Abstract
The aim of this study was to investigate whether vaccination with the sugar-binding domain of FimH (FimH156) was able to protect chickens against avian pathogenic Escherichia coli (APEC). FimH156 was expressed and purified using Ni-NTA affinity chromatography. Binding of FimH156 to mannosylated bovine serum albumin demonstrated that the protein retained its biological activity. Moreover, anti-FimH156 antisera were able to inhibit in vitro binding of E. coli to mannosylated bovine serum albumin. In a first vaccination experiment, FimH156 was administered intramuscularly as a water-in-oil emulsion to specific pathogen free broiler chicks. A predisposing infection with the Newcastle disease virus strain Lasota was administered 3 weeks later, followed 3 days later by an aerosol challenge with the virulent APEC strain CH2. A good anti-FimH156 immunoglobulin (Ig)G immune response was detected in serum, but no protective effects of FimH156 against APEC were seen. In a second experiment, SPF chicks were vaccinated intramuscularly or intranasally with FimH156. Booster vaccinations were administered 20 days later. While the intramuscular immunization yielded a strong IgG response in the serum and trachea, no significant IgA response could be detected in tracheal washes. Intranasal immunization did not yield a significant IgG or IgA response in serum and trachea. No protective effects of the FimH156 could be detected, confirming the results of the first experiment. Thus, although the FimH156 induced a strong immune response, it was unable to protect chickens against APEC.
L'immunisation avec le domaine de fixation du FimH, l'adhésine de fimbriae de type 1, ne protège pas le poulet vis-à-vis d'Escherichia coli pathogène aviaire
Le but de cette étude a été de savoir si la vaccination avec le domaine de fixation du sucre du FimH (FimH 156) est capable de protéger le poulet contre l’Escherichia coli pathogène aviaire (APEC). Le FimH156 a été exprimé puis purifié par chromatographie d'affinité sur résine Ni-NTA. La fixation de FimH156 au BSA mannosylé a montré que la protéine conservait son activité biologique. De plus, le sérum anti- FimH156 a été capable d'inhiber in vitro la fixation de E. coli au BSA mannosylé. Dans un premier essai de vaccination, le FimH156 a été administré par voie intramusculaire sous la forme d'une émulsion eau dans huile à des poulets de chair SPF. Trois semaines plus tard, la souche La Sota du virus de la maladie de Newcastle a été administrée en tant qu'agent infectieux prédisposant. Enfin trois jours après, les animaux ont été éprouvés avec la souche virulent CH2 d'APEC administrée sous forme d'aérosol. Une bonne réponse immunitaire IgG anti-FimH156 a été détectée dans le sérum, mais aucun effet protecteur du FimH156 vis-à-vis de l'APEC n'a été observé. Dans un second essai des poussins SPF ont été vaccinés par voie intramusculaire ou intranasale avec FimH156. Des rappels de vaccination ont été administrés 20 jours plus tard. Alors que l'immunisation intramusculaire a donné une forte réponse en IgG dans le sérum, la trachée, aucune réponse significative en IgA n'a pu être détectée dans les lavages de la trachée. L'immunisation intranasale n'a pas donné de réponse significative en IgG ou IgA au niveau du sérum et de la trachée. Aucun effet protecteur du FimH156 n'a pu être détecté, confirmant les résultats du premier essai. Ainsi, bien que le FimH156 ait induit une forte réponse immunitaire, il n'a pas été capable de protéger les poulets vis-à-vis de l'APEC.
Immunisierung mit der Bindungsdomäne von FimH, dem Adhäsin der Typ 1-Fimbrien, schützt nicht gegen pathogenes Escherichia coli
Ziel dieser Studie war es, zu untersuchen, ob die Vakzination mit der Zucker bindenden Domäne von FimH (FimH156) in der Lage war, Hühner gegen pathogenes aviäres Escherichia coli (APEC) zu schützen. FimH156 wurde exprimiert und mittels Ni-NTA-Affinitätschromatographie gereinigt. Die Bindung von FimH156 an mannosyliertes BSA zeigte, dass das Protein seine biologische Aktivität behalten hatte. Darüber hinaus konnte anti-FimH156-Antiserum in vitro die Bindung von E. coli an mannosyliertes BSA verhindern. In einem ersten Vakzinationsversuch wurde FimH156 als eine Wasser-in-Öl-Emulsion SPF-Broilerküken intramuskular injiziert. Als prädisponierende Infektion wurde drei Wochen später der Stamm Lasota des Virus der Newcastle-Krankheit verabreicht, drei Tage später gefolgt von einer Aerosol-Belastungsinfektion mit virulentem APEC-Stamm CH2. Im Serum wurde eine gute anti-FimH156-IgG-Immunantwort festgestellt, aber eine Schutzwirkung von FimH156 gegen APEC wurde nicht beobachtet. In einem zweiten Versuch wurden SPF-Küken intramuskuär oder intranasal mit FimH156 vakziniert. Eine Boostervakzination erfolgte 20 Tage später. Während die intramuskuläre Immunisierung zu einer hohen IgG-Bildung in Serum und Trachea führte, konnte keine signifikante IgA-Antwort in den Trachealspülproben nachgewiesen werden. Die intranasale Immunisierung induzierte weder eine deutliche IgG- noch IgA-Antwort in Serum und Trachea. Eine Schutzwirkung von FimH156 konnte auch hier nicht ermittelt werden, was die Ergebnisse aus dem ersten Versuch bestätigte. Daraus fogt, dass FimH156, obwohl es eine deutliche Immunantwort induziert, nicht in der Lage ist, Hühner gegen APEC zu schützen.
Inmunización con el dominio de ligamiento de FimH, la adhesina de fimbria tipo 1, no protege a los pollos frente a Escherichia coli patógena
El objetivo de este estudio fue el de investigar si la vacunación con el dominio de ligamiento a un azúcar FimH (FimH156) era capaz de proteger a los pollos frente a Escherichia coli patógena de origen aviar (APEC). La FimH156 se expresó y purificó mediante cromatografía de afinidad Ni-NTA. La unión de la FimH156 a BSA manosilado demostró que la proteína retenía su actividad biológica. Además, el antisuero anti-FimH156 fue capaz de inhibir in vitro la unión de E. coli a BSA manosilado. En un primer experimento de vacunación, se administró FimH156 intramuscularmente en una emulsión de agua en aceite a pollos SPF. Se les infectó tres semanas más tarde con la cepa Lasota de virus de la enfermedad de Newcastle, y, posteriormente, se les desafió con un aerosol que contenía la cepa virulenta CH2 de APEC. Se detectó una buena respuesta immune de IgG anti-FimH156 en el suero, pero no hubo un efecto protector de FimH156 frente a APEC. En un segundo experimento, se vacunaron experimentalmente pollos SPF vía intramuscular o intranasal con FimH156. La revacunación fue administrada 20 días más tarde. Mientras que la inmunización intramuscular resultó en una fuerte respuesta de IgG en suero y tráquea, no se detectó respuesta significativa de IgA en lavados traqueales. La inmunización intratraqueal no resultó en una respuesta significativa de IgG o IgA en suero y tráquea. No se detectaron efectos protectores de FimH156, lo que confirmaba los resultados de los primeros experimentos. Así pues, aunque FimH156 induce una respuesta inmune importante, no es capaz de proteger a los pollos frente a APEC.
