Abstract
Mycoplasma gallisepticum TS-11 vaccine was studied for its safety and protective ability in 49-day-old M. gallisepticum-free and Mycoplasma synoviae-free commercial Tetra SL layer chickens. Sixty birds were distributed into four groups: 15 were unvaccinated but were challenged with M. gallisepticum R-strain, 15 were vaccinated by eye drop and then challenged with virulent M. gallisepticum R-strain 4 weeks post vaccination, 15 were designated as controls without vaccination and challenge, and 15 received TS-11 vaccine but no challenge. Based on the post-challenge clinical signs, body weight gain, gross pathological examination of air sacs and peritoneum, histological examination of the trachea, lung, spleen and liver, and reisolation of mycoplasmas from inner organs, the TS-11 vaccine is safe and does not produce clinical signs, a major decrease of body weight gain or pathological lesions. Vaccination induced a slight serological response to M. gallisepticum antigen in serum plate agglutination and blocking enzyme-linked immunosorbent assay tests and prevented development clinical signs of airsacculitis, peribronchitis and interstitial pneumonia on M. gallisepticum challenge.
Innocuité et efficacité du vaccin Mycoplasma gallisepticum TS-11 pour la protection des futures poules pondeuses vis-à-vis d'une épreuve avec la souche virulente Mycoplasma gallisepticum R
L'innocuité et l'efficacité du vaccin Mycoplasma gallisepticum (MG) TS-11 ont été étudiées chez des futures pondeuses commerciales Tetra SL, indemnes de Mycoplasma gallisepticum et M. synoviae, et âgées de 49 jours. Soixante animaux ont été répartis en quatre groupes : 15 non vaccinés, éprouvés avec la souche MG R; 15 vaccinés par instillation oculaire, éprouvés MG R, 4 semaines après la vaccination; 15 témoins non vaccinés non éprouvés; et 15 vaccinés TS-11, non éprouvés. En se basant sur les symptômes après épreuve, les gains de poids, les lésions macroscopiques des sacs aériens et du péritoine, les lésions histologiques de la trachée, des poumons, de la rate et du foie, et le réisolement des mycoplasmes des organes internes, le vaccin TS 11 n'a pas entraîné de symptômes, de diminution importante des gains de poids ou de lésions pathologiques. La vaccination a induit une réponse sérologique faible vis-à-vis de l'antigène MG détectée par les tests d'agglutination rapide sur lame et ELISA de blocage et a empêché le développement de symptômes d'aérosacculite, de péribronchite et de pneumonie interstitielle après l'épreuve avec MG.
Sicherheit und Wirksamkeit der Mycoplasma gallisepticum-TS-11-Vakzine zum Schutz von Legehennenküken gegen eine Belastungsinfektion mit virulentem Mykoplasma galliseptikum-R-Stamm
Die Mycoplasma gallisepticum (MG)-TS-11-Vakzine wurde hinsichtlich ihrer Sicherheit und ihrer Schutzwirkung in 49-Tage-alten Mycoplasma gallisepticum- und Mycoplsma synoviae-freien kommerziellen Tetra SL-Legehennenküken untersucht. 60 Küken wurden in vier Gruppen unterteilt: 15 blieben unvakziniert, wurden aber mit dem MG-R-Stamm infiziert, 15 wurden mittels Augentropfmethode vakziniert und 4 Wochen später mit dem virulenten MG-R-Stamm belastungsinfiziert, 15 Küken ohne Impfung und Belastungsinfektion dienten als Kontrolltiere und 15 erhielten nur den TS-11-Impfstoff aber keine Belastungsinfektion. Aufgrund der klinischen Untersuchung nach der Belastungsinfektion, der Gewichtsbestimmung, der pathomorphologischen Untersuchung von Luftsäcken und Peritoneum, der pathohistologischen Untersuchung von Trachea, Lunge, Milz und Leber sowie der Reisolierungsversuche von Mykoplasmen aus den inneren Organen kann die TS-11-Vakzine als sicher bezeichnet werden. Sie ruft keine klinischen Symptome, keine bedeutsamen Gewichtsverluste oder pathologische Veränderungen hervor. Die Vakzination induziert eine geringe serologische Immunantwort gegen MG-Antigen in der SPA und im Blocking-ELISA und verhindert die klinischen Symptome von Aerosacculitis, Peribronchitis und interstitieller Pneumonie durch eine Belastungsinfektion.
