–785 Ames Iowa State Press
- Waldenstedt , L . (1998) . Coccidial and clostridial infections in broiler chickens—influence of diet composition . Acta Universitatis Agriculturae Sueciae, Agraria , 88 : 1 – 61 .
- Waldenstedt , L . (2003) . Effect of vaccination against coccidiosis in combination with an antibacterial oregano (Oreganum vulgare) compound in organic broiler production . Acta Agriculturae Scandinavica (section A, Animal Sciences) , 53 : 101 – 109 .
- Waldenstedt , L , Elwinger , K , Hooshmand-Rad , P , Thebo , P and Uggla , A . (1998) . Comparison between effects of standard feed and whole wheat supplemented diet on experimental Eimeria tenella and Eimeria maxima infections in broiler chickens . Acta Veterinaria Scandinavica , 39 : 461 – 471 .
- Waldenstedt , L , Lundén , A , Elwinger , K , Thebo , P and Uggla , A . (1999) . Comparison between a live, attenuated anticoccidial vaccine and an anticoccidial ionophore, on performance of broilers raised with or without a growth promoter, in an initially Eimeria-free environment . Acta Veterinaria Scandinavica , 40 : 11 – 21 .
- Waldenstedt , L , Elwinger , K , Lundén , A , Thebo , P , Bedford , MR and Uggla , A . (2000) . Intestinal digesta viscosity decreases during coccidial infection in broilers . British Poultry Science , 41 : 459 – 464 .
- Waldenstedt , L , Elwinger , K , Lundén , A , Thebo , P and Uggla , A . (2001) . Sporulation of Eimeria maxima oocysts in litter with different moisture contents . Poultry Science , 80 : 1412 – 1415 .
- Waldron , LA , Rose , SP and Kettlewell , PS . (1993) . Difference in productive performance of broiler chickens fed two wheat varieties . Aspects of Applied Biology , 36 : 485 – 489 .
- Wallach , M and Waldenstedt , L . (1999) . Immunity and the effects of removing coccidiostats from poultry feed . World Poultry, Special Supplement Coccidiosis , 3 : 12 – 15 .
- Wallach , M , Smith , NC , Petracca , M , Miller , CM , Eckert , J and Braun , R . (1995) . Eimeria maxima gametocyte antigens: potential use in a subunit maternal vaccine against coccidiosis in chickens . Vaccine , 13 : 347 – 354 .
- Wang GT Davidson J (1992) Comparative efficacy of ionophore/growth promoter combinations for the control of necrotic enteritis in chickens In Proceedings XIX World's Poultry Congress 3 pp. 585–588 Amsterdam The Netherlands
- Watkins , KL , Shryock , TR , Dearth , RN and Saif , YM . (1997) . In-vitro antimicrobial susceptibility of Clostridium perfringens from commercial turkey and broiler chicken origin . Veterinary Microbiology , 54 : 195 – 200 .
- Wicker , DL , Isgrigg , WN , Trammell , JH and Davis , RB . (1977) . The control and prevention of necrotic enteritis in broilers with zinc bacitracin . Poultry Science , 56 : 1229 – 1231 .
- Wijewanta , EA and Seneviratna , P . (1971) . Bacteriological studies of fatal Clostridium perfringens type-A infection in chickens . Avian Diseases , 15 : 654 – 661 .
- Williams , RB . (1986) . On the aetiology of intestinal intussusception in the fowl . Avian Pathology , 15 : 301 – 304 .
- Williams , RB . (1992a) . Differences between the anticoccidial potencies of monensin in maize-based or wheat-based chicken diets . Veterinary Research Communications , 16 : 147 – 152 .
- Williams RB (1992b) The Development, Efficacy and Epidemiological Aspects of Paracox, a New Coccidiosis Vaccine for Chickens Harefield UK Pitman-Moore Europe
- Williams , RB . (1994) . Safety of the attenuated anticoccidial vaccine Paracox in broiler chickens isolated from extraneous coccidial infection . Veterinary Research Communications , 18 : 189 – 198 .
- Williams , RB . (1995) . Epidemiological studies of coccidiosis in the domesticated fowl (Gallus gallus): II. Physical condition and survival of Eimeria acervulina oocysts in poultry-house litter . Applied Parasitology , 36 : 90 – 96 .