Introduction
Escherichia coli is a major pathogen of animals, resulting in both intestinal and extra-intestinal diseases. In poultry, avian pathogenic E. coli (APEC) mostly cause extra-intestinal infections. Following initial colonization of the respiratory tract, infection spreads to the internal organs and is manifested by perihepatitis, pericarditis, peritonitis, salpingitis and septicaemia. In broilers, APEC are often associated with cellulitis of the lower abdomen and thighs, as well as with swollen head syndrome. These disease syndromes often result in carcass condemnation, reduced growth and death, causing substantial economic losses. APEC infection is frequently secondary to a primary predisposing viral (infectious bronchitis virus, Newcastle disease virus) or mycoplasmal infection (Gross, Citation1994; Dho-Moulin & Fairbrother, Citation1999).
A number of putative virulence factors have been discovered in APEC, including iron sequestering systems (aerobactin) (Dho & Lafont, Citation1984), the temperature-sensitive hemagglutinatin (TSH) (Maurer et al., Citation1998), resistance to serum (Dozois et al., Citation1992; Wooley et al., Citation1992) and fimbriae. Fimbriae (pili) are proteinaceous structures on the outer membrane of E. coli that mediate adherence of the bacteria to host epithelial cells, allowing them to overcome resident defence mechanisms of the respiratory tract and more successfully colonize. Type 1 (F1A) fimbriae adhere to mannose residues by means of their adhesin subunit, FimH, and are thought to be involved in the initial respiratory phase of the infection (Gross, Citation1994; Pourbakhsh et al., Citation1997a,Citationb), while P fimbriae, which adhere to digalactoside residues, are believed to play an important role in later phases (Pourbakhsh et al., Citation1997a).
In previous studies (Marc et al., Citation1998; Arné et al., Citation2000) it was demonstrated that fim and fimH deleted mutant APEC strains were still able to infect and colonize chickens to the same extent as the original wild-type strain. This has put the supposed role of type 1 fimbriae in the infection process (Pourbakhsh et al., Citation1997a,Citationb) into question. The adhesin subunit FimH is comprised of 279 amino acids in its mature form and consists of two parts: the amino-terminal sugar binding domain and the carboxyl-terminal pilin domain (Klemm et al., Citation1985; Krogfelt et al., Citation1990).
FimH is required for colonization of the bladder in human urinary tract infections and is therefore a target for vaccine development. Previous studies by Langermann et al. (Citation1997, Citation2000) have shown that vaccination with the stable chaperone-adhesin complex FimH–FimC was able to protect both mice and monkeys against bacterial challenge. The potential use of recombinant FimH in passive immunization trials for the protection of broilers was recently investigated (Kariyawasam et al., Citation2002, Citation2004). No protection was found after transfer of anti-FimH egg yolk antibodies (IgY) to broilers and subsequent challenge, supporting studies showing that FimH is not required in the infection process. However, as this study used denatured FimH, it is possible that the vaccine used induced antibodies that were unable to block adhesion and hence confer protection.
Based on the work of Schembri et al. (Citation2000), we expressed the sugar binding domain of FimH (the first 156 amino acids) and demonstrated that it was soluble and that, after purification, was able to bind to its mannose receptor. The aim of this study was to investigate whether immunization with this active FimH156 was able to protect chickens from APEC infection. Hence, different methods of vaccination as well as different adjuvants were tested using a natural route of infection.
Materials and Methods
Bacterial strains, plasmids and media
APEC strain CH2 (O78) was isolated from an infected chicken and is virulent in experimentally infected chickens (P. van Empel, personal communication). This strain contains the fimH gene, as confirmed using a polymerase chain reaction (PCR) assay, and is able to express type 1 fimbriae, as shown by haemagglutination, using protocols described in Vandemaele et al. (Citation2003a). APEC 1 (Vandemaele et al., Citation2003b) is a virulent serotype O45 strain containing the FimH adhesin. This strain has been transformed with plasmid pEGFP (Clonetech; BD Biosciences, NJ, USA), containing the gene for EGFP. APEC 54 (Vandemaele et al., Citation2003a) is a virulent serotype O2 strain, also containing the FimH adhesin. Plasmid pPCR SCRIPT AMP SK (Stratagene, La Jola, CA, USA) was used for direct cloning of blunt-end PCR products and sequencing, and plasmid pET22b (Novagen, Madison, WI, USA) was used as an expression vector. E. coli strain BL21 (DE3) was used for expression of the pET22b-fimH156 construct. Luria-Bertani broth (Difco; BD Biosciences) was used for growing E. coli strain BL21 and strain CH2 for challenge. When required, ampicillin (100 μg/ml) was added. For the in vitro binding assays, strain APEC 1 was grown without shaking for 24 h at 37°C in Tryptic Soy Broth (with ampicillin), subcultured and grown for an additional 24 h to allow expression of type 1 pili (Vandemaele et al., Citation2003a). During the second 24-h period, IPTG was added to a final concentration of 2 mg/ml to induce EGFP expression.
Newcastle disease virus
Infection with the Newcastle disease virus (NDV) vaccine strain Lasota (Intervet International, Boxmeer, The Netherlands) was used to predispose chickens to colibacillosis. Each chick was administered approximately 2×106.3 median embryo infective doses in phosphate-buffered saline (PBS) by spray.
Plasmid construction
Genomic DNA was extracted (Ausubel et al., Citation1992) from strain APEC 54 and used as a template to amplify the sugar binding domain of fimH (the first 156 amino acids of the mature protein). The primers used were FimH156F (5′-TATCAACCATGGATATC TTCGCCTGTAAAACCGCC-3′) and FimH156R (5′-TGGATACCATGGTTAGTGA TGGTGATGGTGATGGGTACCAGTAGGCACCACCACAT-3′). Primer FimH156F contained an NcoI cleavage site (CCATGG). A hexahistidine affinity tag was incorporated in the reverse primer FimH156R, followed by a stop codon (TAA) and an NcoI cleavage site. PCR was performed in 50 μl reactions containing 0.2 μM each dNTP, Pfu buffer, 0.5 μM FimH156F and 0.5 μM FimH156R, 7.5% dimethylsulphoxide, 2.5 U Pfu DNA Polymerase (Stratagene, La Jolla, CA, USA) and 2 μg genomic DNA. The reaction was incubated at 95°C for 1 min, then through 30 cycles of incubation at 95°C for 30 sec, 58°C for 30 sec and 72°C for 1 min, followed by a final incubation at 72°C for 10 min. The PCR product was extracted from a 2% agarose gel (Qiaquick Gel Extraction Kit; Qiagen, Valencia, USA), and the Stratagene pPCR SCRIPT AMP SK cloning kit (Stratagene) was used to ligate the PCR product into pPCR SCRIPT AMP SK and this used to transform to E. coli XL10. The sequence of the cloned product was confirmed by sequencing using the ABI PRISM Bigdye™ Terminator Cycle Sequencing Ready Reaction Kit (ABI, Foster City, CA, USA), following the manufacturer's manual. Using NcoI, fimH156 was cleaved from this plasmid, ligated to vector pET22b (further referred to as pET22b-fimH156) and used to transform to strain BL21 (DE3) by electroporation.