Seguridad y eficacia de una vacuna TS-11 de Mycoplasma gallisepticum para proteger a pollitas de ponedora frente a un desafío con la cepa virulenta R de Mycoplasma gallisepticum
Se estudió la seguridad y el efecto protector de la vacuna TS-11 de Micoplasma gallisepticum (Mg) en pollitas de ponedoras comerciales Tetra SL de 49 días de vida que eran libres de Mycoplasma gallisepticum y M. synoviae. Se distribuyeron sesenta aves en cuatro grupos: 15 no fueron vacunadas pero sí desafiadas con la cepa R de Mg, 15 fueron vacunadas mediante gota al ojo y posteriormente fueron desafiadas con la cepa R de Mg a las 4 semanas post-vacunación, 15 fueron destinadas a controles no vacunados ni desafiados y otras 15 recibieron la vacuna TS-11, pero no fueron desafiadas. En base a los signos clínicos tras el desafío, la ganancia diaria de peso, el examen anatomopatológico macroscópico de sacos aéreos y peritoneo, el examen histológico de la tráquea, pulmón, bazo e hígado y el reaislamiento de micoplasma a partir de órganos internos, la vacuna TS-11 es segura y no produce signos clínicos, ni una disminución marcada de la ganancia de peso ni lesiones evidentes. La vacunación indujo un respuesta serológica leve al antígeno de Mg en las técnicas de SPA y ELISA de bloqueo y previno el desarrollo de signos clínicos de aerosaculitis, peribronquitis y neumonía intersticial tras el desafío con Mg.
Introduction
Mycoplasma gallisepticum is a major pathogen of poultry worldwide. It causes disease of the respiratory tract, increased mortality, and reduced growth rate. Mycoplasmal salpingitis (Domermuth & Gross, Citation1962) and damage to the shell-producing mechanism (Domermuth et al., Citation1967) may be involved in egg production losses (Ley & Yoder, Citation1997; Kleven, Citation1998; Levisohn & Kleven, Citation2000). M. gallisepticum also leads to deterioration of egg quality (changes in distribution of eggs according to weight and size, egg-shell strength, etc.) (Pruthi & Karole, Citation1981). Mycoplasma infection causes especially severe economic losses in commercial layer flocks (Carpenter et al., Citation1981; Mohammed et al., Citation1987), which generally consist of a huge number of hens housed on individual farms where it is practically impossible to achieve eradication of mycoplasma infection by total depopulation.
Since infected birds laying eggs for human consumption cannot be treated with antibiotics (Anonymous, Citation2001), efforts to reduce egg production losses of layer chickens associated with M. gallisepticum infection resulted in the approval and licensing of three currently available live M. gallisepticum vaccines in the USA a few years ago: FVAX-MG® (Schering-Plough Animal Health, Omaha, Nebraska, USA) (F strain), MYCOVAC® (Intervet Inc., Millsboro, Delaware, USA) (6/85 strain) and Mycoplasma gallisepticum vaccine® (Merial Animal Health, Gainesville, Georgia, USA) (TS-11 strain).
It is important that several questions about the use of these vaccines are clarified for the poultry industry. The issues include pathogenicity (Whithear et al., Citation1990a) and the ability to give protection against virulent strains (Whithear et al., Citation1990b; Barbour et al., Citation2000). Monitoring vaccinated flocks for serological response to vaccination is also a problem because of the low level of antibodies. This appears to have been solved for the TS-11 vaccine by the development of an indirect enzyme-linked immunosorbent assay (ELISA) using monoclonal antibody-purified native pMGA for M. gallisepticum TS-11 strain as coating antigen (Noormohammadi et al., Citation2002a).
The aims of this study were to evaluate the safety and efficacy of the TS-11 M. gallisepticum vaccine in 49-day-old layer pullets against challenge with M. gallisepticum R-strain and to study the serological response of vaccinated birds.
Materials and Methods
Chickens
M. gallisepticum-free and M. synoviae-free commercial Tetra SL layers (Bábolna Agriculture Company) were used. They originated from parent stock that was regularly monitored serologically for mycoplasma infection using stained M. gallisepticum and M. synoviae antigens (Nobilis, Intervet International B.V., Boxmeer, The Netherlands) for the serum plate agglutination (SPA) test. ELISAs were also performed to check for M. gallisepticum and M. synoviae antibodies using MyGa (Czifra et al., Citation1993) and MySy (Zohari et al., Citation1997) test kits (Diagnosticum Rt., Budapest, Hungary). Serological results were negative in all cases. Chicks were obtained at 1 day old and reared in isolation until 49 days of age. From the same hatch an additional 15 1-day-old chicks were slaughtered and examined for pathological lesions characteristic of M. gallisepticum and M. synoviae. Attempts were made to isolate mycoplasmas on medium B (Ernø & Stipkovits, Citation1973) and Frey's medium (Frey et al., Citation1968) or to detect them by polymerase chain reaction (Kempf et al., Citation1993; Lauerman et al., Citation1993) from the trachea, lung and air sac. All tests gave negative results and sera from these chicks were negative for M. gallisepticum and M. synoviae antibodies in the ELISA test.