- Williams RB (1996) The ratio of the water and food consumption of chickens and its significance in the chemotherapy of coccidiosis Veterinary Research Communications 20 437 447 [see also publisher's errata: Veterinary Research Communications, 21, 69]
- Williams RB (1997a) Economic importance of Eimeria mitis, E. praecox and E. acervulina infections in chickens In Proceedings of the VIIth International Coccidiosis Conference p. 39 Oxford UK
- Williams , RB . (1997b) . Laboratory tests of phenolic disinfectants against the chicken coccidium Eimeria tenella . Veterinary Record , 141 : 447 – 448 .
- Williams , RB . (1998) . Epidemiological aspects of the use of live anticoccidial vaccines for chickens . International Journal for Parasitology , 28 : 1089 – 1098 .
- Williams , RB . (1999a) . A compartmentalised model for the estimation of the cost of coccidiosis to the world's chicken production industry . International Journal for Parasitology , 29 : 1209 – 1229 .
- Williams , RB . (1999b) . Anticoccidial vaccines: the story so far . World Poultry, Special Supplement Coccidiosis , 3 : 23 – 25 .
- Williams , RB . (2001) . Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks . International Journal for Parasitology , 31 : 1056 – 1069 .
- Williams , RB . (2002a) . Anticoccidial vaccines for broiler chickens: pathways to success . Avian Pathology , 31 : 317 – 353 .
- Williams , RB . (2002b) . Fifty years of anticoccidial vaccines for poultry (1952–2002) . Avian Diseases , 46 : 775 – 802 .
- Williams , RB . (2003a) . Coccidial and clostridial interactions in broilers vaccinated against coccidiosis . World Poultry, Special Supplement Coccidiosis , 4 : 26 – 28 .
- Williams , RB . (2003b) . Optimizing anticoccidial vaccine efficacy in broilers . World Poultry, Special Supplement Coccidiosis , 4 : 29 – 31 .
- Williams , RB . (2003c) . Anticoccidial vaccines: looking back, looking forward . Zootecnica International , 25 (7/8) : 52, 54–57
- Williams , RB . (2003d) . Anticoccidial vaccination: the absence or reduction of numbers of endogenous parasites from gross lesions in immune chickens after virulent coccidial challenge . Avian Pathology , 32 : 535 – 543 .
- Williams , RB and Andrews , SJ . (2001) . The origins and biological significance of the coccidial lesions that occur in chickens vaccinated with a live attenuated anticoccidial vaccine . Avian Pathology , 30 : 215 – 220 .
- Williams , RB and Catchpole , J . (2000) . A new protocol for a challenge test to assess the efficacy of live anticoccidial vaccines for chickens . Vaccine , 18 : 1178 – 1185 .
- Williams , RB and Gobbi , L . (2002) . Comparison of an attenuated anticoccidial vaccine and an anticoccidial drug programme in commercial broiler chickens in Italy . Avian Pathology , 31 : 253 – 265 .
- Williams , RB , Bushell , AC , Répérant , J-M , Doy , TG , Morgan , JH , Shirley , MW , Yvoré , P , Carr , MM and Frémont , Y . (1996) . A survey of Eimeria species in commercially-reared chickens in France during 1994 . Avian Pathology , 25 : 113 – 130 .
- Williams , RB , Carlyle , WWH , Bond , DR and Brown , IAG . (1999) . The efficacy and economic benefits of Paracox, a live attenuated anticoccidial vaccine, in commercial trials with standard broiler chickens in the United Kingdom . International Journal for Parasitology , 29 : 341 – 355 .
- Williams , RB , Marshall , RN , La Ragione , RM and Catchpole , C . (2003) . A new method for the experimental production of necrotic enteritis and its use for studies on the relationships between necrotic enteritis, coccidiosis and anticoccidial vaccination of chickens . Parasitology Research , 90 : 19 – 26 .
- Yvoré P (1986) Physiology of the host response to coccidial infections In L.R. McDougald, L.P. Joyner & P.L. Long (Eds.) Research in Avian Coccidiosis pp. 148–168 Athens University of Georgia
- Yvoré , P and Mainguy , P . (1972) . Influence de la coccidiose duodenale sur la tenur en carotenoids du serum chez le poulet . Annales de Recherches Vétérinaires , 3 : 381 – 387 .
- Yvoré , P , Dubois , M , Sauveur , B and Aycardi , J . (1972) . Pathogénie de la coccidiose duodénale à Eimeria acervulina . Annales de Recherches Vétérinaires , 3 : 61 – 82 .
- Yvoré , P , Pery , P , Laurent , F and Bessay , M . (1993) . Vaccins anticoccidiens. Bilan et perspectives . Veterinary Research , 24 : 229 – 250 .