Expression and purification
Starter cultures of BL21 (DE3) in LB broth containing ampicillin were incubated at 37°C with shaking at 250 r.p.m. until turbid and then stored at 4°C. The following day, 300 ml LB containing 100 μg ampicillin/ml and 1% glucose was inoculated with the starter cultures (4 ml/100 ml LB broth) and incubated at 30°C with shaking at 250 r.p.m. to an OD600 of 0.6. Expression was then induced by adding IPTG to a final concentration of 1 mM. After 4 h, cells were harvested by centrifugation at 6500× g for 15 min and the bacterial pellet was stored at −20°C until purification. As FimH156 was in the soluble fraction, the protein was purified by Ni-NTA affinity chromatography under non-denaturing conditions. The bacterial pellet was resuspended in 2 vol. Ni-NTA binding buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, 10 mM imidazole). Lysozyme was added to a final concentration of 1 mg/ml and the mixture incubated for 30 min on ice. The lysates were then sonicated on ice and centrifuged twice at 10 000× g for 30 min. The cleared supernatant was mixed with 0.33 vol. Ni-NTA slurry (50 %, Novagen) and incubated at 4°C with shaking for 90 min. The mixture was then applied to a chromatography column and the column then washed twice with 4 ml washing buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, 20 mM imidazole) and protein eluted four times with elution buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, 250 mM imidazole; 0.5 vol. of the Ni-NTA slurry) in tubes already containing the same volume elution buffer (to prevent aggregation due to high concentration). Elution fractions were dialysed extensively against PBS (pH 7.2) using a 10 kDa dialysis membrane (Spectropore; Spectrum Laboratories Inc., CA, USA) to remove imidazole. The presence and purity of FimH156 was checked by sodium dodecylsulphate-polyacrylamide gel electrophoresis (12.5%) and Coomassie blue staining. Western blotting was performed using an anti-His monoclonal antibody (Novagen) to confirm the presence of the His-tagged protein. Protein concentrations were determined using the BCA method (Pierce, Rockford, IL, USA). Freshly prepared FimH156 was used for immunization. Purified and dialysed FimH156 was diluted to 500 μg/ml in PBS, mixed in a 1:1 ratio with glycerol (50 % final concentration), snap-frozen in liquid nitrogen and transferred to −80°C for future use in enzyme-linked immunosorbent assays (ELISAs).
FimH156 activity assay
The activity of the recombinant FimH156 was confirmed by ELISA. ELISA plates were incubated overnight at 4°C with 150 μl mannosylated bovine serum albumin (mBSA) (30 μg/ml in bicarbonate buffer, pH 9.6; Sigma) per well. Plates were washed three times with PBS–Tween (PBST, 0.01 % (v/v) Tween 20 in PBS) and blocked using 0.1% casein in PBS for 1 h at 37°C. After washing with PBST, the recombinant FimH156 (freshly prepared or after storage at −80°C; 12.5 μg/ml in PBS) was added at 150 μl/well and plates were incubated for 2 h at room temperature. Plates were washed and incubated with an anti-His monoclonal antibody (Novagen; 1/2000 in 0.5% bovine serum albumin–PBST (BSA-PBST) for 1 h at room temperature. After washing, a secondary goat anti-mouse horseradish peroxidase (HRP)-labelled antibody (DAKO Cytomation, Glostrup, Denmark; 1/5000 in 0.5% BSA-PBST) was added and the plates incubated for 1 h at room temperature. Bound HRP was detected using the 2,2′-azino-di(3-ethylbenzythiazolin-6-sulfonic acid) (ABTS) substrate and absorbances at 405 nm measured using a Titertek microplate reader (MCC/340 Multiskan; Titertek, AL, USA).
Vaccination experiment 1
Forty-eight specific pathogen free (SPF) broiler chicks (Intervet) were randomly divided into four groups (12 chicks/group initially) and housed in a disinfected stable. Feed and water were supplied ad libitum during the experiment. At 10 days of age (0 days post vaccination [d.p.v.]), chicks in group 1 were vaccinated intramuscularly in the thigh with 500 μl vaccine containing 100 μg FimH156 in a water in oil (w/o) emulsion (45/55, paraffin-based; Intervet). Group 2 received an intramuscular placebo vaccine (sterile PBS in the same w/o emulsion), while groups 3 and 4 were not vaccinated (). Three weeks later (21 d.p.v.), chicks in groups 1, 2 and 3 were spray-infected with the NDV Lasota strain and transferred to SPF isolators. Groups 1 and 2 were mixed and distributed in equal proportions in two isolators to exclude isolator effects. Three days later (24 d.p.v.), the APEC challenge was administered by nebulization of a 10 ml culture of strain CH2 (3×1010 colony-forming units/ml in LB) into each isolator. Birds in groups 3 and 4 were not challenged. Strain CH2 was used because it was a virulent strain, in which the FimH sugar binding domain was identical to that of APEC 54 (on which the construct was based), as demonstrated by sequence analysis. Seven days later (31 d.p.v.), all birds were euthanized by cervical dislocation and autopsied. Scores were determined for macroscopic lesions in the thoracic (0, normal; 1, one air sac very cloudy and presence of fibrinous exudate or limited pinhead-sized foci of fibrinous exudate in both air sacs; 2, both air sacs very cloudy and presence of fibrinous exudate) and abdominal air sacs (0, normal; 1, mild cloudiness and/or pinhead-sized foci of fibrinous exudate; 2, very cloudy with fibrinous exudate), lungs (0, normal; 1, unilateral pneumonia with discoloration and consolidation; 2, bilateral pneumonia), pericardium (0, normal; 1, some cloudy exudate in the pericardium; 2, pericardium filled with large amounts of purulent exudate) and liver (0, normal; 1, some multifocal exudate adherent to surface; 2, extensive layers of exudate or fibrin adherent to the surface). Animals that died during the challenge period were awarded the maximal score of 10 (two per organ). Total lesion scores were calculated for each chicken and medians and lower and upper quartiles were determined for each group. The medians for the total lesion score and the medians for the lesion scores for each organ were analysed to determine whether there were significant differences between groups. Blood was collected on days 10 (0 d.p.v.), 17 (7 d.p.v.), 24 (14 d.p.v.) and 31 (21 d.p.v.), and at autopsy (31 d.p.v.). Serum was collected and stored at −20°C for ELISA. All animal experiments were approved by the ethical committee of the K.U. Leuven.