M. gallisepticum vaccination and challenge
At 49 days (day 0 of the experiment) the chickens were labelled individually in both wings with coloured and numbered wing-tags and their body weight was recorded. They were then distributed into four groups of 15 such that the groups did not differ from each other on average body weight. Each group was placed in a separate room and tended by a different operator. The birds received standard feed and drinking water ad libitum without any antibacterial drugs. Group 1 (G1) was not vaccinated but was challenged (see later) at 77 days of age (day 28 of the experiment). Group 2 (G2) was vaccinated with M. gallisepticum TS-11 at 49 days of age (day 0) and challenged at 77 days. Group 3 (G3) was left unvaccinated and unchallenged as the negative control. Group 4 (G4) was vaccinated as for G2 but unchallenged. Vaccination was performed by eye drop (0.03 ml/chicken) according to the manufacturer's recommendations [Merial Select Inc., Gainesville, Georgia, USA; batch number MA 332, list number TSF-1110, expiry date 12 February 2003, US Vet. Licence number 279, presentation 30 ml (1000 doses) per bottle]. The titre of the vaccine was 4×108 colony-forming units (CFU)/ml. For challenge a 48-h-old broth culture of M. gallisepticum R-strain (3×108 CFU/ml) was used. The chickens were placed into a 220-l box and 10 ml broth culture was sprayed onto them for 100 sec and the total exposure was 20 min. The droplet size was approximately 10 nm.
Evaluation of safety and efficacy of vaccination
Clinical examination
Chickens were observed for clinical signs daily from experimental days 0 to 39. Attention was paid to the development of swelling of the facial skin and eyelids, increase of lacrymation, and congestion of the conjunctival vessels, and respiratory râles. All these signs were scored from 0 to 2 and recorded. The chickens were weighed before vaccination (day 0), 4 weeks after vaccination before challenge (day 28), and on day 38.
Serology
Serological examination was performed by the SPA test using M. gallisepticum stained antigen (batch number 650-1001, expiry date March 2003) and M. synoviae antigen (batch number 71232A, expiry date July 2003) as well as by blocking ELISA with MyGa and MySy kits (batch number 020114, expiry date September 2002 and batch number 020506, expiry date September 2002, respectively). The tests were conducted 14 days and 1 day before vaccination, and on day 28 (before challenge) and day 38. The slide agglutination reactions were scored from 0 to 3.
Gross and histological lesions
On day 40 chickens were euthanized (using sodium-pentobarbital) and the thoracic air sacs and peritoneum were examined for lesions characteristic of M. gallisepticum infection. Lesions were scored from 0 to 4 for left and right thoracic air sacs and peritoneum separately. The maximum score was thus 12/bird. Tracheas, lungs, spleens and livers of chickens were examined histologically following fixation in buffered formalin and staining with haematoxylin and eosin. The thickness (in micrometers) of the tracheal mucous membrane infiltrated by lymphocytes, histiocytes and plasma cells was recorded. Characteristic lesions of the lungs (interstitial pneumonia, lympho-histiocytic and plasmacytic infiltration around bronchi and bronchioles and the presence of catarrhal pneumonia) were scored from 0 to 3. In the liver and spleen, activation of lymphofollicular foci was evaluated from 0 to 3.
Culture and identification of mycoplasma
At the end of the experiment an attempt was made to isolate M. gallisepticum by swabbing the trachea, lungs, air sacs, liver, spleen, kidney and heart of all chickens using medium B (Ernø & Stipkovits, Citation1973) following a standard procedure (Bradbury, Citation1998a). The swabs were plated onto solid medium B and inoculated into 2 ml fluid medium B and incubated in a CO2 incubator at 37°C. Broth cultures were assessed for colour change daily for 7 days, and those showing change were immediately plated onto agar while any remaining broth cultures were plated on day 7. Plates were observed for mycoplasma growth daily for 7 days and then at weekly intervals for a maximum of 3 weeks. Colonies were routinely identified by immunofluorescence (Bradbury, Citation1998b) using anti M. gallisepticum hyperimmune serum prepared in rabbits. Nine isolates from the air sac were tested by polymerase chain reaction according to Liu et al. (Citation2001). The amplicons were digested by AccI restriction endonuclease.
Statistical analysis
Scores of the SPA tests, gross lesions in the air sacs and peritoneum, and the histological lesions of interstitial pneumonia, peribronchitis and catarrhal pneumonia, follicular reactions in the spleen and liver, and isolation rates per groups together describe the state of health of the chickens. Therefore a similarity pattern analysis of different groups was performed by multivar statistics (hierarchical classification and non-metric multidimensional scaling) using Past 0.45 software (Shepard, Citation1962; Kruskal, Citation1964; Podani, Citation1994; Bíró, Citation2003; Rédei et al., Citation2003 Citation2004; Shokri et al., Citation2004). The reason for applying both hierarchical clustering and non-metric multidimensional scaling was to confirm the results obtained by both methods. Average body weight gains and thickness of the tracheal mucous membrane of the groups were analysed by analysis of variance (ANOVA) or by Tukey's pairwise comparisons test. M. gallisepticum ELISA results could be positive, negative or suspicious. Fifteen chickens per group were analysed by M. gallisepticum ELISA, and therefore we compared the frequency of distribution of chickens in these categories expressed by chi-square test.