Table 1. Mortalities and lesion scores in chickens in vaccination experiment 1
Vaccination experiment 2
Seventy-five SPF broiler chicks (Intervet) were randomly allocated to five groups () and housed in a disinfected stable. Feed and water were supplied ad libitum. In this experiment, recombinant FimH156 was combined with the Iscomatrix adjuvant (Bengtsson & Sjölander, Citation1996; Windon et al., Citation2000; Coulter et al., Citation2003) and tested as a parenteral or mucosal vaccine. At 10 days of age (0 d.p.v.), groups 1 and 2 were vaccinated intramuscularly in the thigh (FimH156 and PBS, respectively), while groups 3 and 4 were vaccinated intranasally (50 μl/nostril, FimH156 and PBS, respectively). FimH156 vaccinated birds received 100 μg FimH156 and 50 μg Iscomatrix (in PBS) each. Placebo vaccinated birds received 50 μg Iscomatrix (in PBS) each. Group 5 was not vaccinated. Twenty days later (20 d.p.v.), a booster vaccine was administered in the same manner. Vaccinated birds received 78 μg FimH156 and 75 μg Iscomatrix (in PBS) each, while placebo vaccinated birds received 75 μg Iscomatrix (in PBS) each. At 20 d.p.v., all birds in excess of 12 (two to four randomly selected birds per group, depending on prior mortalities) were euthanized and their tracheas were removed. Tracheal sections 2 cm in length were taken and washed in 1 ml sterile PBS (containing 0.2% sodium azide). After centrifugation and removal of tracheal sections, the supernatants (tracheal washes) were stored at −20°C for ELISA. At 21 d.p.v., all birds were spray-infected with the NDV Lasota strain and transferred to SPF isolators. Groups were mixed to exclude isolator effects. Four days later (25 d.p.v.), they were challenged with the APEC strain CH2 as already described, were euthanized 1 week later (32 d.p.v.) and autopsied. Lesion scores were determined as already described (except that abdominal air sacs were not scored, yielding a total possible score of 8). Tracheas were removed and tracheal washes were performed as already described. Blood was taken on days 10 (0 d.p.v.), 27 (17 d.p.v.) and 42 (32 d.p.v.) from all birds and on day 30 (20 d.p.v.) from the birds from which the tracheas were collected.
Table 2. Mortalities and lesion scores in chickens in vaccination experiment 2
ELISA for anti-FimH156 immunoglobulin G and immunoglobulin A
The anti-FimH156-specific immunoglobulin (Ig)G or IgA concentrations in sera and tracheal washes were determined using a FimH156 ELISA. Coating, blocking and incubation with FimH156 were as already described for the activity assay, but sera from experimental birds were used as primary antibody. Sera were diluted to 1/400 in 0.5% BSA-PBST in the first experiment and to 1/100 in the second experiment. Tracheal washes were used undiluted. After incubation for 1 h at room temperature, the plate was washed three times with PBST and incubated with a HRP-labelled goat anti-chicken IgG antibody (Bethyl, Montgomery, TX, USA; 1/5000 in 0.5% BSA-PBST) or with a HRP-labelled goat anti-chicken IgA antibody (Bethyl; 1/5000 in 0.5% BSA-PBST) for 1 h at room temperature. After washing, bound HRP was detected using 150 μl ABTS substrate per well (KPL, Gaithersburg, MD, USA) and absorbance was determined at 405 nm. Negative controls (no chicken serum, 0.5% BSA-PBST) were incorporated on each plate. The mean of duplicate wells was determined and the value obtained for the negative control was subtracted from this.
Production of polyclonal anti-FimH156 antiserum
New Zealand White rabbits were vaccinated with the purified FimH156. For the primary vaccination, 1 mg FimH156 in complete Freund's adjuvant was administered subcutaneously, followed by an initial booster 7 weeks later with 1 mg FimH156 in incomplete Freund's adjuvant and a second booster 25 weeks later with 0.5 mg FimH156 in incomplete Freund's adjuvant. Blood was sampled at regular time points and the serum was used in the in vitro binding assay. Samples R-s14/16 and R-s14/17 were high-titre anti-FimH156 rabbit sera from 14 days after the first booster from two different rabbits. Sample R-ss10 was collected 10 days after the second booster and sample R-p0 was collected prior to vaccination.
In vitro binding assay
ELISA plates were incubated overnight at 4°C with 100 μl mBSA (Sigma; 75 μg/ml in bicarbonate buffer, pH 9.6) in each well. Plates were then emptied and blocked with 5% BSA in PBS for 1 h at 37°C, followed by three washes with PBS. The presence of type 1 fimbriae on APEC 1 was confirmed by microscopic examination of binding to chicken erythrocytes in the presence and absence of methyl-α-d-mannopyranoside (αMM). The expression of EGFP was confirmed by fluorescence microscopy. For the first experiment, two-fold dilution series of antibodies or αMM were prepared in PBS in a U-shaped 96-well microtitre plate. An equal volume of bacteria (109 colony-forming units/ml in Tryptic Soy Broth or PBS) was added, and the mixture incubated for 90 min at room temperature with gentle rotation. A 40 μl sample of each bacterial suspension was transferred to the washed ELISA plates, to each well of which 60 μl PBS had already been added, and the plates incubated for 2 h at room temperature. Unbound bacteria were removed by three washes with PBS and 100 μl PBS was added to each well. Bound bacteria were detected by determining fluorescence in a Fluoroscan (Thermolab Systems; excitation at 488 nm, detection at 520 nm) in three successive measurements. PBS (without bacteria) was used as a negative control. The three measurements were averaged and the value for the negative control was subtracted from this. The percentage adhesion was calculated relative to an untreated bacterial suspension (bacteria incubated with PBS alone were taken as a reference—100% adhesion). In this first experiment, the anti-FimH156 sera, R-s14/16 and R-s14/17, C610/21, an anti-FimH156 serum from a chicken from the first vaccination experiment (chicken 610 at 21 d.p.v.) and αMM-20 (20% αMM, a known inhibitor of type 1 fimbrial adhesion) were tested. All sera were inactivated by incubation for 25 min at 56°C prior to use. The second experiment was performed as for the first, except that when the plates contained the bacterial suspensions they were incubated for 45 min at 37°C. IgG was purified from the high-titre anti-FimH156 rabbit serum R-ss10 using standard Protein A column chromatography (protocol by Upstate Serologicals Corporation; Norcross, GA, USA). Rabbit pre-immune serum (R-p0) was compared with sample R-ss10 before (R-ss10, unpurified) and after (R-ss10-P, purified, 121.2 μg/ml) isolation of IgG. As a negative control, an irrelevant antibody, KUL01 ascites (Mast et al., Citation1998), was subjected to Protein A purification (KUL01: unpurified, KUL01-P: purified, 108.7 μg/ml). C602/21, a sample of anti-FimH156 serum from a chicken from the first experiment (chicken 602 at 21 d.p.v.), was also used.
Statistical analysis
All statistical analyses were performed using SAS software, version 8.2 (SAS Institute, Cary, NC, USA). Lesion scores were compared using Kruskal–Wallis analysis for differences between multiple groups and the Wilcoxon Rank Sum Test for differences between each pair of groups. IgG and IgA concentrations in serum and tracheal washes were analysed using Proc GLM (multiple two-tailed t test) or the Wilcoxon Rank Sum Test. Fisher's exact test or the Wilcoxon Rank Sum Test were used to compare the morbidity and mortality rates. P≤0.05 was considered significant.
Results
Expression and purification of FimH156
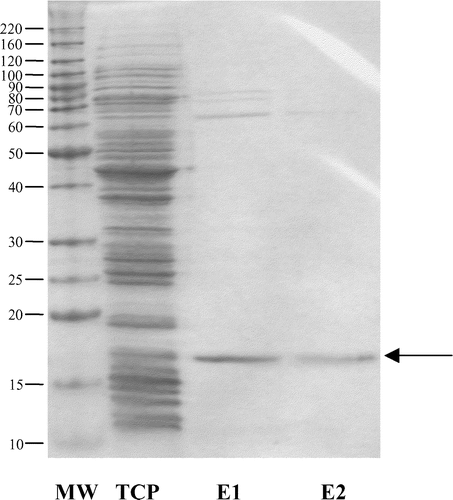
The amino-terminal sugar binding domain of FimH, FimH156, was successfully expressed using pET22b and E. coli strain BL21 (DE3). IPTG induction of expression with 1 mM IPTG at 30°C yielded soluble protein, which was purified using Ni-NTA affinity chromatography under non-denaturing conditions. One major band corresponding to the size of the recombinant protein (17 kDa) was observed in eluted fractions (). Some higher molecular weight bands between 60 and 80 kDa were also seen. Approximately 14.5 mg purified FimH156 was obtained per litre of E. coli culture. Western blotting with an anti-His monoclonal antibody confirmed that the major band was FimH156. In an activity assay, FimH156 was shown to bind to mBSA. FimH156 did not bind to control plates that had not been coated with mBSA. Specific binding of FimH156 to mBSA was used to quantify anti-FimH156 IgG and IgA antibodies, eliminating the potential problem of the contaminating higher molecular weight proteins. FimH156 stored in 50% glycerol at −80°C retained mBSA binding activity for at least 6 months.
Figure 1. Sodium dodecylsulphate-polyacrylamide gel electrophoresis of the purified FimH156. MW, molecular mass (kDa); TCP, total cell protein (soluble+insoluble fraction); E1 and E2, elution fractions 1 and 2 after Ni-NTA purification. E1 and E2 were mixed to give the final vaccine. Arrow, FimH156 (17 kDa).
Vaccination experiment 1
Lesions. Mortality rates and macroscopic lesions are presented in . There was no significant difference in mortality rates between different treatments. Groups 3 and 4 had some slightly affected birds, even though they were not infected. However, the total lesion scores of each of the birds in these groups was less than 1. Most of the sick birds from the infected groups (groups 1 and 2) had high total lesion scores. No significant differences were seen between the total lesion scores of birds in groups 1 and 2 (vaccinated and infected versus unvaccinated and infected, P=0.9398), nor between the two control groups (NDV alone versus no treatment, P=0.5217). Significant differences were seen between the APEC-infected groups (groups 1 and 2) and the uninfected control groups (groups 3 and 4) (group 1 versus group 3, P=5.872×10−4; group 1 versus group 4, P=2.211×10−4; group 2 versus group 3, P=5.954×10−4; and group 2 versus group 4, P=1.694×10−4). Similar results were obtained when lesions scores for individual organs were compared ().
ELISA
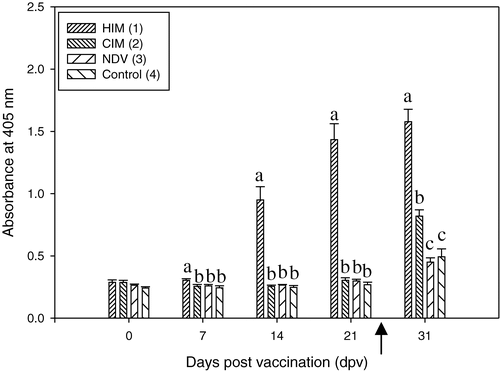
FimH156-specific IgG was detected in serum after IM vaccination (). From 7 d.p.v., there was a significant difference between group 1 (FimH156 vaccinated) and all other groups. Until challenge, there was no difference between the placebo-vaccinated group (group 2) and the control groups (groups 3 and 4). At autopsy, group 1 was still significantly different from all other groups. The placebo-vaccinated and challenged group was also significantly different from the two unchallenged control groups (groups 3 and 4, which did not differ significantly). Serum concentrations anti-FimH156 IgG in the control groups (groups 3 and 4) were significantly higher at autopsy than at earlier times.
Figure 2. Serum IgG response (dilution 1/400) to FimH156 in experiment 1. The bracketed numbers indicate groups, as presented . Significant differences between treatments at each time point are indicated by different lowercase letters. The arrow indicates the time of APEC infection (24 d.p.v.). OD was measured at 405 nm. HIM, FimH156 vaccinated and challenged group; CIM, placebo vaccinated and challenged group; NDV, control group (only received NDV).
Vaccination experiment 2
Lesions. The mortality rates, total lesion scores and lesion scores for individual organs are presented in . No significant differences in mortality rates were seen between the groups (P=0.086).
The total lesion scores and the scores for individual organs of birds vaccinated intramuscularly with FimH156 were not different from those of the placebo vaccinated group. There were also no differences between total lesion scores or individual organ lesion scores of birds vaccinated intranasally with FimH156 and those of placebo vaccinated birds. No clinical signs were observed in the control group. The total lesion scores of birds in the control group were significantly different from those of birds in all other groups. However, there was no difference between scores for liver lesions of birds in the control group and those of birds in groups 1, 3 and 4. There was also no difference between the scores for lung lesions of birds in the control group and those of birds in groups 3 and 4. Total lesion scores (P=0.0234), but not the scores for lesions in individual organs, differed between the vaccinated groups. Total lesion scores (P=0.0808), as well as scores for several of the individual organs (pericardium and lungs, P=0.0436 and P=0.0352, respectively) differed between the two placebo vaccinated groups (groups 2 and 4).