Results
Clinical signs
After the application of TS-11 vaccine, no clinical signs were recorded during the period from day 0 to day 28 in any of the groups. After challenge on day 28, in G1 (non-vaccinated, challenged) slight respiratory râles were recorded in two birds on day 38 and in seven birds on day 39. In G2 (vaccinated, challenged) only one chicken had respiratory râles on day 39. No signs were recorded in G3 and G4.
Body weight gain
The average body weight gains of different groups of chickens between the day of vaccination (day 0) and the day 38 are shown in . Variance analysis of body weight gain is presented in . In the ANOVA test the mean of the all groups was 1.08, the significant difference at P=0.05 and P=0.1 was 0.085 and 0.070, respectively, the coefficient of variation of errors was 10.11%, the F value was 11.62 at P=4.9×10−6 and the Levene's test for homogeneity of variance was P=0.358. Relative weight gains between day 0 and day 38 are presented in . A significant difference was recorded in the G1 (non-vaccinated, challenged group) comparison with the mean of all groups and the other three groups (P=0.05). A significant difference was seen between G4 (vaccinated, non-challenged) and G3 (non-vaccinated, non-challenged) at P=0.1 level, but no difference was observed between groups G2 and G4.
Figure 1. Average body weight gain (g) of different groups of chickens between the day of vaccination (day 0) and day 38. G1, unvaccinated at day 0, challenged (virulent M. gallisepticum) at day 28; G2, vaccinated (TS-11) at day 0, challenged (virulent M. gallisepticum) at day 28; G3, unvaccinated at day 0, unchallenged at day 28; G4, vaccinated (TS-11) at day 0, unchallenged at day 28.
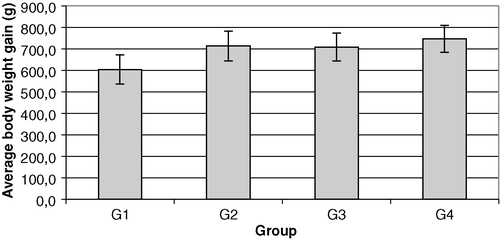
Table 1. Table of variance of relative body weight gain of different groups of chickens between the day of vaccination (day 0) and day 38
Table 2. Relative body weight gain of different groups of chickens between the day of vaccination (day 0) and day 38
Gross lesions
In G1 (non-vaccinated, challenged) six birds showed accumulation of mucus in the paranasal passages, 11 had lesions in the trachea, 14 had airsacculitis, seven had pneumonia, two birds had perihepatitis and one bird had pericarditis. At the same time no exudate in nasal passages, no conjunctivitis and no sinusitis were recorded in G2 (vaccinated, challenged). Only one bird showed slight tracheitis and four showed slight airsacculitis in this group. The lesion score of airsacculitis was only 4 in comparison with a score of 23 in G1. No pathological lesions were recorded in G3 and only one chicken had slight lesions in the trachea in G4 ().
Table 3. Pathological lesions of different groups of chickens on day 40
Histological lesions
After challenge the mucosa of the trachea became very thick in most chickens in G1 ( and ). In addition to this bronchitis, interstitial pneumonia and catarrhal pneumonia developed with scores of 25, 27 and 11, respectively (). Also, in G1 15 birds showed lympho-histiocytic infiltration in the liver and spleen, with scores of 26 and 30, respectively. In the other three groups the number of birds showing bronchitis, peribronchitis, interstitial and catarrhal pneumonia, and lympho-histiocytic infiltration in the liver and spleen and the scores of these lesions were low.
Figure 2. Trachea of a chick challenged by aerosol with M. gallisepticum R-strain. The mucous membrane of the trachea is markedly thickened and infiltrated by lymphocytes, histiocytes and plasma cells. Haematoxylin and eosin staining. Bar marker = 30 µm.
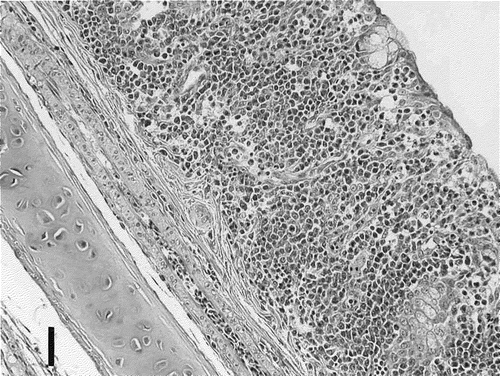
Figure 3. Trachea of a non-challenged control chick. The mucous membrane of the trachea is thin and shows no histological lesions. Haematoxylin and eosin staining. Bar marker = 30 µm.