ELISA
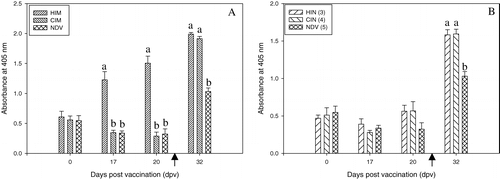
Mean anti-FimH156 IgG concentrations in birds vaccinated intramuscularly with FimH156 were significantly different from those of birds in all other groups at 17 and 20 d.p.v. (). However, at 7 days after challenge (32 d.p.v.), no difference was seen between the challenged groups 1 and 2, which were both significantly different from the unchallenged group (group 5). There was no rise in anti-FimH156 IgG concentrations in the intranasally vaccinated group (group 3) prior to challenge (). No serum was taken between the booster vaccination and challenge, so the effect of the booster could not be evaluated. Seven days after challenge, no difference could be detected between the intranasally vaccinated and the placebo vaccinated birds, which both differed from the unchallenged group (group 5). All groups contained chicks with serum IgG against FimH156 prior to the beginning of the experiment. In the placebo and control groups, the concentrations dropped in these chicks and did not rise in those without detectable anti-FimH156 IgG. However, in group 1 the anti-FimH156 IgG concentrations continued to rise after immunization.
Figure 3. Serum IgG response (dilution 1/100) to FimH156 in experiment 2. 3a: Intramuscularly vaccinated groups compared with controls. 3b: Intranasally vaccinated groups compared with controls. The bracketed numbers indicate groups, as presented in . Arrow, APEC infection at 25 d.p.v. Significant differences between treatments at each time point are indicated by different lowercase letters. OD was measured at 405 nm. HIM, vaccinated intramuscularly with FimH156 and challenged; CIM, vaccinated intramuscularly with placebo and challenged; HIN, vaccinated intranasally with FimH156 and challenged; CIN, vaccinated intranasally with placebo and challenged; NDV, control group (NDV only).
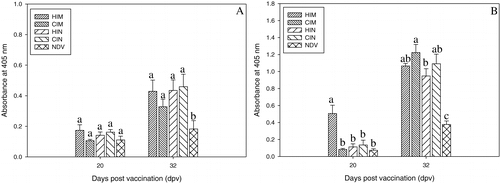
Since the Iscomatrix adjuvant has previously been shown to elicit IgA responses (Windon et al., Citation2000), as well as a systemic response, we also examined the IgA response in tracheal washes at 20 and 32 d.p.v. At 20 d.p.v., two to four birds were selected at random from each group and their tracheas were collected. The anti-FimH156 IgA concentrations were low and at 20 d.p.v. no differences could be detected between the different groups. After challenge (32 d.p.v.), all challenged groups had significantly higher anti-FimH156 IgA concentrations in the trachea than the control group, but no differences could be detected between the challenged groups. Anti-FimH156 IgA concentrations were significantly higher at 32 d.p.v. than at 20 d.p.v. in each group, except for the unchallenged control group. There was also a higher concentration of anti-FimH156 IgG () at 20 d.p.v. in tracheal washes from the intramuscularly vaccinated group than in those from all other groups (group 1 versus group 2, P=0.0004; group 1 versus group 3, P=0.0004; group 1 versus group 4, P=0.001; and group 1 versus group 5, P=0.0003). Tracheal anti-FimH156 IgG concentrations in both intranasally vaccinated groups were low and not different from the control group (P=0.6756). At 32 d.p.v., the tracheal anti-FimH156 IgG concentrations of all challenged groups differed significantly from those of the unchallenged group. With the exception of the control group, at 32 d.p.v. the tracheal anti-FimH156 IgG concentrations in all groups were significantly different from those at 20 d.p.v.
Figure 4. Anti-FimH156 (4a) IgA and (4b) IgG response in tracheal washes from birds in experiment 2. Different letters indicate significant differences between groups at that time point. HIM, vaccinated intramuscularly with FimH156 and challenged; CIM, vaccinated intramuscularly with placebo and challenged; HIN, vaccinated intranasally with FimH156 and challenged; CIN, vaccinated intranasally with placebo and challenged; NDV, control group (NDV only).
In vitro binding assay
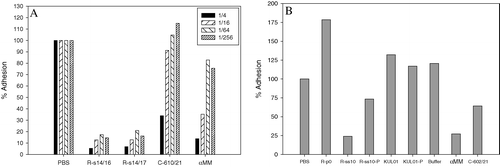
The in vitro binding assay was used to investigate whether antisera against FimH156 were able to inhibit binding of E. coli to mBSA. The virulent APEC strain APEC 1, after transformation with pEGFP, was still able to express type 1 fimbriae, as confirmed by binding to chicken erythrocytes, and was fluorescent after induction. Incubation of bacteria with αMM in the assay inhibited adhesion to mBSA in a concentration-dependent manner. Bacterial binding was not detected in uncoated plates, indicating that adhesion was specifically to mBSA. The polyclonal anti-FimH156 rabbit sera (R-s14/16 and R-s14/17) were of high titre (as determined by ELISA; data not shown) and were able to inhibit binding to mBSA (), even at high dilutions. Sera from vaccinated chickens from experiment 1 were also able to inhibit binding (C610/21 in and C602/21 in ), although inhibition decreased more rapidly at higher serum dilutions, in correlation with the probable lower antibody concentration (since rabbits received primary vaccination and two boosters compared with only a single vaccination in chickens). In order to confirm that inhibition was due to specific antibodies, IgG was purified from high-titre anti-FimH156 rabbit serum (R-ss10) by Protein A column chromatography. This high-titre serum R-ss10 was able to inhibit binding, unlike serum collected prior to vaccination (). The purified IgG was able to inhibit binding, although to lesser extent, probably due to the lower concentration after purification. The elution buffer and an irrelevant antibody KUL01 (purified as well as unpurified) did not inhibit binding. The experiments were repeated using a F1+ GFP-expressing E. coli K12 MG1655 strain (containing plasmid pFPV25; Aertsen et al., Citation2004), and a similar inhibition of adhesion of MG1655 by the rabbit and chicken sera was also observed.
Figure 5. In vitro binding of APEC 1 to mBSA and inhibition by antisera. 5a: Effect of 1/4, 1/16, 1/64 and 1/256 dilutions of serum or αMM. 5b: Sera, elution buffer or aMM were used at a 1/4 dilution. R-s14/16 and R-s14/17, high-titre anti-FimH156 rabbit sera from 14 days after the first booster from two different rabbits. Sample R-ss10 was collected 10 days after the second booster and sample R-p0 was collected prior to vaccination. C610/21 and C602/21, two anti-FimH156 chicken sera from experiment 1 (chickens 610 and 602 at 21 d.p.v.). αMM, 20% methyl-α-d-mannopyranoside. KUL01, an irrelevant antibody (Mast et al., Citation1998). Buffer, the standard protein A elution buffer (to which 1/5 volume neutralization buffer was added) in which purified IgG antibodies were dissolved. R-ss10-P and KUL01-P, the purified IgG from R-ss10 and KUL01, respectively. Percentage adhesion is calculated relative to the untreated bacterial suspension (bacteria incubated in PBS in the assay).