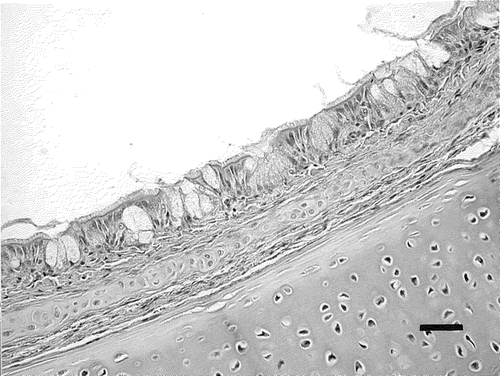
Table 4. Additive scores of histological examinations of lung, spleen and liver of chickens on day 40
The average thicknesses of tracheal mucosa in G1 (non-vaccinated, challenged), G2 (vaccinated, challenged), G3 (non-vaccinated, non-challenged) and G4 (vaccinated, non-challenged) are presented in . By analysing the variance within groups by Levene's homogeneity test, it was established that the data were not homogeneous and therefore ANOVA could not be applied; we therefore performed Tukey's pairwise comparisons. As shown in , significant difference was observed between G1 and the other three groups while no significant difference was seen between G4 (only vaccinated) and G3 (non-vaccinated and non-challenged).
Figure 4. Average tracheal mucosa thickness of different groups of chickens on day 40. G1–G4, see caption to .
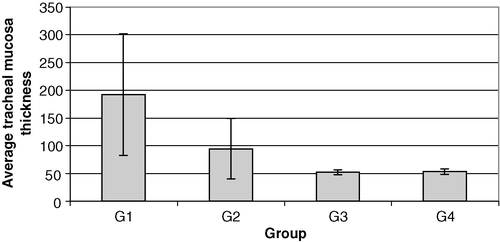
Table 5. Difference in thickness of tracheal mucosa between groups of chickens on day 40 using Tukey's pairwise comparisons
M. gallisepticum isolation
Results of attempts to isolate M. gallisepticum are presented in . In G1 (non-vaccinated, challenged) M. gallisepticum was isolated from 30 out of 45 samples taken from the respiratory tract and from 13 out of 60 samples taken from other organs. Mycoplasma isolation from G2 birds (vaccinated and challenged) was made from eight out of 45 samples from respiratory tract, but not from other organs (liver, spleen, kidney and heart). No mycoplasmas could be detected in chickens in G3 (non-vaccinated unchallenged) or from those in G4 (vaccinated, unchallenged).
Table 6. Mycoplasma reisolation from various organs of chickens at day 40
All isolates tested by immunofluorescence proved to be M. gallisepticum. Eight isolates cultured from the air sac of chickens from G2 (vaccinated, challenged) were confirmed as M. gallisepticum by polymerase chain reaction; however, digestion of amplicons with AccI produced three fragments (231, 156 and 110 base pairs [bp]) characteristic for M. gallisepticum R strains rather than four fragments (341, 231, 156 and 110 bp) that would indicate a mixture of strains R and TS-11 or two (341 and 156 bp) fragments that would indicate fragments of TS-11.
Serology
When tested by SPA 14 days and 1 day before vaccination all chickens were negative for antibodies to M. gallisepticum and M. synoviae. On day 28 (before challenge), 12 and 11 of 15 vaccinated birds in G2 and G4, respectively, had a weak serological response to M. gallisepticum, with a SPA score of 16 and 13 respectively, to M. gallisepticum antigen, compared with 0 in G1 (). After challenge, all chickens in groups G1 and G2 reacted. Group G3 (non-vaccinated, non-challenged) remained negative throughout the experiment. Fourteen of 15 chickens in G4 had produced a slight serological response at 38 days.
Table 7. M. gallisepticum SPA test result of different groups of chickens on days 28 and 38
In the blocking ELISA, all groups were negative 14 days and 1 day before vaccination, while after vaccination some birds in groups G2 and G4 showed suspicious reactions on day 28 (). The number of reactors in G4 had increased by day 38, as had those in G1 and G2 after challenge, while the G3 (non-vaccinated, non-challenged) group did not show any positive reactors. There were statistical differences between G1 and G2 (P=0.016), between G1 and G3 (P=0.028) and between G3 and G4 (P=0.006) at the 95% level based on the number of positive, suspicious and negative reactors ().
Table 8. M. gallisepticum ELISA results of groups of chickens 14 and on day before vaccination and at days 28 and 38
Table 9. Statistical analysis of M. gallisepticum ELISA results of groups of chicken at day 38 using the chi-square test (n=15, degrees of freedom = 2)
All groups were negative by SPA for M. synoviae antibodies on each sampling day except on day 38 when 11 and six birds in challenged groups G1 and G2, respectively, showed weak positive reactions to M. synoviae, but with very low scores.
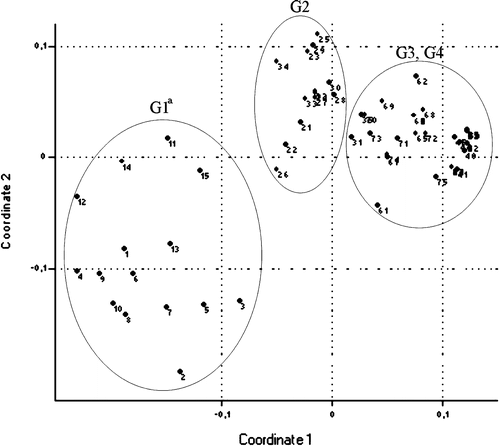
Multivar statistics were performed based on scores of slide agglutinations, gross lesions in the air sacs and peritoneum, histological lesions of interstitial pneumonia, peribronchitis and catarrhal pneumonia, follicular reactions in the spleen and liver, and isolation rates. Based on hierarchical clustering () the groups differ from each other (G1 from animal number 1 to number 15, G2 from animal number 21 to animal number 35, G3 from animal number 41 to animal number 55 and G4 from animal number 61 to animal number 75). Using hierarchical clustering the bird with number 70 from G4 belonged to G2, and birds with numbers 65, 72 and 75 from G4 belonged to G3. Using non-metric multidimensional scaling (Shepard, Citation1962; Kruskal, Citation1964; Podani, Citation1994) (), G3 (non-vaccinated, non-challenged) and G4 (vaccinated, non-challenged) did not differ from each other; however, G1 (non-vaccinated, challenged) and G2 (vaccinated, challenged) were different from each other and from G3 and G4.
Figure 5. Result of hierarchical classification (cluster analysis, euclidean distance) of groups of chickens based on scores of slide agglutinations, gross lesions in the air sacs and peritoneum, the histological lesions of interstitial pneumonia, peribronchitis and catarrhal pneumonia, follicular reactions in the spleen and liver and isolation rates. G1 from animal number 1 to number 15, G2 from animal number 21 to number 35, G3 from animal number 41 to number 55, and G4 from animal number 61 to number 75. G1–G4, see caption to .
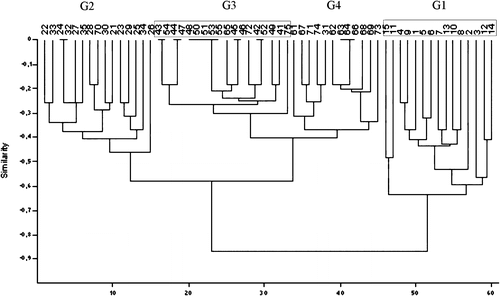
Figure 6. Result of non-metric multi-dimensional scaling (euclidean distance) of groups of chickens based on scores of slide agglutinations, gross lesions in the air sacs and peritoneum, the histological lesions of interstitial pneumonia, peribronchitis and catarrhal pneumonia, follicular reactions in the spleen and liver and isolation rates. G1 from animal number 1 to number 15, G2 from animal number 21 to number 35, G3 from animal number 41 to number 55, and G4 from animal number 61 to number 75. G1–G4, see caption to .
Discussion
After immunizing M. gallisepticum-free and M. synoviae-free chickens with the TS-11 vaccine administered by eye drop, no obvious clinical signs were noted for the 38-day period of observation. Application of the vaccine induced a slight serological response to M. gallisepticum antigen in SPA and blocking ELISA tests, which was observed until the end of the experiment. Vaccination of chickens did not decrease the body weight gain. At postmortem examination of the vaccinated, non-challenged group, very slight airsacculitis was noted in four out of 15 birds, and one bird had slight tracheal inflammation. No differences were observed in the number of chickens affected by M. gallisepticum and the lesion scores (of peribronchitis, interstitial pneumonia, catarrhal pneumonia, and cell reaction of spleen and liver) between G4 (vaccinated, non-challenged) and G3 (non-vaccinated, non-challenged) groups. Only the tracheal mucosa showed increased thickness in comparison with the G3. Based on these data it can be concluded that the TS-11 vaccine is safe for chickens. These results are in agreement with those obtained by Soeripto et al. (Citation1989), Whithear et al. (Citation1990a) and Whithear (Citation1996).
During the 10 days observation following challenge, no important clinical signs due to challenge were noted in G1 (non-vaccinated, challenged), except for mild respiratory râles in some chickens. However, at postmortem examination on day 40 almost all birds in this group showed airsacculitis with lesion scores, and about one-half of them also had tracheitis and pneumonia. Histological examination revealed that almost all birds had bronchitis, peribronchitis, interstitial pneumonia and catarrhal pneumonia with lesion scores. Body weight gain was significantly reduced after challenge. The mucosa of the trachea became approximately four times thicker than that in group G3 (non-vaccinated, non-challenged). Lympho-histiocytic infiltration was observed in the liver as well. All birds became serologically positive to M. gallisepticum and some also showed a slight reaction to M. synoviae antigen.
No respiratory râles or other respiratory signs were recorded in the G2 birds (vaccinated, challenged) after challenge, with the exception of one bird. There was a reduction in the number of birds with tracheitis, airsacculitis, bronchitis, peribronchitis, interstitial pneumonia, catarrhal pneumonia and lympho-histiocytic infiltration in the liver in this group compared with G1 (non-vaccinated, challenged). The thickness of the tracheal mucosa was also markedly less in the vaccinated group. Vaccination also protected the chickens from reduction of body weight gain in comparison with G1 (non-vaccinated challenged). Vaccination completely prevented the colonization of the spleen and liver by mycoplasmas and reduced the presence of mycoplasmas in the respiratory tract. These data support the protective property of the vaccine shown by Whithear et al. (Citation1990a), Turner & Kleven (Citation1998), Barbour et al. (Citation2000) and Branton et al. (Citation2000).