Discussion
The objective of this study was to express soluble and active FimH156 and to test whether vaccination with this protein was able to protect chickens against APEC. In addition to previous studies on FimH (Abraham et al., Citation1988; Sokurenko et al., Citation1995, Citation1998), we recently demonstrated that FimH was highly conserved among avian E. coli strains (> 99% homology), as well as in avian, porcine, bovine and human E. coli, making it an interesting vaccine candidate (Vandemaele et al., Citation2003a, Citation2004). However, as the mature FimH is unstable (Choudhury et al., Citation1999; Schembri et al., Citation2000), it was necessary to express only the sugar-binding domain of FimH, referred to as FimH156 in this study. This approach has previously been used by Schembri et al. (Citation2000), who demonstrated that the recombinant FimH156 was soluble, easy to purify and fully functional, as it retained an ability to bind to mBSA. A similar construct was engineered based on FimH of strain APEC 54 (Vandemaele et al., Citation2003a) and expressed in an E. coli BL21 strain. The resulting FimH156 was detected in the soluble fraction and could be purified using Ni-NTA affinity chromatography. Some higher size contaminants still present after Ni-NTA purification were most probably E. coli chaperones, like DnaK and GroEL, as shown in other studies (Beck & Nassal, Citation2001). The resulting FimH156 was able to bind to mannose, demonstrating its biological activity.
In vitro studies showed that binding of F1+ E. coli strains (virulent strain APEC 1 or E. coli K12 MG1655 strain) was inhibited by both rabbit anti-FimH156 sera and sera from FimH156 vaccinated chickens from experiment 1. Purified IgG from the rabbit serum also inhibited binding, suggesting that the inhibitory effect of the serum was due to the anti-FimH156 antibodies.
Good antibody responses against FimH156 were obtained after a single intramuscular vaccination of chickens. Mast & Goddeeris (Citation1999) have previously shown that it is possible to induce an immune response in chickens at that early age. Upon challenge, all challenged groups had birds with lesions, while no lesions were seen in the unchallenged groups. However, there was no evidence of a protective effect of FimH156 vaccination. As type 1 fimbriae are thought to be mainly active in the respiratory phase of infection (Pourbakhsh et al., Citation1997a,Citationb), it was hypothesized that induction of mucosal antibodies might result in protection.
Intramuscular vaccination with FimH156, adjuvanted with Iscomatrix, resulted in high anti-FimH156 IgG concentrations in serum and tracheal washes. However, no IgA responses were detected in tracheal washes after vaccination. Intranasal vaccination with the FimH156/Iscomatrix mixture did not induce an IgG response in serum or the trachea, nor an IgA response in the trachea. Our findings indicate that the vaccination with FimH156 and Iscomatrix only resulted in a systemic IgG response, but not in a mucosal response, underlining the need for new adjuvants or new vaccination strategies for mucosal vaccination of chickens with proteins. There was no evidence for any protective effect of FimH156 vaccination by either vaccination route, confirming the findings of the first experiment.
Langermann et al. (Citation2000) showed that vaccination with the chaperone-adhesin complex FimC–FimH was able to protect monkeys against bacterial challenge and that there was a correlation with the presence of antibodies in vaginal secretions, rather than in the serum. In contrast, our results are in agreement with previous work (Kariyawasam et al., Citation2004) that found that passive transfer of purified IgY against recombinant full-size FimH did not protect broilers against challenge with homologous or heterologous strains. However, FimH used in this previous study was purified from inclusion bodies in a denatured state and might therefore not have induced protective antibodies.
A rise in anti-FimH156 antibodies in the unchallenged groups was observed after NDV infection. Although no E. coli were administered, NDV may have rendered the birds more susceptible to environmental E. coli, resulting in a low rise in anti-FimH156 antibodies. However, no clinical signs were observed in these unchallenged groups. At the start of the second experiment, all groups contained chicks with serum antibodies against FimH156, but as the concentrations of these antibodies quickly dropped over time in the unvaccinated chicks, and remained low in the seronegative chicks, they were presumed to be of maternal origin. These background antibody levels did not appear to affect immunization with FimH156.
In experiment 2, the birds in the intramuscularly vaccinated group had significantly lower total lesion scores after challenge than those in the intranasally vaccinated group. The group intranasally vaccinated with the placebo also had lower total lesion scores than the group intramuscularly vaccinated with the placebo. As these nasal booster vaccinations both contained the Iscomatrix adjuvant, non-specific immune responses might have been enhanced over the following days and protected the mucosal tract against the spray infection with NDV. Consequently, this booster vaccination might have protected the chickens against the APEC infection.
Our findings indicate that biologically active FimH156 was unable to protect chickens against APEC, notwithstanding a strong immune response. This offers further support for the hypothesis that type 1 fimbriae are not required for APEC infection. Indeed, Arné et al. (Citation2000) demonstrated that a ▵fimH mutant of a virulent APEC strain was a better colonizer of the chicken trachea than the wild-type strain. Considered in combination with similar earlier findings by Marc et al. (Citation1998) using a ▵fim mutant, it appears that type 1 fimbriae and FimH are not required for colonization of the trachea, and could even reduce bacterial persistence.
Translations of the abstract in French, German and Spanish are available on the Avian Pathology website.
Acknowledgments
F.V. was supported by the FWO (Flemish Fund for Scientific Research). The authors thank Carine Borgers and Nyugen Van Thang for skilled technical assistance. They also thank Dr Bram Aertsen and Prof. C. Michiels for the use of the Fluoroscan ELISA reader and for providing E. coli strain K12 MG1655.
References
- Abraham , SN , Sun , D , Dale , JB and Beachey , EH . (1988) . Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae . Nature , 336 : 682 – 684 .
- Aertsen , A , Vanoirbeek , K , De Spiegeleer , P , Sermon , J , Hauben , K , Farewell , A , Nyström , T and Michiels , CW . (2004) . Heat shock protein-mediated resistance to high hydrostatic pressure in Escherichia coli . Applied and Environmental Microbiology , 70 : 2260 – 2266 .
- Arné , P , Marc , D , Brée , A , Schouler , C and Dho-Moulin , M . (2000) . Increased tracheal colonization in chickens without impairing pathogenic properties of avian pathogenic Escherichia coli MT78 with a FimH deletion . Avian Diseases , 44 : 343 – 355 .
- Ausubel FM Brent R Kingston RE Moore DD Seidman JG Smith JA Struhl K (1992) Short Protocols in Molecular Biology 2nd edn New York Greene Publishing Associates and John Wiley & Sons
- Beck , J and Nassal , M . (2001) . Reconstitution of a functional duck Hepatitis B virus replication initiation complex from separate reverse transcriptase domains expressed in Escherichia coli . Journal of Virology , 75 : 7410 – 7419 .
- Bengtsson , KA and Sjölander , A . (1996) . Adjuvant activity of iscoms; effect of ratio and co-incorporation of antigen and adjuvant . Vaccine , 14 : 753 – 760 .