From the data of the experiment presented in this paper it is clear that the application of the TS-11 vaccine induced a weak serological response to M. gallisepticum, which could be detected by both SPA and blocking ELISA. In this respect our data differ from the results obtained by Barbour et al. (Citation2000) and Whithear et al. (Citation1990a Citationb). However, our findings are consistent with the recent results of Noormohammadi et al. (Citation2002b) about the development of antibody after vaccination. This discrepancy could possibly be associated with the actual number of viable TS-11 organisms received by the birds, since a dose-dependent antibody response to the vaccine has already been observed (Whithear et al., Citation1990a Citationb). In the present study the viable cell count of the vaccine was 3×103 CFU/ml just before application. Another explanation might be that other authors used an indirect ELISA while we employed a blocking ELISA, which has been shown to detect antibody responses due to variant M. gallisepticum strains (Czifra et al., Citation1995). In our study we did not determine how long antibody could be detected after vaccination, and in future it would be useful to determine this. Since the serological response was relatively weak compared with that observed in G1 or G2, it can be supposed that natural infection would result in a strong reaction.
Mycoplasmas could be isolated from the respiratory tract of chickens of the vaccinated, challenged group. The vaccine clearly prevented the invasion of mycoplasmas into the liver and spleen of vaccinated chickens, while M. gallisepticum could be reisolated from 13 out of 60 samples originating from the liver, kidney, spleen and heart of the non-vaccinated challenged birds.
Translations of the abstract in French, German and Spanish are available on the Avian Patholgy website.
References
- Anonymous (2001) . Feed Additive Compendium , Minnetonka, MN : Miller Publishing Co
- Barbour , E.K. , Hamadeh , S.K. and Eidt , A. 2000 . Infection and immunity in broiler chicken breeders vaccinated with a temperature-sensitive mutant of Mycoplasma gallisepticum and impact on performance of offspring . Poultry Science , 79 : 1730 – 1735 .
- Bíró , J. 2003 . Temporal–spatial pattern of true bug assemblies (Heteroptera: Gerromorpha, Nepomorpha) in Lake Balaton . Applied Ecology and Environmental Research , 1 : 173 – 181 .
- Bradbury , J.M. 1998a . “ Recovery of mycoplasmas from birds ” . In Mycoplasma Protocols , 1st edn , Edited by: Miles , R. and Nicholas , R. 45 – 51 . Totowa, NJ : Humana Press .
- Bradbury , J.M. 1998b . “ Identification of mycoplasmas by immunofluorescence ” . In Mycoplasma Protocols , 1st edn , Edited by: Miles , R. and Nicholas , R. 119 – 125 . Totowa, NJ : Humana Press .
- Branton , S.L. , Lott , B.D. , May , J.D. , Maslin , W.R. , Pharr , G.T. , Bearson , S.D. , Collier , S.D. and Boykin , D.L. 2000 . The effects of ts-11 strain Mycoplasma gallisepticum vaccination in commercial layers on egg production and selected egg quality parameters . Avian Diseases , 44 : 618 – 623 .
- Carpenter , T.E. , Mallinson , E.T. , Miller , K.F. , Gentry , R.F. and Schwartz , L.D. 1981 . Vaccination with F-strain Mycoplasma gallisepticum to reduce production losses in layer chickens . Avian Diseases , 25 : 404 – 409 .
- Czifra , Gy. , Tuboly , T. , Sundquist , B.G. and Stipkovits , L. 1993 . Monoclonal antibodies to Mycoplasma gallisepticum membrane proteins . Avian Diseases , 37 : 689 – 696 .
- Czifra , Gy. , Kleven , S.H. , Engström , B. and Stipkovits , L. 1995 . Detection of specific antibodies directed against a consistently expressed surface antigen of Mycoplasma gallisepticum using monoclonal blocking enzyme-linked immunosorbent assay . Avian Diseases , 39 : 28 – 31 .
- Domermuth , C.H. and Gross , W.B. 1962 . The production of salpingitis of chickens by Mycoplasma gallisepticum . Avian Diseases , 6 : 499 – 505 .
- Domermuth , C.H. , Gross , W.B. and Dubose , R.T. 1967 . Mycoplasmal salpingitis of chickens and turkeys . Avian Diseases , 11 : 393 – 398 .
- Ernø , H. and Stipkovits , L. 1973 . Bovine mycoplasmas: cultural and biochemical studies I . Acta Veterinaria Scandinavica , 14 : 436 – 449 .
- Frey , M.L. , Hanson , R.P. and Anderson , D.P. 1968 . A medium for the isolation of avian mycoplasmas . American Journal of Veterinary Research , 29 : 2163 – 2171 .
- Kempf , I. , Blanchard , A. , Gesbert , F. , Guittet , M. and Bennejean , G. 1993 . The polymerase chain reaction for Mycoplasma gallisepticum detection . Avian Pathology , 22 : 739 – 750 .
- Kleven , S.H. 1998 . Mycoplasmas in the etiology of multifactorial respiratory disease . Poultry Science , 77 : 1146 – 1149 .
- Kruskal , J.B. 1964 . Nonmetric multidimensional scaling: a numerical method . Psychometrica , 29 : 115 – 129 .
- Lauerman , L.H. , Hoerr , F.J. , Sharpton , A.R. , Shah , S.M. and van Santen , V.L. 1993 . Development and application of a polymerase chain reaction assay for Mycoplasma synoviae . Avian Diseases , 37 : 829 – 834 .
- Levisohn , S. and Kleven , S.H. 2000 . Avian mycoplasmosis (Mycoplasma gallisepticum) . Revue Scientifique et Technique , 19 : 425 – 442 .
- Ley , D.H. and Yoder , H.W. Jr . 1997 . “ Mycoplasma gallisepticum infection ” . In Diseases of Poultry , 9th edn , Edited by: Calnek , B.W. , Barnes , H.J. , Beard , C.W. , Reid , W.M. and Yoder , H.W. 194 – 207 . Ames : Iowa State University Press .
- Liu , T. , García , M. , Levisohn , S. , Yogev , D. and Kleven , S.H. 2001 . Molecular variability of the adhesin-encoding gene pvpA among Mycoplasma gallisepticum strains and its application in diagnosis . Journal of Clinical Microbiology , 39 : 1882 – 1888 .
- Mohammed , H.O. , Carpenter , T.E. and Yamamoto , R. 1987 . Economic impact of MG and MS in commercial layer flocks . Avian Diseases , 31 : 477 – 489 .
- Noormohammadi , A.H. , Browning , G.F. , Cowling , P.J. , O'Rourke , D. , Whithear , K.G. and Markham , P.F. 2002a . Detection of antibodies to Mycoplasma gallisepticum vaccine ts-11 by an autologous pMGA enzyme-linked immunosorbent assay . Avian Diseases , 46 : 405 – 411 .
- Noormohammadi , A.H. , Jones , J.E. , Underwood , G. and Whithear , K.G. 2002b . Poor systemic antibody response after vaccination of commercial broiler breeders with Mycoplasma gallisepticum vaccine ts-11 not associated with susceptibility to challenge . Avian Diseases , 46 : 623 – 628 .
- Podani , J. 1994 . Multivariate Data Analysis in Ecology and Systematics , The Hague : SPB Publishing .
- Pruthi , A.K. and Kharole , M.U. 1981 . Sequential pathology of genital tract in chickens experimentally infected with Mycoplasma gallisepticum . Avian Diseases , 25 : 768 – 778 .
- Shepard , R.N. (1962) . The analysis of proximites: multidimensional scaling with an unknown distance function. I-II Psychometrika , 27 , 125 – 140 , 219–246 .
- Rédei , D. , Gaál , M. and Hufnagel , L. 2003 . Spatial and temporal patterns of true bug assemblages extracted with berlese funnels . Ecology and Environmental Research , 1 : 115 – 142 .
- Rédei , D. , Harmat , B. and Hufnagel , L. 2004 . Ecology of the acalypta species occuring in Hungary (Insecta: Heteroptera: Tingidae) . Ecology and Environmental Research , 2 : 73 – 91 .
- Shokri , M. , Safaian , N. , Ahmadi , M.Z.T. and Amiri , B.J. 2004 . A second look on biogeographical province of Miankaleh Biosphere Reserve . Ecology and Environmental Research , 2 : 105 – 117 .
- Soeripto , Whithear , K.G. , Cottew , G.S. & Harrigan , K.E. (1989) . Virulence and transmissibility of Mycoplasma gallisepticum . Australian Veterinary Journal , 66 , 65 – 72 .
- Turner , K.S. and Kleven , S.H. 1998 . Eradication of live F strain Mycoplasma gallisepticum vaccine using live ts-11 on a multiage commercial layer farm . Avian Diseases , 42 : 404 – 407 .
- Whithear , K.G. 1996 . Control of avian mycoplasmas by vaccination . Review of Science Technology , 15 : 1527 – 1553 .
- Whithear , K.G. , Soeripto , Harrigan , K.E. and Ghiocas , E. 1990a . Safety of temperature sensitive mutant Mycoplasma gallisepticum vaccine . Australian Veterinary Journal , 67 : 159 – 165 .
- Whithear , K.G. , Soeripto , Harrigan , K.E. and Ghiocas , E. 1990b . Immunogenicity of temperature sensitive mutant Mycoplasma gallisepticum vaccine . Australian Veterinary Journal , 67 : 168 – 174 .
- Zohari , S. , Czifra , G. , Kempf , I. , Kaszanyitzky , É. , Rásky , K. & Engström , B.E. (1997) Mycoplasma synoviae-specific monoclonal antibody blocking enzyme-linked immunosorbent assay . In Proceedings of the XIth International Congress of the World Veterinary Poultry Association (p. 285 ). Budapest , Hungary .