- Choudhury , D , Thompson , A , Stojanoff , V , Langermann , S , Pinkner , J , Hultgren , SJ and Knight , SD . (1999) . X-ray structure of the FimC-FimH chaperone–adhesin complex from uropathogenic Escherichia coli . Science , 285 : 1061 – 1066 .
- Coulter , A , Harris , R , Davis , R , Drane , D , Cox , J , Ryan , D , Sutton , P , Rockman , S and Pearse , M . (2003) . Intranasal vaccination with Iscomatrix adjuvanted influenza vaccine . Vaccine , 21 : 946 – 949 .
- Dho , M and Lafont , JP . (1984) . Adhesive properties and iron uptake ability in Escherichia coli lethal and nonlethal for chicks . Avian Diseases , 28 : 1016 – 1025 .
- Dho-Moulin , M and Fairbrother , JM . (1999) . Avian pathogenic Escherichia coli (APEC) . Veterinary Research , 30 : 299 – 316 .
- Dozois , CM , Fairbrother , JM , Harel , J and Bossé , M . (1992) . pap- and pil-related DNA sequences and other virulence determinants associated with Escherichia coli isolated from septicemic chickens and turkeys . Infection and Immunity , 60 : 2648 – 2656 .
- Gross WG (1994) Diseases due to Escherichia coli in poultry In C.L. Gyles (Ed.) Escherichia coli in Domestic Animals and Humans pp. 237–259 Wallingford CAB International
- Kariyawasam , S , Wilkie , BN , Hunter , BD and Gyles , CL . (2002) . Systemic and mucosal antibody responses to selected cell surface antigens of avian pathogenic Escherichia coli in experimentally infected chickens . Avian Diseases , 46 : 668 – 678 .
- Kariyawasam , S , Wilkie , BN and Gyles , CL . (2004) . Resistance of broiler chickens to Escherichia coli respiratory tract infection by passively transferred egg-yolk antibodies . Veterinary Microbiology , 98 : 273 – 284 .
- Klemm , P , Jorgensen , BJ , van Die , I , de Ree , H and Bergmans , H . (1985) . The fim genes responsible for synthesis of type 1 fimbriae in Escherichia coli . Molecular and General Genetics , 199 : 410 – 414 .
- Krogfelt , KA , Bergmans , H and Klemm , P . (1990) . Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia coli type 1 fimbriae . Infection and Immunity , 58 : 1995 – 1998 .
- Langermann , S , Palaszynski , S , Barnhart , M , Auguste , G , Pinkner , JS , Burlein , J , Barren , P , Koenig , S , Leath , S , Hal Jones , C and Hultgren , SJ . (1997) . Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination . Science , 276 : 607 – 611 .
- Langermann , S , Möllby , R , Burlein , JE , Palaszynski , SR , Auguste , CG , DeFusco , A , Strouse , R , Schenerman , MA , Hultgren , SJ , Pinkner , JS , Winberg , J , Guldevall , L , Söderhäll , M , Ishikawa , K , Normark , S and Koenig , S . (2000) . Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli . The Journal of Infectious Diseases , 181 : 774 – 778 .
- Marc , D , Arné , P , Brée , A and Dho-Moulin , M . (1998) . Colonization ability and pathogenic properties of a fim– mutant of an avian strain of Escherichia coli . Research in Microbiology , 149 : 473 – 485 .
- Mast , J and Goddeeris , BM . (1999) . Development of immunocompetence of broiler chickens . Veterinary Immunology and Immunopathology , 70 : 245 – 256 .
- Mast , J , Goddeeris , BM , Peeters , K , Vandesande , F and Berghman , LR . (1998) . Characterisation of chicken monocytes, macrophages and interdigitating dendritic cells by the monoclonal antibody, KUL01 . Veterinary Immunology and Immunopathology , 61 : 343 – 357 .
- Maurer , JJ , Brown , TP , Steffens , WL and Thayer , SG . (1998) . The occurrence of ambient temperature-regulated adhesins, curli, and the temperature-sensitive hemagglutinin Tsh among avian Escherichia coli . Avian Diseases , 42 : 106 – 118 .
- Pourbakhsh , SA , Dho-Moulin , M , Bree , A , Desautels , C , Marineau-Doizé , B and Fairbrother , JM . (1997a) . Localization of the in vivo expression of P and F1 fimbriae in chickens experimentally inoculated with pathogenic Escherichia coli . Microbial Pathogenesis , 22 : 331 – 341 .
- Pourbakhsh , SA , Boulianne , M , Marineau-Doizé , B and Fairbrother , JM . (1997b) . Virulence mechanisms of avian fimbriated Escherichia coli in experimentally inoculated chickens . Veterinary Microbiology , 58 : 195 – 213 .
- Schembri , MA , Hasman , H and Klemm , P . (2000) . Expression and purification of the mannose recognition domain of the FimH adhesin . FEMS Microbiology Letters , 188 : 147 – 151 .
- Sokurenko , EV , Courtney , HS , Maslow , J , Siitonen , A and Hasty , DL . (1995) . Quantitative differences in adhesiveness of type 1 fimbriated Escherichia coli due to structural differences in fimH genes . Journal of Bacteriology , 177 : 3680 – 3686 .
- Sokurenko , EV , Chesnokova , V , Dykhuizen , DE , Ofek , I , Wu , XR , Krogfelt , KA , Struve , C , Schembri , MA and Hasty , DL . (1998) . Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin . Proceedings of the National Academy of Sciences of the USA , 95 : 8922 – 8926 .
- Vandemaele , F , Vandekerchove , D , Vereecken , M , Derijcke , J , Dho-Moulin , M and Goddeeris , BM . (2003a) . Sequence analysis demonstrates the conservation of fimH and variability of fimA throughout Avian Pathogenic Escherichia coli (APEC) . Veterinary Research , 34 : 153 – 163 .
- Vandemaele , FJ , Mugasa , JP , Vandekerchove , D and Goddeeris , BM . (2003b) . Predominance of the papGII allele with high sequence homology to that of human isolates among avian pathogenic Escherichia coli (APEC) . Veterinary Microbiology , 97 : 245 – 257 .
- Vandemaele , F , Hensen , SM and Goddeeris , BM . (2004) . Conservation of deduced amino acid sequence of FimH among Escherichia coli of bovine, porcine and avian disease origin . Veterinary Microbiology , 101 : 147 – 152 .
- Windon , RG , Chaplin , PJ , McWaters , P , Tavarnesi , M , Tzatzaris , M , Kimpton , WG , Cahill , RNP , Beezum , L , Coulter , A , Drane , D , Sjölander , A , Pearse , M , Scheerlinck , J-PY and Tennent , JM . (2000) . Local immune responses to influenza antigen are synergistically enhanced by the adjuvant ISCOMATRIX . Vaccine , 20 : 490 – 497 .
- Wooley , RE , Spears , KR , Brown , J , Nolan , LK and Fletcher , OJ . (1992) . Relationship of complement resistance and selected virulence factors in pathogenic avian Escherichia coli . Avian Diseases , 36 : 679 – 684 .